Abstract
Purpose
To investigate the relationship between MR image contrast associated with Aβ plaques and their histology and compare the histo-pathological basis of image contrast and the relaxation mechanism associated with Aβ plaques in human Alzheimer’s disease and transgenic APP/PS1 mouse tissues.
Materials and Methods
With the aid of the previously developed histological coil, T2*-weighted images and R2* parametric maps were directly compared to histology stains acquired from the same set of Alzheimer’s and APP/PS1 tissue slices.
Results
The electronic microscopy and histology images revealed significant differences in plaque morphology and associated iron concentration between AD and transgenic APP/PS1 mice tissue samples. For AD tissues, T2* contrast of Aβ-plaques was directly associated with the gradation of iron concentration. Plaques with significantly less iron load in the APP/PS1 animal tissues are equally conspicuous as the human plaques in the MR images.
Conclusion
These data suggest a duality in the relaxation mechanism where both high focal iron concentration and highly compact fibrillar beta-amyloid masses cause rapid proton transverse magnetization decay. For human tissues, the former mechanism is likely the dominant source of R2* relaxation; for APP/PS1 animals, the latter is likely the major cause of increased transverse proton relaxation rate in Aβ-plaques. The data presented are essential for understanding the histo-pathological underpinning of MRI measurement associated with Aβ plaques in humans and animals.
Keywords: Beta Amyloid (Aβ) Plaques, MRI microscopy, Iron, APP/PS1 Mouse
Introduction
The formation of amyloid-beta (Aβ) plaques is a neuropathological hallmark and a cardinal feature of Alzheimer’s disease (AD). APP/PS1 transgenic mouse models that mimic the formation of human Aβ plaques in the mouse brain are widely used as an animal model for AD investigations. The development of an imaging technology capable of visualizing and quantifying Aβ plaques in animal models and in the AD brain is critically important for translational, preclinical and clinical research. The ability to delineate Aβ plaques with MRI has been demonstrated ex vivo with human brain samples (1) and in vivo with the mouse model (2–4). Understanding the histological basis of MRI contrast associated with Aβ plaques is essential in this endeavor. To achieve this goal, it is necessary to correlate MRI results with histological stains, which has been technologically challenging because of the limitations in co-registration of planar histology tissue samples with MR images. A prior study involving the innovation of a histological coil has addressed this long-standing difficulty (5). The ability to directly image histological samples is possible when employing the developed histological radio-frequency (RF) coil design. Consequently, MR images and histology data from the same tissue sample can be directly overlaid and compared without uncertainties of co-registration between the two imaging modalities. The goal of the current study is to use this novel technology to a) further optimize the method for routine imaging-histology studies, b) establish the relationship between MR image contrast associated with Aβ plaques and their histology, and c) compare such relationships in human and transgenic APP/PS1 mouse tissues. Furthermore, to ultimately apply the therapies developed with the animal models to human studies, the Aβ plaque MR image-pathology relationship must be validated in humans and compared to transgenic animal data. It is hypothesized within the literature that iron found in and around the amyloid plaques is the dominant cause of the hypo-intensities seen in the MR images. Examination of the relationship between MR contrast due to Aβ plaque and iron deposition both in human AD and the APP/PS1 model is described. The data suggest that iron load alone does not account for the hypo-intensities that are observed in the T2*-weighted images of the animal pathology. The relationship of plaque morphology and overall globular size with their manifestation in the MR image are described. The method developed in this report can be particularly useful for further validation of the histological basis of MR contrast and the development of animal models for other neurological diseases.
Materials and Methods
Human Alzheimer’s and Control Brain Samples
Entorhinal cortex brain tissue samples from clinically and histologically determined AD subjects (n=5) and age-matched controls (n=4) were donated with consent following institutional guidelines and obtained from both internal and external sources (Harvard Brain Tissue Resource Center, McLean Hospital, Belmont, MA). Analysis of the tissue obtained from the brain bank indicates that there was not a significant difference between the age of the subjects upon bereavement. The post mortem index between the tissue harvesting at time of death and utilization in this study was significantly different between the tissue samples, with a slightly longer time period for controls. Tissue from the entorhinal cortex was fully fixed in 4% paraformaldehyde in pH 7.3 phosphate buffered saline (PBS) after which blocks of tissue were cryogenically protected in graded sucrose solutions then sectioned at 60 µm on a cryostat. The sections were prepared for magnetic resonance imaging according to previous methods explained below (5).
Transgenic APP/PS1and Control Mice
Transgenic mice (n=5) inserted with a chimeric amyloid precursor protein (APP) (APPSwe695) and a mutant human presenilin 1 (A246E variant), developed by Borchelt et al. (6,7), were obtained commercially from The Jackson Laboratory (strain type B6C3-Tg(APP695)3Dbo Tg(PSEN1)5Dbo/J). Animals were kept in the animal facility under veterinary care with normal feeding, environmental, and handling conditions. Age-matched cage mate C57BL/6 mice (n=3) were used as a control group. Upon aging naturally until 24 months, animals were euthanized via an intra-parietal injection of sodium pentobarbital (Nembutal, 200 mg/kg) and were transcardially perfused with cold Lactated Ringer's solution (pH 7.4) followed by buffered 4% paraformaldehyde in PBS. Whole brain tissue was harvested fixed in pH 7.3 buffered 4% paraformaldehyde, placed in graded sucrose solutions for cryo-protection, then sections were cut at 60µm and prepared for MR micro-imaging using the protocol below.
Histological slice MRI Scanning Protocols
Histological MRI was accomplished on a 7.0 T magnet (Bruker BioSpin MRI GmbH, Ettlingen, Germany) with the histological coil (5). 60 µm tissue samples were floated in PBS for 30 minutes to allow any residual formalin and sucrose in the tissue to leach out and then encased between the two coverslips. The sample was then placed into the histological RF coil and tuned/matched to 300.48 MHz for micro-MR imaging. For T2* imaging, multi-gradient-echo (MGE) images were acquired in 6h 32m with a TR=718, TE=6.48, FA=30°, 8 echoes with 9ms echo spacing, 64 averages, FOV=23mm × 23mm, and a matrix of 512 × 512 resulting in a final in-plane resolution of 45 µm × 45 µm.
Iron and Amyloid Staining
After histological MR imaging, the 60 µm tissue sections were free floated and rinsed in deionized water (dH2O) for 15 minutes. Tissue sections were co-stained for both iron and beta-amyloid with a Perl’s Prussian Blue stain followed by an aqueous Thioflavin-S stain. The sections were placed in equal volumes of freshly prepared 4% potassium ferrocyanide (#P236, Fisher Scientific, Waltham, MA) and 4% hydrochloric acid (final combined concentrations 2% for each) for 30 minutes under gentle rocking followed by two rinses in dH2O. Intensification of the iron stain was performed with 5 minutes of 3,3'-diaminobenzidine tetrahydrochloride (DAB) staining (#D5637, Sigma, St. Louis, MO) (10 mg dissolved in 15ml of PBS with 16 µl of 30% H2O2) followed by two five-minute rinses in dH2O. Tissue samples were then free floated in 20 ml of 1% Thioflavin-S (#T1892, Sigma, St. Louis, MO) aqueous solution for 5 minutes, followed by differentiation in 70% ethanol for 5 minutes and two five-minute washes in dH2O. Sections were mounted on histological slides with FluorSave Aqueous mounting media (#345789, Calbiochem, San Diego, CA). To test for the possibility of confounding interactions between the two staining methods during co-staining, each was tested on separate tissue samples to determine their individual efficacy and compared to the co-stained sections. A modified Perl’s stain further tested the transgenic animal tissue for minute amounts of iron within the amyloid masses. The stain was adapted and modified from LeVine (8,9) and employs the usage of proteinase K and detergents to break down the periphery of the plaques forming openings in the highly hydrophobic amyloid plaques. This allows the aqueous Perl’s stain to infiltrate into the amyloid mass and is sensitive enough to detect trace amounts of iron in the Aβ plaques.
Microscopy
High resolution microscopy of the tissue sections was performed using a Nikon OptiPhot microscope and Nikon Digital Sight camera using NIS-Elements software. Bright field microscopy under the visible light spectra was used to view iron stains. A FITC fluorescence cube at 430 nm excitation and 550 nm emission was used to visualize Thioflavin-S positive Aβ deposits. Whole tissue image mosaics were created with the 40x magnification bright field of fluorescent images using the Photomerge option in Adobe Photoshop CS (Adobe Systems Incorporated, San Jose, CA, USA). For transmission electron microscopy (TEM), samples were prepared by cutting tissue sections at 60 µm and staining them with Thioflavin-S to elucidate Aβ plaque location. Known regions of Aβ plaque distribution approximately 1 mm × 1 mm were micro-dissected from the sections and processed for electron microscopy following traditional methods.
