Abstract
The chemosensory glomus cells of the carotid body (CB) detect changes in O2-tension. Carotid sinus nerve fibers, which originate from peripheral sensory neurons located within the petrosal ganglion, innervate the CB. Release of transmitter from glomus cells activates the sensory afferent fibers to transmit information to the nucleus of the solitary tract in the brainstem. The ion channels expressed within the sensory nerve terminals play an essential role in the ability of the terminal to initiate action potentials in response to transmitter-evoked depolarization. However, with a few exceptions, the identity of ion channels expressed in these peripheral nerve fibers is unknown. This study addresses the expression of voltage-gated channels in the sensory fibers with a focus on channels that set the resting membrane potential and regulate discharge patterns. Using immunohistochemistry and fluorescence confocal microscopy, potassium channel subunits and HCN (hyperpolarization-activated) family members were localized both in petrosal neurons that expressed tyrosine hydroxylase, and the CSN axons within the carotid body. Channels contributing to resting membrane potential including HCN2, responsible in part for Ih current, and the KCNQ2 and KCNQ5 subunits thought to underlie the neuronal “M current” were identified in the sensory neurons and their axons innervating the carotid body. In addition, the results presented here demonstrate expression of several potassium channels that shape the action potential and the frequency of discharge including Kv1.4, Kv1.5, Kv4.3, KCa (BK). The role of these channels should be considered in interpretation of the fiber discharge in response to perturbation of the carotid body environment.
Keywords: petrosal ganglion, chemoreceptors, ion channels, chemoreflex
Introduction
Maintenance of oxygen homeostasis is mediated in part by the chemosensory cells of the carotid body (CB). The chemosensory cells in the CB, also referred to as glomus cells or Type I cells, are organized into tight clusters surrounded by supportive glia, or Type II cells. Glomus cell clusters are innervated by sensory afferent fibers of the carotid sinus nerve (CSN), which arise from sensory neurons located in the petrosal ganglion. Exposure to hypoxic stimuli results in glomus cell depolarization and release of transmitter substances resulting in CSN fiber activation and signal transmission to central respiratory centers. Much of the information on the contribution of specific ion channels to activity in this afferent limb of the carotid chemoreflex pathway has focused on the properties of channels in the oxygen-sensing glomus cells. However, the ion channels that are present on the sensory afferent terminals are also integral to the process of transmission of sensory information to the central nervous system.
Selective distribution of ion channel proteins to specific regions of a neuron has been widely demonstrated throughout the central and peripheral nervous system. While previous retrograde labeling experiments have identified several voltage-gated K+ (Kv) channel subunits in the soma of petrosal neurons whose axons innervate the carotid body (Andrews and Kunze, 2001), only Kv1.1 (Kline et al., 2005), Task-2 (Yamamoto et al,, 2002), Trek-1 (Yamamoto and Taniguchi, 2006) and Kv1.4 (Sanchez et al. 2002) have been identified in nerve terminals in the carotid body. Of particular importance in this chemoreflex pathway is the role of the ion channels which contribute to the resting membrane potential and those channels that, upon depolarization, control discharge patterns of the sensory terminals. As candidate channel proteins for distribution to the peripheral terminals we focused on the presence of Kv and HCN (hyperpolarization activated) channels that have been identified by immunohistochemical, RT-PCR or electrophysiological studies in the sensory soma in nodose/petrosal complex.
Methods
Carotid body and petrosal ganglion sections
Sprague-Dawley rats, weighing approximately 100g, were anesthetized by halothane or isoflurane inhalation and decapitated. The use of these animals was in accord with NIH guidelines and the approved animal protocols established by Case Western Reserve University’s Animal Resource Center. The bifurcation of the carotid artery was removed and embedded in OCT compound (Tissue-Tek). The bifurcation was sectioned by cryostat and sections were mounted on Fisherbrand Superfrost/Plus slides. The petrosal ganglion was removed together with the nodose. The ganglion was frozen in the same manner as the bifurcation and sectioned by cryostat. The carotid body was sectioned at 10–16μm and the petrosal ganglion at 6μm. Slides containing the carotid bifurcation were placed in the fixative solution (4% paraformaldehyde (PFA) with or without 0.3% Triton-X-100 in 0.1M phosphate buffer, pH 7.4) for twenty minutes and then rinsed in PBS. The petrosal sections were left in fixative overnight and then rinsed in PBS. After fixation, the tissue sections were incubated with blocking solution containing 10% normal donkey serum, 1% bovine serum albumin, and 0.1–0.3% Trition-X-100 in PBS for 30 minutes. Primary antibodies were diluted in PBS containing 1% BSA with or without 0.3%, Triton and tissue sections were incubated with primary Antibodieso vernight at 4°C. Following brief rinses in PBS, the sections were incubated with the corresponding secondary antibodies, diluted in blocking solution, for 90 minutes at room temperature. The tissue sections were rinsed again in PBS and mounted with Prolong Gold mounting media (Molecular Probes). The preadsorbed antibodies were tested at the same concentration as the untreated antibodies on adjacent slices of the carotid body. Both the antibody and the preadsorbed antibody treated slices were imaged with the Leica SP2 spectral confocal microscope at the same gain and offset settings (Figures 1, 4–6). The photomicrographs in Figure 3 were collected using Spot RT Slider Camera (Diagnostic Instruments, Inc) on a Nikon E600 microscope. Contrast and brightness were adjusted in Adobe Photoshop (San Jose, CA).
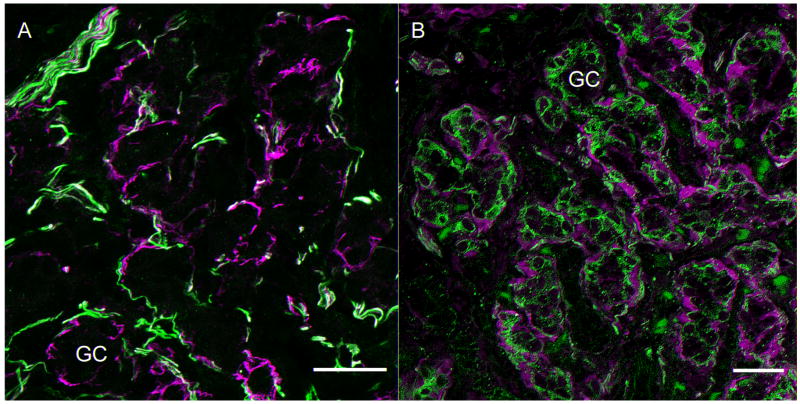
Figure 1. Markers of sensory innervation of Type 1 glomus cell clusters in the carotid body.
