Summary
Ena/VASP proteins are conserved regulators of actin dynamics that have important roles in several physiological processes such as morphogenesis, axon guidance, endothelial barrier function, and cancer cell invasion and metastasis. Although considerable evidence points towards an anti-capping mechanism for Ena/VASP function, some controversy remains. Here, we evaluate the evidence for and against the anti-capping hypothesis, including results from some recent structural and biochemical studies that shed new light on this issue. In addition, we describe several alternate mechanisms that Ena/VASP proteins may utilize to regulate actin dynamics in vivo, including inhibition of branching, bundling and profilin-actin recruitment.
Keywords: Ena/VASP, VASP, Mena, Actin, Capping, Filopodia, Lamellipodia
Introduction
The highly related proteins of the Ena/VASP family – Mena, VASP and EVL – play pivotal roles in cell movement and shape change in vertebrates. Ena/VASP proteins are active in various cell types, including fibroblasts (Bear et al., 2000), endothelial cells (Furman et al., 2007), epithelial cells (Gates et al., 2007; Lawrence et al., 2002; Vasioukhin et al., 2000) and neurons (Lanier et al., 1999; Lebrand et al., 2004; Kwiatkowski et al., 2007); Ena/VASP proteins directly regulate assembly of the actin-filament network, modulate the morphology and behavior of membrane protrusions such as filopodia and lamellipodia (see below), and influence cell motility (Bear et al., 2002; Gertler et al., 1996; Lacayo et al., 2007). VASP also plays an important role in modulating `inside out' agonist-induced integrin activation in platelets (Aszodi et al., 1999; Massberg et al., 2004; Hauser et al., 1999).
Caenorhabditis elegans and Drosophila melanogaster each contain a single Ena/VASP ortholog (Krause et al., 2003); genetic analysis in these systems and in mice revealed roles for Ena/VASP in axon guidance. In mice, genetic studies reveal that Ena/VASP proteins also have crucial roles in neuritogenesis and endothelial barrier formation and neural-tube closure (Furman et al., 2007; Kwiatkowski et al., 2007; Lanier et al., 1999; Menzies et al., 2004). Ena/VASP family members are concentrated at focal adhesions, the distal rim of the lamellipodium and the tips of filopodia in migratory cells (Fig. 1). Therefore, this protein family is perfectly positioned to serve a role in the early rearrangement of the actin cytoskeleton in response to migration cues.
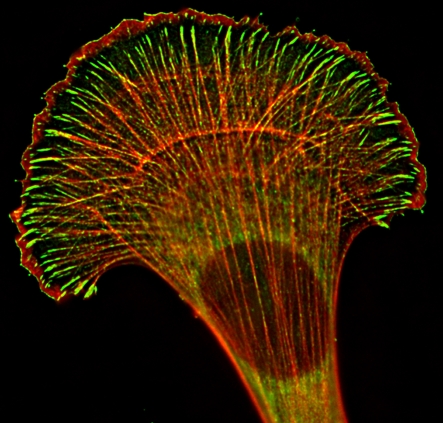
Fig. 1.
Intracellular distribution of Ena/VASP proteins. Immunofluorescence staining for VASP (green) and F-actin (red) in a fibroblast.
Although there is considerable evidence that Ena/VASP proteins act as actin anti-capping proteins (see below), some controversy remains over their mechanism of action. In this Commentary, we evaluate the evidence for and against the anti-capping hypothesis, including results from several recent structural and biochemical studies that shed new light on this issue. In addition, we describe several alternate mechanisms that Ena/VASP proteins may utilize to regulate actin dynamics in vivo, including inhibition of branching, bundling and profilin-actin recruitment.
A primer on the actin cytoskeleton
To set the stage for our discussion of Ena/VASP function, we will provide a simplified overview of the aspects of actin dynamics and some actin-regulatory proteins that are relevant to this Commentary. For a comprehensive treatment of actin dynamics, we refer the reader to two of many excellent reviews on this topic (Chhabra and Higgs, 2007; Pollard and Borisy, 2003).
Actin assembly can drive formation of membrane protrusions such as lamellipodia (which are wide, flat structures containing dense, branched F-actin networks) or filopodia (which are thin cylindrical protrusions comprising bundled, parallel F-actin filaments). The morphology and dynamics of membrane protrusions can be altered by regulation of the length of actin filaments, and of their stability, density and organization (Lacayo et al., 2007; Mogilner, 2006). Regulated actin-filament elongation, branching, bundling, severing and capping help to create the actin network within protrusions (Chhabra and Higgs, 2007; Pollard and Borisy, 2003).
