Summary
Membrane fusion in all eukaryotic cells is regulated by the formation of specific SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complexes. The molecular mechanisms that control this process are conserved through evolution and require several protein families, including Sec1p/Munc18 (SM) proteins. Here, we demonstrate that the mammalian SNARE protein syntaxin 16 (Sx16, also known as Syn16) is a functional homologue of the yeast SNARE Tlg2p, in that its expression fully complements the mutant phenotypes of tlg2Δ mutant yeast. We have used this functional homology to demonstrate that, as observed for Tlg2p, the function of Sx16 is regulated by the SM protein Vps45p. Furthermore, in vitro SNARE-complex assembly studies demonstrate that the N-terminal domain of Tlg2p is inhibitory to the formation of SNARE complexes, and that this inhibition can be lifted by the addition of purified Vps45p. By combining these cell-biological and biochemical analyses, we propose an evolutionarily conserved regulatory mechanism for Vps45p function. Our data support a model in which the SM protein is required to facilitate a switch of Tlg2p and Sx16 from a closed to an open conformation, thus allowing SNARE-complex assembly and membrane fusion to proceed.
Keywords: Membrane fusion, Sec1p/Munc18, SNARE, Syntaxin, Tlg2p
Introduction
The molecular machinery that regulates membrane fusion is conserved throughout the eukaryotic kingdom, with the formation of a functional SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex representing the minimal requirement (Jahn and Scheller, 2006). A SNARE complex consists of a parallel four-helical bundle of SNARE motifs, one of which is contributed by a syntaxin (Jahn and Scheller, 2006). As well as their helical SNARE motif, syntaxins possess an autonomously folded three-helix-bundle domain near their N-terminus; this domain is called the Habc domain (Ungar and Hughson, 2003). For several syntaxins, including the neuronal syntaxin 1a (Sx1a) and the yeast plasma membrane Sso1p, the Habc domain regulates SNARE-complex assembly by binding intramolecularly to the SNARE motif (Dulubova et al., 1999; Misura et al., 2000; Munson et al., 2000). In this closed conformation, the syntaxin is unable to form SNARE complexes, owing to inaccessibility of the SNARE motif. The SNARE motif is released from this inhibition in the open conformation, allowing SNARE-complex assembly to proceed (Dulubova et al., 1999; Misura et al., 2000; Munson and Hughson, 2002).
Regulating SNARE-complex assembly provides a mechanism to regulate membrane fusion. Although the formation of a functional SNARE complex is sufficient to drive membrane fusion in vitro (Parlati et al., 1999), it is clear that other factors, including the Sec1p/Munc18 (SM) proteins, are essential for this process in the cell (Jahn and Scheller, 2006). SM proteins regulate membrane fusion through interaction with SNARE proteins and, like syntaxins, are conserved through evolution (Jahn and Scheller, 2006). We have previously reported a regulatory role for the Saccharomyces cerevisiae SM protein Vps45p (vacuolar protein sorting protein 45) in SNARE-complex assembly by facilitating the switch of its cognate syntaxin Tlg2p (t-SNARE of the late Golgi compartment protein 2; also known as YOLO18C) from a closed to an open conformation (Bryant and James, 2001). Munc18a has also been suggested to regulate Sx1a in this manner (Dulubova et al., 1999), but whether this regulation is conserved across all SM-syntaxin pairs is not clear (Dulubova et al., 2002).
In silico sequence analyses are used to identify homologous proteins across species, and data from different experimental systems are often brought together to describe the function of protein families. Mammalian syntaxin 16 (Sx16, also known as Syn16) was identified as the closest homologue of the yeast syntaxin Tlg2p (Simonsen et al., 1998), and assumptions have been made regarding Sx16 function using experimental evidence obtained from Tlg2p (Dulubova et al., 2002).
Here, we demonstrate that, as well as being an in silico homologue of Tlg2p, Sx16 is a functional homologue of the yeast syntaxin, in that its expression complements mutant phenotypes of yeast cells lacking TLG2. We have used this functional homology, in combination with in vitro SNARE-complex assembly assays, to demonstrate that Vps45p regulates the entry of Tlg2p or Sx16 into functional SNARE complexes by lifting inhibition exerted by the Habc domain of the syntaxin.
Results
Sx16 is a functional homologue of Tlg2p
Sequence analyses identified the yeast syntaxin Tlg2p as the closest S. cerevisiae homologue of the trans-Golgi network (TGN)-localised mammalian Sx16 (Simonsen et al., 1998). To address whether Sx16 is a functional homologue of Tlg2p, we expressed human Sx16 in S. cerevisiae and investigated whether it could perform the same functions as Tlg2p in vivo. For this purpose it was essential that Sx16 was not expressed at grossly elevated levels compared with Tlg2p in wild-type cells, because SNARE proteins can interact promiscuously under non-physiological conditions (Fasshauer et al., 1999; Tsui and Banfield, 2000; Yang et al., 1999). Mammalian syntaxin 4 (Sx4) was also included as an unrelated syntaxin, to address whether any observed functionality of Sx16 was specific to the Tlg2p homologue and could not be performed by any syntaxin expressed at similar levels.
For expression of the mammalian syntaxins in S. cerevisiae, we used the Cu2+-regulated CUP1 promoter (Butt et al., 1984). We constructed a centromeric plasmid encoding an epitope-tagged version of Tlg2p (HA-Tlg2p) from this promoter. When expressed in tlg2Δ yeast cells that were grown in the presence of 50 μM CuCl2, the levels of HA-Tlg2p were comparable to the levels of endogenous Tlg2p (Fig. 1A). Plasmids encoding HA-tagged versions of mammalian Sx16 and Sx4 under CUP1-promoter control produced Sx16 and Sx4 at levels similar to CUP1-expressed HA-Tlg2p (Fig. 1B). Therefore, the levels of Sx16 used in this study are not grossly elevated compared to the endogenous levels of Tlg2p.
Fig. 1.

Expression of Sx16 and Sx4 in S. cerevisiae. (A) tlg2Δ cells (NOzY3) harbouring pMAZ006 (driving HA-Tlg2p production from the CUP1 promoter) or the parent vector (pNB701), and wild-type (WT) cells (SF838-9D harbouring pNB701; `vector') were grown to mid-log phase in synthetic medium lacking uracil but supplemented with 50 μM CuCl2 (SD –ura +50 μM CuCl2). Lysates from these were analysed by immunoblotting for Tlg2p (and the vacuolar ATPase subunit Vph1p to control for equal loading). (B) tlg2Δ cells (NOzY3) harbouring plasmids driving the production of HA-tagged Tlg2p, Sx16 or Sx4 from the CUP1 promoter (pMAZ006, pMAZ002 and pMAZ007, respectively) were grown and processed as for A, except immunoblot analysis was performed using anti-HA antibodies.