A. Neurofilament cocktail (anti-NF 200, 120 and 68) labels major fibers surrounding but seldom penetrating glomus cell clusters (green). Anti-GFAP (Accurate) labels cells that surround the glomus cell clusters (magenta) in close proximity to the nerve fibers. Scale bar= 25μm
B. This panel illustrates the neural innervation stained with anti-peripherin antibody. (green). The fibers surround and infiltrate the clusters of Type I glomus cells. The Type 2 cells surround the Type 1 clusters and are immunolabeled with S100 antibody (magenta, Santa Cruz). Scale bar=20μm. GC=glomus cell cluster.
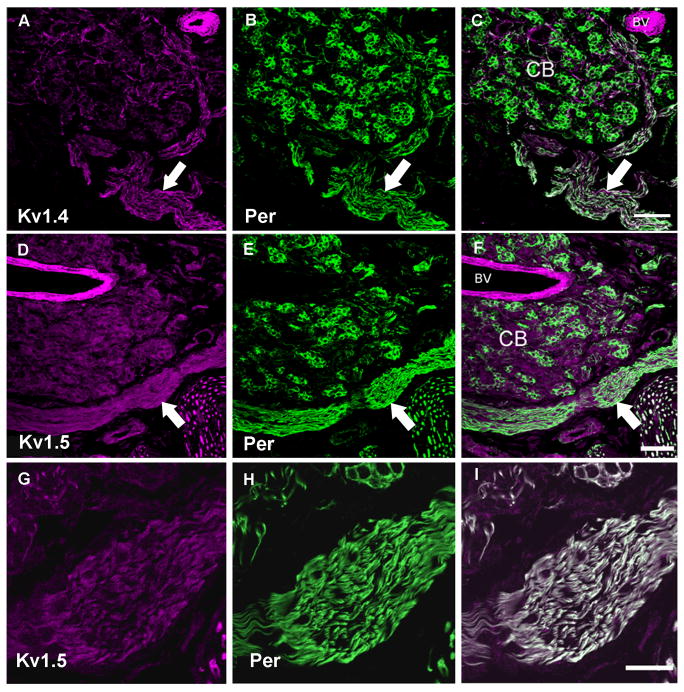
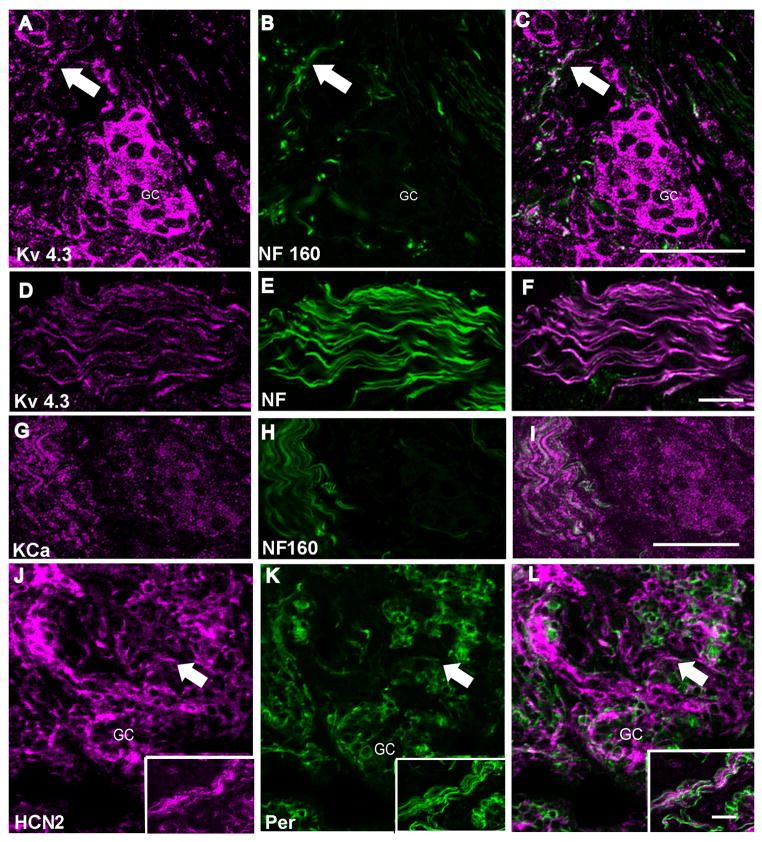
Figure 4. Voltage-gated potassium channel immunoreactivity in fibers of carotid body sections.
In all panels the left image is the ion channel immunoreactivity pseudo-colored in magenta, the center image illustrates anti-peripherin (green). The right image is a merge of the two images. Panel A–C: Low magnification image of carotid body stained with anti-Kv1.4 and anti-peripherin. The carotid sinus nerve enters from below and branches to surround the carotid body. Fine fibers containing Kv1.4 are seen to penetrate the clusters, co-localizing with peripherin within the carotid body in this confocal z-series stack. Scale = 50μm. Panel D–F: Low magnification confocal image of the carotid body and carotid sinus nerve (arrow) immunolabeled with anti-Kv1.5 and anti-peripherin. The glomus cells are also densely labeled with anti-Kv1.5. Scale represents 50 μm. Panel G–I: High magnification of z-series confocal image of the carotid sinus nerve co-localized with anti-Kv 1.5 and anti-peripherin. There is strong co-localization in the nerve as indicated by the white in the merged image. This higher magnification image shows in more detail a small group of glomus cells at the upper region of the image co-localizing Kv1.5 and peripherin with additional labeling within the glomus cells. Scale represents 20μm. CB = carotid body, BV = blood vessel.
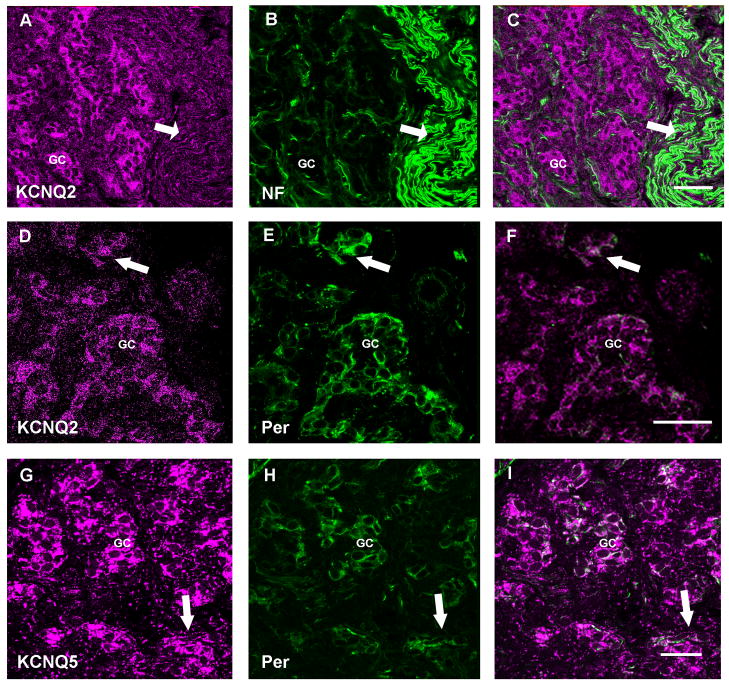
Figure 6. Expression of KCNQ channel protein in carotid body fibers.