Polymerization of growing actin filament `barbed' ends is thought to drive membrane protrusion (Mogilner and Oster, 2003). Spontaneous F-actin assembly is suppressed within cells by proteins that sequester actin monomers; therefore, the generation of barbed ends requires proteins that nucleate new filaments, or that sever or uncap filaments whose growth has been terminated by actin-filament-capping proteins (Pollard and Borisy, 2003). The Arp2/3 complex nucleates new filaments that branch off from the sides of existing filaments (Blanchoin et al., 2000; May et al., 1999; Mullins et al., 1998), preferentially near their barbed ends (Ichetovkin et al., 2002). Arp2/3-mediated nucleation contributes in part to the dense array of branched F-actin networks within the lamellipodium (Bailly et al., 1999; Svitkina and Borisy, 1999); regulated activation of Arp2/3 at the plasma membrane causes nucleation of filaments that elongate transiently (until they are capped), driving lamellipodial protrusion (Pollard and Borisy, 2003). Filopodia arise from lamellipodia, and actin within filopodia is shaped into parallel bundles by the F-actin-bundling protein fascin. The filaments that become bundled by fascin can arise by several mechanisms, including elongation of Arp2/3-nucleated filament or de novo assembly by a different class of actin-nucleating proteins, the formins (Gupton and Gertler, 2007). Formins nucleate linear actin filaments and remain attached to the polymerizing filaments, protecting them from being capped (Chhabra and Higgs, 2007).
Interestingly, protrusive forces produced by Arp2/3-nucleated actin-filament networks also drive the movement of a number of intracellular pathogens, including Listeria monocytogenes (Lambrechts et al., 2008). Listeria co-opt elements of the host-cell cytoskeleton by expressing a protein (ActA) on their surface that contains motifs that bind Arp2/3, actin and Ena/VASP, effectively mimicking several cellular actin-regulatory proteins. ActA activates Arp2/3, causing actin to polymerize on the bacterial surface. Listeria propulsion requires several other proteins that do not bind directly to the bacterium, two of which are germane here – capping protein (CP) and profilin. CP is a barbed-end capping protein and is thought to increase the rate of Listeria motility by enhancing Arp2/3 nucleation (Akin and Mullins, 2008). By capping polymerizing filaments near the bacterium, CP increases the pool of actin monomer that is available for Arp2/3 nucleation. Profilin is an abundant actin-monomer-binding protein that functions to promote formation of ATP-actin monomer by nucleotide exchange. When bound to profilin, actin cannot spontaneously nucleate and can only be added on to the barbed end of growing filaments. Thus, profilin provides a pool of polymerization-competent actin monomer for barbed-end elongation. In addition to binding to actin, profilin binds to proteins that contain a specific polyproline-rich motif (Ferron et al., 2007) that is found in a number of actin-regulatory proteins including Ena/VASP (discussed below). Ena/VASP proteins are not essential for Listeria motility, but stimulate bacterial velocity and enable Listeria to spread from cell to cell. Several mechanisms for Ena/VASP stimulation of Listeria have been proposed, including recruitment of profilin-actin to the bacterium and interactions with the F-actin tail.
Domain structure and interactions of Ena/VASP proteins
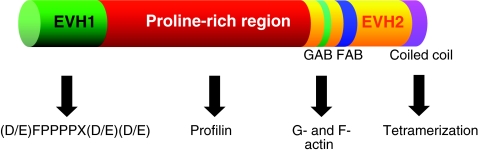
Ena/VASP proteins contain several specific domains that are shared in all family members (Krause et al., 2003) (Fig. 2); these include the N-terminal Ena/VASP homology 1 (EVH1) domain, the proline-rich domain and the C-terminal EVH2 domain. The EVH1 domain binds to a specific proline-rich motif. Functional EVH1-binding sites are found in a number of proteins, including lamellipodin, the axon-guidance receptor Robo and the focal-adhesion proteins zyxin and vinculin. The proline-rich domain, in the middle portion of Ena/VASP proteins, binds to SH3- and WW-domain-containing proteins, and also to profilin. The EVH2 domain binds to G- and F-actin and mediates the tetramerization of Ena/VASP. EVH2-mediated interactions with growing ends of actin filaments are required for efficient targeting of Ena/VASP proteins to lamellipodia and filopodia.
Fig. 2.
Domain structure of Ena/VASP proteins. The EVH1 and EVH2 domains and proline-rich region are indicated. EVH1 mediates protein:protein interactions; most, but not all, EVH1 ligands bind to a motif that has the consensus sequence (D/E)FPPPPX(D/E)(D/E). The proline-rich region harbors binding sites for profilin, including a high-affinity site adjacent to the EVH2 domain. The EVH2 domain contains a G-actin-binding site (GAB), an F-actin-binding site (FAB) and a coiled-coil at the very C-terminus that mediates tetramerization.
Ena/VASP binds to profilin–G-actin complexes through two interfaces simultaneously, a profilin:Ena/VASP interface and a G-actin:Ena/VASP interface. The G-actin in the profilin–G-actin–Ena/VASP complex is oriented towards the F-actin binding motif of Ena/VASP; it is presumably positioned in this way to facilitate its addition to growing filaments (Ferron et al., 2007). The G-actin binding motif in Ena/VASP also stabilizes it at the tips of filopodia (Applewhite et al., 2007). Vertebrate Ena/VASP proteins are in vivo substrates for the cyclic-nucleotide-dependent kinases PKA and PKG; phosphorylation at sites within the EVH2 domain reduces binding of Ena/VASP to G- and F-actin and negatively regulates Ena/VASP function in lamellipodia (Barzik et al., 2005; Harbeck et al., 2000; Lindsay et al., 2007).