MRI and Histology Post Processing
Post processing was performed using in-house software developed with the interactive data language (IDL) 6.1 framework (Research Systems Inc., ITT Industries, Boulder, CO, USA). The program allows for the creation of T2* parameter maps from MGE data with a linear regression method. Plaque regions of interest (ROI’s) were selected by averaging the relaxation of three pixels of interest within each measured plaque. This allowed for the evaluation of relaxation measurements from individual plaques in both human AD and APP/PS1 tissue. ROI’s for non-plaque regions were selected in regions known to be devoid of Aβ plaques according to the Thioflavin-S staining. For co-registration to the staining methods, T2* weighted images were generated by the summation of the amplitude images from all of the echoes. Histology and MRI images were imported into Image J (National Institutes of Health, Bethesda, MD, USA) to measure Aβ plaque diameter. Freehand boundaries were drawn around the plaques and the average pixel length of the major and minor axes from the resulting ellipsoid were used to estimate overall diameter. A micrometer slide was viewed at different magnifications to obtain precise measurements of the pixel to micrometer dimensions for plaque diameter calculation.
Results
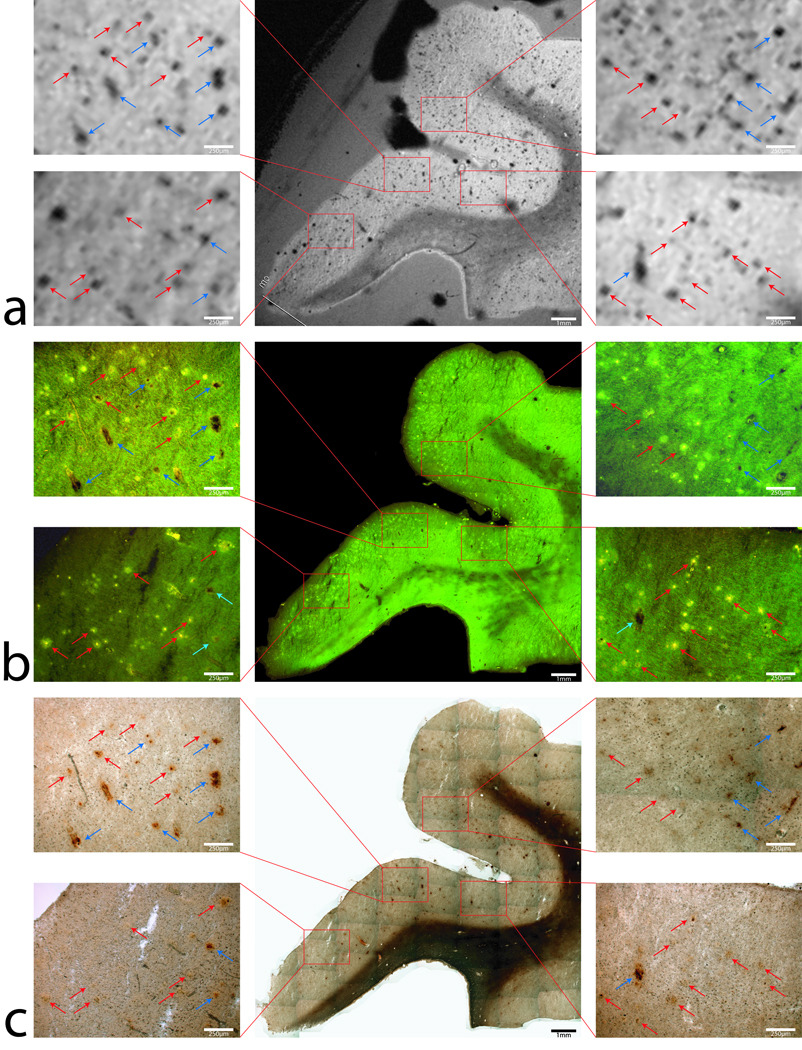
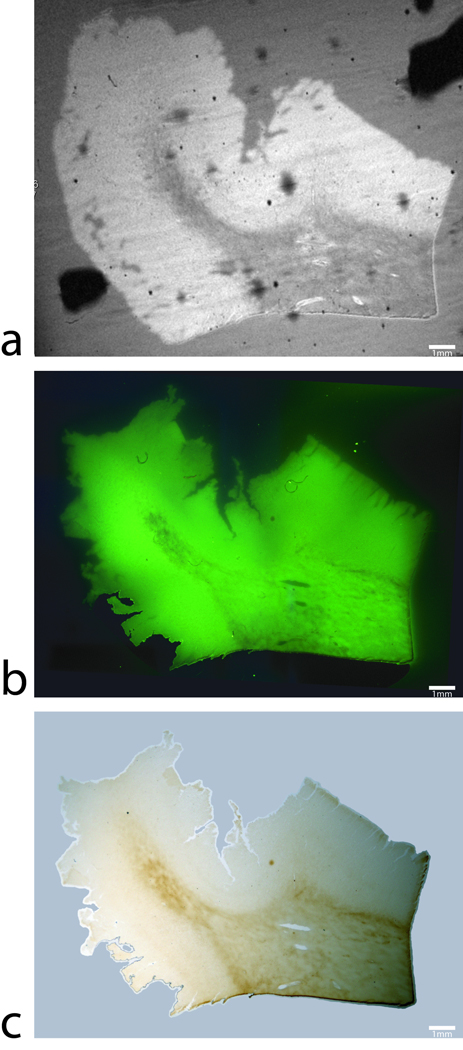
Figure 1 shows a T2* weighted MR image and histological images co-stained with thioflavin-S for Aβ plaques and a traditional Perl’s stain for ferric iron (Fe+3) within the same tissue section from the entorhinal cortex of an AD subject. The image scales of the MR scan and stain mosaic images for Aβ and iron are the same for ease of comparison. For detailed evaluation, 40x magnification images of four selected regions outlined with red boxes are shown on both sides of the corresponding main images. The T2*-weighted images of human Alzheimer’s samples show a loss of signal in focal regions that co-registers remarkably well to the histological iron stain. Many dark hypo-intense spots can clearly be observed in the gray matter in the MR image in Fig. 1a. As seen in the magnification of the thioflavin-S stain images (figure 1b), those hypo-intensities observed in the MR images correspond to either punctate green fluorescent Thioflavin-S positive Aβ-plaques (red arrows) or dark brown patches indicating regions of high focal iron. Focal regions of high iron that are not associated with Aβ plaques can be attributed to locations high in iron such as capillary vessels, hemosiderin (degraded ferritin) or regions of iron oxide (magnetite, hematite or wüstite) deposition (blue arrows) (10,11). Co-staining of Aβ with Thioflavin-S and iron with a Perl’s stain allows the direct comparison of ferric iron concentration in and around the beta-amyloid plaques (Fig. 1c). The Perl’s stain of the tissue section demonstrate focal high iron concentration associated with Aβ plaques in exceptional detail. Many of the Aβ plaques seen in the magnified images appear to have a dark core in their centers which are loaded with a higher concentration of iron, as indicated in the Perl’s stain. This set of images reveals an unprecedented close relationship with MRI contrast between Aβ plaques and brain tissue iron deposition. The minimal AD plaque size diameter that can be resolved with the T2* weighted sequence is 38 µm, demonstrable with the 250 µm scale bars in the figure 1 magnifications. Smaller human AD plaques that do not visibly contain large amounts of iron are not as discernable on the MR images. The high iron distribution within the white matter tracks of the human tissue is consistent with iron found in oligodendrocyte cells making up the myelin sheaths (12). With the histological coil, the relationship between T2* image contrast associated with Aβ plaques and their association with iron deposition are demonstrated unambiguously. The presence of higher iron deposition in the Aβ plaques is a contributing factor to T2* image contrast generation. Regions of gray matter that exhibit high iron content are clearly visible on the MR image with varying degrees of signal dropout differing in size based on the amount of iron present at that location. MR image and histology from a normal brain tissue are shown in Fig. 2 and demonstrate a complete absence of Aβ plaques. The Perl’s stained images of the human control tissue sections demonstrate the known patterns of normal iron staining in regions associated with high iron concentration. Iron stains of the human control tissue (figure 2c) show the expected high diffuse iron in the white matter tracks due to the high iron concentration within the oligodendrocyte cells (13). This known phenomena can be seen with all histological iron stains and in MR images as a signal dropout. Comparison of white matter iron staining between human AD and control samples shows similar iron deposition illustrating negligible iron loss due to formalin leaching. Fluorescent microscopy shows a lack of Thioflavin-S positive Aβ staining, while nonspecific background fluorescence is present. Having neither Aβ plaques nor high focal iron regions, MR images of human control tissue (Fig. 2a) exhibit a lack of hypo-intensities in gray matter except those caused by the highlighted focal iron regions (blue arrows in Fig. 2) and coil noise. Small hypo-intensities that are seen in figure 2 are related to inhomogeneities within the copper foil used in the RF coil, small micro-pockets of air causing artifacts, or small regions of focal iron.
FIG 1.