In all panels the left image is the ion channel immunoreactivity pseudo-colored in magenta, the center image illustrates anti-NF or anti-peripherin (green). The right image is a merge of the two images. Panel A–C: Low magnification confocal z-series demonstrating the immunohistochemical localization of anti-KCNQ2 (Chemicon) with anti-NF. The arrow identifies the carotid sinus nerve. Scale = 40μm. Panel D–F: Single slice high magnification confocal image showing fine fibers with KCNQ2 (Chemicon) and peripherin (Santa Cruz) immunoreactivity in glomus cell clusters. Scale = 40μm. Panel G–I: anti-KCNQ5 overlays with anti-peripherin in fibers (arrow) and in glomus cell clusters in this confocal z-series stack. Scale = 25 μm. GC= Glomus cell clusters.
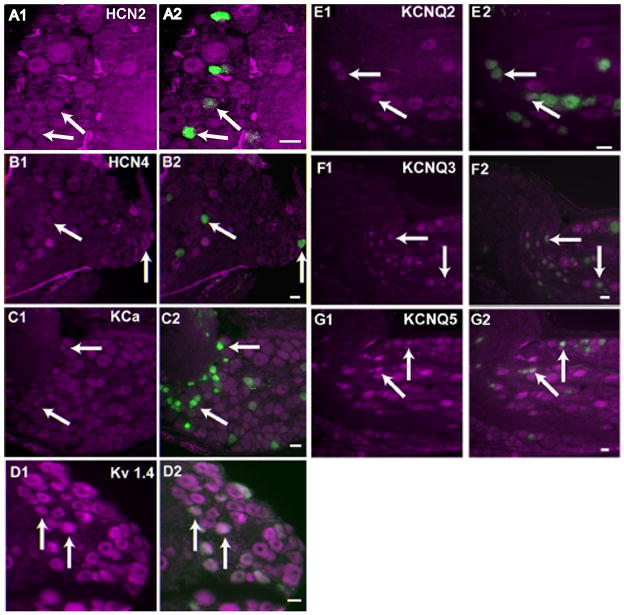
Figure 3. Co-expression of tyrosine hydroxylase with KCNQ2, KCNQ3, KCNQ5, KCa, Kv1.4, HCN2 and HCN4 in neurons of the petrosal ganglion.
The left panel for each antibody demonstrates ion channel immunoreactivity psuedocolored in magenta. The right panels are the resultant overlay of TH immunoreactive neurons (green) whose afferent fibers terminate on the glomus cells. Arrows identify some of the cells exhibiting both TH and the ion channel immunoreactivity. Panel A1, A2: HCN2 (NeuroMab); Panel B1, B2: HCN4; Panel C1, C2: Kca (NeuroMab); Panel D1, D2: Kv1.4; Panel E1, E2 : KCNQ2 (NeuroMab); Panel F1,F2: KCNQ3; Panel G1, G2:KCNQ5. Scale in all the panels = 15um
Antibodies
The antibodies used in this study are described in Table 1. Each description provides the commercial source, the species in which the antibody was raised, whether polyclonal or monoclonal and, where appropriate, the peptide sequences used to generate the antibodies. Where two antibodies against the same protein were used, the staining patterns in the carotid body were the same. Donkey serum and fluorescently conjugated secondary antibodies were obtained from Jackson Immunoresearch (West Grove, PA). All antibodies used against ion channels were examined in western blots of nodose/petrosal tissue as described below.
Table 1.
| Channel | Company and Identification Numben | Host, Immunogen |
|---|---|---|
| Kv1.4 | Upstate (Millipore, Temecula, CA), 5-409, Lot 20734 and 15579 | mouse monoclonal, clone K13/31, seq 13-37aa |
| Kv1.5 | Transduction Labs (BD Biosciences, San Jose, CA), H62520 | mouse monoclonal, clone #8, seq 1-119aa |
| Kv4.3 | Alomone (Jerusalem, Israel), APC-017 Lot AN-05 and 06; | rabbit polyclonal, seq 451-467aa |
| HCN2 | Alomone (Jerusalem, Israel), APC-030 Lot AN-01 and 04; | rabbit polyclonal, seq 147-161aa |
| HCN2 | NeuroMab (Davis, CA), 75-111 | mouse monoclonal, clone N71/37, fusion protein 761-863 aa |
| HCN4 | Alomone (Jerusalem, Israel), APC-052 Lot AN-04; | rabbit polyclonal GST fusion protein, seq 119-155aa |
| KCNQ2 | Chemicon (Millipore, Temecula, CA) AB5577; | rabbit polyclonal, seq 578-593aa |
| KCNQ2 | NeuroMab (Davis, CA), N26A/23 | mouse monoclonal, seq 1-59aa |
| KCNQ3 | Alomone (Jerusalem, Israel), APC-051; | rabbit polyclonal, seq 668-686aa |
| KCNQ5 | Affinity Bioreagents (Golden CO) PA1-941 BR; | rabbit polyclonal seq 880-897aa |
| KCa (BK) | Alomone (Jerusalem, Israel), APC-021, Lot AN-06-07 | rabbit polyclonal, GST fusion protein seq 1098-1196aa |
| KCa (BK) | NeuroMab (Davis, CA) 75-022 | mouse monoclonal, Slo1 clone L6/60; fusion protein seq 690-1196aa |
| NF68 | Novacastra ( Newcastle on TYNE, UK) NCL-NF68 | mouse monoclonal, clone NR4; immunogen, porcine spinal cord |
| NF160 | Sigma (St. Louis, MO) N-5246; | mouse monoclonal, clone NN18; immunogen, purified porcine neurofilament |
| NF200 | Sigma (St. Louis, MO) N-0142; | mouse monoclonal, clone N52; immunogen, carboxyl terminal tail |
| Peripherin | Chemicon (Millipore, Temecula, CA) MAB5380 | mouse monoclonal, clone 7C5; immunogen rat recombinant peripherin |
| Peripherin | Santa Cruz (Santa Cruz, CA) C-19:sc-7604 | goat polyclonal, immunogen, human carboxyl terminal peptide, (TESQKEQRSELDKSSAHSY) 453-471aa |
| GFAP | Accurate Chemicals (Westbury, NY) Axl457; Lot 96 | rabbit polyclonal; immunogen purified bovine spinal cord GFAP |
| GFAP | Sigma (St. Louis, MO) G3893 | mouse monoclonal clone G-A-5; immunogen, purified porcine GFAP |
| S100 | Biomeda (Foster City, CA) 088D | mouse monoclonal clone B32.1; immunogen, bovine S100 protein, recognizes both a and β forms |
Kv1.4 (Upstate, monoclonal) A strong band at ~90kDa and a very weak band at ~80 are present in a Western blot in rat nodose/petrosal tissue. Company data sheet shows a band at 96kDa rat brain.