The anti-capping hypothesis of Ena/VASP function
Ena/VASP proteins have been proposed to act as actin anti-capping proteins. The general idea of anti-capping by Ena/VASP proteins is that the proteins permit actin-filament elongation, even in the presence of high levels of capping proteins. This activity is necessary to allow the formation of the long filaments within cells that contribute to structures such as filopodia. In fact, many cell types, particularly neurons, require Ena/VASP function for filopodium formation (Dent et al., 2007; Lebrand et al., 2004). Ena/VASP supports filopodium formation by antagonizing actin-filament capping activity (Barzik et al., 2005; Bear et al., 2002) and by promoting clustering of filaments at their polymerizing ends (Applewhite et al., 2007).
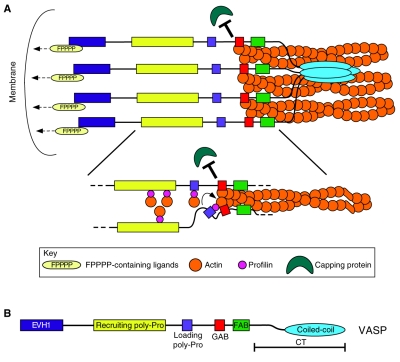
However, there is considerable controversy in this field, some of which arises from confusion over the definition of anti-capping activity. The anti-capping hypothesis of Ena/VASP function specifically states that Ena/VASP proteins associate with elongating actin filaments at or near their rapidly growing (barbed) end in such a way as to block the action of capping proteins that terminate elongation (Fig. 3). It is important to note that this hypothesis does not state that Ena/VASP proteins can remove capping proteins that have become stably associated with filaments (uncapping) nor does it state that Ena/VASP proteins have any effect on the depolymerization of actin filaments from their barbed end (weak capping) nor that Ena/VASP associates processively with the growing actin filaments. One final point about this hypothesis is that anti-capping is not mutually exclusive with other established Ena/VASP functions such as filament clustering, bundling or anti-branching (discussed below).
Fig. 3.
Mechanism of anti-capping by Ena/VASP proteins. (A) Tetramer of VASP in association with a bundle of actin filaments. Interactions between the EVH1 domain of Ena/VASP and FPPPP-motif-containing proteins help to position the Ena/VASP tetramer near the plasma membrane. (B) The profilin–G-actin loading mechanism by which Ena/VASP is thought to supply actin monomers to the barbed ends of filaments while antagonizing the ability of CP to terminate filament elongation.
The anti-capping hypothesis was developed on the basis of results that were obtained by manipulating Ena/VASP activity in fibroblasts (Bear et al., 2002), but it also draws on earlier work about the biochemical function of CP in neutrophil lysates (DiNubile et al., 1995). In the fibroblast system, genetic deletion or sequestration of Ena/VASP proteins led to faster cell motility with more persistent lamellipodial protrusions (Bear et al., 2002). The actin filaments within these lamellipodia were shorter and more highly branched, suggesting that filament capping activity was increased. Conversely, membrane targeting of Ena/VASP protein led to longer filaments with fewer branches. Collectively, these data suggested that Ena/VASP proteins were `anti-capping' factors. In the mid-1990s, Zigmond and colleagues, using neutrophil extracts and permeabilized cells, demonstrated the existence of factors that compete with CP and maintain a pool of extending barbed ends (DiNubile et al., 1995). Although the molecular identity of this factor or factors was not established, the fact that such an activity existed implied that the proposed anti-capping activity of Ena/VASP proteins was plausible.
Further evidence supporting the anti-capping hypothesis
In addition to the data described above, several types of evidence support the anti-capping model for Ena/VASP function, including in vitro assays with purified proteins, structural analysis, cell biological experiments and genetics.
Ena/VASP anti-capping activity has been observed indirectly in pyrene actin polymerization assays (Bear et al., 2002; Barzik et al., 2005) and directly in total internal reflection fluorescence microscopy (TIRF) assays (Pasic et al., 2008) (see Box 1 for more details on these assays). Purified VASP delayed termination of filament growth by heterodimeric CP. The filament elongation rate was enhanced by addition of profilin along with VASP. These data support earlier kinetic experiments in which spectrin–F-actin seeded (`SAS') pyrene-actin polymerization assays were used to test for the antagonism of capping proteins by VASP (Barzik et al., 2005; Bear et al., 2002). VASP increased F-actin polymerization in SAS assays in the presence of CP, an effect that was enhanced by profilin. VASP anti-capping activity was also observed when a CapG, a capping protein unrelated to CP, was used in the assay. The kinetic experiments also demonstrated that the stimulatory effect of profilin on VASP anti-capping required direct profilin:VASP and profilin:G-actin interactions (Barzik et al., 2005; Bear et al., 2002). Importantly, all of these kinetic and visual assays were done under conditions in which VASP exhibited negligible nucleation activity.
Box 1. In vitro techniques for measuring Ena/VASP function
Pyrene actin polymerization assay
In this assay, actin polymerization is measured by the increase in fluorescence in a bulk format such as a cuvette. The fluorescence in this assay arises from the dye, pyrene, which is covalently coupled to a fraction (usually 5-10%) of the total actin in the assay. Upon incorporation of these pyrene-labeled actin monomers into filaments, the fluorescence increases. This assay yields remarkably detailed kinetic information about the extent of total polymerization, but does not give information about the length or architecture of the actin filaments that are formed. In some instances, these reactions are `jump-started' by adding actin filament seeds in the form of spectrin–F-actin seeds (SAS).
