Abstract
As the vertebrate myotome is generated, myogenic precursor cells undergo extensive and coordinated movements as they differentiate into properly positioned embryonic muscle fibers. In the zebrafish, the “adaxial” cells adjacent to the notochord are the first muscle precursors to be specified. After initially differentiating into slow-twitch myosin-expressing muscle fibers, these cells have been shown to undergo a remarkable radial migration through the lateral somite, to populate the superficial layer of slow-twitch muscle of the mature myotome. Here we characterize an earlier set of adaxial cell behaviors; the transition from a roughly 4 × 5 array of cuboidal cells to a 1 × 20 stack of elongated cells, prior to the migration event. We find that adaxial cells display a highly stereotypical series of behaviors as they undergo this rearrangement. Further, we show that the actin regulatory molecule, Cap1, is specifically expressed in adaxial cells and is required for the progression of these behaviors. The requirement of Cap1 for a cellular apical constriction step is reminiscent of similar requirements of Cap during apical constriction in Drosophila development, suggesting a conservation of gene function for a cell biological event critical to many developmental processes.
Keywords: Myotome, Muscle, cap, Adaxial, Apical Constriction, Somite, Zebrafish
Introduction
During embryogenesis, cells undergo a wide variety of largely stereotypical rearrangements and behaviors as they contribute to higher order structures in the developing animal. An extensively studied example of this is the generation of the vertebrate myotome (reviewed in Hollway and Currie, 2005). In all vertebrates, pairs of epithelial somites are formed in a rostral to caudal progression on either side of the neural tube and notochord during the segmentation period. The development of the myotome, the somitic compartment that gives rise to skeletal muscle, has been particularly well studied in aminiotes (Denetclaw and Ordahl, 2000; Kahane et al., 2002; Venters and Ordahl, 2002; Gros et al., 2004). Early on, the ventral portion of the somite de-epithelializes to form the mesenchymal sclerotome, which will give rise to the axial skeleton and ribs. The dorsal portion, termed the dermomyotome, remains epithelial and will give rise to both dermal derivatives and the myotome. The myotome itself is formed by the translocation of newly specified myoblasts from the four lips of the dermomyotome. Initially, pioneer myoblasts emanating from the dorsomedial lip of the dermomyotome populate the myotome and elongate into fibers, providing a scaffold for additional waves of cells from the remaining lips and central sheet to populate and elongate upon, as the myotome matures (reviewed in Kalcheim and Ben-Yair, 2005).
In teleosts, such as the zebrafish, the vast majority of somitic cells give rise to muscle, and thus the somite develops a correspondingly large myotome, with a smaller group of ventral cells specified as sclerotome (Stickney et al, 2000; Morin-Kensicki et al., 2002). In contrast to that of amniotes, the molecular and morphological differentiation of the zebrafish myotome begins early, prior to the epithelialization of somitic border cells. Genes encoding the myogenic regulatory factors such as MyoD and Myf5 are expressed early in the most medial presomitic mesoderm adjacent to the notochord, in rows of pseudo-epithelialized cells referred to as adaxial cells (Thisse et al., 1993; Weinberg et al., 1996; Coutelle et al., 2001). Shortly after somite formation, these adaxial myogenic precursors begin to differentiate alongside the notochord as slow-twitch myosin expressing muscle fibers (Devoto et al., 1996). After a few hours, the majority of these medial slow-twitch muscle fibers undergo a remarkable translocation, migrating radially through the lateral myotome, giving rise to the superficial layer of slow muscle found in the larva and adult (Devoto et al., 1996; Cortes et al., 2003). Directly in the wake of this slow muscle migration the lateral somitic cells elongate into fast-twitch myosin-expressing fibers (Devoto et al., 1996; Blagden et al., 1997; Cortes et al., 2003; Henry and Amacher, 2004). Continued myotomal growth and patterning is achieved through a distinct set of progenitor cells that derive from the anterior somite. These cells appear to represent the zebrafish homolog of the amniote dermomyotome that can contribute further myogenic cells to the maturing myotome including embryonic, larval and adult muscle progenitors (Waterman, 1969; Groves et al., 2005; Devoto et al., 2006; Feng et al., 2006; Hollway et al 2007; Stellabotte et al., 2007).
While the signaling events that pattern the myotome are becoming clear (Brennan et al. 2002; Wolff et al., 2003; Brent and Tabin, 2002), and a description of the cell movements that occur during myotome maturation is becoming comprehensive (reviewed in Hollway and Currie, 2005; Kalcheim and Ben-Yair, 2005) the morphogenetic mechanisms that drive myotome cell movements are less well understood. Previously, we provided evidence for a mechanism driving adaxial cell migration in the zebrafish in which adaxial cells that stably co-express the classical cell adhesion molecules N- and M-Cadherin follow, presumably via homotypic adhesion, a dynamic wave of N- and M-Cadherin co-expression that moves laterally through the somite (Cortes et al., 2003). Further, we have found that the migrating slow fibers provide a necessary cue for the timely elongation of the fast muscle fibers in their wake, although the nature of the signal exchanged between slow fibers and fast-fated myoblasts to drive this elongation remains elusive (Henry and Amacher, 2004). Live embryo imaging and cell tracking analysis has revealed a surprising complexity to the cell movements that underlie the further elaboration of the embryonic myotome of zebrafish, some of which appear to be mediated by Sdf-cytokine directed cell migration (Stellabotte et al., 2007; Hollway et al., 2007). Interestingly, an N-Cadherin based mechanism for myotome cell behaviors has also been reported in the developing avian myotome. Unlike the zebrafish however, it is not required for an early migration of pioneer myoblasts, but for the later segregation of central dermomyotomal daughter cells into myogenic myotomal cells and dermal precursors (Cinnamon et al., 2006).
During the earliest stages of myotome differentiation in the zebrafish, the adaxial cells undergo a rearrangement within the medial portion of the newly forming somite, as a roughly 4 × 5 sheet of cuboidal cells positioned against the notochord is transformed into a single dorsoventral stack of approximately 20 elongated cells (Devoto et al., 1996). Here we characterize the nature of the adaxial cell behaviors during this pre-migratory transformation, and find that they display a highly stereotypical set of morphological changes as they lose their cuboidal, epithelial character, flatten and intercalate between one another, and ultimately make attachments with somite boundaries and elongate next to the notochord. We find that Cap1, an actin regulatory molecule that is specifically enriched in adaxial cells in the early myotome, is required for this progression. Intriguingly, the loss of Cap1 from adaxial cells inhibits their ability to undergo a transient apical constriction, reminiscent of the role of Cap protein in the morphogenetic furrow progression in the Drosophila eye disc, highlighting the Cap protein as a conserved component of a conserved morphogenetic cell behavior critical for a variety of developmental events across species.
