Abstract
New approaches for cardiac repair have been enabled by the discovery that the heart contains its own reservoir of stem cells. These cells are positive for various stem/progenitor cell markers, are self-renewing, and exhibit multilineage differentiation potential. Recently we developed a method for ex vivo expansion of cardiac-derived stem cells from human myocardial biopsies with a view to subsequent autologous transplantation for myocardial regeneration. Here we review the state of the cardiac stem cell field and our own work on cardiosphere-derived stem cells from human hearts. The first of this two-part review outlines emerging preclinical data on the application of cardiac stem cells. Part 2 continues with a discussion of other stem cell sources with clinical potential, a summary of the critical issues surrounding stem cell therapy (with an emphasis on the crucial issue of how cell transplantation may influence arrhythmias), our perception of clinical stem cell trials to date, and the issues facing the clinical application of cardiac stem cells.
Keywords: Cardiac stem cells, Biopsy, Myocardial infarction, Myocardial regeneration
Introduction
This article summarizes the background and content of the Gordon Moe Lecture, which the senior author was honored to present to the Cardiac Electrophysiology Society meeting in November 2005. Much of the work presented at the time was unpublished, hence the delay from that event to the present summary. Even in that interval, the field of cardiac regenerative therapy has advanced in leaps and bounds. What we present here is but a snapshot of this rapidly emerging discipline.
The mammalian heart historically has been viewed as a terminally differentiated organ, formed of anisotropically oriented cardiomyocytes that allow for the conduction of electrical signals and the rhythmic pumping of blood. Conventional wisdom long maintained that, under normal conditions, an adult mammalian cardiomyocyte could not reenter the cell cycle and duplicate or replace itself. Therefore, cardiomyocytes were believed to be subject to decades of use and potential injury with no hope of reprieve. A straightforward quantitative analysis of cell number and organ volume led Quaini et al to undertake a thorough reinvestigation of the cardiomyocyte’s ability for proliferation. In 1994, they demonstrated what appeared to be human cardiomyocytes undergoing mitosis in histologic sections of endomyocardial biopsies obtained from patients with chronic heart failure.1 Cardiomyocytes in the regions surrounding damaged, necrotic areas were identified as mitotic by coexpression of proliferating cell nuclear antigen and α-sarcomeric actin. In a subsequent study, histologic sections of myocardium obtained from patients undergoing cardiac transplantation due to ischemic disease or dilated cardiomyopathy were labeled with propidium iodide and an α-sarcomeric actin antibody. A myocyte mitotic index of 0.0014% in control hearts and 0.013% to 0.015% in diseased hearts was determined by confocal analysis.2 Those initial data were confirmed and amplified in an autopsy study of human hearts3 in which actively dividing cardiomyocytes (evidenced by Ki67 expression, a nuclear antigen expressed in dividing cells4) resulted in mitotic indices of 0.08% and 0.03% in the regions adjacent to and distant from the infarcts, respectively. In principle, mitotic figures in the heart could arise from preformed cardiomyocytes released from cell cycle inhibition or from resident cardiomyocyte precursors or stem cells that become activated. So began the quest for a cardiomyocyte precursor. The question of whether mature mammalian cardiomyocytes can reenter the cell cycle has received less attention, although we have presented preliminary evidence for such “dedifferentiation” in vitro.5
Cardiac stem cell populations
Cardiac stem cells or progenitor cells have since been identified and isolated by several independent groups in adult or postnatal hearts of humans and other mammalian species based on surface expression of stem cell markers traditionally associated with blood- or bone marrow-derived stem cells and even pluripotent embryonic stem cells. Table 1 summarizes the biologic significance of the relevant cell surface markers. The biologic markers are listed horizontally across the top, with details for each (family, ligand, biologic function, and cell type where found) listed below. These various cardiac stem cell populations have each fulfilled one or more criteria of a stem or progenitor cell (multilineage potential, clonogenicity, capacity for self-renewal). Some have been defined by persistence of an antigenically identified population into postnatal or adult heart development, whereas others have been isolated either by antigenic selection for a given surface marker or by virtue of autoproliferative properties in primary culture. Here we review the features and regenerative potential of each proposed cardiac stem cell population. Consideration is given to potential effects of stem cell transplantation on heart rhythm. We review the properties of other stem cell sources being applied clinically for cardiac repair and conclude with a few forward-looking thoughts on the potential of cardiac stem cell therapy for the clinical treatment of heart disease.
Table 1.