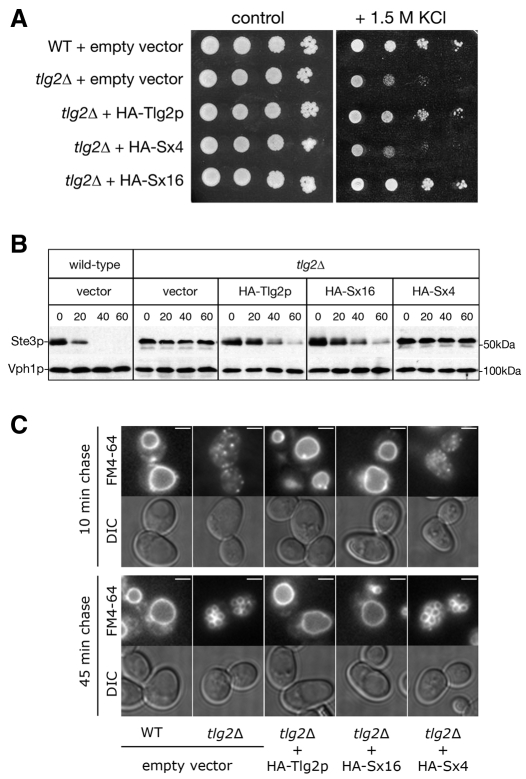
Yeast lacking Tlg2p (tlg2Δ) display multiple endocytic defects (Abeliovich et al., 1998; Holthuis et al., 1998; Seron et al., 1998). tlg2Δ mutants are sensitive to osmotic stress and exhibit a growth defect on high-salt media (Abeliovich et al., 1998; Banta et al., 1988). This growth defect is overcome by expression of either HA-Tlg2p or HA-Sx16, but not HA-Sx4 (Fig. 2A). The endocytic defect of tlg2Δ cells also causes a delay in the degradation of the pheromone receptor Ste3p by vacuolar proteases (Fig. 2B) and of delivery of the lipophilic dye FM4-64 to vacuolar membranes (Fig. 2C). Both of these mutant phenotypes are complemented by expression of either HA-Tlg2p or HA-Sx16, but not HA-Sx4 (Fig. 2B,C). Ste3p is degraded by vacuolar proteases with a half-life of ∼20 minutes in wild-type cells (Davis et al., 1993), but is longer lived in tlg2Δ cells (Fig. 2B). Expression of HA-Sx4 has no effect on the turnover of Ste3p in tlg2Δ cells, whereas expression of either HA-Tlg2p or HA-Sx16 destabilises the receptor (Fig. 2B). FM4-64 is delivered to the vacuolar membrane of wild-type cells during a 10-minute chase period (Fig. 2C; Table 1). tlg2Δ cells require a longer chase period of 45 minutes to deliver FM4-64 to their vacuoles, with the dye localising to punctate structures after the shorter chase time (10 minutes; Fig. 2C; Table 1). Expression of either HA-Tlg2p or HA-Sx16, but not HA-Sx4 complements, not only this delayed delivery of FM4-64 to the vacuolar membrane, but also the fragmented vacuolar morphology phenotype displayed by tlg2Δ cells (Fig. 2C). Collectively the data presented in Fig. 2 demonstrate that heterologous expression of Sx16 complements the endocytic defects of tlg2Δ mutants, whereas expression of Sx4 does not, indicating that mammalian Sx16 can perform the function(s) normally performed by Tlg2p in yeast.
Fig. 2.
Sx16 complements mutant phenotypes of tlg2Δ yeast. The fidelity of the endocytic system of tlg2Δ yeast (NOzY3/4) producing HA-tagged Tlg2p, Sx16 or Sx4 as in Fig. 1 or carrying the empty vector pNB701 was assessed. (A) Aliquots of 5 μl of growing cultures (and tenfold serial dilutions thereof) were spotted onto SD –ura +50 μM CuCl2 plates, containing 1.5 M KCl (high salt) or not (control), and incubated at 30°C for 2-3 days. (B) Delivery of Ste3p-Myc to the vacuole was monitored by following its degradation in the cells described above that also produced Ste3p-Myc from the GAL1 promoter. Ste3p expression was induced during a 3-hour period in galactose medium. The `chase' was initiated by the addition of glucose to 3% (w/v). The initial (0) time point was taken here with subsequent time points taken as indicated (minutes after glucose addition). The amount of Myc-tagged Ste3p at each time point was analysed through immunoblotting (the same filters were also probed for Vph1p to control for equal loading). (C) Uptake of FM4-64 from the cell surface was followed by labelling cells at 0°C and initiating a chase period through incubation in fresh, pre-warmed media for 10 or 45 minutes as indicated. Representative images of the staining displayed by the majority of cells at each time point are shown. Quantification of data obtained from three experiments of this type is shown in Table 1. Scale bars: 2 μm.
Table 1.
Quantification of FM4-64 uptake
|
Mean percentage of cells displaying punctate or vacuolar staining ±
s.e.m.
|
||||||
|---|---|---|---|---|---|---|
|
10-minute chase
|
45-minute chase
|
|||||
| Punctate | Vacuolar | Other | Punctate | Vacuolar | Other | |
| WT (empty vector) | 3±1 | 89±7 | 9±4 | 2±1 | 88±2 | 10±2 |
| tlg2Δ (empty vector) | 65±8 | 22±3 | 11±5 | 8±5 | 87±7 | 6±2 |
| tlg2Δ (HA-Tlg2p) | 16±3 | 68±5 | 14±3 | 7±3 | 83±5 | 11±4 |
| tlg2Δ (HA-Sx16) | 15±4 | 74±8 | 8±4 | 4±1 | 88±5 | 9±5 |
| tlg2Δ (HA-Sx4) | 59±9 | 29±4 | 13±3 | 3±3 | 86±5 | 12±2 |
Note that vacuolar staining represents either the single large vacuole observed in wild-type cells or the fragmented vacuoles observed in tlg2Δ cells. WT, wild type
Regulation of Sx16 by Vps45p
Tlg2p-mediated membrane fusion requires the function of the SM protein Vps45p (Bryant and James, 2001). We have previously demonstrated that interaction between Tlg2p and Vps45p occurs via a pocket-mode of binding that is analogous to that observed in the Sly1p-Sed5p crystal structure (Bracher and Weissenhorn, 2002; Dulubova et al., 2002), in which the N-terminal peptide of the syntaxin inserts into a hydrophobic pocket on the outer surface of the SM protein (Carpp et al., 2006).