T2* weighted MR image (a) and histological images of Thioflavin-S stain for beta-amyloid plaques (b) and (c) Perl’s iron stain of the same tissue section from the entorhinal cortex of an Alzheimer’s disease subject. For detailed comparisons, images with 40x-magnification of four selected regions outlined with red boxes are shown on both sides of the corresponding main images. A large amount of black spots can be seen clearly in the gray matter in the MR image in (a). As seen in Fig 1b in the Thioflavin-S stain images, these black spots are shown either as bright green indicating Aβ-plaques (red arrows) or dark brown indicating small blood vessels (blue arrows). The Perl’s stain of the brain tissue in (c) demonstrates focal high iron concentration in both Aβ-plaques and blood vessels. For the former, a higher iron deposition in Aβ has been previous demonstrated. For the latter, the elevated iron is likely associated with residual blood, ferritin/hemosiderin or magnetite. As seen in Fig. 1c, select hypo-intensities in the MR that are due to beta-amyloid plaques are seen with red arrows while signal dropout due to other focal iron regions are highlighted with blue arrows in the four concurrent image magnifications. The figure illustrates that hypo-intensities seen in the T2* weighted image correlate to plaque location and focal iron concentrations. Iron deposition is present in the beta-amyloid plaques that are viewable in the MR image sets. Scale bars for the magnifications are 250µm and 1mm for the whole images.
FIG 2.
Magnetic resonance image, thioflavin-S and iron stains in human control subjects. (a) An MGE T2* weighted image, (b) thioflavin-S and (c) Perl’s iron stain of the same 60µm thick tissue section from the entorhinal cortex of an Alzheimer’s disease subject. The T2* image shows hypo-intensities that are due to micro pockets of air or coil inhomogeneities. The thioflavin-S stain illustrates a lack of beta-amyloid plaques in the human control subjects. Perl’s staining shows high iron in white matter tracks due to oligodendrocytes and one focal iron region in gray matter that is highlighted with the blue arrow in the three images and can be seen as a signal drop in the MR image. Scale bars in all images are set at 1mm.
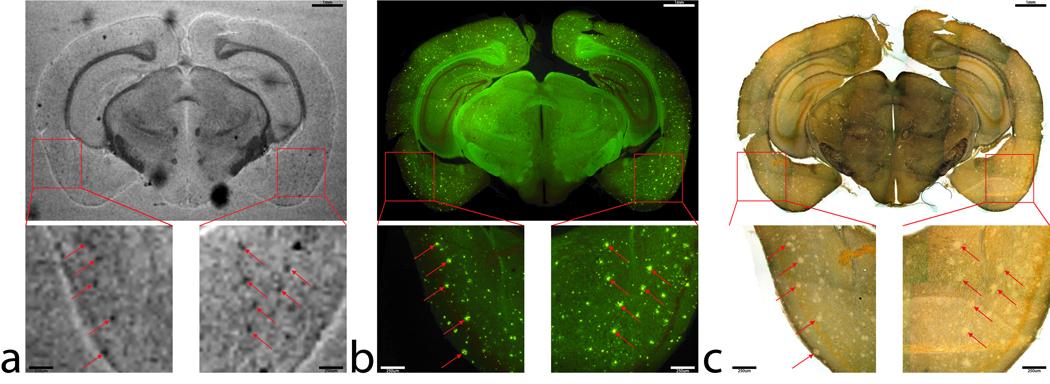
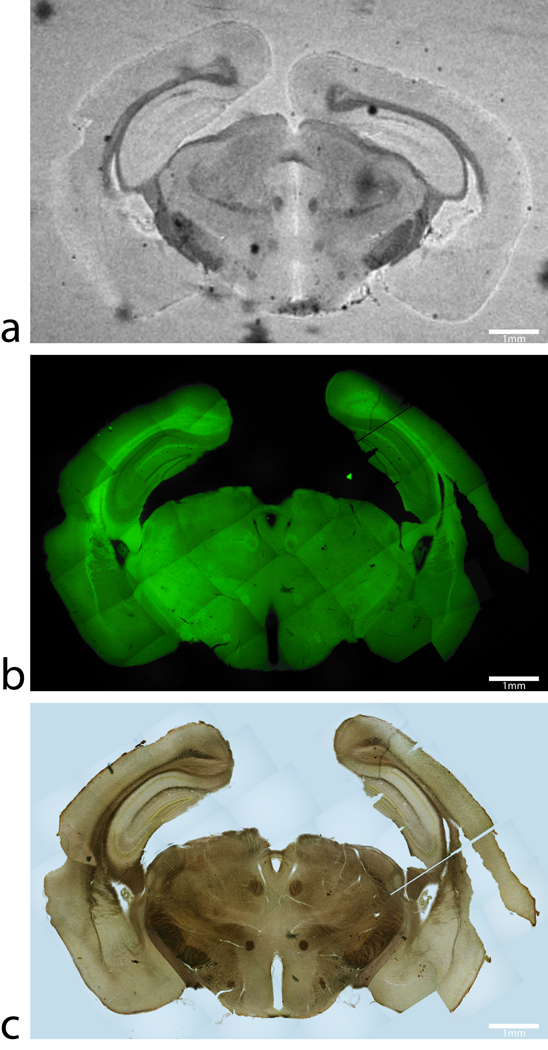
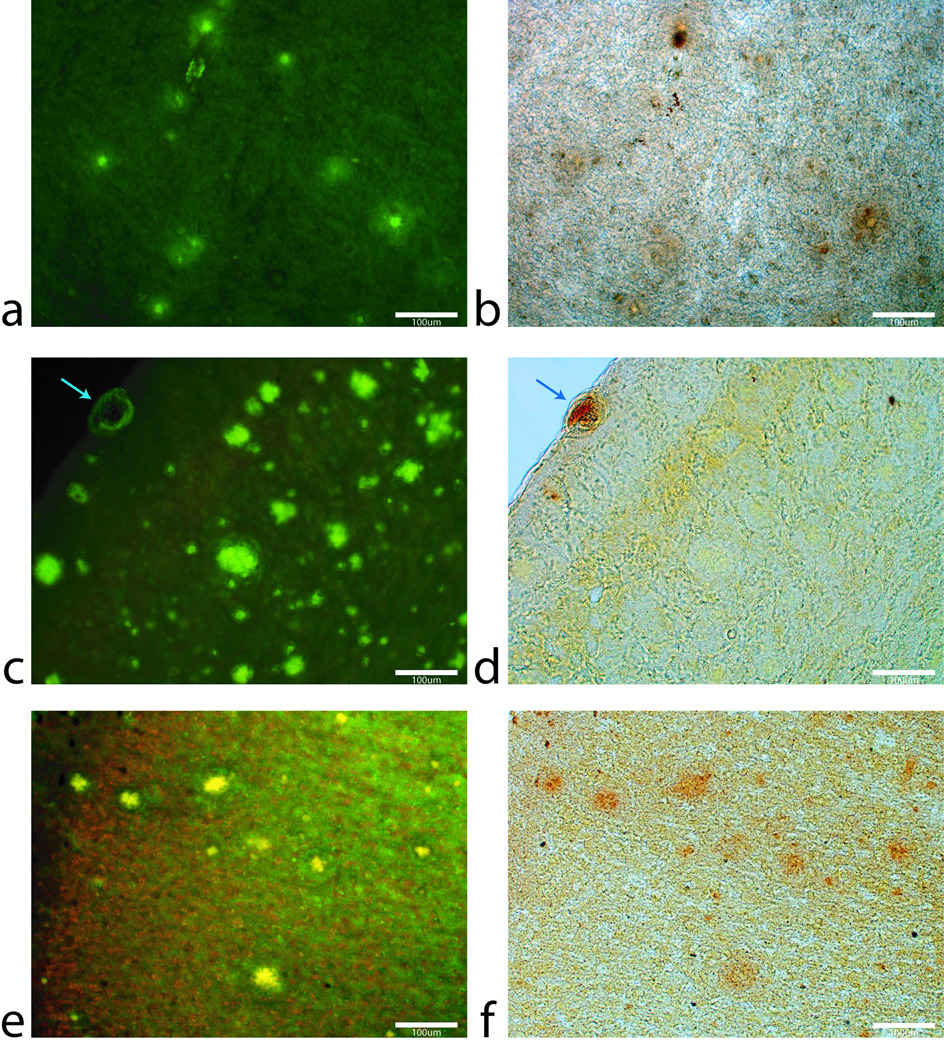
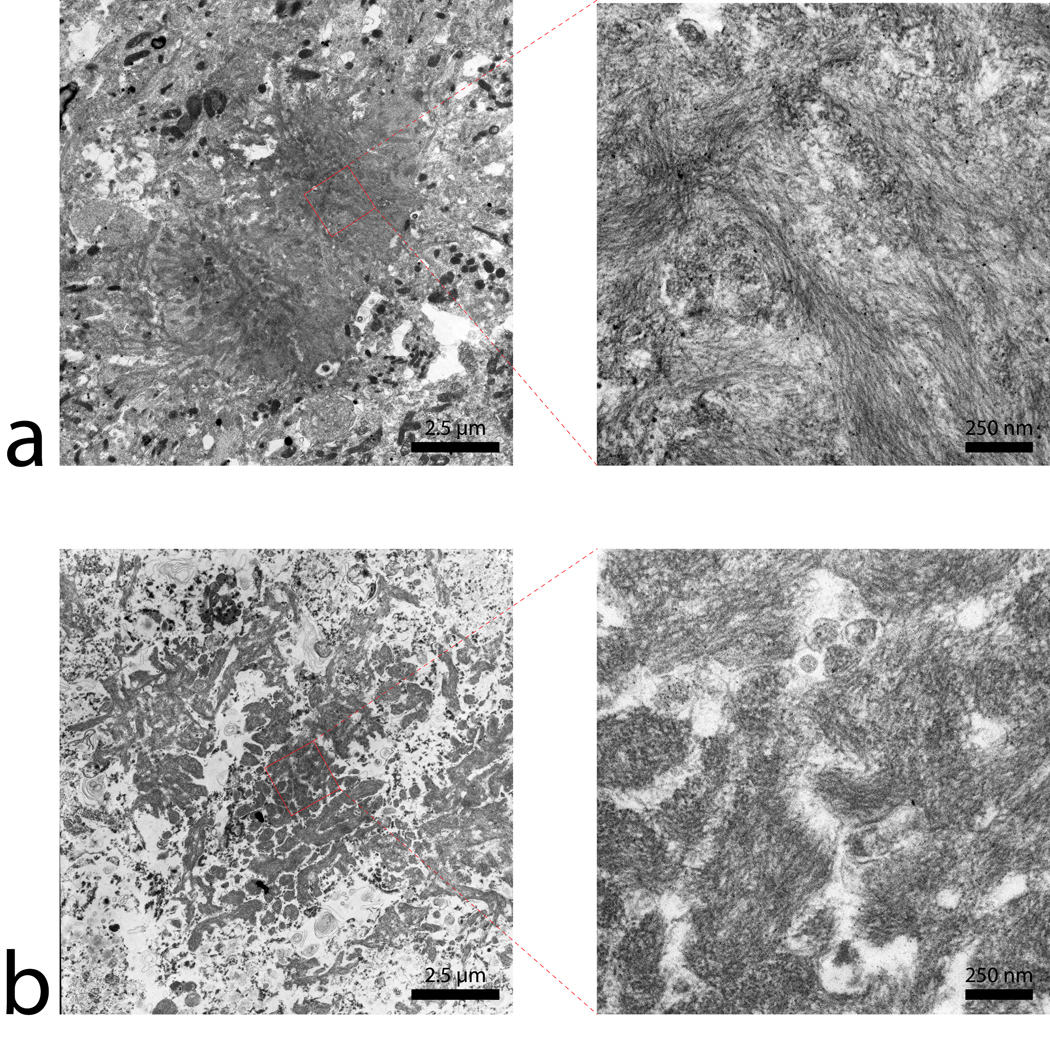
The corresponding images from APP/PS1 and age-matched C57BL/6 control mice tissue sections are shown in figure 3 and figure 4, respectively. The image scales of the animal MR images and histology mosaics for iron and Aβ plaques are the same as in the human data for ease of comparison. The widespread dark spots (red arrows) seen in the human tissue are also shown conspicuously in the T2* image of the transgenic animal tissue within the piriform cortex (Fig. 3a). Thioflavin-S staining (Fig. 3b) confirms that these dark spots are Aβ plaques within the APP/PS1 animal brain. MR imaging of mouse control tissue (Fig. 4a) shows no evidence of focal hypo-intense regions in the neo-cortex. The iron stain of the control mouse tissue in figure 4c shows that there are no visible focal iron regions in the neo-cortex or other gray matter regions. Thioflavin-S staining does not indicate any positive amyloid staining for plaques in mouse control sections apart from the expected non-specific background staining. Regions that are known to be high in iron, such as the substantia nigra and caudate/putamen, stain dark as expected (Fig. 3c) (14). The plaque distribution is consistent with previous studies using the APP/PS1 transgenic mouse line (4,15–17), while plaques are not present in the C57BL/6 control animal tissue. Measurements of the plaques within the APP/PS1 model indicate that MR microscopy observable plaques have a minimum diameter of approximately 40 µm, which is very similar to the human AD samples. Smaller plaques are also visible with Thioflavin-S staining throughout the neo-cortex (barrel fields, somatosensory, motor, and granular cortices), hippocampus, thalamic region, caudate and putamen of the animal brain but are not visible in the MR images. The most prominent difference between the human AD (Fig 1b and c) and APP/PS1 mouse tissues (Fig. 3b and c) involves the focal iron concentration found in the vicinity of the Aβ plaques. Large amounts of focal iron are found within and around the Thioflavin-S positive amyloid plaques in the human AD tissue while very little is seen in the APP/PS1 tissue. In the magnified images, the plaques indicated by the red arrows in figure 3b coincide with the white patches in figure 3c, suggesting lower iron content in these areas. This observation