Summary
The lateral hypothalamic area (LHA) acts in concert with the ventral tegmental area (VTA) and other components of the mesolimbic dopamine (DA) system to control motivation, including the incentive to feed. The anorexigenic hormone, leptin, modulates the mesolimbic DA system, although the mechanisms underlying this control have remained incompletely understood. We show that leptin directly regulates a population of leptin receptor (LepRb)-expressing inhibitory neurons in the LHA, and that leptin action via these LHA LepRb neurons decreases feeding and body weight. Furthermore, these LHA LepRb neurons innervate the VTA, and leptin action on these neurons restores VTA expression of the rate-limiting enzyme in DA production along with mesolimbic DA content in leptin-deficient animals. Thus, these findings reveal that LHA LepRb neurons link anorexic leptin action to the mesolimbic DA system.
Keywords: obesity, tyrosine hydroxylase, VTA, nucleus accumbens
Introduction
The processes that regulate feeding and body weight represent likely points of dysregulation and potential sites for therapeutic intervention in obesity and metabolic disease, but remain incompletely understood. The adipose-derived hormone, leptin, plays a central role in the regulation of energy homeostasis (Friedman, 2002; Myers, Jr., 2004; Morton et al., 2006; Elmquist et al., 2005; Gao and Horvath, 2007; Berthoud, 2007; Myers, Jr. et al., 2009). Leptin activates the long form of the leptin receptor (LepRb) on CNS neurons to mediate most leptin action, including the suppression of feeding (Cohen et al., 2001; de Luca et al., 2005). LepRb-expressing neurons lie in numerous regions involved in the regulation of energy balance, including mediobasal hypothalamic (MBH) “satiety centers” (e.g. the arcuate nucleus (ARC)), and “feeding centers” such as the lateral hypothalamic area (LHA) (Elmquist et al., 1998; Myers, Jr. et al., 2009).
A number of aspects of leptin action in the MBH are beginning to be unraveled, including the role of leptin in regulating LepRb/pro-opiomelanocortin (POMC)-expressing neurons and their opposing LepRb/agouti-related protein/neuropeptide Y (AgRP/NPY)-expressing neurons in the ARC (Morton et al., 2006; Elmquist et al., 2005; Gao and Horvath, 2007; Berthoud, 2007). These neurons contribute to satiety and thus mediate an important component of the anorectic response to leptin, as well as modulating energy expenditure and aspects of glucose homeostasis. Many data suggest that the action of leptin on these LepRb-expressing MBH neurons only accounts for a fraction of leptin action on energy balance, however (Balthasar et al., 2004; Dhillon et al., 2006; Myers, Jr. et al., 2009; van de et al., 2008). Indeed, MBH LepRb neurons represent a minority of LepRb-expressing neurons in the brain (Elmquist et al., 1998; Myers, Jr. et al., 2009). Thus, populations of LepRb neurons in other brain areas are likely to be crucial for leptin action.
In addition to promoting satiety, leptin suppresses the incentive value of food and other rewards (Figlewicz et al., 2006; Fulton et al., 2000)- presumably by modulating components of the mesolimbic dopamine (DA) system, which arises from dopaminergic neurons in the ventral tegmental area (VTA) (Kelley et al., 2005; Nestler, 2005; DiLeone et al., 2003). Indeed, leptin directly regulates a population of LepRb-expressing VTA DA neurons (Figlewicz et al., 2006; Hommel et al., 2006; Fulton et al., 2006). Furthermore, leptin modulates DA-dependent measures of food and drug reward and normalizes the decreased mesolimbic DA content of leptin-deficient animals (Figlewicz et al., 2006; Fulton et al., 2006; Roseberry et al., 2007).
The LHA feeding center also represents a critical component of the mesolimbic reward circuit, and modulates the incentive salience of food and other rewards (Kelley et al., 2005; Nestler, 2005; DiLeone et al., 2003; Harris et al., 2005). Two populations of LHA neurons that express the orexigenic neuropeptides melanin-concentrating hormone (MCH) or the orexins (OX, also known as hypocretins) project widely throughout the CNS, including to the striatum and VTA, respectively, to modulate feeding and measures of reward (Georgescu et al., 2005; Mieda and Yanagisawa, 2002). Leptin inhibits the fasting-induced activity of OX neurons and decreases the expression of MCH and OX, and deletion of MCH improves the obesity of leptin-deficient ob/ob mice (Qu et al., 1996; Segal-Lieberman et al., 2003; Yamanaka et al., 2003). Thus, LHA neurons likely participate in the regulation of energy homeostasis by leptin. The LHA also contains a substantial population of LepRb-expressing neurons, leading us to hypothesize that these neurons might contribute to energy homeostasis and the regulation of the mesolimbic DA system. We have thus examined the role of these LHA LepRb neurons in leptin action.
Results
Leptin directly regulates LepRb-expressing LHA neurons to suppress feeding
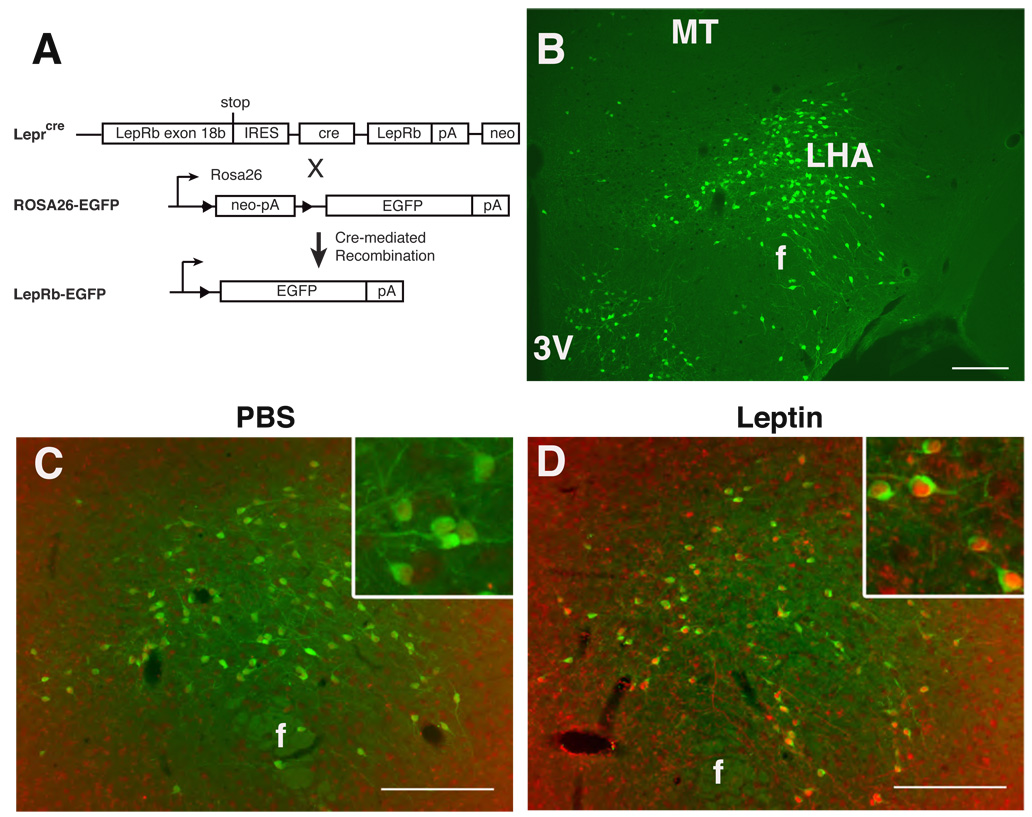
We developed a reporter mouse strain to facilitate the detection and study of LepRb neurons in the LHA and throughout the brain by crossing Leprcre mice (Leshan et al., 2006; Leshan et al., 2009) onto the Gt(ROSA)26Sortm2Sho background, in which cre-mediated recombination promotes the expression of enhanced green fluorescent protein (EGFP) (Mao et al., 1999) (Fig. 1A). Double homozygous (henceforth referred to as LepRbEGFP) mice demonstrate CNS EGFP expression consistent with reported patterns of LepRb expression (Elmquist et al., 1998; Myers, Jr. et al., 2009), including a large population of EGFP-positive neurons in the LHA (Fig. 1B). Activated LepRb directly recruits STAT3 to promote its tyrosine phosphorylation (pSTAT3) (Myers, Jr., 2004). While STAT3 protein expression is widespread, the detection of pSTAT3 immunoreactivity (-IR) during leptin stimulation thus reveals cell-autonomous LepRb signaling (Munzberg et al., 2003). Examination of pSTAT3-IR in LepRbEGFP mice following systemic treatment with leptin to stimulate LepRb revealed that 81±4% of LHA pSTAT3-IR neurons detectably express EGFP, and 87±1% of EGFP-labeled neurons demonstrate pSTAT3-IR following IP leptin treatment (Fig. 1C,D). Thus, EGFP-expressing LHA neurons in LepRbEGFP mice contain functional LepRb that responds to systemically-administered leptin, and EGFP expression in these animals identifies the majority of LHA LepRb neurons.