Materials and Methods
Zebrafish mutant alleles, stocks and husbandry
Wild-type (AB strain) and mutant zebrafish embryos were obtained from natural spawnings of adult fish kept at 28.5°C on a 14h light/10h dark cycle, raised at temperatures between 22°C-30°C, and were staged according to Kimmel et al. (1995). The allele of you-too (yotty119) used in this study has been described previously (van Eeden et al., 1996).
In situ hybridization and immunocytochemistry
Whole mount in situ hybridization was performed as previously described (Jowett, 1999). Alexa Fluor 488 phalloidin was obtained from Molecular Probes and staining was performed as previously described (Daggett et al., 2004). For nuclear staining, embryos were incubated for 30 minutes in 1μg/ml DAPI (Molecular Probes) in PBS subsequent to the phalloidin staining protocol. F59 staining was performed as previously described (Devoto et al., 1996).
Confocal Microscopy and Time-Lapse Analysis
Confocal images were taken on a Leica Confocal microscope and images were processed with NIH ImageJ and Adobe Photoshop. Embryos (8-10 somite stage), including the yolk were bisected, and the dorsal hemispheres were mounted in 80% glycerol onto coverslip-bridged slides for imaging. Trunk and tail portions of 26-somite embryos were dissected from the yolk and imaged laterally between coverslips. To analyze individual adaxial cell behaviors and membrane dynamics in the living embryo, 1-2 cell stage embryos were injected with mRNA encoding a membrane-targeted GFP protein, leading to high-level, mosaic expression. Embryos with few GFP-positive cells in the medial somitic or presomitic mesoderm were selected at the 5-somite stage, and mounted between bridged coverslips in 0.5% agarose. Single-plane capture was performed over the course of 3-5 hours at 1-minute intervals, with occasional interruption for re-focusing, and the captured images were exported and assembled as Quicktime movie files.
Morpholino Design and Injection
The Cap1 morpholino (5’-ATCTGCCATGCCGTCGCCGTGTGAA-3), designed against the ATG region of the cap1 cDNA sequence, a corresponding Cap1 6-basepair mismatch control morpholino (5’-ATgTGCgATcCCGTgGCCcTGTcAA-3) and a 6-basepair mismatch control morpholino of an unrelated cDNA, quo (5’-ACgAGTCgAGAcAGcAAGcGTTgAT-3’), have been previously used and described (Daggett et al., 2004). 3-5 nl of solution containing morpholino (0.2mM Cap1, 0.3mM Cap1 mismatch control, or 0.3mM Quo mismatch control) and rhodamine-dextran (see below) was injected into the yolk just beneath the blastomeres of 1-2 cell stage wild-type embryos. Embryos were allowed to develop at 28.5°C until the blastula stage when they were used for cell transplantation experiments (below). Injection of either Cap1 mismatch or Quo mismatch control morpholinos was used to control for non-specific morpholino effects.
Cell Transplantation
Isochronic transplantations were performed at the blastula stage as previously described (Amacher and Kimmel, 1998). Donor embryos were co-injected at the 1-cell stage with lineage tracer dye (4% tetramethyl rhodamine-dextran) and morpholino oligonucleotides. Cells were removed from donor embryos and placed into the blastoderm margin of unlabeled, wild-type host embryos. Donor embryos were maintained until at least the tailbud stage to confirm the previously described defects in the development of the polster (Daggett et al., 2004). Hosts containing transplanted cells in the mesoderm at the 8-10 somite stage were fixed and processed for staining as described above.
Results and Discussion
Adaxial Cells Undergo a Stereotypical Set of Premigratory Behaviors
We utilized confocal imaging to characterize the adaxial cell behaviors and rearrangements that occur between the commitment of the adaxial cell fate and the onset of their migration, as they transform from a roughly 4 × 5 array to a 1 × 20 stack within the medial somite (Felsenfeld et al., 1991; Devoto et al., 1996; Hirsinger et al., 2004). Zebrafish embryos at the 8-10 somite stage were treated with phalloidin, which labels F-actin specializations and highlights cell shapes, and DAPI, which stains nuclei. Figure 1A indicates the positions along the axis at which adaxial cells of differing maturities were imaged to generate subsequent panels of the figure.
Figure 1.
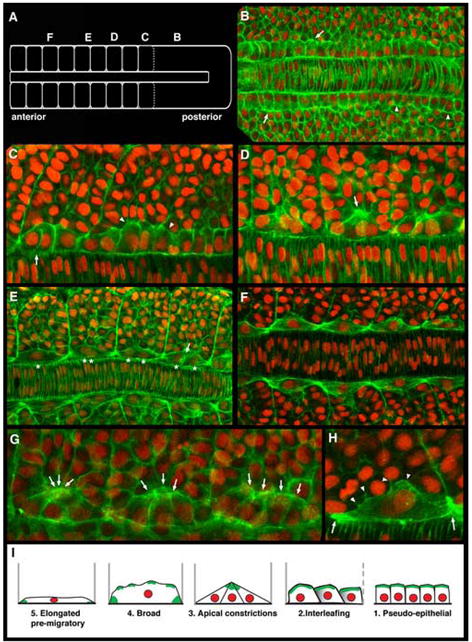
Adaxial cells undergo a series of stereotypical behaviors as the zebrafish myotome matures. Confocal images of adaxial cells at the depth of the notochord within 8-10 somite stage embryos labeled with phalloidin (green) and DAPI (red). Dorsal view, anterior to the left in all panels. (A) Schematic diagram of an 8-somite stage embryo highlighting the regions where subsequent panels (B-F) are centered along the axis. (B) Adaxial cells in the posterior presomitic mesoderm region appear cuboidal (arrowheads) and become more columnar with actin-rich apices in anterior presomitic mesoderm (arrows). (C) As somite boundaries begin to form, adaxial cells begin to lose the columnar shape, displaying more irregular shapes (arrowheads) before becoming more rounded and flattened, and interleafing with one another within the dorsoventral aspect of the forming somite (arrow). (D) As the adaxial cells begin to intercalate, they transiently display actin-rich apical constrictions, with the apices of 2-4 adjacent adaxial cells apparently interacting with one another and with the more lateral cells in the central somite (arrow). (E) Following the release of the apical interactions, adaxial cells continue to broaden, as the number of adaxial cells in a given dorsal ventral section of the somite is ultimately reduced to one. Arrow shows two broad adaxial cells with interacting membranes midway between somite boundaries. Asterisks point out that as somites mature, the nuclei of broadening adaxial cells become superimposed as the adaxial cells ultimately form one dorsoventral stack of cells in the medial somite. (F) Fully intercalated adaxial cells attached to somite boundaries in the anterior somites of an 8-10 somite embryo. (G) Arrows highlight another example of the transition from early interleafing stage adaxial cells with actin-rich apices, to triangular cells displaying apical constrictions is shown across three adjacent somites. (H) Broad, intercalated adaxial cells with thin, lamellar lateral membranes appear to maintain membrane interactions with more lateral somitic cells (arrowheads), and display actin-rich, bipolar somite border attachments (arrows). (I) Schematic summary of the stereotypical behaviors displayed by adaxial cells prior to their migration.