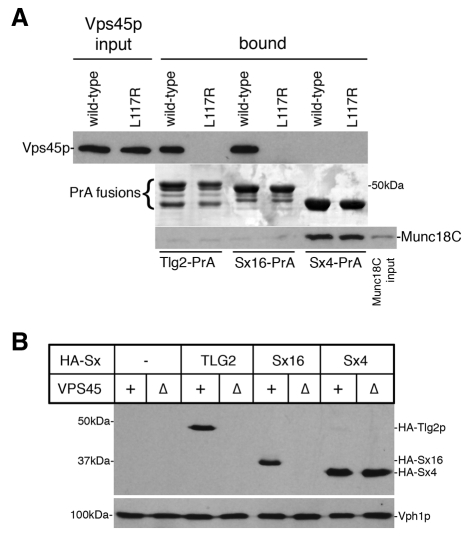
Consistent with its identification as a homologue of Tlg2p, Sx16 has previously been shown to bind to the mammalian homologue of Vps45p (mVps45) and also to recombinant yeast Vps45p in vitro (Dulubova et al., 2002). This interaction is abolished through mutation of the N-terminal peptide of Sx16 (Dulubova et al., 2002), suggesting that it is facilitated through the putative hydrophobic pocket identified in domain 1 of Vps45p (Carpp et al., 2006). Fig. 3A shows that a fusion protein containing the cytosolic domain of Sx16 (Sx16-PrA) captures Vps45p from a yeast cell lysate, but not a mutant that specifically abrogates the pocket-mode of binding between Vps45p and Tlg2p (Vps45pL117R) (Carpp et al., 2006). By contrast, a fusion protein containing the cytosolic domain of Sx4, which binds to the SM protein Munc18c (Aran et al., 2009), does not bind to Vps45p (Fig. 3A). Vps45p is required to stabilise Tlg2p in vivo and, consequently, levels of Tlg2p in vps45Δ cells are greatly reduced compared with wild-type cells (Bryant and James, 2001; Carpp et al., 2007). Levels of Sx4 were similar in vps45Δ and congenic wild-type (VPS45) cells (Fig. 3B). Like the levels of Tlg2p, Sx16 levels were drastically reduced in cells lacking Vps45p (Fig. 3B), indicating that Sx16 requires Vps45p for stability in vivo.
Fig. 3.
Sx16 functions with Vps45p. (A) Tlg2p-PrA, Sx16-PrA and Sx4-PrA immobilised on IgG-Sepharose were incubated with lysates prepared from vps45Δ cells (NOzY1) producing wild-type HA-Vps45p or HA-Vps45pL117R, or with Munc18C purified from E. coli. Bound material was analysed by immunoblotting with anti-HA and -Munc18C antibodies (upper and lower panels, respectively; middle panel shows a Coomassie-stained gel of the immobilised fusion proteins used in the pull-downs). The immunoblot analyses included 10% of the lysates and 2% of the purified Munc18C used. (B) Immunoblot analysis was used to assess the levels of HA-tagged syntaxins present in wild-type (VPS45+; RPY10) or vps45Δ (NOzY2) cells containing plasmids expressing HA-Tlg2p (HA-Sx=TLG2; pMAZ006), HA-Sx16 (HA-Sx=Sx16; pMAZ002) or no HA-tagged syntaxin (HA-Sx= –; pNB701) grown in SD –ura +50 μM CuCl2. Proteins contained within 1 OD600 equivalents of these cells were subject to immunoblot analysis to detect the HA-tagged proteins (and Vti1p as a loading control).
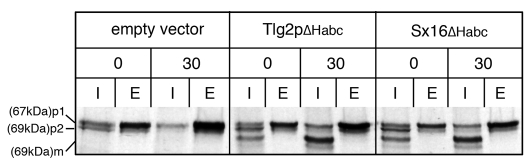
Unlike the full-length protein, a version of Tlg2p lacking the Habc domain (Tlg2pΔHabc) is not downregulated in vps45Δ mutant cells, and is able to form SNARE complexes under these conditions (Bryant and James, 2001). Expression of Tlg2pΔHabc suppresses trafficking defects of vps45Δ cells (Bryant and James, 2001). vps45Δ cells mis-sort the vacuolar hydrolase carboxypeptidase Y (CPY) (Cowles et al., 1994; Piper et al., 1994). In wild-type cells, proCPY (p2) is trafficked from the TGN to the vacuole and processed into its mature form (m). Although vps45Δ mutants secrete ∼70% of their CPY, the CPY that remains intracellular is in the p2 form, reflecting its inability to reach the proteolytically active vacuole. Expression of Tlg2pΔHabc in vps45Δ cells causes the internal CPY to be processed to its m form, demonstrating that the SNARE complexes that it forms in the absence of Vps45p are functional (Bryant and James, 2001). Fig. 4 shows that an analogous version of Sx16 (Sx16ΔHabc) also suppresses the CPY-trafficking defects of vps45Δ cells (note the maturation of the internal CPY). Removal of the Habc domain from Tlg2p and Sx16 would prevent the syntaxins from adopting a closed conformation akin to that adopted by Sx1a and Sso1p (Chen et al., 2008; Dulubova et al., 1999; Misura et al., 2000; Nicholson et al., 1998); therefore, these data support a model in which Vps45p is required for transition of the syntaxin (Tlg2p or Sx16) from a closed to an open conformation to facilitate SNARE-complex assembly.
Fig. 4.
Deletion of the Habc domain from Tlg2p or Sx16 suppresses the CPY-trafficking defect of vps45Δ mutant cells and stimulates SNARE-complex formation. vps45Δ cells producing Tlg2pΔHabc or Sx16ΔHabc (from pSGS043 or pSGS044, respectively) or harbouring the empty vector pVT102U were metabolically labelled for 5 minutes using 35S-methionine. A chase of 30 minutes was initiated by the addition of excess unlabelled methionine. CPY was immunoprecipitated at the start (0) and end (30) of this chase, from both intracellular (I) and extracellular (E) fractions. p1, preproCPY; p2, proCPY; m, mature CPY
Although this model is consistent with the finding that Munc18a is involved in the transition of Sx1a from the closed to the open conformation (Burkhardt et al., 2008; Dulubova et al., 1999), it is in contrast with nuclear magnetic resonance (NMR) spectroscopy studies that suggest that a bacterially produced version of Tlg2p does not adopt a closed conformation (Dulubova et al., 2002). However, the construct used in those studies spans residues 60-283 of Tlg2p, and thus lacks almost half of the SNARE motif, which might destabilise a closed conformation of Tlg2p. Taking this caveat into consideration, along with the data presented in Fig. 4, we favour a model in which both Tlg2p and its functional homologue Sx16 adopt closed conformations that preclude SNARE-complex formation. In support of this, recent evidence shows that multiple regions of the cytosolic domain of Sx16, including the SNARE motif, contribute to its high-affinity binding to mammalian Vps45 (Burkhardt et al., 2008). The simplest interpretation of these data is that, like Sx1a and Sso1p, Sx16 adopts a closed conformation to which Vps45 binds.