Microscopy-based visual assays
In this assay, actin polymerization is monitored directly by observing the formation of individual filaments with TIRF. As in the bulk pyrene assay, a fraction of the actin is labeled with a fluorescent dye in the visible range such as Oregon Green 488 (Molecular Probes). A simpler version of this assay involves stopping diluted polymerization reactions with fluorescent phalloidin (a molecule that binds to the sides of actin filaments and prevents depolymerization) and observing the reaction with TIRF or traditional epifluorescence microscopy.
Reconstituted motility assays
Another assay that is used to probe the role of actin-binding proteins such as Ena/VASP proteins is the in vitro reconstitution assay. In this assay, beads coated with actin nucleators such as ActA are added to complex mixtures of purified proteins or lysates obtained from cells or tissues that contain all the necessary factors to support the motility of the beads. Information about the percentage of motile beads and their motility characteristics such as speed are determined by observing the reactions on a microscope over time.
The ability of Ena/VASP to interact with barbed ends was demonstrated directly using TIRF microscopy (Pasic et al., 2008). In these experiments, VASP was immobilized on a coverslip and preformed, labeled actin filaments were flowed in under conditions that permitted the elongation of barbed, but not pointed, ends. The immobilized VASP captured filament barbed ends; importantly, filaments pre-capped with a barbed-end CP were not captured. These results were consistent with earlier studies indicating that VASP immobilized on beads could capture F-actin with free barbed ends, but not F-actin that had been capped by CP (Bear et al., 2002). Interestingly, barbed-end capture requires only the very C-terminal part of the EVH2 domain, CT (Pasic et al., 2008), a region that lacks the known actin-binding motifs (Bachmann et al., 1999; Harbeck et al., 2000). The CT sequence contains a coiled-coil that tetramerizes in parallel with an unusual right-handed coil (Kuhnel et al., 2004), raising the interesting possibility that this forms a structural interface for binding to barbed ends of F-actin (which also forms a right-handed coil).
The anti-capping activity of Ena/VASP proteins was recently confirmed by the Faix group (Breitsprecher et al., 2008). In these studies, VASP immobilized on beads was capable of supporting continuous elongation of filaments even in the presence of a very high concentration of CP. This arrangement is likely to be similar to physiological situations in which Ena/VASP proteins are tethered at specific locations by interactions with the EVH1 domain and FPPPP-containing ligands. This study did not find anti-capping activity for VASP when it was in solution, as others have; however, this may be due to differences in specific assay conditions or protein preparation or storage conditions (see below).
Several types of cell-biological evidence support the hypothesis that Ena/VASP proteins interact with F-actin barbed ends. In some cell types, including endothelial cells, Ena/VASP localizes in a sarcomeric pattern along stress fibers (Furman et al., 2007; Reinhard et al., 1992). Barbed-end labeling of permeabilized endothelial cells with labeled actin monomer revealed that monomers were inserted into stress fibers adjacent to VASP, reflecting close proximity of VASP to free barbed ends (Furman et al., 2007). In experiments in which Ena/VASP function was blocked, there was a dramatic reduction in the amount of F-actin in stress fibers and in the amount of monomer incorporated into barbed ends. Other data supporting Ena/VASP–barbed-end interactions involve treatment of cells with nanomolar concentrations of cytochalasin D, a drug that blocks barbed ends and displaces Ena/VASP from the tips of protruding lamellipodia in several cell types, including fibroblasts (Bear et al., 2002), MCF7 cells (Scott et al., 2006), B16 melanoma cells (Krause et al., 2004) and keratocytes (Lacayo et al., 2007), indicating that growing barbed ends are required for proper Ena/VASP localization within lamellipodia. Experimental increases or decreases in levels of Ena/VASP in keratocytes resulted in changes in lamellipodial morphology that were consistent with a mathematical model in which Ena/VASP exhibited anti-capping activity (Lacayo et al., 2007). Finally, fluorescence recovery after photobleaching (FRAP) experiments revealed that VASP is stably associated with growing filaments in filopodial tips (Applewhite et al., 2007): co-FRAP of VASP and actin revealed rapid recovery of actin with no recovery of VASP signal, indicating that there is little or no turnover in the VASP pool within the filopodial tip, whereas new actin monomers are continually incorporated at the tip.
Unresolved issues regarding the anti-capping hypothesis
Although most recent studies support the anti-capping hypothesis, there remain a few unresolved issues regarding this idea. For example, early data from one group indicated that VASP had no effect on actin polymerization in the presence of the barbed-end capping protein gelsolin (Boujemaa-Paterski et al., 2001). The authors concluded that VASP did not affect the ability of capping proteins to function. It is worth noting that these reactions also contained ActA and Arp2/3, whose presence would lead to a complex set of nucleation and elongation reactions that may have complicated the interpretation of this experiment. These studies may need to be revisited with techniques such as time-lapse TIRF to understand the effect of VASP in these reactions.
Another issue that remains unresolved is the effect of Ena/VASP proteins on filament elongation, with and without profilin. Some studies have found no effect of VASP alone on filament elongation rates (Barzik et al., 2005; Bear et al., 2002), whereas another finds a significant increase in elongation in the presence of VASP (Breitsprecher et al., 2008). These studies also reached the opposite conclusion about the role of profilin in VASP activity. In one study, profilin increased barbed-end elongation in the presence of VASP (Barzik et al., 2005), whereas the other saw no effect (Breitsprecher et al., 2008). It is unclear why these experiments led to different results, but these discrepancies may reflect differences in how the recombinant VASP was prepared, stored or immobilized on beads; VASP anti-capping activity is quite sensitive to storage conditions (Melanie Barzik, Laboratory of Cell Biology, NHLBI, NIH, Bethesda, MD; personal communication).