Apart from their specific slow muscle fate, adaxial cells have long been distinguished by their pseudoepithelial appearance within the presomitic mesoderm (Thisse et al., 1993; Devoto et al., 1996). Consistent with this, adaxial cells in the posterior presomitic mesoderm of an 8-10 somite stage embryo display a cuboidal morphology, distinct from the adjacent presomitic mesoderm cells (arrowheads, Figure 1B). In the anterior presomitic mesoderm, the adaxial cells appear more columnar, with an apparent apical-basal polarity manifested by basally located nuclei adjacent to the notochord and the increased accumulation of F-actin within the apical cortex of the cells (arrows, Figure 1B). As adaxial cells become incorporated into newly formed somites they undergo a remarkable series of transient, yet stereotypical and identifiable behaviors. In the zone of a newly forming somite, the 4-5 adaxial cells within a given horizontal plane in the dorsoventral axis begin to lose their cuboidal or columnar appearance, become more rounded, and begin to flatten in their dorsoventral aspect. The disc-like cells soon begin to “interleaf” with one another, with more anterior cells appearing to slide over more posterior cells within the row (Figure 1C). Interleafing quickly results in only 2-3 adaxial cells within the plane between somite boundaries. At this point, the adaxial cells transform into a more triangular shape, with their actin-rich apices transiently associated with each other, as well as with the adjacent lateral cells within the somite (Figure 1D, G). The width of the basal membrane domain during apical constriction was on average 4 times the width of the apical domain (n=113 adaxial cells in apical constriction stage somites in 36 embryos). These transient apical constrictions then appear to disperse as the adaxial cells continue to intercalate while broadening both laterally and anteroposteriorally within the plane, with each cell ultimately making an actin-rich attachment to the anterior and posterior somite boundaries, as well as maintaining lamellar interactions with lateral somitic cells (Figure 1E, H). These behaviors lead to a single dorsoventral stack of broadened adaxial cells in the medial portion of the somite (Figure 1F). Finally, in the most anterior somites of an 8-10 somite embryo, the adaxial cells become further elongated and more recognizably fiber-like prior to the initiation of migration a few hours later (data not shown; Devoto et al., 1996; Cortes et al., 2003; Henry and Amacher, 2004). The highly stereotypical and recognizable stages of adaxial cell morphogenesis are schematically summarized in Figure 1I.
As expected, we find that more mature adaxial cells in more anterior somites are further advanced in the series of behaviors than the younger more posterior cells. Upon examination of dorsal-ventral stacks of images (n=13 embryos), we also find that within a single somite, adaxial cell behaviors occur in a largely dorsal to ventral progression. For example, when a more dorsal row of cells is undergoing apical constrictions, a more ventral row in the same somite will only be initiating interleafing behaviors (Figure 2A, A’). Likewise, as a more dorsal cell completes its bipolar attachments to somite boundaries, the cells below it will still be intercalating (Figure 2B, C). While this trend is observed for the majority of the adaxial cell population within a somite, the most dorsal 1-2 layers of cells also appear to lag behind the third or so row of adaxial cells, which initiate the behaviors. Whether this dorsally biased group of initiating adaxial cells represents the muscle pioneer cells, a subset of the adaxial cell fate that is just beginning to become molecularly distinct at this stage (Hatta et al., 1991; Ekker et al., 1992; Wolff et al., 2003), remains to be determined. Interestingly, the dorsal to ventral progression of adaxial cell behaviors in the zebrafish somite is reminiscent of the dorsal to ventral progression of a different set of cell behaviors that has recently been shown to underlie somite rotation in the Xenopus mesoderm (Afonin et al., 2006). In both cases, the nature of the signal that can coordinate cell behaviors in the dorsal-ventral axis of the somite is unknown.
Figure 2.
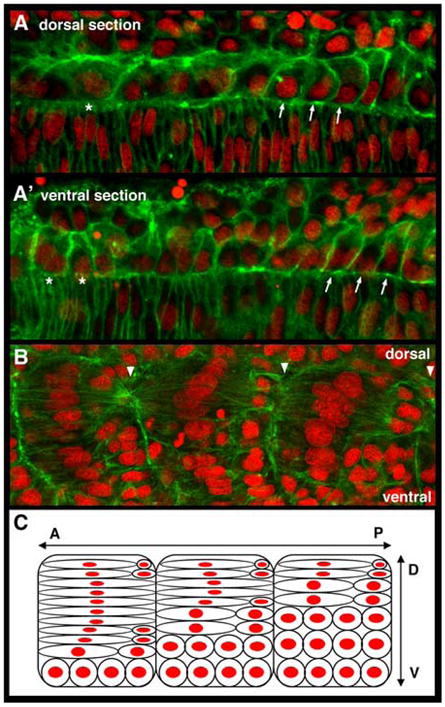
Adaxial cell behaviors occur with a dorsal to ventral progression within the developing myotome. Anterior to left in all panels. (A) In a more dorsal plane of adaxial cells adjacent to the notochord, the more anterior cells are broadening (*) while more posterior cells are undergoing interleafing behaviors (arrows). (A’) In a slightly more ventral plane, the anterior adaxial cells are beginning to lose epithelial shape and to accumulate cortical actin, characteristic of the early interleafing stage (*), while the more posterior adaxial cells still predominantly display a pseudo-epithelial appearance (arrows). (B) A lateral image of 3 adjacent somites, focused just lateral to the notochord surface, shows that in each somite, the more dorsal adaxial cells in the somite have undergone further elongation and intercalation than those more ventral. Arrowheads highlight somite boundaries. (C) Schematic representation of panel B, illustrating both that the majority of more dorsal cells within the myotome are further advanced in their behaviors than the more ventral, and further, that within a given dorsoventral plane, the adaxial cells in more anterior somites are more advanced in their behaviors than those in more posterior somites as is apparent in Figure 1. A, anterior; P, posterior; D, dorsal; V, ventral.