Markers used to identify cardiac stem cells and their biologic functions in human
| SSEA-1 | Oct-3/4 | Isl-1 | c-Kit (CD117, SCFR) |
Sca-1 (Ly 6) |
MDR-1 (Abcb1, Pgp) |
Abcg2 (MXR1, BCRP) |
CD133 (prominin) |
CD90 (Thy-1) |
CD105 (endoglin) |
CD34 | CD31 (PECAM-1) |
CD45 (LCA) |
VEGFR2 (KDR, Flk-1) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Surface carbohydrate |
Homeodomain transcription factor |
LIM/HD proteins |
Tyrosine kinase receptor protein |
GPI-anchored cell surface protein |
ATP-binding cassette transporter |
ATP-binding cassette transporter |
Transmembrane glycoprotein |
GPI-anchored protein |
TGF-β type III receptor | Intercellular adhesion protein and cell surface glycoprotein |
Immunoglobulin superfamily |
Protein tyrosine phosphatase |
Tyrosine kinase receptor protein |
| Ligand | NA | NA | NA | Kit protein | NA | NA | NA | NA | β3 integrin | TGF-β | CD62L (L-selectin) | CD38 | ? | VEGF |
| Biologic function | ? | ? | ? | Tyrosine kinase activity | Modulates lipid raft composition; induces lymphocyte proliferation, myoblast differentiation, proliferation, fusion | Absorption, distribution, and elimination of xenobiotics and drugs | Elimination of xenobiotics and drugs | Specifically localizes to cellular protrusions? | May mediate differentiation of hematopoietic stem cells; mediates adhesion of various white blood cells to activated endothelial cells | Mediates cellular response to TGF-β1 | May mediate attachment of stem cells to bone marrow extracellular matrix or directly to stromal cells | May act as a minor histocompatibility antigen in HLA- identical stem cell transplantation; cell adhesion molecule that plays a key role in leukocyte trafficking across endothelium | An essential regulator of T-cell and B-cell antigen receptor-mediated activation | Regulatory in early vascular embryonic development |
| Positive Cells (normal) | Embryonic stem cell |
Embryonic stem cell |
Interstitial embryonic stem cell (mouse) | Interstitial cells of Cajal, hematopoietic progenitor cells, melanocytes, embryonic brain, endothelium, gonads, mast cells, breast epithelium, germ cells | Hematopoietic, mesenchymal, endothelial precursor/stem cells | Liver cells, kidney cells, gastrointestinal cells, blood-brain barrier cells | Placental syncytiotropho blasts, canalicular liver cells, intestinal epithelial cells, endothelial brain cells | Hematopoietic stem cells, endothelial progenitor cells, neuronal and glial stem cells | Monocytes, endothelial cells, erythroid precursors in marrow, syncytiotrophoblasts | Hematopoietic stem cells, neurons, connective tissue, activated endothelial cells, fibroblasts, keratinocyte stem cells | Dendritic interstitial cells, adult hematopoietic stem cells, neural cells | Hematopoietic cells except mature red blood cells, platelets megakaryocytes; dendritic cells, fibrocytes, thymus | Endothelial cells, platelets, Kupffer cells, T/NK cells, lymphocytes, megakaryocytes, fibroblasts, osteoclasts | Vascular endothelial cells |
? = unknown; ATP = adenosine triphosphate; GPI = glycosylphosphatidylinositol; HLA = human leukocyte antigen; NA = not applicable; NK = natural killer; TGF, transforming growth factor; VEGF = vascular endothelial growth factor.
c-Kit+ cardiac stem cells
The first report of the existence of seemingly primitive cardiac cells in histologic sections of the adult human heart emerged as part of a study of chimerism in the hearts of transplant patients.6 These small interstitial cells could be found expressing c-Kit, MDR-1, or Sca-1 and contained a subset that coexpressed markers of cardiac-specific differentiation (GATA4 or α-sarcomeric actin). These cell populations were markedly increased in transplanted human hearts, and only 12% to 16% could be identified as recipient derived (e.g., blood- or bone marrow-derived cells) by expression of the Y chromosome in males transplanted with female donor hearts. This evidence suggestive of a c-Kit+ resident cardiac stem cell population was explicitly reinforced shortly thereafter in a study of adult rat hearts.7 Analogous c-Kit+ cardiac stem cell populations have been identified in the adult dog8 and mouse,9 in which the majority were found to coexpress c-Kit, MDR-1, and Sca-1, suggesting an overlap in what some have described as distinct cardiac stem cell populations. The rat study first described clusters of c-Kit+ cells, many of which expressed early cardiac-specific transcription factors (Nkx2.5), characterized these cells as blood lineage negative (CD34−, CD45−, CD20−, CD45RO−, CD8−), and demonstrated that single cardiac c-Kit+ cells were self-renewing and clonogenic (expanded in culture after plating one cell per well) as well as multipotent (generating cardiomyocytes, smooth muscle cells, and endothelial cells when placed in differentiation medium containing dexamethasone). c-Kit+ cells were isolated from whole hearts subjected to total cellular digestion and represented approximately 7% of the “small” myocardial cell fraction. c-Kit+ cells were selected using an antibody against c-Kit by a magnetic-activated cell sorting technique and cultured in a base media supplemented with basic fibroblast growth factor, epidermal growth factor, leukemia inhibitory factor, and insulin-transferrin-selenite as a serum substitute. c-Kit+ cells (4 × 105 injected intramyocardially) showed a capacity for efficient myocardial repair when administered in a model of acute myocardial infarction (MI), maintaining improved left ventricular ejection fraction (LVEF) in rats 10 days and 3 weeks after treatment relative to untreated animals (LVEF 45% ± 10% treated vs LVEF 34% ± 3% untreated at 3 weeks [P <.05] compared to sham LVEF 82% ± 5%) and participating in the formation of new cardiomyocytes, smooth muscle cells, and endothelial cells in vivo. Cardiac stem cell populations also are being put to the test in models of chronic MI. Studies have shown that c-Kit+ cardiac stem cells isolated from the adult rat heart home to infarcted areas when delivered via the aortic root during a reperfusion protocol, improving LVEF 5 weeks post-MI (LVEF ≈ 48% treated vs LVEF ≈ 40% control [P <.05] compared to baseline LVEF ≈ 75%), and regenerate muscle and vessels in the absence of cell fusion as demonstrated by the presence of at most two copies of chromosome 12 (detected by fluorescence in situ hybridization) in newly formed green fluorescent protein (GFP)-positive cardiomyocytes.10 This study represents an important step toward clinically relevant application of cardiac stem cells.