Other possible mechanisms of Ena/VASP function
In addition to their proposed anti-capping activity, Ena/VASP proteins have other biochemical activities that may operate in parallel with, or synergistically with, anti-capping to modulate actin dynamics in vivo.
Anti-branching
Several groups have noted that Ena/VASP proteins reduce the frequency of actin-filament branching by the Arp2/3 complex (Bear et al., 2002; Plastino et al., 2004; Samarin et al., 2003; Skoble et al., 2001). The strongest evidence for a direct anti-branching effect comes from the work of Skoble and colleagues (Skoble et al., 2001). They observed that VASP reduced the branching induced by ActA-activated Arp2/3 complex in visual assays. These reactions did not contain capping proteins and the observed anti-branching activity must be independent of its anti-capping function. The mechanism of this direct effect on branching remains unknown, although it is tempting to speculate that Ena/VASP proteins compete with the Arp2/3 complex for actin monomers, which are necessary cofactors for branching. Alternatively, Ena/VASP proteins might act directly to inhibit branching; for instance, they might prevent the docking of the Arp2/3 complex onto the side of a mother actin filament.
Another possible explanation for the observed anti-branching activity of Ena/VASP proteins is through an indirect effect on CP. Akin and Mullins recently described a striking mechanism for controlling the frequency of Arp2/3 branching through the capping of barbed ends (Akin and Mullins, 2008). They demonstrated that increasing capping activity actually increases branching because the levels of actin monomers are locally elevated. Anti-capping factors such as Ena/VASP proteins would therefore be predicted to decrease branching by the reduction of barbed-end capping, which would lead to the increased consumption of monomers through their addition to barbed ends. It has also been proposed that Ena/VASP exerts a catalytic debranching effect on Arp2/3 branches (Plastino et al., 2004; Samarin et al., 2003); however, this idea needs to be confirmed with more direct assays such as TIRF. Future experiments will be required to delineate the precise role of Ena/VASP in Arp2/3 branch dynamics.
Bundling
Ena/VASP proteins bundle actin filaments; this property was identified soon after their discovery and might contribute to the mechanism of anti-capping (Bachmann et al., 1999). Bundling requires both the F-actin-binding activity of Ena/VASP proteins and their tetramerization (both of these activities are mediated by the EVH2 domain) (Fig. 2). The formation of bundles is sensitive to the concentration of salt, which suggests that electrostatic interactions between Ena/VASP proteins and actin filaments contribute to bundling (Barzik et al., 2005). In contrast to other bundling proteins, such as fascin, Ena/VASP proteins are not found along the entire length of actin bundles in vivo. In filopodia, for example, Ena/VASP proteins are restricted to the distal tips of bundles (Lanier et al., 1999). This stable, restricted localization requires the G-actin-binding site of Ena/VASP proteins and probably reflects the interaction between oligomers of Ena/VASP and the barbed ends of actin filaments in the filopodial bundle (Applewhite et al., 2007).
Nucleation
Another biochemical property of Ena/VASP proteins is their ability to nucleate actin filaments in vitro; however, the significance of this effect in vivo remains unclear. Actin-filament nucleation by various Ena/VASP isoforms has been noted by multiple groups using the pyrene actin polymerization assay (Huttelmaier et al., 1999; Lambrechts et al., 2000; Skoble et al., 2001). This nucleation is highly dependent on the salt concentration in the reaction and mainly occurs at sub-physiological salt concentrations. As noted above, this in vitro nucleation effect often complicates the interpretation of pyrene actin polymerization experiments involving VASP. In vivo, Ena/VASP proteins are unlikely to be physiological actin nucleators: mitochondrial targeting of Ena/VASP reveals no accumulation of F-actin (Bear et al., 2000). Similarly, Listeria expressing an ActA mutant protein that recruits Ena/VASP but not Arp2/3 fails to accumulate any detectable F-actin (Lasa et al., 1995; Pistor et al., 2000).
Profilin recruitment
Recent structural and biochemical analyses indicate that VASP harbors a high-affinity profilin-binding `actin-loading site' adjacent to its actin-monomer-binding motif (Chereau and Dominguez, 2006; Ferron et al., 2007). The affinity of profilin–G-actin complexes for the VASP loading site is 7.5 μM, about tenfold higher than that of profilin alone. Interestingly, the presence of the actin-loading site on VASP also doubled the affinity of profilin for G-actin. The structural studies suggest a model in which profilin–G-actin binds to the Ena/VASP loading site in a manner that allows the actin monomer to bind to the Ena/VASP G-actin binding site (which is adjacent to the F-actin-binding motif). Such a model would explain how actin monomers could be transferred efficiently from profilin to the barbed ends of actin filaments while bound to Ena/VASP; elements of this model are similar to those proposed for VASP function in Listeria motility over 10 years ago (Purich and Southwick, 1997; Smith et al., 1996). The model is consistent with the ability of profilin to increase Ena/VASP anti-capping activity by increasing filament-elongation rates (Barzik et al., 2005); it is important to note, however, that profilin is not required for VASP anti-capping activity.