We performed timelapse confocal imaging of adaxial cells expressing a membrane-tethered GFP protein to confirm the behaviors in live embryos and to further examine the membrane dynamics of adaxial cells during this process (Figure 3). In addition to the expected shape transitions, we observed the transient rounding of cells before and after the apical constriction event (Figure 3B-E, Supplementary Movie) and highly active lateral membrane dynamics as cells transition through all the behaviors. Adaxial cells display filopodial and lamellapodial extensions as the entire lateral lamellar surface ruffles and appears to explore its more lateral environment (Fig 3, Supplementary Movie).
Figure 3.
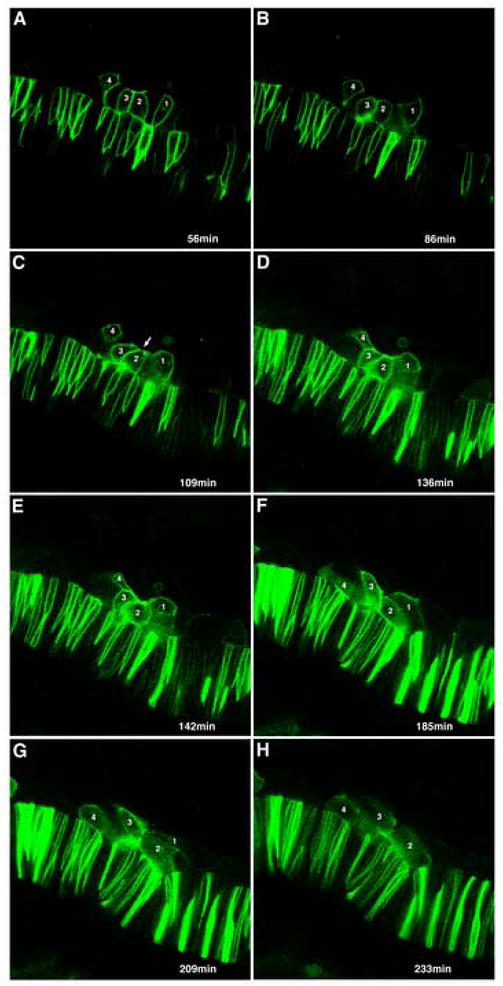
Time-lapse analysis of adaxial cell behaviors. Selected frames from Supplementary movie. Four adaxial cells expressing a membrane-targeted GFP mRNA, that will eventually become incorporated into two adjacent somites, were followed for 4.5 hours. Elapsed time, in minutes, of selected images shown. (A) Adaxial cells (1-4) are initially predominantly pseudo-epithelial, with their basal membranes against the notochord. Columnar shape is lost as adaxial cells become more irregularly shaped and rounded as they begin to interleaf (B), before beginning to make transient apical constrictions (arrow, C). (D-F) Adaxial cell membranes then begin to spread out as cells begin to broaden, with cell 1 showing a broad, lamellar expansion and cell 2 dramatically rounding (E) prior to further elongating. (F, G) Cells maintain broad character and laterally directed lamellar protrusions as they further elongate and intercalate. (H) Cell 3 eventually becomes partially obscured underneath cell 4, while cell 1 becomes intercalated fully beneath cell 2 in an adjacent somite.
Adaxial Cell Rearrangements are Dependent on Hedgehog Signaling
The role of Hedgehog signals emanating from the midline in directing the slow-twitch muscle cell fate in zebrafish is well established (Currie and Ingham, 1996; Blagden et al., 1997; Du et al., 1997; Baressi et al., 2000; Wolff et al., 2003). Recently, it has been demonstrated that the initial induction of the myoD-expressing, morphologically distinct adaxial cells observed in the presomitic mesoderm is Hedgehog-independent, with the further commitment of adaxial cells to the early-differentiating, slow-twitch myosin-expressing cell fate being Hedgehog-dependent (Lewis et al., 1999; Hirsinger et al., 2004). To determine whether the pre-migratory adaxial cell rearrangements we observed are dependent on Hedgehog signaling, we examined them in embryos derived from the yot (you-too) line, which carries a mutation in the Gli2 transcription factor that is activated downstream of Hedgehog-signal transduction, and displays nearly a complete loss of adaxial cell-derived slow muscle cells in the homozygous condition (Lewis et al., 1999; Karlstrom et al., 1999). Consistent with previous studies, adaxial cells in yot mutant embryos still retained a distinguishable pseudo-epithelial character in the presomitic mesoderm (Figure 4A). However as adaxial cells become incorporated into newly forming somites, they fail to display any of the further behaviors such as rounding, apical constriction or broadening, and instead become nearly indistinguishable from neighboring somitic border cells (Figure 4B). In addition, unlike wild-type adaxial cells that show enrichments of F-actin at their apices, morphological specializations, and at somite boundary attachments (Figure 1), F-actin was distributed evenly throughout the cortex of yot adaxial cells both within the presomitic mesoderm and within somites (Figure 4). These results demonstrate that the complex transition from a 4 × 5 array of cuboidal cells to a 1 × 20 stack of broadened adaxial cells that are attached to somite boundaries represents the point where distinct adaxial cell morphogenetic behaviors become Hedgehog-dependent. Furthermore, consistent with the timing of the Hedgehog-dependent onset of slow-twitch myosin expression by adaxial cells (Devoto et al., 1996; Blagden et al., 1997; Bryson-Richardson et al., 2005), these data also confirm that these behaviors are a specific aspect of the slow-twitch muscle cell fate commitment (Hirsinger et al., 2004). Finally, these observations correlate the Hedgehog-dependent cell behaviors with the regulation of actin cytoskeletal dynamics.
Figure 4.