Kv1.5 (Transduction Labs, monoclonal) The Western blot produced a single band at ~70 kDa in rat nodose/petrosal tissue. Company data sheet indicates a band ~80kDa in rat heart
Kv4.3 (Alomone, polyclonal) Western produced bands at ~65kDa and ~45kDa in rat nodose/petrosal tissue. The latter was previously reported as a degradation product (Zicha et al., 2004). The company data sheet indicates band in rat brain at ~65kDa Adsorption: All staining was abolished in sections of rat carotid body when 3μg peptide/1μg antibody were incubated together for one hour prior to use.
KCNQ2 (Chemicon, polyclonal) The Western blot analysis gave a strong band at ~ 100kDa in both rat nodose/petrosal tissue and rat brain. A faint band at twice the size ~200kDa is also present. No blot was provided in company data sheet. A band is expected at about 95kDa. Adsorption: All staining was abolished in sections of rat carotid body when 1μg peptide/1μg antibody were incubated together for one hour.
KCNQ2 (NeuroMab, monoclonal) A band at ~85kDa is recognized in our Western blot of rat nodose/petrosal tissue. A second light band about twice the size occurs at ~ 170kDa. No blot was provided in the company data sheet although it states a 95kDa band is recognized.
KCNQ3 (Alomone, ployclonal) There are two bands in the Western blot, one is just below 100 and the other at ~ 60kDa in rat nodose/petrosal tissue. Company data sheet shows the same two bands in rat brain. Adsorption: All staining was abolished in sections of rat carotid body when 1μg peptide/1μg antibody were incubated together for one hour.
KCNQ5 (Affinity Bioreagents, polyclonal). Our Western blot shows two bands, at 95kDa and 60kDa, in rat nodose/petrosal tissue. The expected size is about 100kDa. Company data sheet states the antibody detects band at 125kDa in rat brain. In rat brain we also detected a band ~130kDa as well as the two bands that appeared in the nodose/petrosal tissue. Our lower band (60kDa) may be a degradation product. Peptide antigen was not available.
BKCa (Alomone, polyclonal) Our Western blot analysis gives a strong band at 130kDa and a weaker band at about 95kDa in rat nodose/petrosal tissue. Company data sheet shows the 130 kDa band rat brain. Detailed studies indicate several bands may occur as degradation products between 70 and 130kDa (Knaus et al., 1995) or as splice variants (Chen et al., 200). Adsorption: All staining was abolished in sections of rat carotid body when 3μg peptide/1μg antibody were incubated together for one hour.
BKCa (NeuroMab, monoclonal) The Western blot gave a strong band at ~130kDa and weaker at ~95kDa in rat nodose/petrosal tissue. Company data sheet shows a band ~120kDa in rat brain. Detailed study indicate several bands may occur as degradation products between 70 and 130kDa (Knaus et al., 1995).
HCN2 (Alomone, polyclonal) Western blot shows a single band at ~90–95 kDa in rat nodose/petrosal tissue. Western blot provided in the company data sheet gives a similar band.
HCN2 (NeuroMab, monoclonal) Western blot gave one strong band at 115–120kDa and weaker band at 80kDa in rat nodose/petrosal tissue. Company data sheet also shows two bands, at ~120kDa and ~105kDa in rat brain.
HCN4 (Alomone, polyclonal) In the Western blot there are two bands at ~150kDa and 85kDa in rat nodose/petrosal tissue. Company data sheet does not show a Western blot but other studies report the higher band 140–160kDa in rat brain (Muto et al., 2007, using an Alomone antibody and Notoni and Shigemoto, 2004 using their own well characterized antibody). Adsorption: All staining was abolished in sections of carotid body with 1μg peptide/1μg antibody.
Peripherin (Santa Cruz, polyclonal) The company shows a single band at about 55kDa in a Western blot from cultured PC12 cells grown as a monolayer and a doublet near the same molecular weight from PC12 cells grown in suspension. According to the company, the sequence of the immunizing peptide is provided in Baiou et al. 2007.
Neuronal and non-neuronal marker antibodies. Western blots were not performed on these antibodies which were prepared against purified proteins. Anti-Neurofilament 68, anti-Neurofilament 160 and anti-Neurofilament 200 are markers of nerve fibers in carotid body and elsewhere as shown in Buniel et al., 2003; Doan et al., 2004 and Kline et al., 2005. Peripherin labels nerve fibers in the carotid body including those attached to isolated glomus cell clusters as shown by Buniel et al., 2003. GFAP and S100 are markers for Type II supporting cells as shown previously by Chou et al. (1998), and Kondo et al. (1982). We have also shown this in carotid body in Buniel et al., 2003 and Kline et al., 2005.
PCR amplification of nodose/petrosal ganglia Kv1.4, Kv1.5 and Kv4.3 α-subunit cDNA fragments
Nodose/petrosal ganglia and brain from adult rats (6–8 weeks old) were excised and stored at −70 °C. mRNA was isolated using the MicroPoly (A) Pure kit (Ambion) following the manufacturers instructions. Poly A+ mRNA was quantified by spectrophotometric absorbance at 260 nm and stored in aliquots at − 70 °C until its use.
Primer design
Pairs of specific primers were designed to amplify unique DNA fragments corresponding to specific regions of rat Kv1.4, Kv1.5 and Kv4.3 by reverse transcriptase polymerase chain reaction (RT-PCR). Primer sequence and location in published cDNA sequence in GenBank of the National Center for Biotechnology This information is provided in Table 2.