Although these data support a model in which Vps45p facilitates a switch of Tlg2p (and Sx16) from a closed to an open conformation, another possibility is that an unidentified inhibitory factor binds the N-terminal domain of Tlg2p to prevent SNARE-complex assembly in the absence of Vps45p (Dulubova et al., 2002). To distinguish between these two models, we set out to functionally test whether Tlg2p adopts a closed conformation that hinders its ability to form SNARE complexes. To this end, we compared the abilities of the intact cytosolic domain of Tlg2p and a version lacking the Habc domain (Tlg2pΔHabc) to form SNARE complexes in vitro. The cytosolic domain of Tlg2p binds the v-SNARE Snc2p in the presence of the cytosolic domains of the partner SNAREs Tlg1p and Vti1p (Carpp et al., 2006). Fig. 5A shows that Tlg2pΔHabc forms Snc2p-containing SNARE complexes more efficiently than full-length Tlg2p, reflected by the increased amount of Snc2p bound to Tlg2pΔHabc at the 20-minute time point. Following a longer incubation (120 minutes), full-length Tlg2p and Tlg2pΔHabc bind similar amounts of Snc2p, indicating that their affinities for Snc2p have not been changed, but rather that the rate of SNARE assembly is increased by removal of the Habc domain of Tlg2p.
Fig. 5.
SNARE-complex formation is stimulated by both deletion of the Habc domain from Tlg2p, and by Vps45p. (A) The ability of Tlg2p-GST, or a version lacking the Habc domain (Tlg2pΔHabc-GST), to form SNARE complexes in vitro was assessed by incubating the GST fusions (purified from E. coli and immobilised on glutathione-Sepharose) with tenfold molar excesses of His6-tagged versions of Snc2p and Vti1p, and the untagged Sx8-Tlg1p chimera [all purified from E. coli as described (Paumet et al., 2005)]. After incubation for 20 or 120 minutes at 4°C with continuous mixing, Sepharose beads were harvested by centrifugation at 1000 g for 1 minute and washed extensively with binding buffer. Immunoblot analysis was used to assess the amount of Snc2p bound to the Tlg2p-GST fusions (lower panel; a sample of the input cognate SNAREs was included in this analysis). Upper panel shows a Coomassie-stained gel of the Tlg2p-GST fusions that were incubated either with (+) or without (–) the cognate SNARE-binding proteins. (B) The effect of adding purified His6-tagged Vps45p on the ability of Tlg2p-GST and Tlg2pΔHabc-GST to form SNARE complexes in vitro during a 20-minute incubation was assessed by performing the experiment presented in A in the absence or presence of a tenfold molar excess of His6-tagged Vps45p (over Tlg2p-GST and Tlg2pΔHabc-GST).
Because the SNARE assembly experiment was performed with purified proteins produced in Escherichia coli, the inhibitory effect of the Habc domain on SNARE-complex assembly is not due to the binding of another factor as the only proteins present during the incubation were the four SNARE-complex components. Thus, the data presented in Fig. 5A indicate that, as observed for Sx1a and Sso1p (Dulubova et al., 1999; Nicholson et al., 1998), the Habc domain of Tlg2p inhibits the ability of the syntaxin to form SNARE complexes. Taken in conjunction with the observation that removal of the Habc domain from Tlg2p in vivo bypasses the requirement of SNARE-complex formation, and membrane traffic, on Vps45p (Bryant and James, 2001), this supports a model whereby the SM protein activates the syntaxin for entry into functional SNARE complexes. Our finding that this also holds true for the function of Sx16 (Fig. 4), which has also been suggested to adopt a closed conformation (Burkhardt et al., 2008), indicates that this regulatory mechanism is conserved through evolution.
To directly test whether Vps45p can alleviate the kinetic delay that the presence of the Habc domain exerts on the ability of Tlg2p to form SNARE complexes, we added purified Vps45p to our in vitro SNARE-complex assembly assay. Fig. 5B shows that, whereas the addition of Vps45p has no effect on the amount of Snc2p bound by Tlg2pΔHabc, addition of the SM protein increases the amount bound by full-length Tlg2p during a 20-minute incubation. In the presence of Vps45p, the amount of Snc2p bound by full-length Tlg2p after 20 minutes was similar to that bound by Tlg2pΔHabc during the same time period. This finding that the kinetic delay of SNARE-complex formation displayed by Tlg2p in vitro can be relieved by either removal of the Habc domain or the addition of Vps45p supports a model in which the SM protein facilitates a switch of the syntaxin from a closed to an open conformation.
Discussion
Elucidating the conserved mechanisms that regulate SNARE-complex formation has proved challenging. Structural studies have revealed that the Habc domains of Sx1a and Sso1p form intramolecular interactions with the SNARE motif in a closed conformation and inhibit SNARE-complex formation (Dulubova et al., 1999; Misura et al., 2000; Munson et al., 2000), but the function of this domain in other syntaxins is less clear. We have previously demonstrated that removal of the Habc domain from Tlg2p suppresses the CPY-trafficking defects of yeast cells lacking the SM protein Vps45p (Bryant and James, 2001). This is consistent with a model in which Tlg2p adopts a closed conformation that is incompatible with SNARE-complex formation in the absence of Vps45p, and suggests that, in a manner analogous to that suggested for Munc18a (Dulubova et al., 1999; Misura et al., 2000), Vps45p is involved in the transition of the syntaxin from the closed to the open conformation. However, these data cannot discount the possibility that an unidentified inhibitory factor might bind to the N-terminal portion of Tlg2p and prevent SNARE-complex formation as suggested by Dulubova and colleagues (Dulubova et al., 2002). If Vps45p were involved in releasing such an inhibition to promote SNARE-complex assembly, Tlg2pΔHabc would not be able to bind this inhibitory factor and thus would not be subject to the same regulation by Vps45p (Dulubova et al., 2002).
Here, we demonstrate that the presence of the Habc domain of Tlg2p results in a kinetic delay on SNARE-complex formation (Fig. 5A). Because this experiment was performed using only purified SNARE proteins, the reduced ability of the Tlg2p cytosolic domain to bind its SNARE binding partners, when compared with a version lacking the Habc domain, is not caused by the binding of an inhibitory factor. Thus, as observed for that of Sx1a and Sso1p (Dulubova et al., 1999; Nicholson et al., 1998), the Habc domain of Tlg2p directly regulates the ability of the syntaxin to form SNARE complexes. These data strongly suggest that Tlg2p adopts a closed conformation akin to that observed for Sx1a and Sso1p (Dulubova et al., 1999; Misura et al., 2000; Munson et al., 2000). To investigate this, we tested for direct interactions between the Habc and SNARE-motif regions of Tlg2p using several approaches, including in vitro mixing experiments with purified proteins and yeast two-hybrid assays, but have been unable to observe any significant binding (data not shown). This was not particularly surprising because the intramolecular interaction that stabilises the closed conformation of Sso1p (which adopts a tightly closed conformation) is rather weak in trans (Munson et al., 2000; Nicholson et al., 1998). This is a consequence of the physiological function of this interaction; when the Habc and SNARE domains are present on the same polypeptide (i.e. in cis), the two elements are at extremely high effective concentrations. It is therefore crucial that the Habc–SNARE-motif interaction has an effectively low affinity, so that it can be released, to allow SNARE-complex assembly in a biologically relevant timescale. Definitive proof of whether Tlg2p does adopt a closed conformation will require high-resolution structural analyses and/or large-scale mutational analyses, and is a goal of future research.