Figure 1. LepRbEGFP mice reveal a large population of leptin-responsive LHA LepRb neurons.
(A) Schematic diagram demonstrating cre-mediated EGFP expression in LepRb expressing cells of LepRbEGFP mice. (B) Immunofluorescent detection of EGFP (green) in the LHA of LepRbEGFP mice. (C,D) Immunohistochemical detection of pSTAT3-IR (red nuclei) and EGFP (green) in the LHA of LepRbEGFP mice following IP treatment with vehicle (C) or leptin (5 mg/kg, 2 hours) (D). Insets: higher magnification view of labeled LHA neurons. Scale bars = 10 µm. 3V= third cerebral ventricle; MT= mammilothalamic tract; f= fornix.
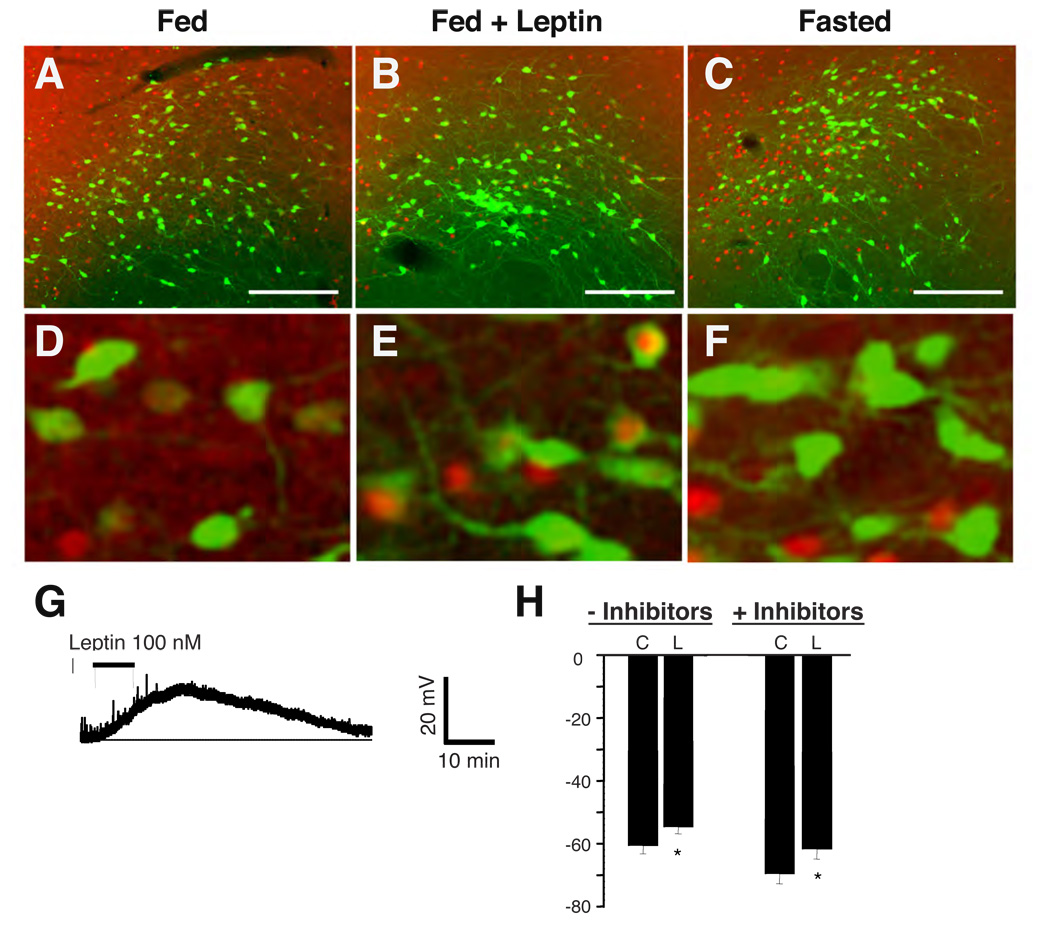
In order to determine the regulation of LHA LepRb neurons by leptin, we examined c-fos-IR (FosIR; a marker of neuronal activation (Hoffman et al., 1993)) in EGFP-expressing LHA neurons from LepRbEGFP mice following fasting or systemic leptin treatment (Figure 2A–F). A modest number of LHA LepRb neurons from ad libitum-fed, vehicle-treated animals exhibit FosIR, and fasting tended to decrease this number (ad libitum 12±3% vs. fasted 5±1%, p=0.05). In contrast, leptin treatment of ad libitum-fed animals induces FosIR (PBS = 12±3% vs. leptin = 41±7%, p<0.05) in EGFP-expressing LHA neurons of LepRbEGFP mice, demonstrating that systemic leptin treatment activates a substantial number of LHA LepRb neurons.
Figure 2. Leptin directly regulates the activity of LHA LepRb neurons in LepRbEGFP mice.
(A–F) Immunohistochemical detection of FosIR (red nuclei, pseudocolored) and immunofluorescent detection of GFP (green) in LepRbEGFP animals following ad libitum feeding (A,D) or fasting for 36 hours (C,F) followed by treatment with vehicle, or ad libitum-fed animals following leptin treatment (B,E) (5 mg/kg, IP 4 hours). Scale bars = 10 µm. Panels D–F show higher magnification images of a-c, respectively. (G,H) Electrophysiologic response of LHA LepRb neurons to leptin. EGFP-expressing LHA neurons were identified under light and fluorescent microscopy for recording under current clamp conditions and examined for their electrophysiologic response to leptin. One population of neurons were depolarized in response to leptin (G). (H) Graphs of the response of neurons that were depolarized in response to leptin in the absence (-Inhibitors) or presence (+Inhibitors) of inhibitors of synaptic transmission. Error bars represent the SEM.