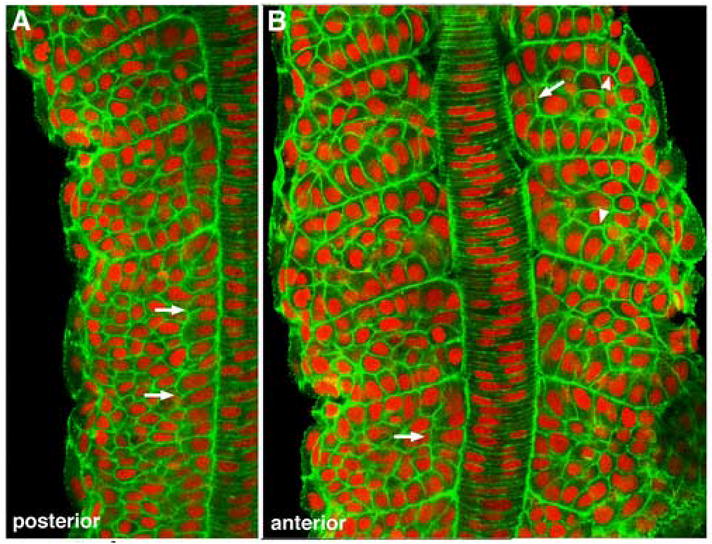
Adaxial cell behaviors are dependent on Hedgehog signaling. (A) Anterior presomitic and posterior somitic mesoderm in a 10-somite yot-/- embryo. (B) More anterior somites of the same embryo. Anterior is up in both panels. Adaxial cells (arrows) in the presomitic mesoderm of yot mutants are distinguishable by their columnar appearance (A), but fail to accumulate apical actin or to undergo any intercalation or broadening behaviors as somites begin to form (B), and quickly become indistinguishable from other somitic cells (arrowheads).
The Expression of the Actin Regulatory Molecule Cyclase-Associated Protein 1 is Enriched in Adaxial Cells as they Undergo Rearrangement Behaviors
The complex behaviors and actin-based cellular specializations displayed by slow-twitch muscle cell precursors raised the possibility that the morphogenetic aspect of their fate is controlled by the deployment of specific regulators of cell morphology. In an in situ hybridization screen for tissue specific modulators of the cytoskeleton, we previously identified cyclase-associated protein 1 (cap1) as displaying developmentally-restricted expression patterns that included adaxial cells (Daggett et al., 2004). Earlier in zebrafish development, cap1 expression is required in the anterior mesendodermal cells of the gastrula for their proper arrangement and anterior migration as the embryonic axis extends (Daggett et al., 2004). Cap has been well studied during Drosophila development, where it is required to regulate apical actin filament growth and drive apical cellular constriction in cells during both morphogenetic furrow progression in the eye disc and the formation of the follicular epithelium (Benlali et al., 2000; Baum and Perrimon, 2001). Cap1 is thus an attractive candidate for mediating aspects of adaxial cell behaviors during myotome development.
The expression of cap1 in adaxial cells is first detectable by in situ hybridization in the presomitic mesoderm at the tailbud stage (data not shown), at a time when adaxial cells are becoming committed to their fate and display the characteristic cuboidal shape adjacent to the notochord (Hirsinger et al., 2004). cap1 is continually expressed in adaxial cells as somites form and throughout mid-somitogenesis stages as adaxial cells rearrange prior to migration (Figure 5). From the tailbud stage until approximately the 10-somite stage, cap1 transcripts are highly enriched in adaxial cells (Figure 5A). After the 10-somite stage, while adaxial cell expression persists, further expression begins to appear in the overlying ectoderm (Figure 5B). Adaxial cap1 expression in anterior somites becomes less discernable after the 15-somite stage, just prior to the initiation of adaxial cell migration, as additional non-somitic cap1 expression domains continue to appear (data not shown).
Figure 5.
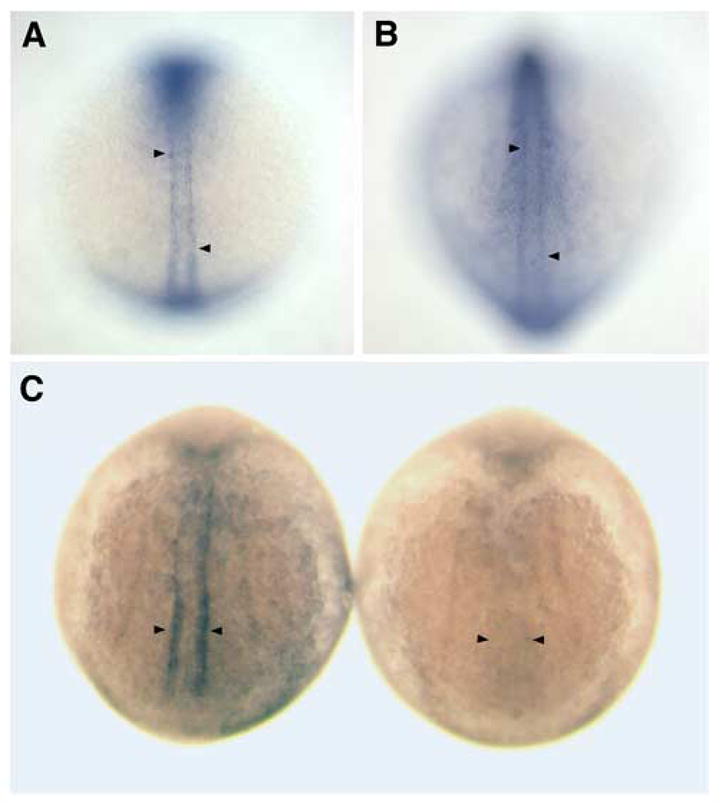
cap1 expression is enriched in adaxial cells prior to and during their pre-migratory behaviors and is dependent on Hedgehog signaling. (A) In a 5-somite stage wild-type embryo, cap1 is expressed specifically in the adaxial cells of both newly formed somites (upper arrowhead) and the presomitic mesoderm (lower arrowhead). (B) At the 12-somite stage cap1 continues to be expressed within the adaxial cells of somitic and presomitic mesoderm (arrowheads), and is becomes increasingly upregulated in the overlying ectoderm. (C) 10-somite stage embryos showing cap1 expression in wild-type (left) and yot mutant (right) embryos. cap1 is not expressed in the adaxial cells of yot mutants (arrowheads), which are deficient in Hedgehog signal transduction.
If cap1 plays a critical role in the Hedgehog-dependent regulation of adaxial cell rearrangements, cap1 expression in adaxial cells might be expected to be Hedgehog-dependent. Indeed, cap1 expression is absent from adaxial cells in approximately 25% of the embryos derived from intercrosses of yot heterozygotes (Figure 5C), while its expression in other domains, such as the anterior mesendoderm, is unaffected (data not shown; Daggett et al., 2004).