Sca-1+ cardiac stem cells
A population of Sca-1+ cardiac stem cells was independently identified as a subgroup of cells (constituting ≈ 14%) isolated in the noncardiomyocyte cell fraction of the adult mouse heart in a whole heart digestion.11 Sca-1+ cells were isolated by antibody targeting and magnetic sorting. Freshly isolated Sca-1+ cells coexpressed CD31 and CD38, lacked c-Kit, CD34, CD45, Flk-1, and Flt-1 and were blood lineage negative. Sca-1+ cells were 120-fold enriched for the side population (SP) phenotype. This study reported that greater than 93% of SP cells were, in fact, Sca-1+. Expression of telomerase reverse transcriptase, which has been associated with self-renewal potential, was restricted to the Sca-1+ fraction of noncardiomyocytes and was absent in the Sca-1− fraction. Freshly isolated Sca-1+ cells did express the early cardiac-specific transcription factors GATA4, Mef2C, and Tef-1 but did not express Nkx2.5 or genes encoding cardiac sarcomeric proteins. Sca-1+ cells could be cultured in base media supplemented with 10% serum on a fibronectin-coated surface. These cells did not spontaneously differentiate in vitro, but, when stimulated with the cytosine analog 5-azacytidine in culture, a fraction expressed α-sarcomeric actin, cardiac troponin I, Nkx2.5, and myosin heavy chain. In a mouse model of ischemia-reperfusion, Sca-1+ cells (106 injected intravascularly) had engrafted at a much higher rate than Sca-1− cells after 2 weeks and could be found forming new cardiomyocytes. The authors reported a contribution of cell fusion in about 50% of new cardiomyocytes (as evidenced by coexpression of β-gal and Cre in R26R recipient mice given Sca-1+ cells isolated from the α-MHC-Cre mouse).
A subsequent study by Matsuura et al12 reported that oxytocin treatment of adult mouse-derived Sca-1+ cells was able to produce spontaneously beating cells that exhibited calcium transients responsive to stimulation by isoproterenol, demonstrating the ability of Sca-1+ cardiac stem cells to adopt a functional cardiomyocyte phenotype in vitro. Wang et al13 demonstrated that the Sca-1+CD31− subpopulation isolated from the adult mouse heart adopted cardiomyocyte and endothelial properties in vitro. Differentiation media (containing many cardiogenic agents, including 5-azacytidine) induced cardiomyogenic differentiation evidenced by expression of Nkx2.5 and GATA4, and further coculture with primary cardiomyocytes led to the expression of cardiac sarcomeric proteins. Microtubule formation was also seen when Sca-1+CD31− cells were cultured in Matrigel, suggestive of endothelial potential. This study also demonstrated an important reparative role of Sca-1+CD31− cells. Sca-1+CD31− cell number increased immediately after acute MI, suggesting a role for these cells in normal cardiac repair. Furthermore, when Sca-1+CD31− cells (106) were injected intramyocardially into the acutely infarcted mouse heart, animals had a significantly higher LVEF at 2 and 3 weeks post-MI than did Sca-1−CD31− and vehicle-injected controls (LVEF ≈ 38% vs LVEF ≈ 33% and LVEF ≈ 31% [P <.001] compared to control LVEF ≈ 58%), and engrafted cells formed new cardiomyocytes and endothelial cells in vivo.
SP cardiac stem cells
Like other adult organs, the heart contains a population of putative progenitor cells termed side population cells, which are characterized by the ability to efflux metabolic markers such as Hoechst due to high expression of membrane pumps encoded by the multidrug-resistance genes.14,15 Hierlihy et al14 were the first to demonstrate the presence of a verapamil-sensitive SP cell in the postnatal mouse heart. Shortly thereafter, Martin et al15 attributed the cardiac SP phenotype to Abcg2 protein expression and showed that a population of SP cells persisted into adulthood in the mouse. When selected, adult mouse SP cells also could differentiate into α-actinin-positive cells when cocultured with primary cardiomyocytes. A phenotypic analysis of adult mouse cardiac SP cells revealed that these cells were Sca-1high, c-Kitlow, CD34low, and CD45low. Further fractionation and functional characterization of the adult cardiac SP population has since been performed.16 Pfister et al16 noted that 84% of cardiac SP cells are Sca-1+ and 75% CD31+. They demonstrated that the greatest potential for cardiomyogenic differentiation lies in Sca-1+CD31− SP cells, which make up only 10% of the SP population. A fraction of Sca-1+CD31− SP cells elicited spontaneous contractions when cocultured with primary cardiomyocytes, and functional coupling was demonstrated as synchronous calcium cycling between neighboring cells. Oyama et al17 reported a comparable characterization and similar capacity for adopting a functional cardiac phenotype for cardiac SP cells isolated from the neonatal rat heart. Most importantly, this study showed that GFP-labeled neonatal SP cells home to the cryoinjured heart and form new cardiomyocytes, fibroblasts, endothelial cells, and smooth muscle cells, the first evidence of the regenerative potential of cardiac SP cells. In the adult human heart, Abcg2 expression has been ascribed to CD31+ cells primarily lining vessel walls,18 perhaps hinting toward a largely endothelial fate. Human Abcg2 mRNA levels were 2.5-fold increased in cardiomyopathic hearts compared with normal hearts, leaving open the possibility of SP cell activation or proliferation in response to heart disease as a potential reparative process.