In order to determine whether leptin regulates the activity of LHA LepRb neurons directly, we examined the electrophysiologic response of LHA LepRb neurons to leptin in acute hypothalamic slice preparations from LepRbEGFP mice (Fig. 2G,H). EGFP fluorescence identified LepRb-expressing LHA neurons for recording in current clamp configuration. As in vivo, where leptin treatment caused an approximately 30% increase in FosIR colocalization with LHA LepRb neurons, leptin (100 nM) depolarized 34% of LHA LepRb neurons (p <0.05) (Fig 2G,H and Supplemental Table 1). Furthermore, the presence or absence of inhibitors of synaptic transmission did not alter the percentage of cells depolarized by leptin, suggesting that the depolarizing effect of leptin on this subset of LHA LepRb neurons is direct. We additionally observed that some LHA LepRb neurons were hyperpolarized by leptin; the presence of inhibitors did not alter the proportion of cells responding to leptin (Supplemental Table 1). Thus, leptin acts directly upon LHA LepRb neurons to regulate their membrane potential. Also, multiple subpopulations of LHA LepRb neurons display different electrophysiologic responses to leptin and could thus serve distinct neural functions in leptin action.
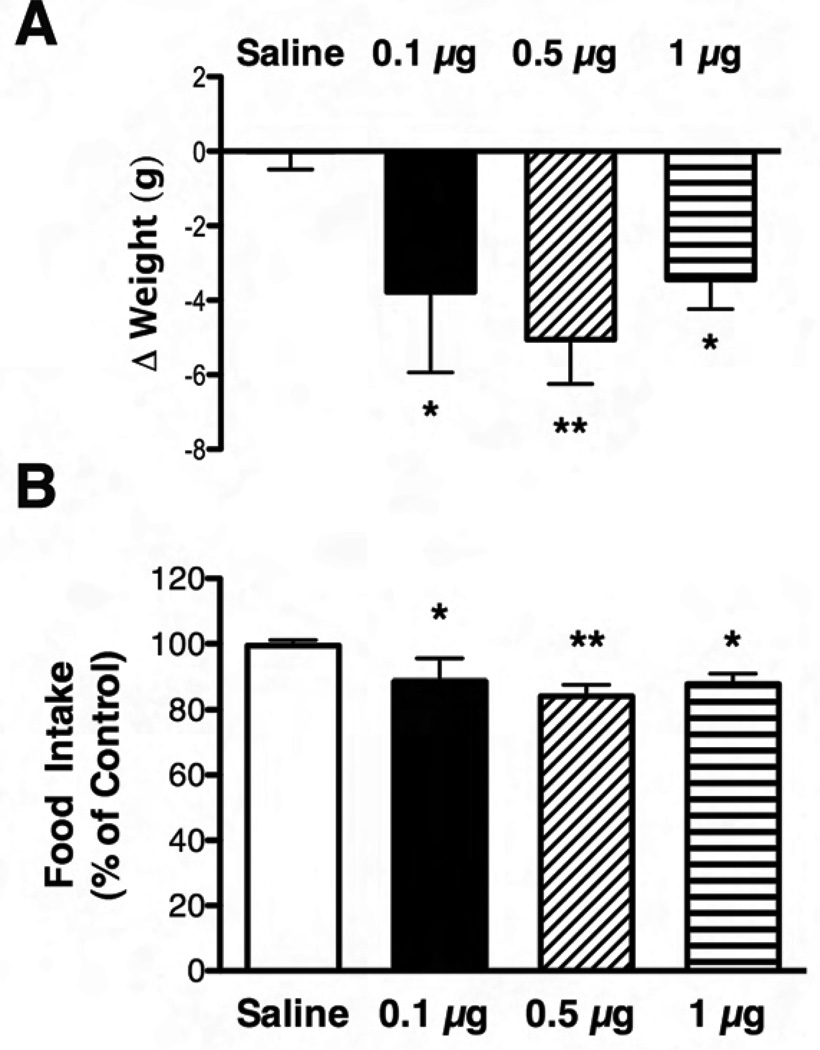
In order to determine the role for LHA LepRb neurons in leptin action in vivo, we examined the effect of direct leptin injection into the LHA on food intake and body weight of Long-Evans rats (Fig. 3). Cannulae were placed unilaterally into the LHA of the rats. Following recovery, we administered saline, 0.1 µg, 0.5 µg or 1 µg of leptin via the cannula and monitored food intake and body weight over the subsequent 24 hours. We chose these doses based upon the similarity of the lower doses to those used by others for demonstrating site-specific leptin effects in the rat brain (Hommel et al., 2006). The upper dose was chosen to lie below the minimum effective ICV dose for decreased feeding (Seeley et al., 1996). All three doses of unilateral intra-LHA leptin decreased weight and food intake (p<0.05 and p<0.01) over 24 hours compared to saline treatment (Fig. 3A,B). In addition to examining cannula placement in these animals, we analyzed pSTAT3-IR in the brains of animals that received the lowest dose of leptin, to confirm the restriction of leptin action to the region of the cannula (Supplemental Fig. 1). This analysis revealed that leptin-induced pSTAT3-IR was mainly confined to the ipsilateral LHA, with no increase in pSTAT3-IR in the ARC, VTA, or other distant regions. Thus, these data suggest that leptin action via LHA LepRb neurons is sufficient to suppress food intake and weight gain in normal rats. While we did not examine the detailed timing of the leptin response for all doses, the highest dose of leptin did not produce a significant reduction in food intake before 4 hours (data not shown). This delayed timing is consistent with the role of leptin as a long-term, rather than acute, signal of energy status.
Figure 3. Response of rats to intra-LHA leptin treatment.
Change in weight (A) and total food intake (B) in Long Evans rats in response to 24h treatment with intra-LHA saline (white bars) or 0.1 µg (black bars), 0.5 µg (diagonally hatched bars) or 1 µg leptin (horizontally hatched bars). Food intake over 24 hours is plotted as percent of intake following saline injection; weight data are plotted as change in weight over 24 hours. Average values +/−SEM are shown; *p<0.05, **p<0.01 compared to saline by one way ANOVA with Dunnett post test.