Cap1 is Required for the Progression of Early Adaxial Cell Behaviors
To test whether the expression of cap1 in adaxial cells is required for the progression of adaxial cell behaviors we performed loss of function analyses. Previously we showed that loss of Cap1 by morpholino-mediated knockdown led to defects in the migration of anterior mesendodermal cells, which specifically express cap1 in the late gastrula, with secondary consequences on the convergent extension movements of more posterior mesoderm (Daggett et al., 2004). Defects in convergent extension lead to abnormal cellular distributions and tissue morphology in both axial and paraxial mesoderm that would likely confound any analysis of individual adaxial cell morphologies in early segmentation stage embryos (reviewed in Tada et al., 2002). In addition, convergent extension defects have been shown to interfere with the normal specification of slow-twitch muscle precursors in the developing myotome (Yin and Solnica-Krezel, 2007). Thus to directly address the cell-autonomous requirements of Cap1 in adaxial cells we performed mosaic analyses by transplanting small numbers of cells from morpholino-injected donor embryos into wild-type hosts, and then determined the ability of the Cap1-deficient donor cells to undergo normal cell behaviors when they contributed to the adaxial cell lineage (Figure 6). We assessed adaxial cell shape and behavior by comparing the morphology of transplanted cap1-deficient transplanted cells with that of the adjacent and/or contralateral wild-type adaxial cells in fixed embryos.
Figure 6.
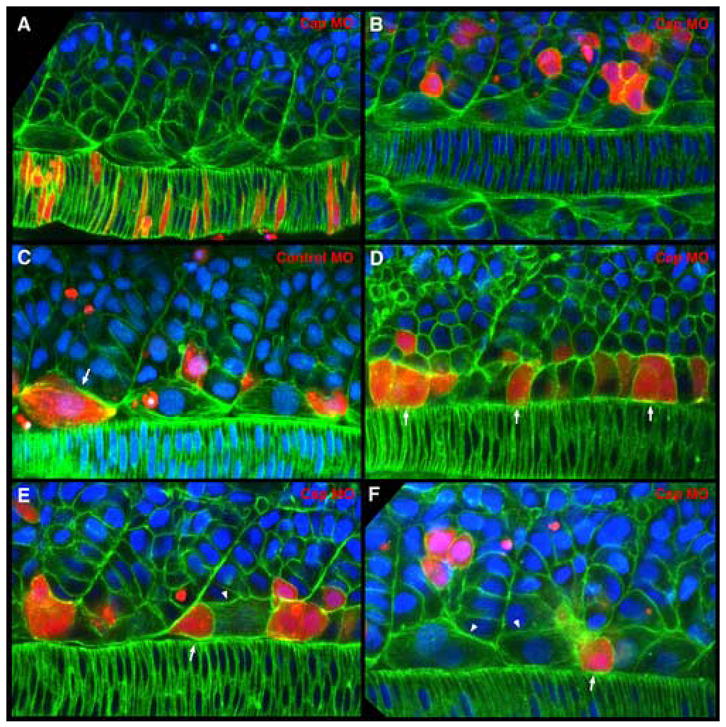
Cap1 is required for adaxial cells to form apical constrictions, intercalate and broaden. Cells from embryos co-injected with rhodamine-dextran and translation blocking or mismatch control morpholinos were transplanted into presumptive mesodermal precursor regions of wild type embryos at the late blastula stage. Confocal images of adaxial cells at the mid-notochord level of fixed 8-10 somite stage embryos labeled with phalloidin (green) and DAPI (blue). Rhodamine-dextran labels transplanted cells (red). Dorsal view with anterior to the left in all panels. (A, B) Injection of Cap1 morpholino does not cause defects in non-adaxial mesoderm cell behaviors. (A) Cap1 morpholino-containing notochord cells undergo normal intercalation behaviors and are otherwise indistinguishable from wild-type neighbors. (B) Cap1 morpholino-containing cells in the more lateral somite display the same shapes and distributions as their wild type neighbors. (C) Adaxial cells containing mismatch control morpholinos are able to complete the typical array of cell behaviors, becoming broadened and making somite attachments (arrow) in a manner indistinguishable from their wild-type neighbors. (D) Cap1-deficient adaxial cells display the normal cuboidal and columnar cells shapes in presomitic mesoderm (arrows). (E, F) Cap1-deficient adaxial cells fail to make apical constrictions, to intercalate normally or to broaden and make bipolar somite boundary attachments. Instead they display an isolated rounded appearance (arrows in E, F) among neighboring wild-type adaxial cells that have broadened (arrowheads in E, F).
When Cap1-deficient cells contributed to either the notochord or non-adaxial somitic cells, both tissues that do not significantly express Cap1 at these stages, they appeared indistinguishable from their wild-type neighbors (100%, n=58 cells in 22 embryos), demonstrating that the Cap1 morpholino itself does not cause non-specific defects in cell morphology in mesodermal tissues (Figure 6A, B). Likewise, when cells containing a “mismatch” control morpholino contribute to the adaxial lineage, they behave normally, broadening and forming somite border attachments (Figure 6C), further demonstrating an absence of non-specific effects on adaxial cells (100%, n=13 cells in 7 embryos). Cap1-deficient adaxial cells still display the pseudo-epithelial morphology typical within the presomitic mesoderm, and also can begin the subsequent loss of cuboidal morphology (Fig 6D; 97%, n=35 cells in 24 embryos). However, Cap1-deficient adaxial cells appear to arrest their behaviors at this point, as none were observed making the transient apical constrictions, and very few progressed to the broad morphology or made somite border attachments (3%, n=37 cells in 27 embryos). Instead Cap1-deficient adaxial cells retain a rounded morphology, adjacent to one somite boundary or the other, and appear to lack any localized accumulations of F-actin (Figure 6E, F). It is possible that the arrested “round” phenotype of transplanted Cap1-deficient cells, a phenotype not observed in yot(gli2) mutants, results from the presence of neighboring wild-type adaxial cells. The observations that Cap1 is required for the progression of early adaxial cell behaviors, that these behaviors are absent in yot mutants, and that cap1 is not expressed in yot mutants, suggest that the lack of Cap1 is responsible for the lack of adaxial cell behavior in yot and other Hedgehog-signaling mutants (Lewis et al., 1999; Hirsinger et al., 2004). It remains to be seen however if other Hedgehog-dependent gene products absent in yot adaxial cells contribute to the cell behaviors or whether Cap1 is by itself sufficient to drive them in cells deficient in the Hedgehog response. Together, these results demonstrate that Hedgehog-dependent Cap1 is required cell-autonomously for adaxial cells to progress through the apical constriction phase and to further broaden and make somite border attachments in a normal manner.