Table 2.
| Channel | Sense Primer | Anti-sense Primer | Internal Nucleotides |
|---|---|---|---|
| Kv1.4 (accession number RNRCK4) | nucleotides 1797–1823 5′-CAG CAG CAG GCT ATG TCC TTT GCC ATC- 3′ | nucleotides 2340–2315 5′-CCA TCT CTA GAT ACT CTG ACT TGT CC – 3′ | nucleotides 2005–2021 5′-AGG CAG ATG AAC CTA CCA CCC ATT TC-3′ |
| Kv1.5 (accession number RATKV1AA) | nucleotides 2146–2171 5′-GCA GTA GTC ACT ATG ACC ACT GTA G- 3′ | nucleotides 2560–2539 5′-TGT TTC ACG GCT AGT GTC CAG ACA G-3′ | nucleotides 2383–2407 5′-TCA ACG GAA GGT CAG CTG CAG CAA G-3 |
| Kv4.3 (accession number RNU42975) | nucleotides 1273–1294 5′-GAA TTC TTT AGC AGG ATC TAC CAT CAG-3′ | nucelotides1969–1949 5′-GTC GAC TTA CAA GAC AGA GAC CTT GAC- 3′ | nucleotides 1650–1676 5′-GTA GCA AGA AGA CCA CGC ACC TGC-3′ |
RT-PCR
Poly-A+ mRNA (100 ng) was heat denatured at 70 °C for 5 min and then reverse transcribed into first-strand cDNA using a mixture of random hexameric, unlabelled deoxynucleotides and MuLV Reverse Transcriptase (First-Strand cDNA Synthesis Kit, Perkin-Elmer) at 42 °C for 1 h. The first-strand cDNA products were used directly as templates for PCR amplification. The amplifications were performed using the following cycling program: one cycle (2 min at 95 °C); 35 cycles (15 s at 94 °C, 15 s at 55 °C, 1 min at 68 °C); one cycle (10 min at 72 °C). PCR products, were resolved by electrophoresis on 1.2 % agarose gels, and transferred to BrightStar-Plus nylon membranes (Ambion). Following transfer, the membrane was baked at 80°C in a vacuum oven. Channel-specific PCR products were identified by hybridization to radiolabeled internal oligonucleotide specific for Kv1.4, Kv1.5 and Kv4.3. The internal oligonucleotides are provided in Table 2. Radiolabeling was accomplished with T4 Polynucleotide Kinase in the presence of [γ-32P]ATP. After the blots were prehybridized in Ultrasensitive Hybridization Solution (Ambion) at 44 °C for 1 h, [32P]-labeled probe (at 106 c.p.m. ml−1) was added and hybridized at 44 °C for up to 16 h. After hybridization, the blots were washed first with 2 × SSC (150 mM NaCl, 15 mM sodium citrate)/0.1 % sodium dodecyl sulphate (SDS) at room temperature followed by a more stringent wash with 0.1 SSC/0.1 % SDS at 44 °C. Blots were exposed to BioMaxMS film (Kodak) at −80 °C with an intensifying screen.
Results
We examined the distribution of the ion channels in the major nerve bundles as well as the fibers that branch to surround and penetrate the glomus cell clusters. Many proteins that were potentially useful in identifying carotid body nerve fibers (for instance, tyrosine hydroxylase, PGP9.5, neuron specific enolase and substance P) are present in both nerve fibers and glomus cells making clear identification of the fibers within and around the glomus cells difficult. At least two proteins, neurofilament and peripherin were confined to the nerve fibers and were used to identify the carotid sinus nerve fibers entering the carotid body and/or penetrating the glomus clusters. An anti-neurofilament cocktail (NF) containing anti-NF200, NF160, and NF68 labels fibers within the nerve bundles and the branches surrounding the glomus cell clusters but does not often extend to the final terminal regions (Fig 1A) within the clusters. Anti-peripherin, an antibody against intermediate filament protein, identified fibers found not only in the main nerve bundles but also in fibers surrounding and penetrating the glomus cell clusters as indicated by the extensive innervation of the glomus cell clusters in Figure 1B. A previous study showed that isolated Type 1 glomus cells did not express peripherin. (Buniel et al., 2003) The carotid body section was also labeled with anti-GFAP (Fig 1A) or anti-S100 (Fig 1B) to identify Type 2 sustentacular cells surrounding the glomus clusters (Kondo et al., 1982; Chou et al., 1998; Nurse and Fearon, 2002). Other non-neuronal cells present in these images, in addition to Type 2 cells, may also be labeled by anti-GFAP and or anti-S100.
RT-PCR and/or electrophysiological data have been previously reported in the soma of visceral sensory neurons for ten of the twelve channels examined in the present study (Hay and Kunze, 1994; Doan and Kunze, 1999; Glazebrook et al., 2002; Wladyka and Kunze, 2006). For this reason they were likely candidates for distribution to the peripheral axons. We verified the expression in the soma of the remaining three channels, Kv1.4, Kv1.5 and Kv4.3, using a second method. Substantiating RT-PCR data from nodose/petrosal ganglia are presented in Figure 2.
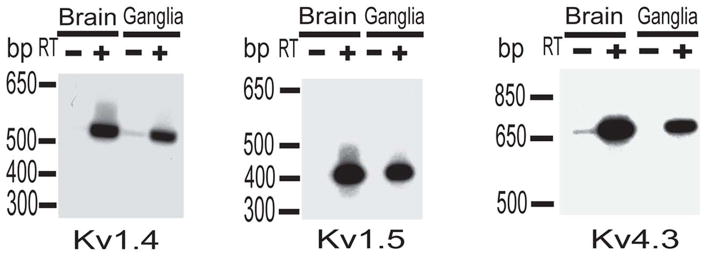
Figure 2. Expression of Kv1.4, Kv1.5 and Kv4.3 channel mRNAs in brain and nodose/petrosal ganglia.
Oligonucleotide primers designed to specifically amplify Kv1.4, Kv1.5 and Kv4.5 channels were used in RT-PCR reactions from the rat ganglia and, for comparison, brain Poly A+ RNA. As a control, first-strand cDNA reactions were performed with (+) or (−) reverse transcriptase (RT). In both reactions, brain and ganglia, the (–)RT lanes had no signal. Expected size of the cDNA fragments was 543bp, 414bp and 690bp for Kv1.4, Kv1.5 and Kv4.3 respectively.
Voltage and/or calcium-gated potassium channels in petrosal neurons projecting to carotid body
Tyrosine hydroxylase expressing petrosal neurons have been shown to project to the carotid body and to innervate glomus cell clusters (Finley et al; 1992). An illustration of co-localization of TH with Kv1.5 and with Kv4.3 had previously been published in soma of petrosal neurons (Andrews and Kunze, 2001). We extended this to demonstrate the presence of Kv1.4, KCa (BK), HCN2, HCN4, KCNQ2, KCNQ3 and KCNQ5 in tyrosine hydroxylase-containing neurons in the petrosal ganglion sections (Fig. 3).