LHA LepRb neurons are distinct from known orexigenic LHA neurons
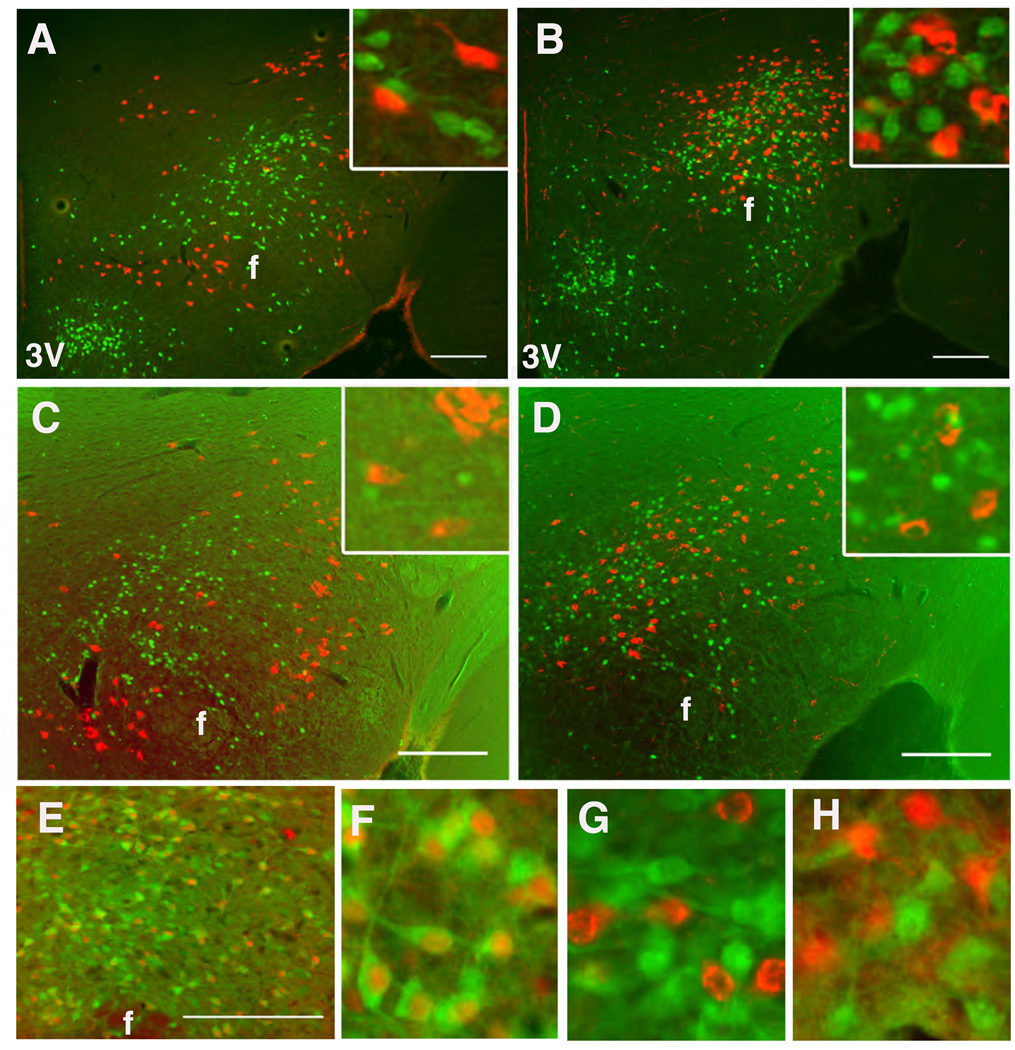
We examined the potential expression of LepRb in known populations of orexigenic LHA neurons by examining the colocalization of EGFP with MCH and OX in LepRbEGFP mice. This analysis revealed no detectable expression of LepRb/EGFP in MCH or OX neurons in untreated (not shown) or colchicine-treated animals (Fig. 4A,B). Furthermore, pSTAT3-IR is not found in MCH or OX neurons in normal mice following treatment with high-dose ICV leptin (3 µg) to promote LepRb-mediated pSTAT3 in all neurons containing functional receptor (Fig. 4C,D).
Figure 4. Neurotransmitter content of LHA LepRb Neurons.
(A,B) Immunofluorescent detection of EGFP (green) and MCH (A, red) or OX (B, red) in the LHA of colchicine-treated LepRbEGFP mice. (C,D) Immunohistochemical detection of pSTAT3-IR (green nuclei, pseudocolored) and MCH (C, red) or OX (D, red) following ICV leptin treatment for 1 hr. (E) Immunohistochemical detection of pSTAT3 (red, pseudocolored) and immunofluorescent detection of EGFP (green) in Gad1EGFP mice. (F) Digital zoom of (E). (G,H) immunofluorescent detection of EGFP (green) and OX (red (G)) or MCH (red (H))in mice that express EGFP in GAD67 neurons. Scale bars = 10 µm. 3V= third cerebral ventricle; f= fornix
To understand the neurochemical mediators by which LHA LepRb neurons might contribute to leptin action, we investigated their neurotransmitter content. Reasoning that the LHA LepRb neurons are distinct from OX neurons, which are known to be glutamatergic/excitatory (Rosin et al., 2003), we analyzed the potential GABAergic/inhibitory nature of the LHA LepRb neurons by utilizing Gad1EGFP mice (which express EGFP in neurons that contain GAD1- an enzyme that synthesizes the inhibitory neurotransmitter, GABA)(Abe et al., 2005) to examine the potential colocalization of leptin-stimulated pSTAT3-IR in EGFP-expressing GABA neurons. All LHA pSTAT3-IR/LepRb neurons in the Gad1EGFP mice contain EGFP, revealing the GABAergic nature of the LHA LepRb neurons (Fig. 4E,F). By contrast, adjacent OX and MCH neurons do not co-express EGFP/GAD1 (Fig. 4G,H).
Thus, LHA LepRb neurons represent a unique population of neurons distinct from previously described OX- and MCH-neurons in the LHA. The inhibitory nature of LHA LepRb neurons contrasts with the excitatory properties of some orexigenic LHA neurons. Similarly, while leptin activates at least some LHA LepRb neurons, it suppresses the fasting-induced activity of OX neurons (Yamanaka et al., 2003). Thus, these findings are also consistent with opposite roles in the regulation of feeding for LHA LepRb neurons (which decrease feeding in response to LHA leptin) compared to the orexigenic OX neurons.
LHA LepRb neurons innervate the VTA
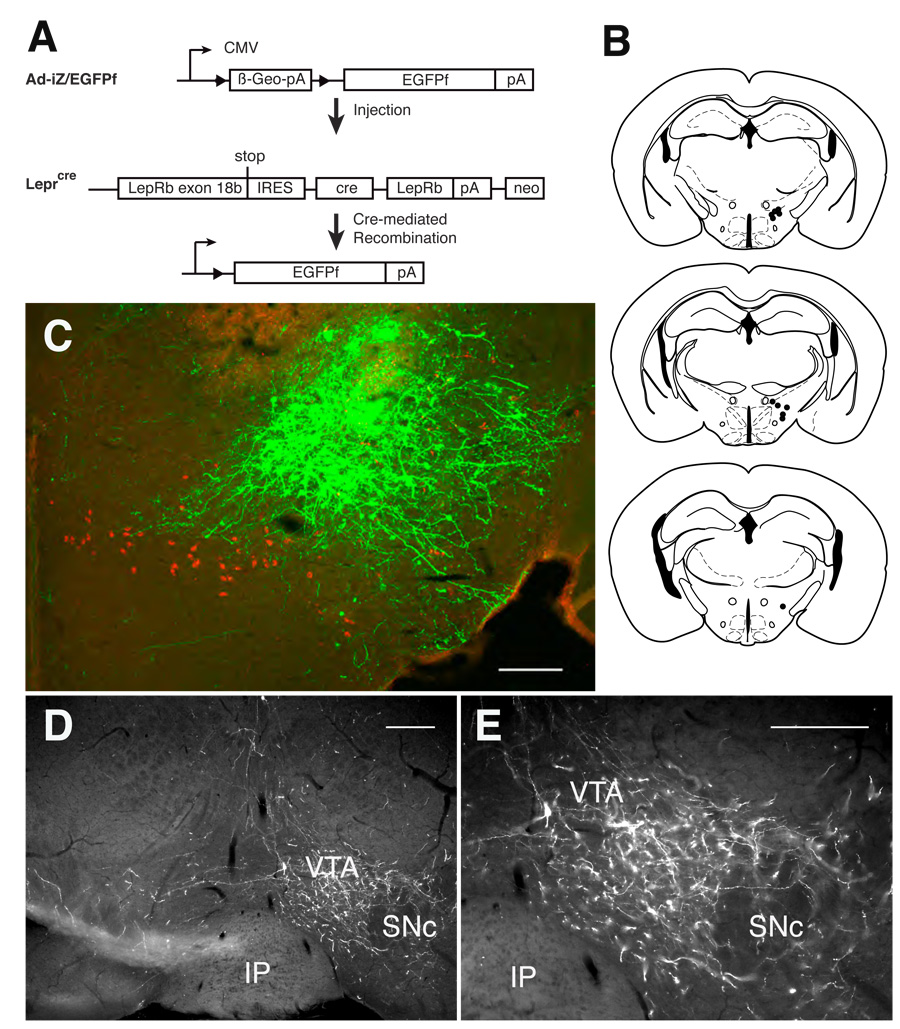
Conventional anterograde tracing studies have demonstrated the connection of LHA neurons with multiple brain centers within and outside of the hypothalamus, including the VTA, NAc, and other areas (Swanson et al., 2005). Such studies do not differentiate the projections of LepRb neurons from those of other LHA neuronal populations, however. To define projections from LepRb-expressing soma in the LHA, we thus merged the use of molecular tracers with the cre-inducible system (for LepRb-specificity) and adenoviral stereotaxic injection (for anatomic specificity) to generate the adenoviral vector, Ad-iZ-EGFPf (Fig. 5A) (Leshan et al., 2009). This vector mediates the cre-inducible expression of farnesylated EGFP (EGFPf), which localizes to the membrane, effectively labeling even very long axonal projections (Zylka et al., 2005; Leshan et al., 2009).