Cardiosphere-derived cardiac stem cells
In 2004, Messina et al19 reported the first isolation of cardiac stem cells from human myocardium. This study showed that a heterogenous population of cells, including cardiac stem cells identified by c-Kit and Sca-1 expression as well as subpopulations of CD34+ and CD31+ cells (potentially endothelial progenitor cells that had taken up residence in the heart), were spontaneously shed from human surgical specimens as well as from embryonic and adult mouse myocardial tissue maintained in primary culture. When placed in suspension culture in a base media supplemented with basic fibroblast growth factor, epidermal growth factor, cardiotrophin-1, thrombin, and B27 as a serum substitute, these cells spontaneously organized into spherical multicellular clusters, thus named cardiospheres. The c-Kit+ population increased as cellular proliferation occurred within the core of the sphere as evidenced by bromodeoxyuridine incorporation. Both human and mouse cardiosphere-derived cells were shown to be clonogenic in vitro when cardiospheres were dissociated and plated as single cells. This study also showed that cardiospheres provide an environment in which some cardiac stem cells become partially differentiated cardiomyocytes and endothelial cells. Immunohistochemical analysis revealed expression of sarcomeric proteins, such as troponin I or myosin heavy chain, and vascular proteins, such as kinase domain receptor, primarily in the cardiosphere surface cells, whereas c-Kit expression was maintained in proliferative cardiosphere core cells. The most obvious evidence of cardiomyogenic differentiation (previously shown only in embryonic stem cell culture) was the demonstration of beating cardiospheres in vitro, either in coculture with neonatal rat cardiomyocytes (human cardiospheres) or spontaneously (embryonic mouse cardiospheres). Human cardiospheres were delivered intramyocardially into immunodeficient mice, and significant functional improvement was seen 3 weeks later (fractional shortening 37% in cardiosphere-injected group vs 18% in control group [P <.05] compared to sham 59%) along with vigorous engraftment and cardiogenesis. The cardiosphere culture method has since been used by independent groups to enrich c-Kit+ cardiac stem cells20 and Sca-1+ cardiac stem cells21 alike for further analysis.
Isl-1+ cardiac stem cells
An Isl-1+ cell population has been shown to contribute to the development of parts of the embryonic mouse heart, notably the outflow tract, both atria, right ventricle, and portions of the left ventricle.22 Laugwitz et al23 demonstrated the presence of an Isl-1+ population of cardiac stem cells in the postnatal mouse, rat, and human heart, most prominently in the outflow tract and right atrium. Further studies showed that Isl-1 marks a population of multipotent cardiac stem cells contributing to smooth muscle, endothelial, and pacemaker cell lineages in the embryonic and postnatal heart24,25 and implicate Isl-1+Nkx2.5+Flk-1+ cells as the most primitive cardiac stem cells. No compelling evidence for an Isl-1+ population in the adult heart has been presented.
SSEA-1+ cardiac stem cells
An SSEA-1+ cardiac stem cell has been identified in the neonatal and adult heart. Ott et al26 described a cell characterized by expression of this marker traditionally associated with embryonic stem cells, in the rat but not in humans (despite the article’s misleading title). In the neonatal rat heart, SSEA-1+ cells coexpressed Nkx2.5, GATA4, and cardiac myosin heavy chain, whereas in the adult rat heart, SSEA-1+ cells lacked cardiac-specific markers in vivo. SSEA-1+ cells isolated from the adult heart by harsh digestion of tissue fragments (in order to eliminate cardiomyocytes) did coexpress Oct3/4, initially lacked c-Kit and Sca-1, and could be expanded in vitro in suspension over a mesenchymal cell feeder layer obtained from the same digestion. Approximately 4.5 × 105 suspension cells could be generated in 90 days from approximately 1 g of tissue. Over time, SSEA-1 expression decreased, Flk-1 expression increased, transient coexpression of Abcg2 and Sca-1 was observed in Flk-1+ cells, transient coexpression of c-Kit was observed as well, but only 50% of c-Kit+ cells coexpressed Flk-1. Finally, expression of Nkx2.5, GATA4, and Isl-1 (a cardiac stem cell marker present only in the embryonic and postnatal periods) could be seen in some cells, indicating cardiac commitment, and these populations increased in number over time. When placed in differentiation media in the absence of a mesenchymal cell layer or in coculture with primary cardiomyocytes, SSEA-1+ cells underwent further cardiomyogenic differentiation and were found to express α-sarcomeric actin or cardiac myosin heavy chain and to form beating colonies. When SSEA-1+ cells were transplanted into the 2-week-old infarcted rat heart (106 cells injected intramyocardially), functional improvement relative to control was seen 1, 3, and 5 weeks later (LVEF ≈ 57% in treated vs LVEF ≈ 28% in control at 3 weeks [P <.05] compared to 2-week baseline LVEF ≈ 36%), SSEA-1+ cells also proved capable of forming new cardiomyocytes and endothelial cells in the infarct area. The authors propose that SSEA-1 identifies the most primitive cardiac stem cells present in the adult mammalian heart and suggest that c-Kit, Sca-1, and Abcg2 identify cardiac stem cells at later stages of cardiac-specific differentiation.
These cumulative data from independent laboratories strongly support the notion that the adult mammalian heart contains populations of cardiac stem cells. These studies establish a successful treatment paradigm in rodents: cardiac stem cells isolated from the heart and delivered to ischemic areas will orchestrate cardiac regeneration and maintain global cardiac function.
Clinical potential of cardiac stem cells
Stem cells are present in many organs, where they offset physiologic cell death and, to variable degrees, mediate tissue repair in pathologic states. It now is apparent that the adult mammalian heart possesses limited self-renewal potential attributable to small clusters of resident cardiac stem cells found throughout the myocardium. Human cardiac stem cells are believed to be involved in maintaining myocardial homeostasis throughout life but seem to be incapable of counteracting massive degenerative events such as MI. Application of ex vivo expanded human cardiac stem cells is an obvious approach to bolster the heart’s inherent repair capacity. Not only have many populations of cardiac stem cells shown efficacy in animal models of heart disease, but all have shown an ability to generate the major constituents of the myocardium either in vitro or in vivo. However, a clinically applicable method for isolating and expanding human cardiac stem cells for the purpose of application during an injury event was lacking until recently.