Voltage and/or calcium-gated potassium channels in carotid body nerve fibers
Kv1.4 Kv1.4 immunoreactivity in sensory nerves was strong, shown here co-localizing with peripherin (Fig 4A–C) in both the nerve bundles and in the fibers surrounding and penetrating the glomus cell clusters. It was not present in the Type 1 glomus cells.
Kv1.5 This immunoreactivity co-localized with peripherin in the major fiber bundles and in fibers penetrating the glomus cell clusters (Fig 4D–I). It also appeared as weak labeling in glomus cells (seen at higher magnification in upper part of Fig 4G). It was present in cells outside the glomus as indicated by staining outside the peripherin-enclosed cluster complexes. Kv1.5 immunoreactivity is also present in blood vessels in the carotid body.
Kv4.3 This channel which underlies a 4-AP- and TEA-resistant rapidly activating transient current was present in the major fiber bundles co-localizing well with NF (Fig 5D–F). Because it was also present within the glomus cells (Fig 5A–C) as previously reported in rabbit (Sanchez et al., 2002), it was not possible to clearly identify nerve fibers coursing among the glomus cells.
Figure 5. Distribution of Kv4.3, KCa, and HCN2 immunoreactivity in the carotid body nerve fibers.
In all panels the left image is the ion channel immunoreactivity pseudo-colored in magenta, the center image illustrates anti-NF, anti-NF160 or anti-peripherin (green). The right image is a merge of the two images. Panel A–C is a confocal Z-series stack demonstrating the distribution of anti-NF160 and anti-Kv4.3. The arrow illustrates one example of co-localization in fibers in the carotid body. The individual glomus cells are highly immunoreactive for Kv4.3 making it impossible to distinguish between innervating fibers and cell bodies within the cluster. Scale = 50μm. Panel D–F is a high magnification confocal z-series of the carotid sinus nerve as it enters the carotid body where there is almost total overlay of Kv4.3 and the neurofilament cocktail. Scale = 20μm. Panel G–I, a single confocal image from a z-series, demonstrates at high magnification KCa channel immunoreactivity in a group of carotid sinus nerve fibers adjacent to a glomus cell cluster. The glomus cell cluster shows very punctuate labeling in this single image. Similar results were obtained with the two antibodies listed in Table 1. Scale = 50μm. Panel J–L is a low magnification confocal slice showing the co-localization of anti-HCN2 (Alomone) and anti-peripherin (Santa Cruz). The inset shows a labeled group of CSN fibers. HCN2 immunolabeling does not appear to include the glomus cells although it co-localizes with peripherin in the fibers on the edge of the cluster and, in a few fibers, appears to penetrate the clusters. However, it is most intense in surrounding cells that do not label with anti-peripherin, probably Type II cells. Scale = 25μm. GC=glomus cell cluster.
KCa (Kca1.1): In the carotid body the KCa immunoreactivity co-localized with anti-NF in the axonal bundles innervating the carotid body (Fig 5G–I). Within the glomus cell clusters the immunoreactivity was generally punctuate and widespread making it difficult to distinguish individual fibers among the glomus cells.
HCN (Hyperpolarization-activated cation) channels
Previous studies of HCN2 and HCN4 identified both immunoreactivity and ionic currents that are small in amplitude but important in resting membrane control in visceral sensory neurons (Doan et al. 2004). The localization of both antibodies in TH-containing petrosal neurons (Fig. 3) indicated that these proteins might distribute to the axons. HCN2 immunoreactivity in the fibers was the strongest of the HCN subunits we examined (Fig 5J–L, inset). It localized with peripherin surrounding glomus cell clusters, was not present in the glomus cells and was most abundant in cells outside glomus clusters. HCN4 labeling was weak and punctuate could not conclusively be associated with nerve fibers (not shown) in carotid body sections.
KCNQ2, KCNQ3, KCNQ5 (“M current” channels)
We evaluated the KCNQ family members, KCNQ2, KCNQ3, KCNQ5 (Kv7.2, 7.3, 7.5) which have recently been shown to be present in visceral sensory neurons using both current recordings and immunohistochemistry (Wladyka and Kunze, 2006). KCNQ2 was present in NF-labeled fibers in branches of the CSN and in the glomus cells (Fig 6A–F). Again, when immunoreactivity was strong within the glomus cells we were unable to distinguish the fibers within the labeled clusters. Although anti-KCNQ3 was present in TH petrosal neurons, labeling of nerve fibers was weak and punctate and could not be clearly associated with specific fibers (not shown). KCNQ5 was present in nerve fibers and co-localized well with peripherin in the fibers within the glomus cell clusters (Fig 6G–I).
Other channels
Five of twelve channels present in the TH containing petrosal soma could not be identified in the peripheral nerve bundles or terminals. In addition to HCN4 and KCNQ3 mentioned earlier, we were unable to definitively localize Kv1.2, Kv1.6, Kv2.1 to fibers within the nerve bundles or fibers within the glomus clusters although the immunoreactivity is present in TH expressing petrosal neurons (Andrews and Kunze, 1999). We surmise that these channels are either not distributed to the terminals or are not expressed in sufficient quantity to be detectable with the present techniques.
Discussion
The present studies allow us to include the role of the channels in carotid body fibers in addressing the overall response to the neural transmitters released from the glomus cells in response to hypoxic stimuli. Verna (1979) summarizes the data supporting the position that most, if not all, of the fibers ending on glomus Type 1 cells are sensory afferents. The fibers innervating the glomus cell clusters are thought to be those containing TH (Finley et al 1992). Although the immunoreactivity for the channels reported here was identified in TH-containing petrosal neurons and in the large nerve bundles innervating the carotid body, in many cases we were unable to definitively localize the immunoreactivity to the nerve fibers within the glomus cell clusters because it was either masked by the presence of the channel protein in the glomus cells, was present in low concentration or did not extend to the terminals. The presence of immunoreactivity in nerve fibers outside the glomus cell clusters could indicate innervation of other structures within the carotid body such as the blood vessels.
In previous studies immunoreactivity for Kv3.4, Kv4.3 (Sanchez et al., 2002), Kv3.1, Kv3.2, Kv3.3 (Perez-Garcia et al., 2004), KCa (Nurse and Fearon, 2002) have been identified in glomus cells. In situ hybridization revealed mRNA for Kv4.1 (Sanchez et al., 2002) in isolated glomus cells. This study adds KCNQ2, KCNQ5 and Kv1.5 to the list of channels in the glomus cells.