Augmenting formin function
Experiments in Dictyostelium indicate that the orthologs of the formin mDia2 and VASP are both required for filopodium formation: deletion of either gene ablates filopodium formation (Schirenbeck et al., 2006). Further experiments indicated that the F-actin binding site in Dictyostelium VASP was required for it to support the formation of filopodia. Together, these results, along with in vitro data on actin bundling, led Schirenbeck and coworkers to propose that the sole function of VASP in filopodium formation in Dictyostelium was to bundle filaments that were nucleated and/or elongated by the activity of Dia2. As the anti-capping activity of VASP requires its ability to bind to F-actin, the aforementioned experiments do not distinguish between a role for VASP in bundling, anti-capping or both. Work in mammalian cells indicates that filament bundling and anti-capping by Ena/VASP both contribute to filopodium formation (Applewhite et al., 2007); Ena/VASP is likely both to protect filaments from capping and to cause them to cluster at their growing barbed ends, after which fascin bundles them along their length (Svitkina et al., 2003; Vignjevic et al., 2003). Additionally, the Dictyostelium results are not transferable to mammalian systems because, in mammals, mDia2 and Ena/VASP can form filopodia independently of one another. In particular, in neurons that are genetically deficient in all three Ena/VASP proteins and lack filopodia (Dent et al., 2007), filopodium formation can be rescued by expression of mDia2. It is, however, possible that other formin family members have a role in filopodium formation in conjunction with Ena/VASP. Recent work in Drosophila suggests that the formin subfamily member DAAM is localized along filopodia and shows some overlap with Ena at the filopodial tip (Matusek et al., 2008). Further experiments will be required to dissect the functional interactions between Ena/VASP and formin proteins in mammalian systems.
Conclusions and perspectives
On the basis of the current published literature, the anti-capping model of Ena/VASP function seems to be the simplest explanation for many of the known cell-biological and biochemical properties of this protein family. By extension, anti-capping could also account for the demonstrable ability of Ena/VASP to reduce the branching density of filaments; it might do this by maintaining free barbed actin-filament ends that would compete with Arp2/3 for actin-monomer binding. A clearer understanding of how Ena/VASP functions to promote the formation of longer, less-branched networks will require structural information showing exactly how Ena/VASP interacts with F-actin. Furthermore, visual assays with Arp2/3 will help clarify how Ena/VASP exerts its anti-branching activity. Finally, the mechanism underlying the ability of VASP (and probably Mena and EVL) to modulate integrin activation is poorly understood; it is unclear whether the effects of Ena/VASP proteins on adhesion are linked to anti-capping or represent a distinct molecular activity. Resolving these and other outstanding questions about Ena/VASP function will allow a greater insight into actin-based motility and the physiological processes that depend on precisely controlled actin dynamics.
We thank Melanie Barzik for sharing unpublished observations, for assistance with figures and for the image in Fig. 1. J.E.B. is supported by NIH grant GM 083035 and F.B.G. by GM 58801. Deposited in PMC for release after 12 months.
References
- Akin, O. and Mullins, R. D. (2008). Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell 133, 841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applewhite, D. A., Barzik, M., Kojima, S., Svitkina, T. M., Gertler, F. B. and Borisy, G. G. (2007). Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol. Biol. Cell 18, 2579-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszodi, A., Pfeifer, A., Ahmad, M., Glauner, M., Zhou, X. H., Ny, L., Andersson, K. E., Kehrel, B., Offermanns, S. and Fassler, R. (1999). The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 18, 37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann, C., Fischer, L., Walter, U. and Reinhard, M. (1999). The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J. Biol. Chem. 274, 23549-23557. [DOI] [PubMed] [Google Scholar]
- Bailly, M., Macaluso, F., Cammer, M., Chan, A., Segall, J. E. and Condeelis, J. S. (1999). Relationship between Arp2/3 complex and the barbed ends of actin filaments at the leading edge of carcinoma cells after epidermal growth factor stimulation. J. Cell Biol. 145, 331-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzik, M., Kotova, T. I., Higgs, H. N., Hazelwood, L., Hanein, D., Gertler, F. B. and Schafer, D. A. (2005). Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J. Biol. Chem. 280, 28653-28662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear, J. E., Loureiro, J. J., Libova, I., Fassler, R., Wehland, J. and Gertler, F. B. (2000). Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101, 717-728. [DOI] [PubMed] [Google Scholar]
- Bear, J. E., Svitkina, T. M., Krause, M., Schafer, D. A., Loureiro, J. J., Strasser, G. A., Maly, I. V., Chaga, O. Y., Cooper, J. A., Borisy, G. G. et al. (2002). Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509-521. [DOI] [PubMed] [Google Scholar]