Human cardiosphere-derived cardiac stem cells
We postulated that cardiospheres generated from nonsurgical myocardial specimens might prove a useful source for autologous cardiac stem cell therapy and began a proof-of-concept study that was recently published.27 Cardiospheres previously had been successfully generated from human surgical specimens.19 Our study took an important next step toward developing a clinically applicable cardiosphere-based method. We chose to work with biopsy specimens obtained from the septal wall using a bioptome introduced via the right femoral vein and advanced to the right ventricle with fluoroscopic monitoring. This procedure for obtaining percutaneous endomyocardial biopsy specimens is conducted routinely in an outpatient setting, is minimally invasive, and poses relatively few risks for patients.28,29 We chose the cardiosphere culture method because sphere culture offers a convenient means for expanding cardiac progenitors ex vivo given a small amount of starting tissue material. In fact, spontaneous sphere formation by progenitor cells is not a phenomenon unique to progenitors isolated from the heart. Sphere formation also has been observed with neural,30 retinal,31 mammary,32 kidney,33 and prostate34 progenitors and is thought to play a role in determining cell phenotype and survival.35
We were highly successful using the cardiosphere method. All but one of our initial 70 specimens (21.0 ± 1.9 mg) taken from adults aged 20 to 80 years yielded cardiosphere-derived cells. We demonstrated that cardiospheres grown from adult human endomyocardial biopsies had characteristics like those previously described (Figure 1a).19 Cardiospheres were readily generated and could be further expanded as a monolayer of cardiosphere-derived cells. A single experimental biopsy specimen yielded 1.7 ± 0.4 million cardiosphere-derived cells in about 45 days, a workable number of cells that can be multiplied several-fold by simply increasing the number of biopsies obtained at the outset. A subset of these cells maintained expression of c-Kit (but were MDR-1, Sca-1, and Abcg2 negative) after extended culture (Figures 1b and 1c). This population was complemented by a mesenchymal-like population of cardiosphere-derived cells (CD105+CD90+) likely serving as a feeder layer to the more primitive cell populations during ex vivo proliferation (Figure 1b). Cardiosphere-derived cells were shown to express connexin43 in discrete plaques localized to cell-cell junctions, suggesting that these cells were electrically and metabolically coupled to one another and may integrate efficiently into a myocardial syncytium. Like their mother cardiospheres, when cocultured with primary cardiomyocytes the cardiosphere-derived cells behaved as functional cardiomyocytes. Spontaneous repetitive action potentials and synchronous calcium cycling were observed in a small percentage of cocultured cardiosphere-derived cells, demonstrating the capacity for complete cardiomyogenic differentiation. Whether cardiosphere-derived cells could mediate functional improvement in an immunodeficient mouse model of acute MI then was tested. Proximal ligation of the left anterior descending coronary artery reduced baseline LVEF from 80% to 45% 2 days after surgery. A quantity of 105 cardiosphere-derived cells proved to be therapeutically effective when compared to both a fibroblast cell control and a vehicle cell control group. Echocardiographic measurements performed 3 weeks after MI and simultaneous cell delivery revealed a significantly higher LVEF in the cardiosphere-derived cell-treated group (42.8% ± 3.3%) compared to either the fibroblast-treated group (25.0% ± 2.0%) or the phosphate buffered saline (PBS)-treated group (26.0% ± 1.8%; Figure 2a). Histology revealed that human cardiosphere-derived cells engrafted well in these mice and increased the amount of viable myocardium remaining within the infarct (Figures 2b and 2c). One week after delivery, cardiosphere-derived cells had distributed themselves within the MI area, forming islands or continuous bands of human tissue. Furthermore, several cardiosphere-derived cells could be identified as differentiated cardiomyocytes, endothelial cells, and smooth muscle cells. This work introduced the prospect for autologous cardiac stem cell therapy without the need for surgical tissue harvesting.
Figure 1.
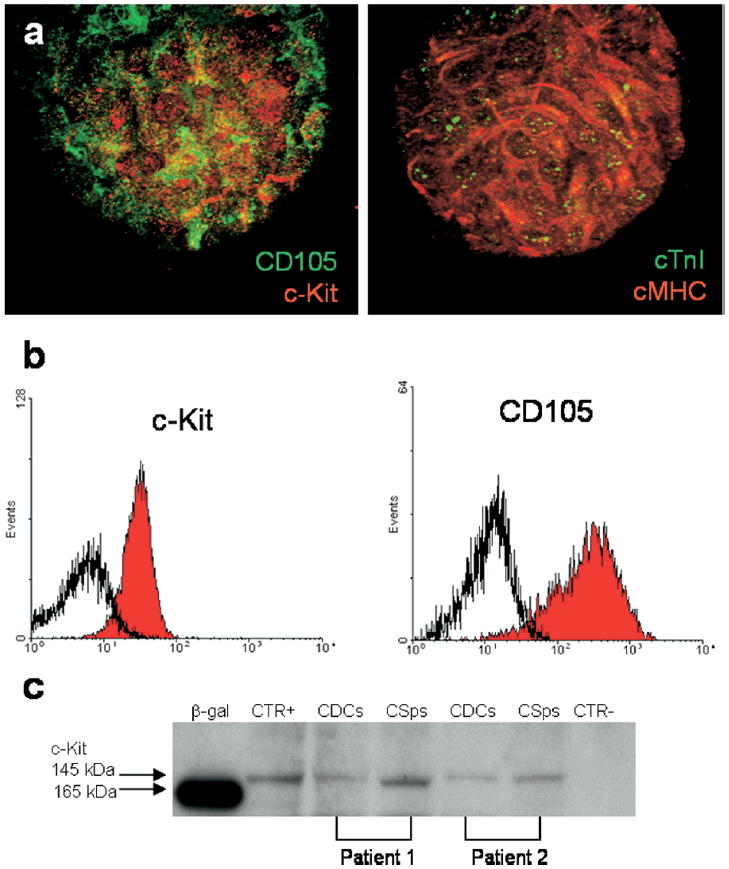
a: Confocal analysis of human cardiospheres derived from percutaneous endomyocardial biopsy specimens. Expression of cytoplasmic cardiac markers are shown: cardiac myosin heavy chain (cMHC, red) and cardiac troponin I (cTnI, green). Expression of stem cell and mesenchymal markers are shown: c-Kit (red) and CD105 (green). b: c-Kit and CD105 expression are maintained after cardiosphere expansion as shown by example flow cytometric histograms. c: c-Kit expression demonstrated by western blot analysis for two different patients. Positive control (CTR+) and negative control (CTR−) were performed using the c-Kit–positive cell line TF1 and the c-Kit-negative cell line MCF7, respectively. CDCs = cardiosphere-derived cells; CSps = cardiospheres.