Following the early Cap1-dependent adaxial cell behaviors documented here, the majority of wild-type adaxial cells undergo a well characterized migration event through the lateral myotome to populate the superficial slow fiber (SSF) domain, while a minority remain medial and differentiate into the muscle pioneer (MP) cluster associated with the horizontal myoseptum (Devoto et al., 1996; Cortes et al., 2003; Hatta et al., 1991; Ekker et al., 1992). To determine whether Cap1 and the early adaxial cell behaviors are required for these later events, we performed mosaic analyses as above and analyzed embryos at the 26-somite stage, when adaxial cell migration in trunk somites is complete. By labeling the slow-twitch muscle population with the F59 antibody (Devoto et al., 1996), we found that Cap1-deficient cells in a wild-type environment can express slow-twitch myosin and contribute normally to the SSF and MP domains (Supplementary Figure 1, Supplementary Table 1). These results suggest that while Cap1 is critical for the normal rearrangements of differentiating adaxial cells in the early somite, Cap1-deficient adaxial cells retain the ultimate capacity to differentiate as slow muscle and migrate as the somite matures, illustrating both compartmentalization and robustness within the critical event of myotome development. We have previously shown that adaxial cell migration is initially disrupted in Notch pathway mutants, but recovers over time, and that the accompanying myotome boundary recovery event is dependent upon Hh signals (Henry et al., 2005). Whether Cap1 is required for proper morphology of migrating adaxial cells, or for precise timing of the migration event, are still open questions.
In Drosophila, Cap acts in concert with Profilin to control actin filament growth and regulate apical constrictions of cells in the morphogenetic furrow of the developing eye disc (Benlali et al., 2000), in addition to controlling actin filament formation at apical cell junctions in the follicular epitheilium (Baum and Perrimon, 2001). Intriguingly, apical constriction in the morphogenetic furrow is required for proper Hedgehog signaling to neighboring cells, potentially by restricting signaling to the vicinity of the apices and limiting more long range effects (Benlali et al., 2000). As Hedgehog signaling from the midline is thought to provide quantitatively and temporally distinct signals across the zebrafish myotome to influence cell fates (Wolff et al., 2003; Feng et al., 2006) it will be interesting to see whether the apical constriction of adaxial cells driven by Cap1 plays modulatory roles in the determination of myotomal cell fates in addition to its role in adaxial cell rearrangements.
Previously we showed that Cap1 is required for proper both the regulation of cortical actin and directed cell movements during the migration of anterior mesoderm in the zebrafish gastrula (Daggett et al., 2004). Here we demonstrate a second specific requirement for Cap in zebrafish, the progression of adaxial cell behaviors during myotome maturation. Considered alongside the role of Cap in Drosophila, the failure of Cap1-deficient adaxial cells to form transient apical constrictions suggests that Cap proteins may have a conserved function in controlling actin dynamics to create this particular cellular specialization in a variety of developmental events across diverse species. More broadly, a fundamental role for cellular apical constriction in embryonic morphogenesis during developmental stages such as gastrulation and neurulation has long been appreciated (Odell et al., 1981), and recent work has begun to uncover the molecular mechanisms that drive it (Chrisholm, 2006; Pilot and Lecuit, 2005). During Drosophila gastrulation, apical constriction is regulated by the folded gastrulation (fog) pathway, which directs actomyosin contractions. In vertebrates, the PDZ domain protein Shroom promotes apical positioning of an actomyosin network and apical constriction via the Rap1 GTPase during neural tube closure (Haigo et al., 2003; Hildebrand, 2005). In C. elegans, non-canonical Wnt-signaling has been implicated in regulating apical constriction during the ingression of cells in early gastrulation events (Lee et al., 2006). Thus Cap is one of a growing number of molecules and pathways implicated in regulating embryonic morphogenesis by promoting specific cell behaviors that effect larger scale tissue rearrangements.
Supplementary Material
Acknowledgments
We thank Jennifer St. Hilaire, Emily Janus and Kimberly Blum for excellent zebrafish care and technical support. We thank Holly Aaron at the Molecular Imaging Center at Berkeley for imaging advice. We are grateful to Emilie Delaune-Henry for helpful comments on the manuscript. This work was supported by an NIH grant (GM61952-01) and a Pew Scholar Award to S.L.A., an NIH NRSA fellowship (GM70081-03) to D.F.D., SFSU Sabatical and NIH MBRS SCORE (S06 6M52588) to C.R.D. and support to P.D.C. via the MRC UK and NHMRC, AUS Grant 404806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonin B, Ho M, Gustin JK, Meloty-Kapella C, Domingo CR. Cell behaviors associated with somite segmentation and rotation in Xenopus laevis. Dev Dyn. 2006;235:3268–79. doi: 10.1002/dvdy.20979. [DOI] [PubMed] [Google Scholar]
- Amacher SL, Kimmel CB. Promoting notochord fate and repressing muscle development in zebrafish axial mesoderm. Development. 1998;125:1397–406. doi: 10.1242/dev.125.8.1397. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–99. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Baum B, Perrimon N. Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat Cell Biol. 2001;3:883–90. doi: 10.1038/ncb1001-883. [DOI] [PubMed] [Google Scholar]
- Benlali A, Draskovic I, Hazelett DJ, Treisman JE. act up controls actin polymerization to alter cell shape and restrict Hedgehog signaling in the Drosophila eye disc. Cell. 2000;101:271–81. doi: 10.1016/s0092-8674(00)80837-5. [DOI] [PubMed] [Google Scholar]
- Blagden CS, Currie PD, Ingham PW, Hughes SM. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997;11:2163–75. doi: 10.1101/gad.11.17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C, Amacher SL, Currie PD. Somitogenesis. Results Probl Cell Differ. 2002;40:271–97. doi: 10.1007/978-3-540-46041-1_14. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Curr Opin Genet Dev. 2002;12:548–57. doi: 10.1016/s0959-437x(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Bryson-Richardson RJ, Daggett DF, Cortes F, Neyt C, Keenan DG, Currie PD. Myosin heavy chain expression in zebrafish and slow muscle composition. Dev Dyn. 2005;233:1018–22. doi: 10.1002/dvdy.20380. [DOI] [PubMed] [Google Scholar]
- Chisholm AD. Gastrulation: Wnts signal constriction. Curr Biol. 2006;16:R874–6. doi: 10.1016/j.cub.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Cinnamon Y, Ben-Yair R, Kalcheim C. Differential effects of N-cadherin-mediated adhesion on the development of myotomal waves. Development. 2006;133:1101–12. doi: 10.1242/dev.02291. [DOI] [PubMed] [Google Scholar]