seems to be in opposition to what has been demonstrated in the human AD tissues. No dark iron cored plaques were observed in the transgenic animal histological images compared to the human AD sections. This is also at seen at higher magnification, as illustrated at 100x in figure 5. Aβ plaques in human AD tissue appear to be small cored plaques surrounded by large diffuse coronas while the plaques in the APP/PS1 mouse tissue exhibit larger dense cores made of compact fibrillar Aβ protein deposition with a smaller diffuse corona region. The Alzheimer’s tissue samples demonstrate the localization of iron (Fig. 5b) within the amyloid plaque mass (Fig. 5a). Transgenic animal tissue shows a high concentration of large amyloid deposits while co-staining with the traditional Perl’s ferric iron stain does not show regions of high iron within the these plaques. Staining capillaries high in iron serves as a positive control for the effectiveness of the traditional Perl’s stain for detecting iron, indicated by arrows in figures 5c and 5d. Minor digestion of the Aβ plaque periphery in the APP/PS1 tissue and staining with the modified Perl’s technique are shown in figures 5e and 5f. There is visible evidence of trace amounts of iron in the transgenic animal plaques that was not stainable with the traditional method. The iron load within the APP/PS1 plaques is very minute compared to the human AD tissue. Staining the human AD tissue (not shown) with the modified Perl’s technique rendered the samples completely brown, which is indicative of iron throughout the tissue samples. There is a possibility that the protein digestion also exposed the ferrihydrite and magnetite core of the ferritin complexes in these samples allowing the Prussian blue reaction to occur throughout the sample (18). This result rendered the microscopic detection of focal iron around Thioflavin-S plaques impractical for human AD sections stained in this manner. The iron stain of both APP/PS1 and control animal tissue does show appropriate tissue iron distribution as evident in the known high iron brain structures such as the substantia nigra, striatum and white matter tracks (19). High magnification 6000x and 46,000x transmission electron microscopy images of amyloid plaques from both APP/PS1 and human AD can be seen in figure 6a and b, respectively. Each plaque in figure 6a and b is similar in size, approximately 10 µm in diameter. The 6000x images include the whole plaque within the viewing plane and illustrate that Aβ plaques in the APP/PS1 model have a denser overall structure then the plaques found in human AD. Higher 46,000x magnification of the outlined regions shows fibrillar Aβ strands in both AD and APP/PS1 tissue and further demonstrates the difference in density between AD and APP/PS1 plaques.
FIG 3.
Co-registration of (a) magnetic resonance images, (b) beta-amyloid and (c) iron stains in the APP/PS1 mouse model. (a) An MGE T2* weighted image of a 60µm slice from a APP/PS1 mouse brain a approximately −2.92mm Bregma. Final in-plane image resolution was 45µm × 45 µm. Image magnifications (40×) of the selected regions of interest within the left and right piriform cortex are seen below. Hypo-intensities are noted by red arrows. (b) Thioflavin-S fluorescent mosaic image of the same tissue section in 1a. Beta-amyloid staining is evident and can be seen as the bright green positions. The same regions as in 1a have been magnified with the arrows pointing towards the plaques responsible for the T2* weighted hypo-intensities. (c) Perl’s iron stain with the same magnifications and regions of interest arrows as in 1a and 2a. The figure illustrates that the hypo-intensities seen in the T2* weighted image are in the same region as large beta-amyloid plaques approximately 50 – 60 µm in diameter. Iron deposition is not present at the plaque locations, as they seem to be regions of low iron concentration compared to the surrounding gray matter tissue. Scales bars for the magnifications are 250 µm and 1mm for the whole image.
FIG 4.
Magnetic resonance image, thioflavin-S and iron stains from a control C57BL/6 mouse. (a) An MGE T2* weighted image, (b) thioflavin-S, and (c) Perl’s iron stain of same 60 µm thick section of tissue from a C57BL/6 control mouse at approximately −2.80mm Bregma. The T2* weighted image shows hypo-intensities and iron staining at regions of known high iron concentration such as the substantia nigra, white matter tracks and the caudate/putamen. Thioflavin-S staining reveals only non-specific background staining with no beta-amyloid plaques in the control animals. There are no MR hypo-intensities that are associated with positive thioflavin-S staining. Scale bars in all images are 1mm.