Figure 2.
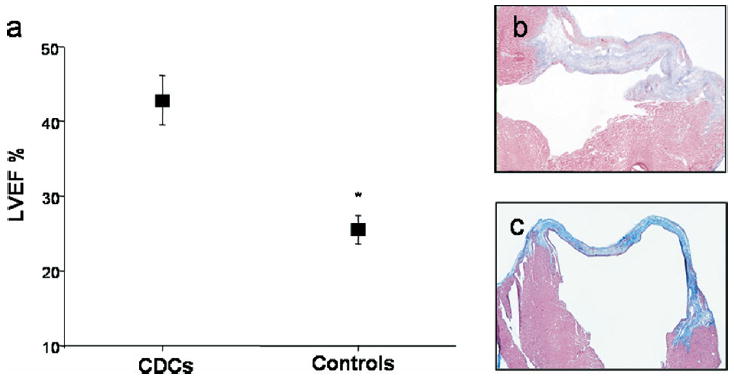
a: Left ventricular ejection fractions (LVEF) in the three experimental groups (control group summarizes phosphate buffered saline (PBS)-injected group and fibroblast-injected group) 3 weeks after myocardial infarction was created and cells were injected. *P <.01. b, c: Representative Masson trichrome-stained sections from infarcted hearts, explanted 3 weeks after injection of cardiosphere-derived cells (b) or PBS (c). CDCs = cardiosphere-derived cells.
We have since collected unpublished data that demonstrate engraftment and functional preservation in mice for up to 6 weeks after MI.36 The 6-week end-point is relatively long term for a mouse model (>10% of the typical 1-year murine life span), yet remarkably the cardiosphere-derived cell benefit persisted. We also have since demonstrated the presence of human growth factors in the mouse myocardium that can elicit prosurvival and proangiogenic effects in vivo.37 A robust paracrine response of human cardiosphere-derived cells was observed in the immediate post-MI period, which declined over time but remained out to 3 weeks after MI. The factors produced by cardiosphere-derived cells that we have identified thus far can help prevent cell apoptosis and begin the endogenous repair response.
Human c-Kit+ cardiac stem cells
Cardiac stem cells have been successfully isolated from surgical specimens taken from the human heart by selection for c-Kit+ cells either from an enzymatic digestion of the whole specimen or from cells grown out from the specimen in explant culture.38 Specimens (≈ 30 mg) were obtained from patients undergoing cardiac surgery, but no information was available regarding the atrial or ventricular origin of the specimen or regarding the patients themselves. c-Kit+ cells constituted approximately 1.1% of cells obtained during enzymatic digestion and approximately 1.6% of approximately 6,000 outgrowth cells (or ≈ 100 cells per specimen). Outgrowth cells were isolated approximately 3 weeks after the start of explant culture and reportedly underwent approximately 24 population doublings to reach passage 8 (to generate ≈170 million cells) in an assumed 30 additional days (as the reported doubling time for a c-Kit+ clone was ≈ 30 hours). c-Kit+ cells at passage 8 reportedly did not reach replicative senescence (as indicated by telomere length >5.0 kbp in approximately 70% of cells, where 2.0 kbp is the predicted critical limit) and maintained expression of c-Kit in approximately 70% of cells. c-Kit+ human cardiac stem cells were clonogenic in culture (with 0.8% efficiency) and capable of multilineage differentiation in vivo. Furthermore, functional coupling was demonstrated between GFP-labeled human c-Kit+ cells and cardiomyocytes in vitro and in vivo. Human c-Kit+ cells (≈ 80,000) mediated an effect on LVEF in nude rats 3 weeks after cell delivery (LVEF ≈ 48% treated vs LVEF ≈ 36% untreated). Differentiation of c-Kit+ cells occurred in the absence of cell fusion by fluorescence in situ hybridization for human and rodent X chromosomes (lack of colocalization indicated differentiation without fusion) as well as by delivery of Cre recombinase-expressing cells into a heart expressing loxP-flanked GFP (lack of GFP expression in human-derived cardiomyocytes, endothelial cells, and smooth muscle cells indicated differentiation without fusion). This study provides important data regarding the potential for ex vivo expansion of human c-Kit+ cardiac stem cells. The authors estimate that cells could undergo 50 population doublings before replicative senescence is reached, making possible the generation of 1015 c-Kit+ cells from a small surgical specimen. Furthermore, this study provides evidence that human cardiac stem cells generate new cardiomyocytes and vessels without undergoing fusion with host cells, demonstrating a true capacity for cardiac differentiation. This feature of the cardiac stem cell may be a defining characteristic, distinguishing the cardiac stem cell from populations of bone marrow-derived cells purported by some to undergo cell fusion as opposed to transdifferentiation in vivo.
Preliminary preclinical cardiac stem cell studies
Thus far, we have discussed studies showing that human cardiosphere-derived cells and c-Kit+ cardiac stem cells can reliably be generated from biopsies taken from patients and that they can elicit functional benefits in small animal models of heart disease. On the path to clinical application, the feasibility, safety, and efficacy will need to be demonstrated in a large animal model of heart disease using autologous cells. Preliminary preclinical data from relevant large animal models and autologous cardiac stem cells are rapidly emerging.39-42 These studies are tackling the issues of autologous tissue harvesting and cell delivery via a clinically relevant route to a well-established infarct.