- Blanchoin, L., Amann, K. J., Higgs, H. N., Marchand, J. B., Kaiser, D. A. and Pollard, T. D. (2000). Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404, 1007-1011. [DOI] [PubMed] [Google Scholar]
- Boujemaa-Paterski, R., Gouin, E., Hansen, G., Samarin, S., Le Clainche, C., Didry, D., Dehoux, P., Cossart, P., Kocks, C., Carlier, M. F. et al. (2001). Listeria protein ActA mimics WASp family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry 40, 11390-11404. [DOI] [PubMed] [Google Scholar]
- Breitsprecher, D., Kiesewetter, A. K., Linkner, J., Urbanke, C., Resch, G. P., Small, J. V. and Faix, J. (2008). Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 27, 2943-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereau, D. and Dominguez, R. (2006). Understanding the role of the G-actin-binding domain of Ena/VASP in actin assembly. J. Struct. Biol. 155, 195-201. [DOI] [PubMed] [Google Scholar]
- Chhabra, E. S. and Higgs, H. N. (2007). The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110-1121. [DOI] [PubMed] [Google Scholar]
- Dent, E. W., Kwiatkowski, A. V., Mebane, L. M., Philippar, U., Barzik, M., Rubinson, D. A., Gupton, S., Van Veen, J. E., Furman, C., Zhang, J. et al. (2007). Filopodia are required for cortical neurite initiation. Nat. Cell Biol. 9, 1347-1359. [DOI] [PubMed] [Google Scholar]
- DiNubile, M. J., Cassimeris, L., Joyce, M. and Zigmond, S. H. (1995). Actin filament barbed-end capping activity in neutrophil lysates: the role of capping protein-beta 2. Mol. Biol. Cell 6, 1659-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron, F., Rebowski, G., Lee, S. H. and Dominguez, R. (2007). Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 26, 4597-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman, C., Sieminski, A. L., Kwiatkowski, A. V., Rubinson, D. A., Vasile, E., Bronson, R. T., Fassler, R. and Gertler, F. B. (2007). Ena/VASP is required for endothelial barrier function in vivo. J. Cell Biol. 179, 761-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates, J., Mahaffey, J. P., Rogers, S. L., Emerson, M., Rogers, E. M., Sottile, S. L., Van Vactor, D., Gertler, F. B. and Peifer, M. (2007). Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development 134, 2027-2039. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Niebuhr, K., Reinhard, M., Wehland, J. and Soriano, P. (1996). Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 87, 227-239. [DOI] [PubMed] [Google Scholar]
- Gupton, S. L. and Gertler, F. B. (2007). Filopodia: the fingers that do the walking. Sci STKE 2007, re5. [DOI] [PubMed] [Google Scholar]
- Harbeck, B., Huttelmaier, S., Schluter, K., Jockusch, B. M. and Illenberger, S. (2000). Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem. 275, 30817-30825. [DOI] [PubMed] [Google Scholar]
- Hauser, W., Knobeloch, K. P., Eigenthaler, M., Gambaryan, S., Krenn, V., Geiger, J., Glazova, M., Rohde, E., Horak, I., Walter, U. et al. (1999). Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc. Natl. Acad. Sci. USA 96, 8120-8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttelmaier, S., Harbeck, B., Steffens, O., Messerschmidt, T., Illenberger, S. and Jockusch, B. M. (1999). Characterization of the actin binding properties of the vasodilator-stimulated phosphoprotein VASP. FEBS Lett. 451, 68-74. [DOI] [PubMed] [Google Scholar]
- Ichetovkin, I., Grant, W. and Condeelis, J. (2002). Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr. Biol. 12, 79-84. [DOI] [PubMed] [Google Scholar]
- Krause, M., Dent, E. W., Bear, J. E., Loureiro, J. J. and Gertler, F. B. (2003). Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 19, 541-564. [DOI] [PubMed] [Google Scholar]
- Krause, M., Leslie, J. D., Stewart, M., Lafuente, E. M., Valderrama, F., Jagannathan, R., Strasser, G. A., Rubinson, D. A., Liu, H., Way, M. et al. (2004). Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell 7, 571-583. [DOI] [PubMed] [Google Scholar]
- Kuhnel, K., Jarchau, T., Wolf, E., Schlichting, I., Walter, U., Wittinghofer, A. and Strelkov, S. V. (2004). The VASP tetramerization domain is a right-handed coiled coil based on a 15-residue repeat. Proc. Natl. Acad. Sci. USA 101, 17027-17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski, A. V., Rubinson, D. A., Dent, E. W., Edward van Veen, J., Leslie, J. D., Zhang, J., Mebane, L. M., Philippar, U., Pinheiro, E. M., Burds, A. A. et al. (2007). Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron 56, 441-455. [DOI] [PubMed] [Google Scholar]
- Lacayo, C. I., Pincus, Z., VanDuijn, M. M., Wilson, C. A., Fletcher, D. A., Gertler, F. B., Mogilner, A. and Theriot, J. A. (2007). Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLoS Biol. 5, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts, A., Kwiatkowski, A. V., Lanier, L. M., Bear, J. E., Vandekerckhove, J., Ampe, C. and Gertler, F. B. (2000). cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem. 275, 36143-36151. [DOI] [PubMed] [Google Scholar]
- Lambrechts, A., Gevaert, K., Cossart, P., Vandekerckhove, J. and Van Troys, M. (2008). Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 18, 220-227. [DOI] [PubMed] [Google Scholar]
- Lanier, L. M., Gates, M. A., Witke, W., Menzies, A. S., Wehman, A. M., Macklis, J. D., Kwiatkowski, D., Soriano, P. and Gertler, F. B. (1999). Mena is required for neurulation and commissure formation. Neuron 22, 313-325. [DOI] [PubMed] [Google Scholar]