Our contention that Tlg2p adopts a closed conformation is supported by a recent study that used isothermal titration calorimetry to monitor the binding of mammalian Vps45p to the Tlg2p homologue, Sx16 (Burkhardt et al., 2008). This study demonstrated that regions of Sx16 outside the N-terminal peptide, previously thought to be sufficient for binding to Vps45p (Dulubova et al., 2002), contribute to the high-affinity interaction with Vps45p. By analysing the binding of various truncations of Sx16 to Vps45p, and comparing their interaction to the binding of corresponding regions of Sx1a to Munc18a, the authors concluded that the C-terminal portion of the SNARE motif interacts with mVps45p in a manner equivalent to the interaction of Sx1a with the inner cavity of the arch-shaped Munc18a (Burkhardt et al., 2008; Misura et al., 2000).
Sx16 was previously identified as the in silico homologue of Tlg2p (Simonsen et al., 1998). We have now demonstrated that Sx16 is also a functional homologue of Tlg2p, in that its expression complements many of the trafficking phenotypes displayed by yeast cells lacking Tlg2p. We used this functional homology to demonstrate that, in the same way observed for Tlg2p, removal of the Habc domain of Sx16 suppresses the trafficking defects of yeast cells lacking the SM protein Vps45p. As discussed above, these data support a model in which the SM protein functions to alleviate an inhibitory effect of SNARE-complex formation exerted by the Habc domain of both Tlg2p and Sx16. Thus, we conclude that the inhibition of SNARE-complex assembly by the Habc domain is conserved through evolution and is utilised by endocytic, as well as exocytic, SNAREs, indicating that it represents a central regulatory mechanism.
Our finding that the addition of purified Vps45p relieves the kinetic delay on SNARE-complex assembly that is observed for the full-length cytosolic domain of Tlg2p (compared with that lacking the Habc domain) indicates that the SM protein enhances SNARE-complex formation by facilitating a switch of the syntaxin from the closed to the open conformation, or by stabilising the open conformation. Although it is tempting to speculate that this represents a conserved function of SM proteins, and indeed has been suggested for other members of the family (Dulubova et al., 1999), it is clear that more work needs to be carried out to determine whether this is the case. In contrast to our finding that expression of versions of Tlg2p or Sx16 that are unable to adopt closed conformations suppresses the trafficking defects of vps45Δ cells, expression of open-syntaxin (UNC-64) mutants does not rescue unc-18 Caenorhabditis elegans mutants (Weimer et al., 2003), perhaps arguing for other essential functions of the SM protein. It is, however, worth noting that the secretion of neurotransmitter, controlled by the UNC-64–UNC-18 interaction is probably subject to more layers of regulation than the transport through the endosomal system controlled by Tlg2p (or Sx16)-Vps45p. It is therefore possible that, although all SM proteins function to `open' their cognate syntaxins, those that function in highly regulated transport steps do so in conjunction with other factors.
Materials and Methods
Yeast strains and plasmids
The yeast strains and plasmids used are listed in Tables 2 and 3.
Table 2.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| NOzY1 | MATα ura3-52 leu2-3 112 his4-519 ade6 gal2 pep4-3 vps45Δ::Kanr | (Bryant and James, 2001) |
| NOzY2 | MATα ura3-52 leu2-3 112 his4-519 ade6 gal2 vps45Δ::Kanr | (Bryant and James, 2001) |
| NOzY3 | MATα ura3-52 leu2-3 112 his4-519 ade6 gal2 pep4-3 tlg2Δ::Kanr | (Bryant and James, 2001) |
| NOzY4 | MATα ura3-52 leu2-3 112 his4-519 ade6 gal2 tlg2Δ::Kanr | (Bryant and James, 2001) |
| RPY10 | MATα ura3-52 leu2-3 112 his4-519 ade6 gal2 | (Piper et al., 1994) |
| SF838-9D | MATα ura3-52 leu2-3 112 his4-519 ade6 gal2 pep4-3 | (Rothman and Stevens, 1986) |
SF838-9D, NOzY1, NOzY2, NOzY3 and NOzY4 are congenic to RPY10
Table 3.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pCOG070 | Yeast expression plasmid (2μ, URA3) encoding wild-type HA-Vps45p | (Carpp et al., 2006) |
| pCOG071 | As pCOG070, encoding HA-Vps45pL117R | (Carpp et al., 2006) |
| pCOG022 | E. coli expression vector encoding two IgG-binding domains of protein A (PrA) | (Carpp et al., 2006) |
| pCOG025 | E. coli expression vector encoding Tlg2p-PrA | (Carpp et al., 2006) |
| pALA1 | E. coli expression vector encoding Sx16-PrA | This study |
| pFB09/1 | E. coli expression vector encoding Sx4-PrA | This study |
| pNB701 | Yeast expression plasmid (CEN, URA3) encoding Pho8p from the CUP1 promoter | This study |
| pMAZ002 | Yeast expression plasmid (CEN, URA3) encoding HA-Sx16 from the CUP1 promoter | This study |
| pMAZ007 | Yeast expression plasmid (CEN, URA3) encoding HA-Sx4 from the CUP1 promoter | This study |
| pMAZ006 | Yeast expression plasmid (CEN, URA3) encoding HA-Tlg2p from the CUP1 promoter | This study |
| pSL2099 | Yeast expression plasmid (CEN, LEU2) encoding Ste3p-Myc from the GAL1 promoter | (Davis et al., 1993) |
| pVT102U | Yeast expression plasmid (2 μ, URA3) harbouring the ADH1 promoter | (Vernet et al., 1987) |
| pSGS043 | Yeast expression plasmid (2 μ, URA3) encoding Tlg2p lacking the N-terminal 230 residues (Tlg2pΔHabc) from the ADH1 promoter | This study |
| pSGS044 | Yeast expression plasmid (2 μ, URA3) encoding Sx16 lacking the N-terminal 205 residues (Sx16ΔHabc) from the ADH1 promoter | This study |
| pCMD008 | E. coli expression vector encoding Tlg2p-GST | This study |
| pCMD007 | E. coli expression vector encoding Tlg2pΔHabc-GST | This study |
| pCMD004 | E. coli expression vector encoding a chimeric SNARE motif made from Tlg1p and Sx8 as described by Paumet et al. (Paumet et al., 2005) tagged with GST | This study |
| pJM132 | E. coli expression vector encoding His6-Vti1p | (McNew et al., 2000) |
| pJM81 | E. coli expression vector encoding His6-Snc2p | (McNew et al., 2000) |
| pNB710 | E. coli expression vector encoding His6-Vps45p | (Carpp et al., 2006) |
A detailed account of plasmids made during the course of this study can be found in the Materials and Methods
Antibodies
Antibodies against Tlg2p and Vti1p have been described (Bryant and James, 2001). Antibodies against Snc2p were raised in rabbits immunised with peptides corresponding to residues 12-26 and 72-86 of Snc2p. Antibodies against Vph1p (10D7-A7-B2) and the HA (3F10) and Myc (9E10) epitope tags were from Molecular Probes, Roche and Sigma, respectively.