Channels involved in setting the resting membrane potential in the soma of the sensory cells
Members of two families of channels that have been shown to contribute to the resting membrane potential of the soma of visceral sensory neurons are also present in the peripheral terminal regions. HCN channels are mixed cation channels permeable to both potassium and sodium. The HCN current has a reversal potential in the range of −35 to −40mV. These channels are active at the resting membrane potential in visceral sensory neurons and their current offsets hyperpolarizing potassium current. Block of the channels by cesium drives the membrane potential toward EK as the influence of potassium channels predominates (Doan and Kunze, 1999). While we were unable to obtain definitive localization of HCN4 within the carotid body, HCN2 provided a clear staining pattern co-localizing with peripherin around glomus cell clusters and also in cells surrounding the glomus cell clusters that did not stain with anti-peripherin. The immunoreactivity for HCN2 is of interest because HCN2 is modulated by cAMP. Transmitters such as dopamine which is released from the glomus cells may alter cAMP levels in the terminals via postsynaptic D1/D2 receptors (Bairam et al 2003; Czyzyk-Krzeska, 1992; Kline et al., 2002) and thus regulate the terminal membrane potential through HCN2. This is in line with the modulatory role of dopamine proposed in a recent review (Iturriaga and Alcayaga, 2004).
The group of KCNQ2, KCNQ3 and KCNQ5 subunits is thought to comprise the “M current” which is suppressed by one of the transmitter substances released by the glomus cells, acetylcholine acting via a muscarinic receptor. These potassium channels are active in the soma at the resting membrane potential (−65 to −50mV, Wladyka and Kunze, 2006). At the soma, block of the current depolarizes the membrane potential, increases the input resistance and increases the excitability of the cell in response to a depolarizing stimulus. Acetylcholine through nicotinic receptors generally increases activity in the chemoreceptor afferents while the role of muscarinic receptors is less clear although they appear to modulate the nicotinic response in carotid body (for review, Iturriaga and Alcayaga, 2004). Shirahata et al. (2004) reported the presence of M1 muscarinic acetylcholine receptors on the nerve terminals in the carotid body of cat.
The data in the present study when taken together with reports of Task-2 (Yamamoto et al,, 2002) and Trek-1 (Yamamoto and Taniguchi, 2006), both potassium channels expected to be active at the resting membrane potential, provide a composite description of the channels controlling the resting membrane potential in the terminal fibers. To our knowledge there is only one report of intracellular recording from nerve fibers within the carotid body. In their technically demanding study, Hayashida et al. (1980) reported resting potentials of about −36 mV although they believed this value was somewhat low because of the inevitable damage produced by penetrating fibers of small dimensions. Nevertheless, it is reasonable to assume that the resting potential in the nerve endings is set to an intermediate value between the reversal potential for potassium channels and that of the mixed Na and K conductance of HCN channels depending on their relative contributions.
Channels shaping the frequency of discharge in response to depolarization
The pattern and frequency of discharge of the afferent fibers, responding to transmitter release from the glomus cells, will be influenced by the potassium channels Kv1.1, identified previously in terminal fibers (Kline et al. 2005) and by those identified here, Kv1.4 (see also Sanchez et al., 2002), Kv1.5, Kv4.3 and KCa. These channels regulate discharge through control of the duration of the action potential, the after-hyperpolarization and refractory period. These data can be incorporated in the development of models of the response to depolarization as is being done for the somal currents (Schild et al, 1994, 1997).
Potential for oxygen sensitivity
Several ion channels proposed as sensors of oxygen in either carotid body glomus cells or other tissues are present on the nerve fibers entering the carotid body and enveloping and penetrating the glomus cell clusters including Kv1.4 (Duprat et al., 1995), Kv1.5 (Hulme et al., 1999; ), Kv4.3 (Sanchez et al., 2002) Kca (Riesco-Fagundo et al., 2001, Lewis et al.2002), HCN (Mironov et al., 2001). Do these channels respond to hypoxia in the nerve terminal? While it has not been technically possible to address the oxygen sensitivity of the carotid body nerve fibers in situ, there is one report that tackles this question with an innovative preparation. In a co-culture of glomus cells innervated by petrosal neurons, hypoxia was only effective in eliciting discharge in the sensory afferent if co-cultured with glomus cells but not alone (Zhong et al, 1997). This suggests that the fibers are not sensitive to the reduced oxygen and rules out a direct effect of oxygen on the channel proteins. However, in most reported cases of sensitivity to hypoxia the production of a substance by a hypoxic stimulus is necessary to initiate the channel response. What occurs within or around the fibers ending in situ during hypoxia remains to be determined and leaving open the question of oxygen sensitivity of the fibers.