- Lasa, I., David, V., Gouin, E., Marchand, J. B. and Cossart, P. (1995). The amino-terminal part of ActA is critical for the actin-based motility of Listeria monocytogenes; the central proline-rich region acts as a stimulator. Mol. Microbiol. 18, 425-436. [DOI] [PubMed] [Google Scholar]
- Lawrence, D. W., Comerford, K. M. and Colgan, S. P. (2002). Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am. J. Physiol. Cell Physiol. 282, C1235-C1245. [DOI] [PubMed] [Google Scholar]
- Lebrand, C., Dent, E. W., Strasser, G. A., Lanier, L. M., Krause, M., Svitkina, T. M., Borisy, G. G. and Gertler, F. B. (2004). Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 42, 37-49. [DOI] [PubMed] [Google Scholar]
- Lindsay, S. L., Ramsey, S., Aitchison, M., Renne, T. and Evans, T. J. (2007). Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239. J. Cell Sci. 120, 3011-3021. [DOI] [PubMed] [Google Scholar]
- Massberg, S., Gruner, S., Konrad, I., Garcia Arguinzonis, M. I., Eigenthaler, M., Hemler, K., Kersting, J., Schulz, C., Muller, I., Besta, F. et al. (2004). Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood 103, 136-142. [DOI] [PubMed] [Google Scholar]
- Matusek, T., Gombos, R., Szecsenyi, A., Sanchez-Soriano, N., Czibula, A., Pataki, C., Gedai, A., Prokop, A., Rasko, I. and Mihaly, J. (2008). Formin proteins of the DAAM subfamily play a role during axon growth. J. Neurosci. 28, 13310-13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R. C., Hall, M. E., Higgs, H. N., Pollard, T. D., Chakraborty, T., Wehland, J., Machesky, L. M. and Sechi, A. S. (1999). The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr. Biol. 9, 759-762. [DOI] [PubMed] [Google Scholar]
- Menzies, A. S., Aszodi, A., Williams, S. E., Pfeifer, A., Wehman, A. M., Goh, K. L., Mason, C. A., Fassler, R. and Gertler, F. B. (2004). Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J. Neurosci. 24, 8029-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner, A. (2006). On the edge: modeling protrusion. Curr. Opin. Cell Biol. 18, 32-39. [DOI] [PubMed] [Google Scholar]
- Mogilner, A. and Oster, G. (2003). Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophys. J. 84, 1591-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, R. D., Heuser, J. A. and Pollard, T. D. (1998). The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 95, 6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasic, L., Kotova, T. and Schafer, D. A. (2008). Ena/VASP proteins capture actin filament barbed ends. J. Biol. Chem. 283, 9814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistor, S., Grobe, L., Sechi, A. S., Domann, E., Gerstel, B., Machesky, L. M., Chakraborty, T. and Wehland, J. (2000). Mutations of arginine residues within the 146-KKRRK-150 motif of the ActA protein of Listeria monocytogenes abolish intracellular motility by interfering with the recruitment of the Arp2/3 complex. J. Cell Sci. 113, 3277-3287. [DOI] [PubMed] [Google Scholar]
- Plastino, J., Olivier, S. and Sykes, C. (2004). Actin filaments align into hollow comets for rapid VASP-mediated propulsion. Curr. Biol. 14, 1766-1771. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D. and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. [DOI] [PubMed] [Google Scholar]
- Purich, D. L. and Southwick, F. S. (1997). ABM-1 and ABM-2 homology sequences: consensus docking sites for actin-based motility defined by oligoproline regions in Listeria ActA surface protein and human VASP. Biochem. Biophys. Res. Commun. 231, 686-691. [DOI] [PubMed] [Google Scholar]
- Reinhard, M., Halbrugge, M., Scheer, U., Wiegand, C., Jockusch, B. M. and Walter, U. (1992). The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 11, 2063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarin, S., Romero, S., Kocks, C., Didry, D., Pantaloni, D. and Carlier, M. F. (2003). How VASP enhances actin-based motility. J. Cell Biol. 163, 131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirenbeck, A., Arasada, R., Bretschneider, T., Stradal, T. E., Schleicher, M. and Faix, J. (2006). The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc. Natl. Acad. Sci. USA 103, 7694-7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. A., Shewan, A. M., den Elzen, N. R., Loureiro, J. J., Gertler, F. B. and Yap, A. S. (2006). Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol. Biol. Cell 17, 1085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoble, J., Auerbuch, V., Goley, E. D., Welch, M. D. and Portnoy, D. A. (2001). Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 155, 89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. A., Theriot, J. A. and Portnoy, D. A. (1996). The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J. Cell Biol. 135, 647-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T. M. and Borisy, G. G. (1999). Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T. M., Bulanova, E. A., Chaga, O. Y., Vignjevic, D. M., Kojima, S., Vasiliev, J. M. and Borisy, G. G. (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160, 409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin, V., Bauer, C., Yin, M. and Fuchs, E. (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209-219. [DOI] [PubMed] [Google Scholar]
- Vignjevic, D., Yarar, D., Welch, M. D., Peloquin, J., Svitkina, T. and Borisy, G. G. (2003). Formation of filopodia-like bundles in vitro from a dendritic network. J. Cell Biol. 160, 951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]