Fluorescence microscopy
FM4-64 [N-(3-triethylammoniumpropyl)-4-(p-diethyl-hexatrienyl)pyridinium-dibromide] was used to examine the endocytic pathway essentially as described by Vida and Emr (Vida and Emr, 1995), with some minor modifications. Briefly, cells were incubated with 30 μM FM4-64 for 30 minutes at 0°C. A chase period (at 30°C) was initiated by resuspending harvested cells in pre-warmed media for 10 or 45 minutes as indicated. The chase period was terminated by the addition of NaN3 to 15 mM, and cells were kept on ice prior to visualisation by fluorescence and differential interference contrast (DIC) microscopy. To quantify the results from these experiments, the staining observed in 300 cells, located in four to six random fields, was scored as punctate, vacuolar or `other' (with the last category including cells displaying faint or no staining).
Vacuolar delivery of Ste3p
The delivery of a Myc-tagged version of Ste3p to the vacuole, and its subsequent degradation, was followed as previously described by Davis and colleagues (Davis et al., 1993) with some minor modifications. Briefly, a 3-hour `pulse' of receptor synthesis was induced by the addition of galactose (to 2% w/v) to exponential cultures of cells carrying the GAL1-driven Ste3p-Myc-encoding construct (pSL2099) growing in minimal media containing raffinose (2% w/v) as a carbon source (lacking leucine for selection of pSL2099). Following this, the `chase' period was instigated by the addition of glucose to 3% (w/v). The first (0) time point was taken immediately after the addition of glucose and subsequent time points were taken as indicated. The amount of Myc-tagged Ste3p at each time point was analysed through immunoblotting using antibodies that specifically recognise the Myc epitope.
Pulse-chase radiolabelling and immunoprecipitation of CPY
The fate of newly synthesised CPY was followed by immunoprecipitation of the protein from intracellular and extracellular fractions of cells that had incorporated radiolabelled methionine into proteins synthesised during a 5-minute time period as previously described (Piper et al., 1994).
In vitro binding assays and SNARE-complex assembly
The binding of Vps45p and Munc18C to Staphylococcus aureus protein A (PrA) fusion proteins was assessed as previously described (Carpp et al., 2006) using Vps45p provided by yeast cell lysates and Munc18C purified from E. coli (Aran et al., 2009). The ability of Tlg2p-GST or Tlg2pΔHabc-GST to form SNARE complexes was monitored essentially as described (Carpp et al., 2006). GST-fusion proteins purified from E. coli and immobilised on glutathione-Sepharose were incubated with tenfold molar excesses of His6-tagged versions of Snc2p and Vti1p and untagged Sx8-Tlg1p chimera (generated through thrombin cleavage of a GST fusion) [all purified from E. coli as described (Paumet et al., 2005)]. Where indicated, purified His6-tagged Vps45p (Carpp et al., 2006) was also added to tenfold molar excess. Following incubation for 20 or 120 minutes at 4°C with continuous mixing. Sepharose beads were harvested by centrifugation at 1000 × g for 1 minute. Following extensive washing with binding buffer, bound material was subjected to SDS-PAGE followed by immunoblot analysis. Note that these experiments were performed using a Sx8-Tlg1p chimera because it does not contain the regulatory sequence found within the SNARE motif of Tlg1p, but forms functional SNARE complexes with Tlg2p, Vti1p and Snc2p (Paumet et al., 2005). This enabled us to examine the influence of the Habc domain of Tlg2p on SNARE-complex assembly in the absence of this second, distinct regulatory switch (Paumet et al., 2005).
Construction of plasmids and recombinant-protein production
All DNA manipulations were performed using standard procedures (Sambrook and Russel, 2001).
pALA1, encoding the cytosolic domain of Sx16 fused at the C-terminus to two IgG-binding domains of PrA under the control of the T7 promoter, was constructed by amplifying sequence encoding residues 1-269 of Sx16A from a cDNA containing the entire Sx16A coding sequence [provided by Harald Stenmark, Norwegian Radium Hospital (Simonsen et al., 1998)] using oligonucleotide primers 5′-AGATCTATGGCCACCAGGCGTTTAACC-3′ and 5′-CTCGAGCTTCCGATTCTTCTTTTGATACTG-3′. The resulting 918-bp PCR product was subcloned into the BglII-XhoI sites of pCOG022 to create pALA1.
pFB09/1, encoding the cytosolic domain of Sx4 fused at the C-terminus to two IgG-binding domains of PrA under the control of the T7 promoter, was constructed by subcloning the fragment of DNA encoding the cytosolic domain of Sx4 (residues 1-273) from a plasmid encoding a C-terminally tagged GST-Sx4 fusion (Aran et al., 2009) as a NdeI-XhoI fragment into pCOG022.
pNB701, the parent vector used for the heterologous expression of genes in yeast from the inducible CUP1 promoter, was created by using site-directed mutagenesis to remove a SalI site present in the polylinker of a plasmid encoding Pho8p under CUP1 regulation [provided by Rob Piper, University of Iowa, IA; this plasmid contains the PHO8 ORF immediately preceded by the CUP1 promoter in pRS316 (Sikorski and Hieter, 1989)]. This allows the majority of the PHO8 ORF to be removed from the plasmid by digestion with XhoI and SalI. Homologous recombination can then be used to place the coding sequence of interest immediately downstream of the CUP1 promoter. This can be achieved by using PCR amplification to add sequence complementary to the 3′ end of the CUP1 promoter to the 5′ end of the sequence, and to the 5′ end of the 3′ UTR of PHO8 at the 3′ end of the sequence. The resultant product can then be used to repair pNB701 digested with XhoI and SalI by co-transformation of the PCR product and the gapped plasmid into any S. cerevisiae ura3 auxotroph (this study used SF838-9D).