Figure 5. Ad-iZ/EGFPf-mediated tracing reveals projection of LHA LepRb neurons to the VTA.
(A) Schematic diagram showing cre-mediated expression of EGFPf in infected LepRb-expressing neurons following stereotaxic injection of Ad-iZ/EGFPf into Leprcremice. (B) Summary of Ad-iZ/EGFPf injection sites for the eleven (11) cases utilized for the study. (C) Immunofluorescent detection of EGFPf (green) and MCH (red) in the LHA of an example of a correctly targeted intra-LHA injection of Ad-iZ/EGFPf in the Leprcremice. (D) Immunofluorescent detection of EGFPf-containing projections in the VTA of Leprcre mice following intra-LHA injection of Ad-iZ/EGFPf. (E) Higher magnification of (D). Scale bars = 10 µm. VTA= ventral tegmental area; SNc- substantia nigra pars compacta; IP= interpeduncular tubercle.
We administered Ad-iZ/EGFPf into the LHA of Leprcre mice (Fig. 5B) and perfused them for immunofluorescent analysis 5 days later. While intra-LHA administration of Ad-iZ/EGFPf produced copious EGFPf-expression in the LHA of Leprcre mice (Fig. 5C), no EGFPf expression was detected in wild-type animals (data not shown); nor was EGFPf detected in MCH- or OX-expressing neurons of Leprcre mice (supplemental Fig. 2), confirming the cre-specificity of EGFPf expression.
To determine the brain regions innervated by LHA LepRb neurons, we analyzed EGFPf-containing projections in Leprcre animals (n=11) where the injection site and EGFP-immunoreactive soma were confined to the dorsal perifornical area of the LHA (Fig. 5B,C). While the DMH, ARC, and other hypothalamic areas contained few or no LHA LepRb-originating EGFPf projections, in addition to the copious number of neurites observed in the LHA itself the VTA contained dense EGFPf projections ipsilateral to the injection site (Fig. 5D,E). Other regions caudal to the LHA also received some projections, but no projections to the striatum (including the NAc) or other rostral regions were observed (Supplemental Fig. 2).
To verify the results of this anterograde tract-tracing method, we utilized retrograde tracing with fluorogold (FG) in LepRbEGFP mice to verify the projection of LHA LepRb neurons to the VTA (Supplemental Fig. 3). Intra-VTA FG labeled many LHA neurons including LepRb-expressing LHA neurons, while LepRb-containing neurons in other hypothalamic regions (such as the ARC) did not accumulate FG from the VTA. Thus, LHA LepRb neurons project caudally to innervate the VTA, and may play a unique role among hypothalamic LepRb-expressing neurons in the regulation of the mesolimbic DA system. We also examined leptin-induced cFosIR in the FG-treated LHA LepRb neurons (Supplemental Fig. 3): Most FG-labeled EGFP neurons did not contain cFosIR. In contrast, strong cFosIR was noted in non FG-labeled EGFP neurons, suggesting that most VTA-projecting LHA LepRb neurons are not leptin-activated LHA LepRb neurons.
LHA LepRb neurons regulate mesolimbic DA production
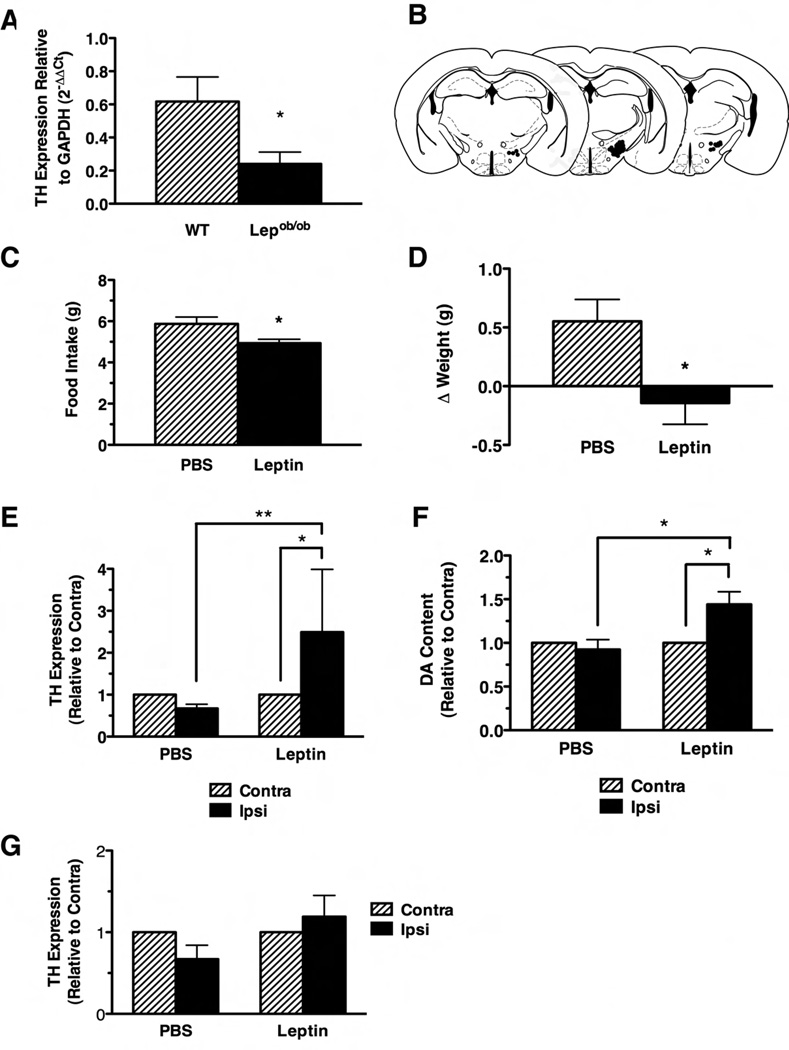
While the administration of exogenous leptin (even at supraphysiologic doses) often evokes only modest effects in normal leptin-replete animals, leptin deficiency promotes dramatic alterations in CNS function and mammalian physiology that are readily reversed by the normalization of leptin levels. For instance, exogenous leptin does not increase hypothalamic Pomc mRNA expression in normal fed animals, although such treatment restores the diminished Pomc expression of leptin-deficient Lepob/ob animals (Schwartz et al., 1997). Reasoning that Lepob/ob animals have impaired VTA expression of tyrosine hydroxylase (TH, the rate-limiting enzyme in DA production) and decreased mesolimbic DA content that is normalized by systemic leptin treatment (Figure 6A and (Fulton et al., 2006)) and that LHA LepRb neurons are well-placed to regulate the VTA and mesolimbic DA system in response to leptin, we examined the ability of LHA leptin action to regulate energy balance and normalize these parameters in Lepob/ob mice (Figure 6B–F). We utilized unilateral intra-LHA cannulae to deliver a small dose of leptin (250 pg- designed to approximate circulating leptin levels in the volume of distribution of the LHA) to these Lepob /obanimals. This leptin treatment stimulated pSTAT3-IR only in the LHA on the same side as the cannula (and not in the MBH), demonstrating the specificity of this treatment for the LHA LepRb neurons (Supplemental Fig. 4).