FIG 5.
High magnification (100×) images of beta-amyloid plaques in both human Alzheimer’s entorhinal cortex (a,b) and APP/PS1 animals piriform cortex (c,d,e,f). The thioflavin-S stain (a) and traditional Perl’s stain (b) illustrate a close relationship between beta-amyloid plaques and focal iron deposition in Alzheimer’s disease. This relationship between plaques (c) and iron (d) is not clearly seen in the APP/PS1 animals with traditional Perl’s staining. The arrow in (c) and (d) illustrate iron deposition in a capillary of the APP/PS1 animal demonstrating the positive stain for iron. Staining with a modified Perl’s technique (e,f) via degradation of the plaques’ periphery proteins allows the aqueous stain to penetrate the plaques more readily. There is an indication of minute amounts of iron in the transgenic animal plaques that is not perceivable with the traditional Perl’s stain. Differences in plaque morphology between the AD and APP/PS1 animals is evident in images (a) and (c,e). The human AD plaques have a dense core of fibrillar amyloid protein with a halo of amyloid protein around them that is less susceptible to thioflavin-S staining. APP/PS1 Aβ plaques show a larger and denser thioflavin-S positive core with a smaller halo region around them. The Perl’s stains indicate that high concentrations of iron found throughout the human AD plaques that associated with the amyloid protein or within cells such as microglia inside the space the plaque has occupied. Compared to the human AD plaques, the APP/PS1 images show a reduction in focal iron within the plaques that is diffusely found throughout the plaque. Scale bars for all images are 100µm.
FIG 6.
Transmission electron microscope images of plaques in (a) APP/PS1 and (b) human AD tissue. The 6000× magnification images of the plaques include the whole plaque within the imaging plane. The plaque diameter for both APP/PS1 and AD tissue is approximately the same, at 10 µm. The lower magnification image illustrates the denser overall structure of the beta-amyloid plaques in the APP/PS1 compared to the human AD tissue. The human AD plaques have numerous gaps present between the fibril bundles throughout them that are rarely found in the transgenic mouse plaques. 46,000× magnification of the outlined regions reveal differences in the fibrillar orientation of the beta-amyloid strands in the APP/PS1 and AD plaques. Magnification of the outlined regions also illustrates the denser nature of the APP/PS1 plaques while the human AD tissue has gaps between amyloid strand clusters.
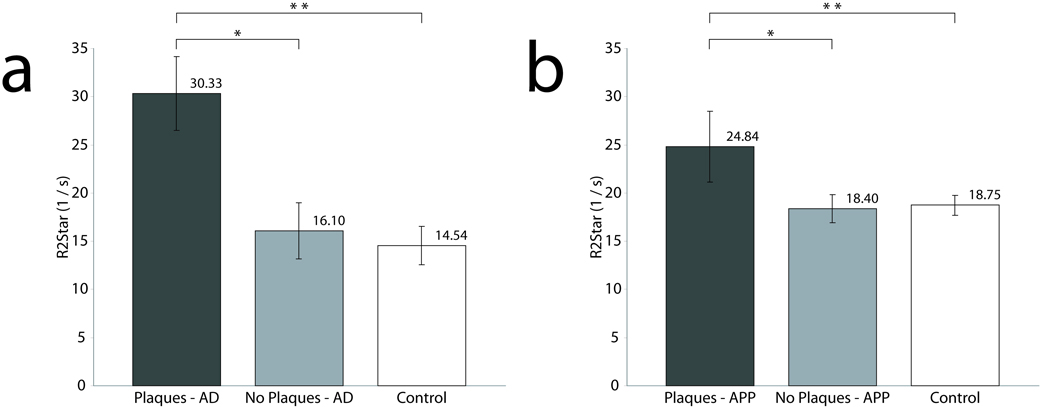
For quantitative MRI studies, Fig. 7a graphically shows average relaxation values for regions of interest that included and excluded Aβ plaques within the AD sample and regions within the gray matter of the control sample. Average R2* measurements for the AD tissue samples were 30.33 ± 3.86 (1/s) in regions with plaques and 16.10 ± 2.92 (1/s) in regions without plaques. Control tissue R2* relaxation rate was 14.54 ± 2.01 (1/s). R2* relaxation rates for the regions with plaques were significantly higher (p<0.0001) than both the controls and regions without plaques. Relaxation measurements in regions without plaques were not significantly different from control tissue. Average relaxation values for gray matter regions in the APP/PS1 mice that included or excluded Aβ plaques and control animals are seen in figure 7b. Average R2* measurements for APP/PS1 were 24.84 ± 3.67 (1/s) in regions with plaques and 18.40 ± 1.47 (1/s) in regions without plaques. C57BL/6 controls had an average R2* relaxation of 18.75 ± 1.03 (1/s). Regions with plaques in the APP/PS1 animals had a significantly higher R2* relaxation compared to both control tissue and regions without plaques (p<0.0001). Measurements of R2* relaxation were not significantly different between regions of interest in the transgenic animal devoid of plaques and control mouse tissue.
FIG 7.
Bar graphs of average R2* rates from ROI’s with plaques, without plaques and control tissue within human (a) and mouse tissue (b). The R2* relaxation rate of plaque ROI’s in the AD tissue is significantly higher than both regions without plaques and control tissue sections. A similar trend is found in mouse data. There is also a higher R2* relaxation rate for the plaque ROI’s in AD than in the APP/PS1 mouse, hypothesized to be due to higher iron in the AD plaques as shown with the Perl’s stain in Fig. 5 (b,d and e).
Discussion
Understanding the relationship between beta-amyloid plaque pathology in AD and the associated MRI contrast is fundamentally important for MRI study of AD pathology. This work demonstrates the ability to image Aβ plaques in histology sections of both human AD and amyloid-generating APP/PS1 transgenic mice brains. The histological MR images taken at a field strength of 7.0 T clearly show high resolution details of Aβ plaques in human and animal samples. Previous MRI studies of Aβ plaques have been carried out with blocks of human AD brain tissue samples (1). With this approach, accurately matching histological sections of the whole tissue block with the MRI image slice is difficult. Using the histological RF coil, MRI and histological analyses were performed on the same tissue sample so that tissue pathology and associated MRI contrast changes could be precisely compared. This capability allowed us to establish a one-to-one relationship between MRI parameters and histology data acquired from human AD and APP/PS1 transgenic mouse tissue samples. Such a relationship is important for the interpretation of MRI findings in the AD brain and its animal models. Additionally, the information acquired with this technology is valuable in the development of potential therapies for AD research and investigations of other pathologies.
The MR imaging of amyloid plaques in transgenic APP/PS1 mice has been pioneered by several groups using both in vivo and ex vivo imaging methodologies (4,15,16). The hypo-intensities in the MR images of APP/PS1 animal tissue that coincide with the plaques are similar in signal dropout and size compared to MR images of human AD plaques (Fig. 1 and Fig. 3). The cause for the hypo-intensities seen in T2* and T2 weighted images of APP/PS1 mice thus has been believed to be caused by high iron accumulation in the plaques (4,15,16). However, when viewing the iron and Aβ stained images in Fig. 3 and Fig. 5, the iron concentration is significantly reduced in the APP/PS1 Aβ plaques compared to the human AD tissue in Fig. 1c, while Aβ-plaques in APP/PS1 and human AD samples are perceptibly the same when viewed with MR images. To confirm that the staining methods did not cause aberrant interactions with one another, independent iron and amyloid stains were performed yielding the same appearance as co-staining procedures. To understand this unexpected result, notice that APP/PS1 plaques in Fig. 3b and Fig. 5 show an extremely dense nature, which suggested that the current traditional Perl’s staining method might not be able to stain the iron inside these plaques. The hydrophobic nature of the plaques coupled with the acidic aqueous based Perl’s stain could prevent the Prussian blue reaction from occurring if the stain cannot infiltrate into the plaque. A modified Perl’s stain (8) was tested that utilizes protein degradation and detergents to break down the outer surface of the plaques to allow better peripheral penetration of the aqueous stain. With the modified stain, the images in Fig. 5f indeed exposed minute focal iron distributions within the APP/PS1 transgenic animal plaques, but much less than that in the human AD plaques. As a control condition, application of the modified stain to human AD tissues resulted in dark brown staining throughout the entire tissue sample (not shown). This could be due to staining of not only the free labile iron pool but additionally iron bound inside ferritin cores that can be stained only when the proteins are digested open with the modified staining procedure. The above observations suggested that iron in the animal plaques is significantly less than that in the human plaques and the plaques in the animals are much denser than those in human samples. Since MR images of the plaques in both human and animals appeared essentially the same, the differing characteristics of the animal plaques raised a fundamental question as to the cause of the MR image contrast associated with the animal plaques.