To express the human Sx16A ORF in yeast the oligonucleotide primers 5′-GATATTAAGAAAAACAAACTGTACAATCAATCAATCAATCATCACATAAAATGGCCCACCAGGCGTTTAACCGAC-3′ and 5′-ATTATAACGTATTAAATAATATGTGAAAAAAGAGGGAGAGTTAGATAGGATTATCGAGACTTCACGCCAACGAG-3′ were used to amplify the coding sequence from a cDNA provided by Harald Stenmark, Norwegian Radium Hospital (Simonsen et al., 1998). The resulting PCR product consisted of the Sx16A ORF flanked by sequences homologous to the 3′ end of the CUP1 promoter and the proximal end of the 3′ UTR of PHO8 (underlined in the oligonucleotide sequences). This fragment was then used to repair pNB701 digested with XhoI and SalI as described above. Site-directed mutagenesis using the oligonucleotides 5′-AATCAATCATCACATAAAATGTACCCATACGATGTTCCGGATTACGCTGCCACCAGGCGTTTAACC-3′ and 5′-GGTTAAACGCCTGGTGGCAGCGTAATCCGGAACATCGTATGGGTACATTTTATGTGATGATTGATT-3′ was subsequently used to insert sequence encoding the HA epitope (YPYDVPDYAA; bold in the oligonucleotide sequences) immediately after the Sx16A initiating codon, thus creating pMAZ002.
pMAZ007 was constructed by amplifying the mouse Sx4 ORF from a cDNA kindly provided by Gwyn Gould, University of Glasgow, UK using the oligonucleotide primers 5′-GATATTAAGAAAAACAAACTGTACAATCAATCAATCAATCATCACATAAAATGTACCCATACGATGTTCCGGATTACGCTCGCGACAGGACCCACGAG-3′ and 5′-ATTATAACGTATTAAATAATATGTTGAAAAAAGAGGGAGAGTTAGATAGGATTATCCAACGGTTATGGTGATGC-3′. The resulting PCR product contains sequence encoding Sx4 with an HA-tag immediately after the initiating methionine (encoded by the bold sequence in the oligonucleotide sequence) flanked by sequence homologous to the 3′ end of the CUP1 promoter and the proximal end of the 3′ UTR of PHO8 (underlined in the oligonucleotide sequences). This fragment was then used to repair pNB701 digested with XhoI and SalI as described above.
To create pMAZ006, which drives the production of a HA-tagged version of Tlg2p from the CUP1 promoter, the oligonucleotide primers 5′-GATATTAAGAAAAACAAACTGTACAATCAATCAATCAATCATCACATAAAATGTACCCATACGATGTTCCGGATTACGCTTTTAGAGATAGAACTAATTTATTTTT-3′ and 5′-ATTATAACGTATTAAATAATATGTTGAAAAAAGAGGGAGAGTTAGATAGGATCAAAGTAGGTCATCCAAAGCAT-3′ were used to amplify the TLG2 ORF from pYCG-YOL18c (Seron et al., 1998). The resulting PCR product contains sequence encoding Tlg2p with a HA-tag immediately after the initiating methionine (encoded by the bold sequence in the oligonucleotide sequence) flanked by sequence homologous to the 3′ end of the CUP1 promoter and the proximal end of the 3′ UTR of PHO8 (underlined in the oligonucleotide sequences). This fragment was then used to repair pNB701 digested with XhoI and SalI as described above.
pSGS043, which drives the production of a version of Tlg2p lacking the N-terminal 230 residues (Tlg2pΔHabc) from the ADH1 promoter was constructed by using the oligonucleotide primers 5′-AATCTAGATGTACCCATACGATGTTCCGGATTACGCTACGTTGCAGAGACAGCAACAG-3′ and 5′-AACTCGAGTCAAAGTAGGTCATCCAAAGC-3′ to amplify the appropriate region of the TLG2 ORF from pYCG-YOL18c (Seron et al., 1998). The resulting product was subcloned into the XbaI-XhoI sites of pVT102u (Vernet et al., 1987) to generate pSGS043.
pSGS044, which drives the production of a version of Sx16 lacking the N-terminal 205 residues (Sx16ΔHabc) from the ADH1 promoter was constructed in a similar manner to pSGS043 using the oligonucleotide primers 5′-AATCTAGATGTACCCATACGATGTTCCGGATTACGCTTTAGTTCTGGTGGACCAGAAC-3′ and 5′-AACTCGAGTTATCGAGACTTCACGCC-3′ to generate a PCR product from pMAZ002. This product was subcloned into the XbaI-XhoI sites of pVT102u (Vernet et al., 1987) to generate pSGS044.
pCMD008, encoding the cytosolic domain of Tlg2p carrying a C-terminal GST tag was constructed as follows. DNA encoding residues 1-309 of Tlg2p was subcloned from pCOG025 (Carpp et al., 2006) as an NdeI and XhoI fragment into pETDuet-1-GST [pETDuet-1-GST was constructed by replacing DNA encoding the C-terminal S-tag in pETDuet-1 (Novagen) with sequence encoding GST as a XhoI and PacI fragment].
pCMD007, encoding the cytosolic domain of Tlg2p lacking the Habc domain (Tlg2pΔHabc) carrying a C-terminal GST tag was constructed by using the oligonucleotide primers 5′-CTCGAGGTAGTGTGTTGCTTTATTCAAC-3′ and 5′-CATATGACGTTGCAGAGACAGCAACAG-3′ to amplify sequence encoding residues 231-309 of Tlg2p from pYCG-YOL18c (Seron et al., 1998). This was subcloned as an NdeI and XhoI fragment into pETDuet-1-GST (described above).
pCMD004, encoding a GST-tagged protein harbouring a chimeric SNARE motif made from Tlg1p and Sx8 that forms bona fida SNARE complexes with Tlg2p, Vti1p and Snc2p (Paumet et al., 2005) was created by PCR SOEing using external oligonucleotides that incorporate BamHI and EcoRI sites (5′-GGATCCTTGGGTTTCGATGAGATCCG-3′ and 5′-GAATCCTCAAGCAATGAATGCCAAAACTAA-3′) and overlapping internal oligonucleotides (5′-GTCCATCCCTCGTCCATATTATCCAACAATTGTCCCTGTTCGTCCAGTTCATTCCCAATCT-3′ and 5′-AAGCAAATGGGCCAGGAGATTGGGAATGAACTGGACGAACAGGGACAATTGTTGGATAATATG-3′). The product of this reaction was subcloned into pGEX6P-1 (Amersham Biosciences). Cleavage of the N-terminal GST tag was achieved using PreScission Protease (GE Healthcare Life Sciences).
Plasmids pJM132 and pJM81 were used to produce His6 versions of the cytosolic domains of Vti1p and Snc2p, respectively (McNew et al., 2000).