Figure 6. Energy balance, VTA TH mRNA Expression, and NAc DA content in Lepob/ob mice after intra-LHA leptin treatment.
(A) TH mRNA levels in the VTA of normal C57/Bl6 mice (WT, hatched bar) and Lepob/ob mice (solid bar). TH expression for each genotype is shown relative to GAPDH expression (calculated by the 2−ΔΔCt method) +/−SEM; *p<0.05. (B) Summary of LHA cannulation sites in the Lepob/ob mice included in the study. 24-hour food intake (C) and body weight change (D) in Lepob/ob mice in response to intra-LHA PBS (hatched bars) or leptin (solid bars). All data are plotted as average +/− SEM; *p<0.05. VTA TH mRNA expression relative to GAPDH (E) and NAc DA content (F) contralateral (hatched bars) and ipsilateral (solid bars) in Lepob/ob mice mice treated with 24 hours of intra-LHA PBS or leptin. Values are shown relative to the (unperturbed) contralateral side,+/− SEM; *p<0.05, **p<0.01. (G) Expression of TH mRNA relative to GAPDH in Lepob/ob mice mice treated with 24 hours of intra-VTA PBS or leptin. Data are plotted as in E. Significance determined by one way ANOVA with Bonferroni post-tests.
We measured food intake and body weight over 24 hours of treatment in these animals (Figure 6C,D). As predicted based upon the response of normal rats to leptin in the LHA, intra-LHA leptin treatment of Lepob/ob animals attenuated their food intake over the 24 hour treatment period and suppressed weight gain (p<0.05), confirming that the absence of leptin action via LHA LepRb neurons contributes to the dysregulated feeding and energy balance of leptin-deficient animals. While we examined food intake at times earlier than 24 hours, intra-LHA leptin did not significantly alter feeding at these shorter times (data not shown), consistent with the role of leptin as a long-term signal of energy stores.
We also examined the effect of intra-LHA leptin treatment on VTA TH expression and NAc DA content (Figure 6E,F). The unilateral nature of both the LHA LepRb→ VTA projections (Figure 5) and the leptin treatment dictates the confinement of the leptin effect to the side ipsilateral to the cannula; we thus compared these parameters ipsilateral and contralateral to the cannula. This approach also increases the power of the experiment by permitting a “within-mouse” comparison, minimizing the effects of potential between-animal variation in TH mRNA and DA content. Intra-LHA PBS treatment tended to modestly decrease ipsilateral compared to contralateral VTA TH expression (Fig, 6E PBS-treated samples), potentially consistent with minor disruption of LHA tissue (and thus the LHA → VTA projections) by the cannula. By contrast, leptin increased VTA TH mRNA expression ipsilateral to the cannula by approximately 2.5-fold (p<0.05) (Fig. 6E), as well as increasing ipsilateral NAc DA content by approximately 40% (p<0.05) (Fig. 6F), indicating that the LHA → VTA circuit is largely intact and that LHA leptin action regulates these important parameters of mesolimbic DA system function. While the approximately 2.5-fold increase in VTA TH mRNA expression ipsilateral to the cannula in intra-LHA leptin-treated Lepob/ob animals (Figure 6E) was similar in magnitude to the increased total TH expression of wild-type compared to Lepob/ob animals (Figure 6A), intra-VTA leptin administration did not significantly alter VTA TH mRNA expression (Figure 6G). Collectively, these data indicate that intra-LHA (but not intra-VTA) leptin modulates the TH expression in Lepob/ob mice. Thus, leptin action specifically via LHA LepRb neurons promotes VTA TH gene expression and mesolimbic DA content and regulates the mesolimbic DA system.
Discussion
Overall, our findings reveal that leptin action via GABAergic LHA LepRb neurons suppresses feeding. We also show that these neurons densely innervate the VTA and that leptin action via LHA LepRb neurons (but not via VTA LepRb neurons) increases VTA TH expression and mesolimbic DA content of leptin-deficient animals. Thus, leptin action via LHA LepRb neurons modulates the mesolimbic DA system and decreases feeding. The role of the mesolimbic DA system in mediating the action of leptin in the LHA is an important area for future study.
While the finding that increased VTA TH expression (and mesolimbic DA production/content) decreases food intake contravenes the widely-held notion that NAc dopamine release promotes feeding, increased DA does not always correlate with increased feeding; cocaine and amphetamines promote the accumulation of extracellular DA in the NAc, but blunt feeding. Indeed, DA in the mesolimbic DA system regulates the incentive value of many behaviors, not just feeding, and leptin would be predicted to differentially regulate the components of the mesolimbic DA system that encode feeding compared to reproductive behavior, for instance. Furthermore, while we were not able to examine activity in these experiments, it is also possible that the regulation of mesolimbic DA content by leptin modulates activity, in addition to feeding. Also, DA action in the dorsal striatum has been suggested to regulate feeding (Palmiter, 2007), and the role of leptin in the modulation of this system has yet to be explored.
It is also important to note that while our data demonstrate a role for leptin in promoting DA production, it is possible that DA release is regulated differently. The regulation of DA content (rather than DA release) by leptin is more consistent with the chronic/tonic nature of leptin action, since leptin conveys a signal of long-term energy stores and does not fluctuate greatly on an acute basis. This long-term modulation of DA content (rather than acute release) by LHA LepRb neurons may also serve to prevent large acute changes in mesolimbic DA input that might otherwise occur with alterations in nutritional status; such changes might produce undesirable long-term consequences in the mesolimbic DA system, as seen with drugs of abuse.
Each population of LepRb-expressing neurons throughout the brain presumably mediates a distinct aspect of overall leptin action (Myers, Jr. et al., 2009). For instance, MBH LepRb neurons in the ARC and VMH appear to regulate satiety, some aspects of energy expenditure, and glucose homeostasis (Morton et al., 2006; Elmquist et al., 2005), while our present data suggest that LHA LepRb neurons contribute to the actions of leptin on the mesolimbic DA system. Leptin also acts directly upon a population of LepRb-expressing neurons in the VTA (Fulton et al., 2006; Roseberry et al., 2007; Hommel et al., 2006), inhibiting the activity of VTA DA neurons and decreasing food intake (Hommel et al., 2006). In contrast, systemic leptin increases VTA TH expression and NAc DA content in Lepob/ob animals (Fulton et al., 2006; Roseberry et al., 2007). While VTA LepRb neurons may contribute to leptin action, our present findings that LHA, but not VTA, leptin administration in Lepob/ob mice increases VTA TH expression (and NAc DA) toward normal levels suggest that leptin action via LHA LepRb neurons regulates VTA TH expression and the mesolimbic DA system.