It is well known that T2 and T2* relaxation and contrast can be altered in a variety of manners. Iron bound within the ferrihydrite-like core of ferritin, hemosiderin and assorted a number of iron oxide mineral deposits are found in sufficient quantities in the brain to alter MRI contrast (20–24). As a ferromagnetic ion, ferric iron (Fe+3) is known to cause a shortening of T2 relaxation due to the creation of microscopic magnetic field inhomogeneities that de-phase the signal of water protons in the vicinity (19,25,26). Thus, ferric iron in brain tissue acts as a natural contrast agent causing faster proton T2 and T2* relaxation. There is some evidence to support that small amounts of ferrous iron (Fe+2) is also stained with the Perl’s stain reaction (27). The effect of ferrous iron on T2* and T2 relaxation is much less pronounced than that caused by the ferric form (28) (our laboratory’s unpublished data). Thus, the signal hypo-intensities seen in the MR images associated with focal iron regions is likely dominated by the ferric form. One other important factor associated with the MRI appearance of plaques is their size under given experimental conditions (pixel resolution, TE/TR times, etc.). The minimal plaque size viewable in our MR images of the APP/PS1 mice was similar to the AD tissue images, about 40 µm and 38 µm, respectively. Thus, due to the similarity in the plaque size for both species, plaque diameter alone does not account for the hypo-intensities seen in the APP/PS1 data. This is also supported by the fact that human AD plaques of a similar diameter with low observable iron are marginally visible on the MR images, while APP/PS1 plaques with a significantly less iron association are clearly discernable on the MR images. Magnification of the thioflavin-S positive plaques (100x seen in figure 5 and figure 6000x & figure 46000x in figure 6) shows a distinct difference in plaque morphology between AD and APP/PS1 mice. Differences between human AD and the APP/PS1 Aβ plaques are most noticeable in the TEM magnifications. The human AD plaques consist of fragmented patches with random fibrillar orientation in the amyloid core while the structure of transgenic mouse plaques appears to be a highly packed aggregation of long and oriented fibrils. The decreased density of the amyloid core creates gaps that are prevalent in the human AD plaques while rarely found in the transgenic mouse plaques. The APP/PS1 mice Thioflavin-S data illustrates that, while the plaques are a similar size to those in AD tissue, the plaques have a larger center core of dense compact amyloid. In previous studies, comparison of the structure of the amyloid fibril in the transgenic APP/PS1 model and human AD is subtly different due to post-translational modifications leading to alterations in the Aβ molecule (29–31). Plaques found in the APP/PS1 mouse model have a variable distribution of both human and mouse amyloid in the plaques. Diffuse plaques have an intermingled distribution of both human and mouse amyloid while dense plaques are composed of human amyloid cores surrounded by mouse amyloid (32). Thioflavin-S is known to bind to fibrillar but not to diffuse Aβ deposits (33,34). The difference in Thioflavin-S staining between human AD and APP/PS1 mice could be due to the different primary fibrillar or diffuse composition of the plaques. Many of the amino-acid residue positions within the Aβ protein are hydrophobic and are sufficient for amyloidal fibril formation (35,36). With the additional two hydrophobic amino acids found in the Aβ42, it aggregates more readily than Aβ40 (37). These views suggest that the hydrophobic regions are at least partially responsible for the fibril formation (38). The morphology of the mouse plaques with their large globular nature and the dense center core seem to be responsible for the T2* relaxation associated with these plaques. Unlike smaller proteins, the amyloid mass found in the animal plaques is rigid and fixed in the tissue resulting in a situation where the protein mass would behave similar to a polymer-like solid. This creates a number of consequences that may affect relaxation in the plaques. First, it sets up an environment where a large amount of available surface area is present inside the plaque mass along the component Aβ fibrils. Water molecules then become bound to the hydrophilic regions of the fibril axis while repelled by the hydrophobic regions. Water in the immediate vicinity of the hydrophilic regions is bound via hydrogen bonding such that it is rotationally or irrotationally bound. Layers of water are affected as they diffuse near the bound water. Cross-relaxation between the protons of bound water and the protein molecules by proton-proton magnetization exchange could lead to a more rapid relaxation of water proton. As such, the relaxation of bound water on the Aβ fibril chains would act as a relaxation sink for water moving in the surrounding area (39,40). As a result, T2 relaxation shortening occurs. Secondly, the magnetic susceptibility differences between the highly compact Aβ protein mass and surrounding issues could induce static dephasing in addition to that caused by deposited iron. Thirdly, tissue hydration could be also a significant contributing factor for plaque contrast. The dense formation of the animal plaques as indicated in the TEM image limits free water from accessing their core. As shown by histology results, staining iron in the plaques in the APP/PS1 mouse was difficult following standard methodology without protein degradation treatment. These experimental data suggest that access of free water molecules to the animal plaque is very limited. For human samples, the gaps seen between the Aβ patches (Fig. 6b) would accommodate more free water pools. The transverse relaxation of these water molecules is shortened by the magnetic inhomogeneities caused by ferromagnetic iron in the plaques. Thus, with these observations we would suggest a dual relaxation mechanism for the generation Aβ plaques T2* contrast associated with both the compact fibrillar protein mass and iron deposition. The focal iron concentration could play a dominant role for rapid T2* relaxation in human AD plaques while the increased fibrillar density and compacted morphology would be more likely a major factor for T2* shortening in the APP/PS1 transgenic plaques. Although the potential relaxation mechanisms in Aβ plaques discussed above are preliminary and speculative, it is important to realize that alternative relaxation mechanisms must be considered. To thoroughly develop and validate the model of relaxation in Aβ plaques, more experimental studies are required in future investigations. In addition, caution should be used when comparing ex vivo R2* contrast obtained in the fixed tissue samples and extrapolating this to in vivo conditions (5).
The quantitative comparison of the R2* maps from different tissue types supports our hypothesis in regard to the relaxation mechanism associated with Aβ plaques. The mean R2* for the selected amyloid plaques in the AD tissues (Fig. 7a) is on average 90% greater than that from the ROI’s without plaques and control tissue. Regions in AD gray matter with no Aβ plaques and in control tissue samples (Fig. 7a) have no statistical difference in relaxation time. However, there is a trend of faster R2* relaxation rates in regions without plaques throughout the Alzheimer’s tissue compared to control tissue samples. This is indicative of a higher overall iron concentration in the AD neural tissue compared to the control tissue, possibly due to a systemic inability to regulate iron properly within the brain. Iron staining of the tissue samples supports this, which shows an overall increase in high focal iron in the AD tissue samples that is not seen in the control tissue. In the mouse tissue samples (Fig. 7b), the mean R2* for the selected amyloid plaques is on average 56% higher than that in the ROI’s without plaques and in the control tissue. The mean R2* of regions without plaques and of control tissue are nearly identical, indicative of similar tissue fixation across tissue sub-types and consistent preparation of tissue samples. This information alleviates the confounding possibility that separate tissue handling procedures are the source of the observed MR contrast differences. Comparison of R2* in ROI’s with plaques in both human and APP/PS1 animals tissues reveal that the human plaques have a significantly higher R2* value. It is conceivable that human plaques have a duality of relaxation where both high focal iron concentration and fibrillar Aβ masses cause rapid proton transverse magnetization decay. APP/PS1 plaques exhibiting slower decay due to the reduced concentration of iron is a reasonable explanation for this result. The R2* for normal human tissues also appeared to be higher than that of animal tissue. This is consistent with the overall higher iron content in human neural tissue compared to the APP/PS1 animal samples. Iron staining for regions without plaques in the gray matter of APP/PS1 tissue and mouse control tissue samples exhibit similar patterns of iron staining.