In summary, a complement of voltage-gated ion channels has been identified within the fibers of the carotid body. These provide the basis for including a discussion of the role of the sensory afferent fibers in analyzing the response of the afferent limb of the carotid chemoreflex pathway to changes in arterial PO2. Since the electrical properties of the terminals themselves cannot yet be technically addressed, one potential approach to understanding the contribution of the carotid body fibers in the transduction process is to develop a testable model based on the kinetic features of the channels as characterized in the soma
Acknowledgments
Supported by a grant from the National Heart Lung and Blood Institute HL25830
Reference List
- Andrews EM, Kunze DL. Voltage-gated K+ channels in chemoreceptor sensory neurons of rat petrosal ganglion. Br Res. 2001;897:199–203. doi: 10.1016/s0006-8993(01)02121-7. [DOI] [PubMed] [Google Scholar]
- Baiou D, Santha P, Avelino A, Charrua A, Bacskai T, Matesz K, Cruz F, Nagy I. Neurochemical characterization of insulin receptor-expressing primary sensory neurons in wild-type and vanilloid type 1 transient receptor potential receptor knockout mice. J Comp Neurol. 2007;503:334–347. doi: 10.1002/cne.21389. [DOI] [PubMed] [Google Scholar]
- Bairam A, Carroll JL, Labelle Y, Khandjian EW. Differential changes in dopamine D2- and D1-receptor mRNA levels induced by hypoxia in the arterial chemoreflex pathway organs in one-day-old and adult rabbits. Biol Neo. 2003;84:222–231. doi: 10.1159/000072306. [DOI] [PubMed] [Google Scholar]
- Buniel M, Schilling WP, Kunze DL. Distribution of transient receptor potential channels in the rat carotid chemosensory pathway. J Comp Neurol. 2003;464:404–413. doi: 10.1002/cne.10798. [DOI] [PubMed] [Google Scholar]
- Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem. 2005;280:33599–60. doi: 10.1074/jbc.M505383200. [DOI] [PubMed] [Google Scholar]
- Chou CL, Sham JS, Schofield B, Shirahata M. Electrophysiological and immunocytological demonstration of cell-type specific responses to hypoxia in the adult cat carotid body. Brain Res. 1998;789:229–23. doi: 10.1016/s0006-8993(97)01472-8. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Lawson EE, Millhorn DE. Expression of D2 dopamine receptor mRNA in the arterial chemoreceptor afferent pathway. J Auton Nerv Syst. 1992;41:31–39. doi: 10.1016/0165-1838(92)90124-y. [DOI] [PubMed] [Google Scholar]
- Doan TN, Kunze DL. Contribution of the hyperpolarization activated current (IH) to the resting membrane potential of neonatal rat nodose sensory neurons. J Physiol. 1999;514:125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan T, Stephans K, Glazebrook PA, Ramirez A, Andresen MC, Kunze DL. Differential distribution and function of hyperpolarization-activated channels (IH) in sensory neurons and mechanosensitive fibers. J Neurosci. 2004;24:3335–3343. doi: 10.1523/JNEUROSCI.5156-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JC, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neuroscience. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- Glazebrook PA, Ramirez AN, Schild JH, Shieh CC, Doan T, Wible BA, Kunze DL. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol. 2002;541:467–482. doi: 10.1113/jphysiol.2001.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Bao W, Wang Z, Nattel S. Comparison of ion-channel subunit expression in canine cardiac Purkinje fibers and ventricular muscle. Circ Res. 2002;91:790–797. doi: 10.1161/01.res.0000039534.18114.d9. [DOI] [PubMed] [Google Scholar]
- Hay M, Kunze DL. Calcium-activated potassium channels in rat visceral sensory afferents. Br Res. 1994;639:333–336. doi: 10.1016/0006-8993(94)91749-3. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Koyano H, Eyzaguirre C. An intracellular study of chemosensory fibers and endings. J Neurophysiol. 1980;44:1077–1088. doi: 10.1152/jn.1980.44.6.1077. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K+ channels expressed in the pulmonary vasculature. Circ Res. 1999;85:489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J. Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Br Res Rev. 2004;47:46–45. doi: 10.1016/j.brainresrev.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Izal-Azcárate A, Belzunegui S, Sebastián WS, Garrido-Gil P, Vázquez-Claverie M, López B, Marcilla I, Luquin MA. Immunohistochemical characterization of the rat carotid body. Respir Physiol Neurobiol. 2008;161:95–9. doi: 10.1016/j.resp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kline DD, Buniel MC, Glazebrook P, Peng YJ, Ramirez-Navarro A, Prabhakar NR, Kunze DL. Kv1.1 deletion augments the afferent hypoxic chemosensory pathway and respiration. J Neurosci. 2005;25:3389–3399. doi: 10.1523/JNEUROSCI.4556-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of tractus solitarius J. Neurophysiology. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Eberhart A, Koch RO, Munujos P, Schmalhofer WA, Warmke JW, Kaczorowski GJ, Garcia ML. Characterization of tissue-expressed alpha subunits of the high conductance Ca(2+)-activated K+ channel. J Biol Chem. 1995;270:22434–2243. doi: 10.1074/jbc.270.38.22434. [DOI] [PubMed] [Google Scholar]
- Kondo H, Iwanaga T, Nakajima T. Immunocytochemical study on the localization of neuron-specific enolase and S-100 protein in the carotid body of rats. Cell Tissue Res. 1982;227:291–295. doi: 10.1007/BF00210887. [DOI] [PubMed] [Google Scholar]
- Lewis A, Peers C, Ashford ML, Kemp PJ. Hypoxia inhibits human recombinant large conductance, Ca(2+)-activated K(+) (maxi-K) channels by a mechanism which is membrane delimited and Ca(2+) sensitive. J Physiol. 2002;540:771–780. doi: 10.1113/jphysiol.2001.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Richter DW. Hyperpolarization-activated current Ih, in inspiratory brainstem neurons and its inhibition by hypoxia. Eur J Neurosci. 2000;12:520–526. doi: 10.1046/j.1460-9568.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Muto T, Ueda N, Opthof T, Ohkusa T, Nagata K, Suzuki S, Tsuji Y, Horiba M, Lee JK, Honjo H, Kamiya K, Kodama I, Yasui K. Aldosterone modulates I(f) current through gene expression in cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H2710–2718. doi: 10.1152/ajpheart.01399.2006. [DOI] [PubMed] [Google Scholar]
- Nurse CA, Fearon IM. Carotid body chemoreceptors in dissociated cell culture. Microsc Res Tech. 2002;59:249–255. doi: 10.1002/jemt.10199. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia MT, Colinas O, Miguel-Velado E, Moreno-Dominguez A, Lopez-Lopez JR. Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J Physiol. 2004;557:457–471. doi: 10.1113/jphysiol.2004.062281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O(2) modulates large-conductance Ca(2+)-dependent K(+) channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res. 2001;89:430–436. doi: 10.1161/hh1701.095632. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Lopez-Lopez JR, Perez-Garcia MT, Sanz-Alfayate G, Obeso A, Ganfornina MD, Gonzalez C. Molecular identification of Kvalpha subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid. J Physiol. 2002;542:369–382. doi: 10.1113/jphysiol.2002.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A and C type nodose sensory neurons: Model interpretations of dynamic discharge characteristics. J Neurophys. 1994;71:2338–2358. doi: 10.1152/jn.1994.71.6.2338. [DOI] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and Modeling Study of Na Current Heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophys. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Shirahata M, Hirasawa S, Okumura M, Mendoza JA, Okumura A, Balbir A, Fitzgerald RS. Identification of M1 and M2 muscarinic acetylcholine receptors in the cat carotid body chemosensory system. Neurosci. 2004;128:635–644. doi: 10.1016/j.neuroscience.2004.06.068. [DOI] [PubMed] [Google Scholar]
- Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575:175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna A. Ultrastructure of the carotid body in the mammals. Int Rev Cytol. 1979;60:271–330. doi: 10.1016/s0074-7696(08)61265-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kummer W, Atoji Y, Suzuki Y. TASK-1, TASK-2, TASK-3 and TRAAK immunoreactivities in the rat carotid body. Br Res. 2002;950:304–307. doi: 10.1016/s0006-8993(02)03181-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Taniguchi K. Expression of tandem P domain K+ channel, TREK-1, in the rat carotid body. J Histochem Cytochem. 2006;54:467–472. doi: 10.1369/jhc.5A6755.2005. [DOI] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J Physiol. 1997;503:599–612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561:735–48. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]