- Cortes F, Daggett D, Bryson-Richardson RJ, Neyt C, Maule J, Gautier P, Hollway GE, Keenan D, Currie PD. Cadherin-mediated differential cell adhesion controls slow muscle cell migration in the developing zebrafish myotome. Dev Cell. 2003;5:865–76. doi: 10.1016/s1534-5807(03)00362-9. [DOI] [PubMed] [Google Scholar]
- Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–89. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Coutelle O, Blagden CS, Hampson R, Halai C, Rigby PW, Hughes SM. Hedgehog signalling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev Biol. 2001;236:136–50. doi: 10.1006/dbio.2001.0193. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–5. doi: 10.1038/382452a0. [DOI] [PubMed] [Google Scholar]
- Daggett DF, Boyd CA, Gautier P, Bryson-Richardson RJ, Thisse C, Thisse B, Amacher SL, Currie PD. Developmentally restricted actin-regulatory molecules control morphogenetic cell movements in the zebrafish gastrula. Curr Biol. 2004;14:1632–8. doi: 10.1016/j.cub.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Denetclaw WF, Ordahl CP. The growth of the dermomyotome and formation of early myotome lineages in thoracolumbar somites of chicken embryos. Development. 2000;127:893–905. doi: 10.1242/dev.127.4.893. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Melancon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–80. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Stoiber W, Hammond CL, Steinbacher P, Haslett JR, Barresi MJF, Patterson SE, Adiarte EG, Hughs SM. Generality of vertebrate developmental patterns: evidence for a dermomyotome in fish. Evolution & Development. 2006;8:101–110. doi: 10.1111/j.1525-142X.2006.05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Devoto SH, Westerfield M, Moon RT. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J Cell Biol. 1997;139:145–56. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker M, Wegner J, Akimenko MA, Westerfield M. Coordinate embryonic expression of three zebrafish engrailed genes. Development. 1992;116:1001–10. doi: 10.1242/dev.116.4.1001. [DOI] [PubMed] [Google Scholar]
- Felsenfeld AL, Curry M, Kimmel CB. The fub-1 mutation blocks initial myofibril formation in zebrafish muscle pioneer cells. Dev Biol. 1991;148:23–30. doi: 10.1016/0012-1606(91)90314-s. [DOI] [PubMed] [Google Scholar]
- Feng X, Adiarte EG, Devoto SH. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev Biol. 2006;300:736–746. doi: 10.1016/j.ydbio.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Gros J, Scaal M, Marcelle C. A two-step mechanism for myotome formation in chick. Dev Cell. 2004;6:875–82. doi: 10.1016/j.devcel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Groves JA, Hammond CL, Hughes SM. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development. 2005;132:4211–22. doi: 10.1242/dev.01958. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Hatta K, Bremiller R, Westerfield M, Kimmel CB. Diversity of expression of engrailed-like antigens in zebrafish. Development. 1991;112:821–32. doi: 10.1242/dev.112.3.821. [DOI] [PubMed] [Google Scholar]
- Henry CA, Amacher SL. Zebrafish slow muscle cell migration induces a wave of fast muscle morphogenesis. Dev Cell. 2004;7:917–23. doi: 10.1016/j.devcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Henry CA, McNulty IM, Durst WA, Munchel SE, Amacher SL. Interactions between muscle fibers and segment boundaries in zebrafish. Dev Biol. 2005;287:346–60. doi: 10.1016/j.ydbio.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hirsinger E, Stellabotte F, Devoto SH, Westerfield M. Hedgehog signaling is required for commitment but not initial induction of slow muscle precursors. Dev Biol. 2004;275:143–57. doi: 10.1016/j.ydbio.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Hollway G, Currie P. Vertebrate myotome development. Birth Defects Res C Embryo Today. 2005;75:172–9. doi: 10.1002/bdrc.20046. [DOI] [PubMed] [Google Scholar]
- Hollway GE, Bryson-Richardson RJ, Berger S, Cole NJ, Hall TE, Currie PD. Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev Cell. 2007;12:207–219. doi: 10.1016/j.devcel.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Jowett T. Analysis of protein and gene expression. Methods Cell Biol. 1999;59:63–85. doi: 10.1016/s0091-679x(08)61821-x. [DOI] [PubMed] [Google Scholar]
- Kahane N, Cinnamon Y, Kalcheim C. The roles of cell migration and myofiber intercalation in patterning formation of the postmitotic myotome. Development. 2002;129:2675–87. doi: 10.1242/dev.129.11.2675. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Ben-Yair R. Cell rearrangements during development of the somite and its derivatives. Curr Opin Genet Dev. 2005;15:371–80. doi: 10.1016/j.gde.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–93. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lee JY, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr Biol. 2006;16:1986–97. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KE, Currie PD, Roy S, Schauerte H, Haffter P, Ingham PW. Control of muscle cell-type specification in the zebrafish embryo by Hedgehog signalling. Dev Biol. 1999;216:469–80. doi: 10.1006/dbio.1999.9519. [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Melancon E, Eisen JS. Segmental relationship between somites and vertebral column in zebrafish. Development. 2002;129:3851–60. doi: 10.1242/dev.129.16.3851. [DOI] [PubMed] [Google Scholar]
- Odell GM, Oster G, Alberch P, Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446–62. doi: 10.1016/0012-1606(81)90276-1. [DOI] [PubMed] [Google Scholar]
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–94. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- Stellabotte F, Dobbs-McAuliffe B, Fernandez DA, Feng X, Devoto SH. Dynamic somite cell rearrangements lead to distinct waves of myotome growth. Development. 2007;134:1253–7. doi: 10.1242/dev.000067. [DOI] [PubMed] [Google Scholar]
- Stickney HL, Barresi MJ, Devoto SH. Somite development in zebrafish. Dev Dyn. 2000;219:287–303. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1065>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–60. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–15. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nusslein-Volhard C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–64. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Venters SJ, Ordahl CP. Persistent myogenic capacity of the dermomyotome dorsomedial lip and restriction of myogenic competence. Development. 2002;129:3873–85. doi: 10.1242/dev.129.16.3873. [DOI] [PubMed] [Google Scholar]
- Waterman RE. Development of the lateral musculature in the teleost, Brachydanio rerio: a fine structural study. Am J Anat. 1969;125:457–93. doi: 10.1002/aja.1001250406. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–80. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Wolff C, Roy S, Ingham PW. Multiple muscle cell identities induced by distinct levels and timing of Hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–81. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- Yin C, Solnica-Krezel L. Convergence and extension movements mediate the specification and fate maintenance of zebrafish slow muscle precursors. Dev Biol. 2007;304:141–55. doi: 10.1016/j.ydbio.2006.12.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