These findings generate several important questions regarding the difference between Aβ plaques found naturally occurring in the human Alzheimer’s brain and transgenically introduced into the APP/PS1 animal model. Previous studies have revealed that Aβ-plaques in APP/PSI animal show numerous differences from human AD plaques, including plaque morphology and composition (31,41). The difference in morphology and the reduction of iron staining in the plaques raises the question for translating the APP/PS1 model’s data to human AD as it is hypothesized that there is a link between Aβ plaques and elevated iron in the brain tissue as the cause of oxidative stress leading to cellular neurotoxicity (42,43). The relationship of iron to plaque generation is not currently well understood within the literature and is a subject of much investigation. An imbalance in the iron regulatory system and a dysfunctional difference in mineralization of iron within the ferritin core in AD subjects has been previously demonstrated (11,44). It is largely unknown whether the initial generation of the plaque causes the aggregation of iron or if aberrant regulation of extra- or intra-cellular iron stores cause the formation of the Aβ protein around it. There is general agreement that the location of Aβ plaques in human AD brain tissue coincides with focal iron deposits. Iron associated with plaques can accumulate from a number of potential sources. As a metalloprotein, free iron will bind to Aβ fibrils and collect within the plaques. Iron from ferritin, and its degradation to hemosiderin, can originate from nearby neurons and microglial cells that have migrated to the Aβ plaques and can become part of the plaque mass (45–47). Biogenic magnetite found proportionally higher in AD ferritin cores than normal aged human neural tissue is known to be accumulate within Aβ plaques (10,11). While it is unknown precisely how Aβ plaque aggregation occurs, it is known that metal ions do play a role in Aβ fibril formation. The conversion of Aβ’s secondary protein structure to a β-sheet has been shown to occur in the presence of divalent metal ions, including ferrous iron (48). The component fibrils of the Aβ plaques are known metalloproteins and possess metal chelation regions in their amino-acid sequence (49). The MR and histological images in Fig. 1 demonstrate that Aβ plaques are consistently associated with elevated iron load in and around their vicinity in human AD brain tissue. Research has shown that the co-localization of iron with the human Aβ plaques is accompanied by endoplasmic reticulum stress induced apoptosis, DNA oxidation and cellular damage in cells adjacent to plaques. Conversely, in regions where Aβ plaques accumulate alone without iron there is no indication of oxidative stress or apoptosis pathway activation (50). It has been demonstrated that the toxicity of Aβ is amplified upon the direct interaction of iron ions with the Aβ peptide, while unbound iron itself has no effect upon toxicity (51). This strongly suggests that Aβ plaques by themselves are not toxic to cells adjacent to plaques, whereas iron accumulation in and around plaques is essential for neuronal damage. The morphology and associated iron dissimilarity between the human AD and transgenic APP/PS1 plaques raises a question regarding differing amyloidal genesis processes for each species. In the APP/PSI mouse model used here, plaques are produced in the brain as early as six months after birth and are believed to continually grow throughout the life span of the animal (52). Current understanding of human amyloidal genesis suggests that plaque generation occurs over years, perhaps decades, and places the plaques into discrete classes based upon the morphology of the plaque and surrounding tissue. It is hypothesized that plaques start out as a seed formations of Aβ peptide and eventually become much more compact and dense over time (53). The APP/PS1 model generates plaques at a rapid pace and iron staining indicates a reduced amount of iron associated with these plaques compared to AD tissue. It is plausible that the rapid fibrillarization from Aβ oligomers into compacted fibrillar plaques could lead to less of iron deposition within the plaques in the APP/PS1 model.
In summary, imaging of thin slices of tissue samples with the aid of the histological coil allows the one-to-one comparison of tissue pathology as seen in histological stains and different MRI methodologies. With this technique, the relaxation mechanisms for Aβ plaques in human Alzheimer’s disease and the APP/PS1 mouse model were investigated with respect to their morphology and relationship with iron deposition. The histology stains of AD and APP/PS1 tissue samples were directly compared to T2*-weighted images and R2* parametric maps. The histological iron stains presented here support the hypothesis that iron associated with the Aβ plaques in the human AD samples plays a major role in generation of MRI T2* contrast. Histological data from previous studies using post mortem human AD tissue (14,45,54,55) also support the notion that focal iron load in Aβ plaques is the dominant cause for faster T2 and T2* decay. The electron microscopy and histology data revealed that there are important differences in plaque morphology and associated iron concentration between transgenic APP/PS1 mice and human AD tissue samples. In the Alzheimer’s tissue, beta-amyloid plaques with high iron concentration are clearly visible in the T2*-weighted images while others of similar size with less focal iron are not as discernable. In the APP/PS1 animal tissue, large plaques are equally observable as human plaques in MR imaging while iron load is significantly less than human plaques. This suggests that there is a degree of difference between the amyloid plaques in the APP/PS1 mouse model and human Alzheimer’s, in respect to their morphology and relationship with in vivo iron stores. The increased transverse proton relaxation rate in Aβ plaques in animals is likely caused mainly by the interactions of water with the highly compacted amyloid fibril mass. The improved resolution of the ex vivo data set allows for the detailed comparison between tissue histology and MR contrast that is not possible with current lower resolution clinically based protocols. The extrapolation of microscopic MR images to in vivo applications is promising in the near future considering the rapid increase in achievable image resolution with the development of higher magnetic fields and the usage of parallel imaging technology (56). The data presented in this report are essential for understanding the histo-pathological underpinning of MRI measurement of Aβ plaques in humans and animal models for both current and future MR applications.
Acknowledgements
The authors would like to thank Dr. Michael Garwood of the CMRR at the University of Minnesota for his insightful discussion in regard to the transverse relaxation mechanism and Roland Myers of the Electron Microscopy Core Facility for help with the transmission electron microscope data collection.
Funding for this project has been made available in part through the Bioengineering Research Partnership – NIH grants 5R01EB000459-4 and R01AG027771, the George M. Leader Foundation and, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds.
References
- 1.Benveniste H, Einstein G, Kim KR, Hulette C, Johnson GA. Detection of neuritic plaques in Alzheimer's disease by magnetic resonance microscopy. Proc Natl Acad Sci U S A. 1999;96(24):14079–14084. doi: 10.1073/pnas.96.24.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Garwood M, Wengenack TM, Borowski B, Curran GL, Lin J, Adriany G, Grohn OH, Grimm R, Poduslo JF. In vivo visualization of Alzheimer's amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn Reson Med. 2004;52(6):1263–1271. doi: 10.1002/mrm.20266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Marjanska M, Wengenack TM, Reyes DA, Curran GL, Lin J, Preboske GM, Poduslo JF, Garwood M. Magnetic resonance imaging of Alzheimer's pathology in the brains of living transgenic mice: a new tool in Alzheimer's disease research. Neuroscientist. 2007;13(1):38–48. doi: 10.1177/1073858406295610. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Adriany G, Poduslo JF. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer's transgenic mice. J Neurosci. 2005;25(43):10041–10048. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meadowcroft MD, Zhang S, Liu W, Park BS, Connor JR, Collins CM, Smith MB, Yang QX. Direct magnetic resonance imaging of histological tissue samples at 3.0T. Magn Reson Med. 2007;57(5):835–841. doi: 10.1002/mrm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13(6):159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 7.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 8.LeVine SM. Iron deposits in multiple sclerosis and Alzheimer's disease brains. Brain Res. 1997;760(1–2):298–303. doi: 10.1016/s0006-8993(97)00470-8. [DOI] [PubMed] [Google Scholar]
- 9.LeVine SM. Oligodendrocytes and myelin sheaths in normal, quaking and shiverer brains are enriched in iron. J Neurosci Res. 1991;29(3):413–419. doi: 10.1002/jnr.490290317. [DOI] [PubMed] [Google Scholar]
- 10.Collingwood JF, Chong RK, Kasama T, Cervera-Gontard L, Dunin-Borkowski RE, Perry G, Posfai M, Siedlak SL, Simpson ET, Smith MA, Dobson J. Three-dimensional tomographic imaging and characterization of iron compounds within Alzheimer's plaque core material. J Alzheimers Dis. 2008;14(2):235–245. doi: 10.3233/jad-2008-14211. [DOI] [PubMed] [Google Scholar]
- 11.Quintana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, Ferrer I, Arranz R, Patino C. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer's disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol. 2006;153(1):42–54. doi: 10.1016/j.jsb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Gerber MR, Connor JR. Do oligodendrocytes mediate iron regulation in the human brain? Ann Neurol. 1989;26(1):95–98. doi: 10.1002/ana.410260115. [DOI] [PubMed] [Google Scholar]