Cardiosphere-derived cardiac stem cells
We have developed a model of subacute ischemic cardiomyopathy in pigs that provides preclinical data relevant to a potential clinical study.40,42 To more accurately model human adults, this model was created in adult miniature swine. Pigs were subjected to an anterior MI using an angioplasty balloon inflated in the mid-left anterior descending coronary artery for 2.5 hours, after which the balloon was deflated and the ischemic territory reperfused. After reperfusion, a right ventricular biopsy sample was obtained using a standard clinical cardiac bioptome introduced via the right internal jugular vein. The animals were allowed to recover while cardiosphere-derived cells were isolated from their biopsy samples. Four weeks after infarction, the animals underwent cardiac magnetic resonance imaging (MRI) for assessment of infarct size, global and regional function, chamber volumes, and perfusion. Several days later, the animals underwent repeat cardiac catheterization. Pretreatment hemodynamics were assessed, and autologous cardiosphere-derived cells or placebo (carrier solution alone) were infused into the left anterior descending coronary artery via angioplasty balloon. After cardiosphere-derived cell or placebo infusion, the pigs were followed for 8 more weeks, undergoing repeat MRI 8 weeks after cell delivery. After the last MRI, the animals were brought back to the catheterization laboratory, where they underwent catheterization for assessment of infarct vessel patency and hemodynamics, electrophysiologic study for determination of inducibility of ventricular tachycardia, and then were sacrificed for histology of heart, lung, liver, spleen, and kidneys.
Preliminary unpublished data from our laboratory has shown success in generating autologous pig cardiosphere-derived cell stocks (14.2 ± 3.1 million cardiosphere-derived cells in ≈ 23 days)40 using a modified cardiosphere culture method. Notably, starting material for this study was safely scaled up by approximately 4.5-fold compared to the original pilot human study,27 whereas yield was approximately 8.4-fold higher in half the original time period. Using the modified culture method, subsequent human specimen yields tended to be 10-fold higher than those obtained with the original method. In a preliminary study designed to track cell engraftment, autologous pig cardiosphere-derived cells were transduced with a lentiviral construct encoding a nuclear-targeted lacZ. Animals were subjected to the usual protocol, given cardiosphere-derived cells, and sacrificed solely for histology. X-gal staining of myocardial tissue sections showed evidence of cell survival and engraftment in the MI border zone and islands within the scar 8 weeks after intracoronary delivery. The lacZ-labeled autologous cardiosphere-derived cells had differentiated into cardiomyocytes and endothelial cells as evidenced by morphology and α-sarcomeric actin expression.40 A separate preliminary study has allowed us to define an optimal cardiosphere-derived cell dosage, one that results in measurable acute engraftment and minimizes further injury during intracoronary delivery. For the pivotal study, all animals undergo biopsy and autologous cardiosphere-derived cells are generated. Cells are not genetically altered in any way. Animals are randomly assigned to receive either cardiosphere-derived cells or placebo at the time of infusion. Several safety and efficacy end-points are being examined, including (1) laboratory values of hematologic, hepatic, and renal function of cardiac enzymes, (2) inducibility of ventricular arrhythmias at time of sacrifice, (3) adverse events, (4) body weight, (5) gross and microscopic tissue analysis at autopsy, (6) cardiac hemodynamics, and (7) MRI parameters of infarct size and left ventricular function and volumes at time of sacrifice. Preliminary data presented as abstracts to date have shown the following: (1) no clinically significant laboratory abnormalities in either the cell therapy group or the placebo group, (2) provocable arrhythmia commonplace in both placebo (as expected from prior studies43,44) and cell-treated animals, (3) low spontaneous mortality in both groups, (4) body weights increasing in both groups over the study period, (5) pathologic evidence of infarcts and some heart failure but no differences between experimental groups, no evidence of tumor formation or inflammation in the heart, and no off-site detrimental effects in the cell therapy group, (6) beneficial hemodynamic changes signifying improved contractility and enhanced lusitropy in the cell therapy group,42 and (7) a reduction in infarct size as a percentage of LV mass in the cell therapy group at the study’s 8-week end-point.42 These preclinical data demonstrate that cardiosphere-derived cells are safe in a large animal model and may be an effective therapy in the setting of ischemic dysfunction following MI.
c-Kit+ cardiac stem cells
Similarly, surgical samples taken from the atrial appendages are being used to obtain c-Kit+ cardiac stem cells from pigs during experimentally induced ischemia-reperfusion injury.39,41 Cells were isolated using a primary explant technique. c-Kit+ cardiac stem cells and other cardiac progenitors identifiable by GATA4 expression migrated out of small surgical tissue samples maintained in culture. c-Kit+ cells were selected from among the migratory populations by magnetic sorting and reportedly constituted 30% of the 2,000 to 5,000 cells obtained in 3 to 4 weeks, or 600 to 1500 cells. Selected cells were further expanded in culture with a doubling time of approximately 48 hours at both early (P4) and late (P11) passages. After culture expansion, cells lacked hematopoietic markers, were clonogenic, were multipotent in vitro after exposure to differentiation medium, were replication competent out to passage 9 as evidenced by retention of telomerase activity, and were karyotypically normal out to passage 16. The therapeutic potential of autologous c-Kit+ cardiac stem cells currently is being examined in the pig ischemia-reperfusion model. Pigs were subjected to a 90-minute coronary occlusion, during which time atrial tissue samples were obtained, and then allowed to reperfuse for 2 to 4 months. c-Kit+ cells were delivered via the mid-left anterior descending coronary artery with the aid of a balloon catheter advanced through the right carotid artery. Animals were examined by echocardiography and subjected to hemodynamic testing prior to cell infusion and 1 month after cell infusion. Treated animals (at the time of the last preliminary report) were showing improved thickening fraction (≈32% vs ≈13% for control [P <.05], with baseline ≈25%) as well as improved maintenance of left ventricular end-diastolic pressure (≈12 mmHg vs ≈16 mmHg [P <.05], with sham ≈8 mmHg). The animals were sacrificed 1 month after cell infusion, and histology was performed. Cells had not been genetically labeled and so could not be explicitly tracked, but large regions of regenerated myocardium were evident in the infarct region of treated animals as demonstrated by expression of Ki67 in cells positive for α-sarcomeric actin. This study validates the therapeutic potential of c-Kit+ cardiac stem cells in a clinically relevant model.