LHA and VTA LepRb neurons may regulate distinct components of the mesolimbic DA system, however, and LHA LepRb neurons may be divisible into distinct subpopulations (e.g., based on their electrical response to leptin and/or projection targets). As only some LHA LepRb neurons project to the VTA, others might project locally within the LHA to modulate the function of OX or MCH neurons, both of which are inhibited by leptin (Jo et al., 2005; Yamanaka et al., 2003). The unique roles played by these subpopulations of LHA LepRb neurons, as well as those played by VTA LepRb neurons, remain unclear, but could include the requirement for leptin to differentially regulate specific populations of midbrain DA neurons and/or to distinctly modulate the incentive for feeding relative to other behaviors. Going forward, it will be important to determine how these various populations of mesolimbic DA system-interacting LepRb neurons differ in terms of their wiring and the control of different aspects of mesolimbic DA signaling.
Overall, our present observations reveal that the LHA LepRb neuronal population represents a major link between anorectic leptin action and the mesolimbic DA system. These findings reveal important mechanisms that underlie the regulation of the mesolimbic DA system by a crucial signal of energy stores. In the future, it will be crucial to address the potential dysregulation of these neurons in states of obesity.
Methods
Materials
Leptin was purchased from NHPP (Los Angeles, CA).
Mouse Strains
The generation of LeprCre mice has been described previously (Leshan et al., 2006). To generate LepRbEGFP mice, we crossed LeprCre/+ with Gt(ROSA)26Sortm2Sho purchased from Jackson laboratories and then intercrossed their double heterozygous progeny to generate double homozygous LeprCre/Cre;Gt(ROSA)26Sortm2Sho/tm2Sho (LepRbEGFP) mice, which were propagated by intercrossing. C57/Bl6 (WT) and Lep ob/ob mice were purchased from Jackson Laboratories and Gad1EGFP mice were propagated at Yale University. All other mice were housed in a 12h light/12h dark cycle and cared for by ULAM at the University of Michigan or similar facilities at AECOM. All animals had ad libitum access to food and water, except in experiments where mice were fasted before perfusion. All care and procedures for mice were in accordance with the guidelines and approval of the University of Michigan, AECOM, or Yale University University Committee on Use and Care of Animals (UCUCA).
Immunohistochemistry and Immunofluoresence
Treatment with ICV colchicine (10 µg) to concentrate neuropeptides in the soma for some experiments was for 2d prior to perfusion. Adult mice were anesthetized with an overdose of intraperitoneal (IP) pentobarbital and transcardially perfused with 4% paraformaldehyde or 10% neutral buffered formalin and processed for pSTAT3 or c-fos immunoreactivity by DAB as described (Munzberg et al., 2007). DAB staining was pseudocolored using Photoshop software to appear colored in images. All other antibodies were subsequently added and visualized via immunofluorescent secondary detection using species-specific Alexa-488 or −568 antibodies (Invitrogen, 1:200) and were processed and imaged as previously (Munzberg et al., 2007). Antibodies used were GFP (Abcam 1:1000), Orexins (Calbiochem, 1:2000 Orexin A + 1:30 Orexin B) and MCH (Phoenix 1:200).
Electrophysiology Slice preparation
Transverse brain slices were prepared from LepRbEGFP mice at postnatal age 28–35 days. Animals were anesthetized with a mixture of ketamine and xylazine. After decapitation, the brain was transferred into a sucrose-based solution bubbled with 95%O2−5% CO2 and maintained at ~3°C. This solution contained (mM): sucrose 248; KCl 2; MgCl2 1; KH2PO4 1.25; NaHCO3 26 and glucose 10. Transverse coronal brain slices (200 µM) were prepared using a Vibratome (Leica VT1000S). Slices were equilibrated with an oxygenated artificial cerebrospinal fluid (aCSF) for > 1 hour prior to transfer to the recording chamber. The slices were continuously superfused with aCSF at a rate of 2ml/min containing (in mM); NaCl 113, KCl 3, NaH2PO4 1, NaHCO3 26, CaCl2 2.5, MgCl2 1 and glucose 5 in 95% O2/ 5% CO2 at room temperature. Membrane potentials of LepRbEGFP neurons were measured 5 min after the onset of recordings and at the maximum response to leptin. Students’ t-test was used to test for significance between control and leptin-treated samples. Data were considered significant for p<0.05.
Rat Cannulation, Microdissection and Analysis
Male Long-Evans rats (250–300 g, Harlan, Indianapolis, IN) were individually housed on a 12-hour light/dark cycle (1:00 PM lights off). All care and procedures were in accordance with the guidelines and approval of the University of Cincinnati Committee on Use and Care of Animals (UCUCA). Under ketamine/xylazine anesthesia, rats were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the incisor bar set at −0.3 mm. The skull was exposed via a 2mm midline incision, and the periosteum was gently scraped to the side. A 26-gauge guide cannula (Plastics One, Roanoke, VA) was lowered toward the left lateral hypothalamus at the following coordinates: AP −2.30 mm, ML −1.80 mm, and DV −7.80 mm. The guide cannula was cemented in place via dental acrylic and anchor screws and fitted with an obturator that extended 1.0 mm below the guide cannula. On the day of the experiment, rats were weighed and food removed from cages and weighed at 8:00 AM. At 9:00 AM, rats received a 0.2 µl intra-LHA infusion of saline or either 0.1 µg, 0.5 µg or1.0 µg recombinant mouse leptin (Calbiochem, San Diego, CA) over 2 minutes. The 1 µg dose of leptin was chosen because it is below the threshold dose required to observe an anorectic response to ICV leptin (Seeley et al., 1996), while the lower 0.1 µg and 0.5 µg leptin doses are similar to those utilized for the analysis of leptin action in the VTA (Hommel et al., 2006). Infusions were made using a microinfusion pump (Harvard Apparatus, Hollison, MA) holding a 25-µl Hamilton syringe (Hamilton Company, Reno, NV) attached via saline-filled polyethylene tubing to a 33-gauge internal cannula (Plastics One, Roanoke, VA) that extended 1.0 mm below the guide cannula. Food was returned to cages at 1:00 PM and food intake and body weight change was measured at 24 hours.. After 72h (by which time the leptin effects on food intake and body weight are extinguished), the treatment groups were switched and data collected as above. To verify cannulation site, rats were anesthetized and their brains removed to a rat coronal brain matrix (1mm divisions) to isolate the hypothalamus, which was then placed in 4% paraformaldehyde for fixation at 4°C for 72h, followed by cryoprotection in 30% sucrose and microtome sectioning. A few rats were perfused with 4% paraformaldehyde and immunostained for pSTAT3 to visualize the spread of leptin-induced pSTAT3-IR within the brain. Hypothalamic sections from each animal were immunostained for OX to verify the cannulation site relative to the LHA OX/LepRb-rich region and rats were included in the study if cannulae were placed in the LHA. Exclusion by these criteria, and utilization of the cross-over treatment paradigm, resulted in saline n=50 rats, 0.1 µg leptin n= 16 rats, 0.5 µg leptin n = 16 rats and 1 µg leptin n= 18 rats for the feeding and weight measures.
LepRb Projection Tracing
For the generation of Ad-iZ/EGFPf, the floxed β-geo cassette from pCALL (kindly provided by Corrine Lobe, Toronto, CA) was excised and inserted upstream of the MCS from pShuttleCMV (He et al., 1998) to generate pShuttle-iZ. The coding sequence of EGFP from pEGFP (Invitrogen) was amplified with the addition of sequences encoding a COOH-terminal farnesylation by standard PCR and subcloned into the MCS of pShuttle-iZ to generate pShuttle-iZ/EGFPf, which was purified, linearized and utilized to generate the Ad-iZ/EGFPf adenovirus as previously described. Concentrated adenoviral stocks were generated and purified as previously described (Morton et al., 2003).