- 13.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17(2):83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Switzer RC., 3rd The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11(3):595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 15.Dhenain M, El Tanni El, El Tayara N, Wu TD, Guegan M, Volk A, Quintana C, Delatour B. Characterization of in vivo MRI detectable thalamic amyloid plaques from APP/PS1 mice. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Vanhoutte G, Dewachter I, Borghgraef P, Van Leuven F, Van der Linden A. Noninvasive in vivo MRI detection of neuritic plaques associated with iron in APP[V717I] transgenic mice, a model for Alzheimer's disease. Magn Reson Med. 2005;53(3):607–613. doi: 10.1002/mrm.20385. [DOI] [PubMed] [Google Scholar]
- 17.Falangola MF, Ardekani BA, Lee SP, Babb JS, Bogart A, Dyakin VV, Nixon R, Duff K, Helpern JA. Application of a non-linear image registration algorithm to quantitative analysis of T2 relaxation time in transgenic mouse models of AD pathology. J Neurosci Methods. 2005;144(1):91–97. doi: 10.1016/j.jneumeth.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvez N, Fernandez B, Sanchez P, Cuesta R, Ceolin M, Clemente-Leon M, Trasobares S, Lopez-Haro M, Calvino JJ, Stephan O, Dominguez-Vera JM. Comparative structural and chemical studies of ferritin cores with gradual removal of their iron contents. J Am Chem Soc. 2008;130(25):8062–8068. doi: 10.1021/ja800492z. [DOI] [PubMed] [Google Scholar]
- 19.Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA. MRI of brain iron. AJR Am J Roentgenol. 1986;147(1):103–110. doi: 10.2214/ajr.147.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Gossuin Y, Hautot D, Muller RN, Pankhurst Q, Dobson J, Morris C, Gillis P, Collingwood J. Looking for biogenic magnetite in brain ferritin using NMR relaxometry. NMR Biomed. 2005;18(7):469–472. doi: 10.1002/nbm.983. [DOI] [PubMed] [Google Scholar]
- 21.Gossuin Y, Roch A, Muller RN, Gillis P. Relaxation induced by ferritin and ferritin-like magnetic particles: the role of proton exchange. Magn Reson Med. 2000;43(2):237–243. doi: 10.1002/(sici)1522-2594(200002)43:2<237::aid-mrm10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Bulte JW, Vymazal J, Brooks RA, Pierpaoli C, Frank JA. Frequency dependence of MR relaxation times. II. Iron oxides. J Magn Reson Imaging. 1993;3(4):641–648. doi: 10.1002/jmri.1880030414. [DOI] [PubMed] [Google Scholar]
- 23.Vymazal J, Brooks RA, Baumgarner C, Tran V, Katz D, Bulte JW, Bauminger R, Di Chiro G. The relation between brain iron and NMR relaxation times: an in vitro study. Magn Reson Med. 1996;35(1):56–61. doi: 10.1002/mrm.1910350108. [DOI] [PubMed] [Google Scholar]
- 24.Vymazal J, Zak O, Bulte JW, Aisen P, Brooks RA. T1 and T2 of ferritin solutions: effect of loading factor. Magn Reson Med. 1996;36(1):61–65. doi: 10.1002/mrm.1910360111. [DOI] [PubMed] [Google Scholar]
- 25.Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23(1):1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Bizzi A, Brooks RA, Brunetti A, Hill JM, Alger JR, Miletich RS, Francavilla TL, Di Chiro G. Role of iron and ferritin in MR imaging of the brain: a study in primates at different field strengths. Radiology. 1990;177(1):59–65. doi: 10.1148/radiology.177.1.2399339. [DOI] [PubMed] [Google Scholar]
- 27.Meguro R, Asano Y, Iwatsuki H, Shoumura K. Perfusion-Perls and -Turnbull methods supplemented by DAB intensification for nonheme iron histochemistry: demonstration of the superior sensitivity of the methods in the liver, spleen, and stomach of the rat. Histochem Cell Biol. 2003;120(1):73–82. doi: 10.1007/s00418-003-0539-y. [DOI] [PubMed] [Google Scholar]
- 28.Thomas LO, Boyko OB, Anthony DC, Burger PC. MR detection of brain iron. AJNR Am J Neuroradiol. 1993;14(5):1043–1048. [PMC free article] [PubMed] [Google Scholar]
- 29.Bussiere T, Bard F, Barbour R, Grajeda H, Guido T, Khan K, Schenk D, Games D, Seubert P, Buttini M. Morphological characterization of Thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance. Am J Pathol. 2004;165(3):987–995. doi: 10.1016/s0002-9440(10)63360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson JA, Burns DK. Mouse models of Alzheimer's disease: a quest for plaques and tangles. Ilar J. 2002;43(2):89–99. doi: 10.1093/ilar.43.2.89. [DOI] [PubMed] [Google Scholar]
- 31.Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Groen T, Kiliaan AJ, Kadish I. Deposition of mouse amyloid beta in human APP/PS1 double and single AD model transgenic mice. Neurobiol Dis. 2006;23(3):653–662. doi: 10.1016/j.nbd.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Stopa B, Piekarska B, Konieczny L, Rybarska J, Spolnik P, Zemanek G, Roterman I, Krol M. The structure and protein binding of amyloid-specific dye reagents. Acta Biochim Pol. 2003;50(4):1213–1227. [PubMed] [Google Scholar]
- 34.Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, Olschowka JA, Fonseca MI, O'Banion MK, Tenner AJ, Lemere CA, Duff K. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease. Am J Pathol. 2001;158(4):1345–1354. doi: 10.1016/S0002-9440(10)64085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorevic PD, Castano EM, Sarma R, Frangione B. Ten to fourteen residue peptides of Alzheimer's disease protein are sufficient for amyloid fibril formation and its characteristic x-ray diffraction pattern. Biochem Biophys Res Commun. 1987;147(2):854–862. doi: 10.1016/0006-291x(87)91008-4. [DOI] [PubMed] [Google Scholar]
- 36.Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Dobeli H, Schubert D, Riek R. 3D structure of Alzheimer's amyloid-beta(1–42) fibrils. Proc Natl Acad Sci U S A. 2005;102(48):17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Liu J, McCallum SA, Yang D, Wang C. Methyl dynamics of the amyloid-beta peptides Abeta40 and Abeta42. Biochem Biophys Res Commun. 2007;362(2):410–414. doi: 10.1016/j.bbrc.2007.07.198. [DOI] [PubMed] [Google Scholar]
- 38.Kim W, Hecht MH. Generic hydrophobic residues are sufficient to promote aggregation of the Alzheimer's Abeta42 peptide. Proc Natl Acad Sci U S A. 2006;103(43):15824–15829. doi: 10.1073/pnas.0605629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fullerton GD, Potter JL, Dornbluth NC. NMR relaxation of protons in tissues and other macromolecular water solutions. Magn Reson Imaging. 1982;1(4):209–226. doi: 10.1016/0730-725x(82)90172-2. [DOI] [PubMed] [Google Scholar]
- 40.Fullerton GD, Finnie MF, Hunter KE, Ord VA, Cameron IL. The influence of macromolecular polymerization of spin-lattice relaxation of aqueous solutions. Magn Reson Imaging. 1987;5(5):353–370. doi: 10.1016/0730-725x(87)90125-1. [DOI] [PubMed] [Google Scholar]
- 41.Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143(2):461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Perry G, Cash AD, Smith MA. Alzheimer Disease and Oxidative Stress. J Biomed Biotechnol. 2002;2(3):120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MA. Oxidative stress and iron imbalance in Alzheimer disease: how rust became the fuss! J Alzheimers Dis. 2006;9(3 Suppl):305–308. doi: 10.3233/jad-2006-9s334. [DOI] [PubMed] [Google Scholar]
- 44.Connor JR, Menzies SL. Cellular management of iron in the brain. J Neurol Sci. 1995;134 Suppl:33–44. doi: 10.1016/0022-510x(95)00206-h. [DOI] [PubMed] [Google Scholar]
- 45.Connor JR, Snyder BS, Beard JL, Fine RE, Mufson EJ. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer's disease. J Neurosci Res. 1992;31(2):327–335. doi: 10.1002/jnr.490310214. [DOI] [PubMed] [Google Scholar]
- 46.Wegiel J, Wang KC, Imaki H, Rubenstein R, Wronska A, Osuchowski M, Lipinski WJ, Walker LC, LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging. 2001;22(1):49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 47.Mehlhase J, Gieche J, Widmer R, Grune T. Ferritin levels in microglia depend upon activation: modulation by reactive oxygen species. Biochim Biophys Acta. 2006;1763(8):854–859. doi: 10.1016/j.bbamcr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Miura T, Mitani S, Takanashi C, Mochizuki N. Copper selectively triggers beta-sheet assembly of an N-terminally truncated amyloid beta-peptide beginning with Glu3. J Inorg Biochem. 2004;98(1):10–14. doi: 10.1016/j.jinorgbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Smith DG, Cappai R, Barnham KJ. Biochim Biophys Acta. 2007. The redox chemistry of the Alzheimer's disease amyloid beta peptide. [DOI] [PubMed] [Google Scholar]
- 50.Ghribi O, Golovko MY, Larsen B, Schrag M, Murphy EJ. Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem. 2006;99(2):438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- 51.Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30(4):447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 52.Wengenack TM, Whelan S, Curran GL, Duff KE, Poduslo JF. Quantitative histological analysis of amyloid deposition in Alzheimer's double transgenic mouse brain. Neuroscience. 2000;101(4):939–944. doi: 10.1016/s0306-4522(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 53.Satou T, Cummings BJ, Head E, Nielson KA, Hahn FF, Milgram NW, Velazquez P, Cribbs DH, Tenner AJ, Cotman CW. The progression of beta-amyloid deposition in the frontal cortex of the aged canine. Brain Res. 1997;774(1–2):35–43. doi: 10.1016/s0006-8993(97)81684-8. [DOI] [PubMed] [Google Scholar]
- 54.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3(1):41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- 55.Connor JR, Snyder BS, Arosio P, Loeffler DA, LeWitt P. A quantitative analysis of isoferritins in select regions of aged, parkinsonian, and Alzheimer's diseased brains. J Neurochem. 1995;65(2):717–724. doi: 10.1046/j.1471-4159.1995.65020717.x. [DOI] [PubMed] [Google Scholar]
- 56.Nakada T, Matsuzawa H, Igarashi H, Fujii Y, Kwee IL. In vivo visualization of senile-plaque-like pathology in Alzheimer's disease patients by MR microscopy on a 7T system. J Neuroimaging. 2008;18(2):125–129. doi: 10.1111/j.1552-6569.2007.00179.x. [DOI] [PubMed] [Google Scholar]