We thank Rob Piper, George Sprague, Jr and Harald Stenmark for reagents, and Robert Insall for help with microscopy. pFB09/1 was constructed by Fiona Brandie. This work was supported by the BBSRC (project grant 17/C19548 to N.J.B., studentships to M.S.S., C.M. and D.K.), the Wellcome Trust (studentship to L.N.C.) and the NIH (GM068803 to M.M.). N.J.B. is a Prize Fellow of The Lister Institute of Preventative Medicine. Deposited in PMC for release after 6 months.
References
- Abeliovich, H., Grote, E., Novick, P. and Ferro-Novick, S. (1998). Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem. 273, 11719-11727. [DOI] [PubMed] [Google Scholar]
- Aran, V., Brandie, F. M., Boyd, A. R., Kantidakis, T., Rideout, E. J., Kelly, S. M., Gould, G. W. and Bryant, N. J. (2009). Characterisation of two distinct binding modes between Syntaxin 4 and Munc18c. Biochem. J. 419, 655-660. [DOI] [PubMed] [Google Scholar]
- Banta, L. M., Robinson, J. S., Klionsky, D. J. and Emr, S. D. (1988). Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107, 1369-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher, A. and Weissenhorn, W. (2002). Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 21, 6114-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N. J. and James, D. E. (2001). Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 20, 3380-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt, P., Hattendorf, D. A., Weis, W. I. and Fasshauer, D. (2008). Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 27, 923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt, T. R., Sternberg, E. J., Gorman, J. A., Clark, P., Hamer, D., Rosenberg, M. and Crooke, S. T. (1984). Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc. Natl. Acad. Sci. USA 81, 3332-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpp, L. N., Ciufo, L. F., Shanks, S. G., Boyd, A. and Bryant, N. J. (2006). The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J. Cell Biol. 173, 927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpp, L. N., Shanks, S. G., Struthers, M. S. and Bryant, N. J. (2007). Cellular levels of the syntaxin Tlg2p are regulated by a single mode of binding to Vps45p. Biochem. Biophys. Res. Commun. 363, 857-860. [DOI] [PubMed] [Google Scholar]
- Chen, X., Lu, J., Dulubova, I. and Rizo, J. (2008). NMR analysis of the closed conformation of syntaxin-1. J. Biomol. NMR 41, 43-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles, C. R., Emr, S. D. and Horazdovsky, B. F. (1994). Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J. Cell Sci. 107, 3449-3459. [DOI] [PubMed] [Google Scholar]
- Davis, N. G., Horecka, J. L. and Sprague, G. F., Jr (1993). Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 122, 53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova, I., Sugita, S., Hill, S., Hosaka, M., Fernandez, I., Sudhof, T. C. and Rizo, J. (1999). A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 18, 4372-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova, I., Yamaguchi, T., Gao, Y., Min, S. W., Huryeva, I., Sudhof, T. C. and Rizo, J. (2002). How Tlg2p/syntaxin 16 `snares' Vps45. EMBO J. 21, 3620-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer, D., Antonin, W., Margittai, M., Pabst, S. and Jahn, R. (1999). Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 274, 15440-15446. [DOI] [PubMed] [Google Scholar]
- Holthuis, J. C., Nichols, B. J., Dhruvakumar, S. and Pelham, H. R. (1998). Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 17, 113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, R. and Scheller, R. H. (2006). SNAREs-engines for membrane fusion. Nat. Rev. Mol. Cell. Biol. 7, 631-643. [DOI] [PubMed] [Google Scholar]
- McNew, J. A., Parlati, F., Fukuda, R., Johnston, R. J., Paz, K., Paumet, F., Sollner, T. H. and Rothman, J. E. (2000). Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153-159. [DOI] [PubMed] [Google Scholar]
- Misura, K. M., Scheller, R. H. and Weis, W. I. (2000). Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404, 355-362. [DOI] [PubMed] [Google Scholar]
- Munson, M. and Hughson, F. M. (2002). Conformational regulation of SNARE assembly and disassembly in vivo. J. Biol. Chem. 277, 9375-9381. [DOI] [PubMed] [Google Scholar]
- Munson, M., Chen, X., Cocina, A. E., Schultz, S. M. and Hughson, F. M. (2000). Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat. Struct. Biol. 7, 894-902. [DOI] [PubMed] [Google Scholar]
- Nicholson, K. L., Munson, M., Miller, R. B., Filip, T. J., Fairman, R. and Hughson, F. M. (1998). Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat. Struct. Biol. 5, 793-802. [DOI] [PubMed] [Google Scholar]
- Parlati, F., Weber, T., McNew, J. A., Westermann, B., Sollner, T. H. and Rothman, J. E. (1999). Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA 96, 12565-12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumet, F., Rahimian, V., Di Liberto, M. and Rothman, J. E. (2005). Concerted auto-regulation in yeast endosomal t-SNAREs. J. Biol. Chem. 280, 21137-21143. [DOI] [PubMed] [Google Scholar]
- Piper, R. C., Whitters, E. A. and Stevens, T. H. (1994). Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur. J. Cell Biol. 65, 305-318. [PubMed] [Google Scholar]
- Rothman, J. H. and Stevens, T. H. (1986). Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell 47, 1041-1051. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. E. and Russel, D. W. (2001). Molecular Cloning: A Laboratory Manual, 3rd Edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Seron, K., Tieaho, V., Prescianotto-Baschong, C., Aust, T., Blondel, M. O., Guillaud, P., Devilliers, G., Rossanese, O. W., Glick, B. S., Riezman, H. et al. (1998). A yeast t-SNARE involved in endocytosis. Mol. Biol. Cell 9, 2873-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S. and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., Bremnes, B., Ronning, E., Aasland, R. and Stenmark, H. (1998). Syntaxin-16, a putative Golgi t-SNARE. Eur. J. Cell Biol. 75, 223-231. [DOI] [PubMed] [Google Scholar]
- Tsui, M. M. and Banfield, D. K. (2000). Yeast Golgi SNARE interactions are promiscuous. J. Cell Sci. 113, 145-152. [DOI] [PubMed] [Google Scholar]
- Ungar, D. and Hughson, F. M. (2003). Snare protein structure and function. Annu. Rev. Cell Dev. Biol. 19, 493-517. [DOI] [PubMed] [Google Scholar]
- Vernet, T., Dignard, D. and Thomas, D. Y. (1987). A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52, 225-233. [DOI] [PubMed] [Google Scholar]
- Vida, T. A. and Emr, S. D. (1995). A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128, 779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer, R. M., Richmond, J. E., Davis, W. S., Hadwiger, G., Nonet, M. L. and Jorgensen, E. M. (2003). Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 6, 1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B., Gonzalez, L., Jr, Prekeris, R., Steegmaier, M., Advani, R. J. and Scheller, R. H. (1999). SNARE interactions are not selective. Implications for membrane fusion specificity. J. Biol. Chem. 274, 5649-5653. [DOI] [PubMed] [Google Scholar]