For tracing studies, Leprcre mice were anesthetized with an isofluorane vaporizer and fixed in a stereotaxic apparatus. Coordinates to the LHA, relative to Bregma, were A/P: −1.34, M/L: −1.13, D/V: −5.20, in accordance with The Mouse Brain in Stereotaxic Coordinates by KBJ Franklin and G. Paxinos, Eds., Academic Press, San Diego, CA, 1997. An access hole was drilled in this spot, through which a guide cannula with a stylet was inserted and lowered to the D/V coordinate. The stylet was then replaced by an injector and 250–500 nL of Ad-iZ-EGFPf was injected into the tissue via a 0.5 µl Hamilton syringe at a rate of 100 nL per min. After 10 min to allow for tissue absorption, the injector and cannula were removed and the skull and the incision closed with wound clips. Mice received post-surgical analgesia (Buprenex) and were housed for 5 days in the animal facility, after which they were perfused and processed for immunofluoresence.
Lepob/ob Cannulation, Microdissection and qPCR and DA Analysis
Twenty male Lepob/ob mice (12 wk, 49–61 g) were anesthetized and a stereotaxic frame was utilized to locate the LHA, as described above. A 26-gauge guide cannula with a 4.4 mm projection below the pedestal was lowered into the LHA site, the pedestal affixed to the skull with surgical adhesive and cemented in place, and then the wound was closed around the cannula with sutures. A dummy injector with a 5.2 mm projection was placed in the guide cannula and allowed to remain through the recovery period. Mice received post-surgical analgesia (Buprenex) and were single-housed for 9 d to recover, during which daily food intake and weight were monitored. On the day before treatment. mice were mock treated, which consisted of replacing the dummy with an un-loaded injector and tubing to simulate the treatment conditions—this was called the baseline day. On the following day (treatment day) the dummy was replaced with an injector with a 5.2 mm projection used to deliver either 250 nL of sterile PBS or leptin (0.001 ng/nL, thus each dose = 0.25 ng) every 12 hours for 24 hours, during which food intake and weight were monitored. Mice were then treated once more and 2 hours later were anesthetized and their brains removed to a rodent coronal brain matrix (1 mm divisions) for microdissection. The ipsilateral and contralateral VTA and NAc were microdissected and frozen on dry ice. The hypothalamus was removed, frozen on dry ice and then placed in 4% paraformaldehyde for fixation at 4°C for 72 hours, followed by cryoprotection in 30% sucrose and microtome sectioning. Hypothalamic sections were immunostained for OX to verify the cannulation site relative to the LHA OX/LepRb-rich region and for pSTAT3 to verify that leptin-induced pSTAT3 activation was confined to the LHA. Mice were excluded from analysis if cannulae were misplaced, if they failed to gain weight and eat normally prior to the study, or if mice exhibited significant pSTAT3 outside of the LHA. Exclusion by these criteria left PBS n=5 mice and leptin n= 6 mice for the feeding and weight measures. Food intake is plotted as g of food injested over the 24h treatment period +/− SEM. Weight is plotted in g as the weight at the end of the 24h treatment period minus the weight at the beginning of treatment +/− SEM.
RNA was prepared from microdissected VTA using Trizol (Invitrogen) and 1 µg samples were converted to cDNA using the Superscript First Strand Synthesis System for RT-PCR (Invitrogen). Sample cDNAs were analyzed in triplicate via quantitative RT-PCR for GAPDH and TH (Applied Biosystems) using an Applied Biosystems 7500. Relative mRNA expression values are calculated by the 2−ΔΔCt method, with normalization of each sample ΔCt value to the contralateral side ΔCt; thus TH expression in the contralateral side is set to 1, and the ipsilateral TH expression of each sample is relative to its contralateral side. Data are ploted as the average fold expression +/− SEM. A sample was lost in processing, thus PBS n=5, leptin n=6.
For determination of DA content, ipsilateral and contralateral NAc tissues were weighed, sonicated in 88 µL of 10% trichloroacetic acid/0.1% sodium bisulfite, and centrifuged for 10 min at 5000 X g at 4°C. The resulting supernatant was shaken for 10 min with 75 µL isooctane, centrifuged for 5 min at 13,000 X g and the aqueous layer was removed and frozen at −80°C until further processing. The same volume (10–40 µ1) of ipsilateral and contralateral sample for each mouse was diluted with 0.05N HClO4 containing 200 µg/L dihydroxybenzylamine as an internal standard with 1.6 mM ethylenediaminetetraacetic acid, 8 mM sodium metabisulfite in a final volume of 200 µl. Samples were then filtered (0.22 µm filters) and DA concentrations were determined using HPLC with electrochemical detection (Coulochem II; ESA, Waltham, MA), as described (Hu and Becker, 2003). For each sample, the contralateral and ipsilateral values were normalized to the contralateral value. Thus, DA expression in the contralateral side is set to 1, and the ipsilateral DA expression of each sample is relative to its contralateral side. Data are ploted as the average fold expression +/− SEM.
Cannulation of male Lepob/ob mice in the VTA was performed essentially the same as described for the LHA. VTA coordinates relative to Bregma were A/P: −3.2, - M/L: −0.5, D/V: −4.3 , in accordance with The Mouse Brain in Stereotaxic Coordinates by KBJ Franklin and G. Paxinos, Eds., Academic Press, San Diego, CA, 1997. VTA cannulated mice were treated similar to LHA-cannualted mice over 24h, and VTA RNA was isolated and analyzed for TH expression (PBS n =7 mice, leptin n=9 mice).
Statistics
Students’ t-test was used to compare two test groups as computed with Excel. One way ANOVA with post-testing was used for multiple comparisons using In Stat software for Macintosh.
Supplementary Material
Acknowledgements
We thank Gary Schwartz, Bob Kennedy, Maura Perry, and members of the Myers lab for helpful discussions. Supported by the Michigan Diabetes Research and Training Center and Michigan Comprehensive Diabetes Center and grants from the American Diabetes Association and American Heart Association (MGM), the NIH (MGM, CJR, JBB), and the Obesity Society (GML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abe H, Yanagawa Y, Kanbara K, Maemura K, Hayasaki H, Azuma H, Obata K, Katsuoka Y, Yabumoto M, Watanabe M. Epithelial localization of green fluorescent protein-positive cells in epididymis of the GAD67-GFP knock-in mouse. J Androl. 2005;26:568–577. doi: 10.2164/jandrol.04157. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin Receptor Signaling in POMC Neurons Is Required for Normal Body Weight Homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin. Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Naleid AM, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60:S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua SC, Jr, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, Munzberg H, Myers MG., Jr Leptin receptor signaling and action in the central nervous system. Obesity. (Silver. Spring) 2006;14 Suppl 5:208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring cre recombinase-mediated DNA excisions in mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Yanagisawa M. Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr. Opin. Neurobiol. 2002;12:339–345. doi: 10.1016/s0959-4388(02)00331-8. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Niswender KD, Rhodes CJ, Myers MG, Jr, Blevins JT, Baskin DG, Schwartz MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fak/fak) rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr., Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek J, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behavior. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci. 2007;27:7021–7027. doi: 10.1523/JNEUROSCI.1235-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Van Dijk G, Campfield LA, Smith FJ, Burn P, Nelligan JA, Bell SM, Baskin DG, Woods SC, Schwartz MW. Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm. Metab Res. 1996;28:664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Bates S, Myers MG, Jr, Flier JS, Maratos-Flier E. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci. Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- van de WE, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.