Part 2 of this review will discuss the clinical application of stem cells in general and the primary safety issues associated with cell therapy and will include a commentary on the potential of cardiac stem cells for clinical use.
Acknowledgments
Supported by the National Institutes of Health to Dr. Marbán, the Donald W. Reynolds Foundation to Dr. Marbán, and the Pasteur-Foundation Cenci Bolognetti to the Department of Experimental Medicine, University of Rome “La Sapienza” to Dr. Giacomello. Capricor has licensed cardiac stem cell technology from Johns Hopkins University and the University of Rome. Dr. Ruckdeschel Smith is an employee of Capricor. Dr. Marbán holds equity in Capricor. Capricor has provided no funding for any research conducted in this study.
References
- 1.Quaini F, Cigola E, Lagrasta C, et al. End-stage cardiac failure in humans is coupled with the induction of proliferating cell nuclear antigen and nuclear mitotic division in ventricular myocytes. Circ Res. 1994;75:1050–1063. doi: 10.1161/01.res.75.6.1050. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Leri A, Finato N, et al. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 4.Scholzen T, Endl E, Wohlenberg C, et al. The Ki-67 protein interacts with members of the heterochromatin protein 1 (HP1) family: a potential role in the regulation of higher-order chromatin structure. J Pathol. 2002;196:135–144. doi: 10.1002/path.1016. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Chang KC, Delgado CC, et al. Do cardiac stem cells arise from cardiomyocyte dedifferentiation? Circ Res. 2006;99:2. [Google Scholar]
- 6.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 7.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 8.Linke A, Muller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Hu Q, Nakamura Y, et al. The role of the Sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 14.Hierlihy AM, Seale P, Lobe CG, et al. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 15.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Pfister O, Mouquet F, Jain M, et al. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 17.Oyama T, Nagai T, Wada H, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meissner K, Heydrich B, Jedlitschky G, et al. The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J Histochem Cytochem. 2006;54:215–221. doi: 10.1369/jhc.5A6750.2005. [DOI] [PubMed] [Google Scholar]
- 19.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 20.Yamabi H, Lu H, Dai X, et al. Overexpression of integrin-linked kinase induces cardiac stem cell expansion. J Thorac Cardiovasc Surg. 2006;132:1272–1279. doi: 10.1016/j.jtcvs.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Tang YL, Shen L, Qian K, et al. A novel two-step procedure to expand cardiac Sca-1+ cells clonally. Biochem Biophys Res Commun. 2007;359:877–883. doi: 10.1016/j.bbrc.2007.05.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai CL, Liang X, Shi Y, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moretti A, Caron L, Nakano A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Liang X, Najafi N, et al. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott HC, Matthiesen TS, Brechtken J, et al. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract. 2007;4(Suppl 1):S27–S39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 27.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy RE, 3rd, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 29.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol. 1992;19:43–47. doi: 10.1016/0735-1097(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 31.Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 32.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver JA, Maarouf O, Cheema FH, et al. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson DA, Xin L, Lukacs RU, et al. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates RC, Edwards NS, Yates JD. Spheroids and cell survival. Crit Rev Oncol Hematol. 2000;36:61–74. doi: 10.1016/s1040-8428(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 36.Smith RR, Leppo MK, Chimenti I, et al. Human cardiosphere-derived cells engraft long-term in infarcted mice and preserve left ventricular function. Circulation. 2007;116:II–130. [Google Scholar]
- 37.Chimenti I, Smith RR, Leppo MK, et al. Secretion of pro-survival and proangiogenic growth factors in vitro and in vivo by cardiac progenitor cells from human biopsies. Circ Res. 2007;101:1209. [Google Scholar]
- 38.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolli R. Translational studies of cardiac stem translational studies of cardiac stem cells for regeneration in acute and cells for regeneration in acute and chronic myocardial chronic myocardial infarction. American Heart Association Scientific Sessions, Plenary Sessions. 2005 [Google Scholar]
- 40.Johnston P, Sasano T, Mills K, et al. Isolation, expansion and delivery of cardiac derived stem cells in a porcine model of myocardial infarction. Circulation. 2006;114(Suppl II):II–125. [Google Scholar]
- 41.Bearzi C. Identification and characterization of cardiac stem cells in the pig heart. American Heart Association Scientific Sessions, Plenary Sessions. 2006 [Google Scholar]
- 42.Johnston P, Sasano T, Mills K, et al. Autologous cardiac-derived stem cells decrease infarct size compared to placebo in a porcine model of ischemic cardiomyopathy. Circ Res. 2007;101:6. [Google Scholar]
- 43.Sasano T, McDonald AD, Kikuchi K, et al. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat Med. 2006;12:1256–1258. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashikaga H, Sasano T, Dong J, et al. Magnetic resonance-based anatomical analysis of scar-related ventricular tachycardia: implications for catheter ablation. Circ Res. 2007;101:939–947. doi: 10.1161/CIRCRESAHA.107.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]


