Abstract
The holy grail for HIV vaccine development is an immunogen that elicits persisting antibodies with broad neutralizing activity against field strains of the virus. Unfortunately, very little progress has been made in finding or designing such immunogens. Using the SIV model, we have taken a markedly different approach: delivery of an adeno-associated virus (AAV) gene transfer vector to muscle for the expression of antibodies or antibody-like immunoadhesins having predetermined anti-SIV specificity. With this approach, anti-SIV molecules are endogenously synthesized in myofibers and passively distributed to the circulatory system. Using such an approach in monkeys, we have now generated long-lasting neutralizing activity in serum and observed complete protection against intravenous challenge with virulent SIV. In essence, this strategy bypasses the adaptive immune system and holds significant promise as a novel approach to an effective HIV vaccine.
Development of an HIV vaccine is proving to be a daunting task. In the 25 years since the discovery of HIV, hundreds of vaccine candidates have been vetted in a variety of animal models. Many have also been tested in early phase human clinical trials with mostly disappointing results. Two vaccine approaches, each targeting a different arm of the adaptive immune response, have been evaluated in large efficacy trials. Both failed to protect vaccine recipients from infection, and neither diminished viral replication after infection 1-4. While there are other candidates in the pipeline, it seems unlikely that a dramatic breakthrough is imminent.
These sobering observations underscore the tremendous hurdles that must be overcome to develop an effective HIV vaccine 5-9. Foremost among these hurdles is the inability to induce antibodies that neutralize a wide array of HIV field isolates. Such antibodies are rarely found in the sera of long-term infected humans 10,11, and only a handful of broadly neutralizing human monoclonal antibodies have been isolated 12-15. Therefore, one can conclude that broadly neutralizing antibodies are both relatively rare and difficult to elicit, even after acute and chronic natural infection. It seems unlikely that we will improve upon natural responses with contrived immunogens, at least in the near term.
Passive immunization schemes using neutralizing antibodies have protected monkeys from SIV or SHIV challenge infections 16-18. Unfortunately, an injection of antibodies every few weeks is neither practical nor cost effective as a large-scale human vaccine approach. Sanhadji et al.19 showed some years ago that sustained delivery of antibodies could be achieved in mice by implanting collagen-encapsulated 3T3 fibroblasts that had been transduced ex vivo with a retroviral vector carrying an antibody gene. Anti-HIV antibodies that seeped out of the implant decreased viral burden in HIV-1-infected, humanized immunodeficient mice.
Our interest in adeno-associated virus (AAV) vectors caused us to consider such vectors as a means to deliver anti-HIV antibodies directly to muscle, thereby avoiding ex vivo manipulations. In this scheme, the antibody gene of choice is packaged into an AAV vector, which is then delivered by direct intramuscular injection. Thereafter, antibody molecules would be endogenously synthesized in myofibers and passively distributed to the circulatory system. In a proof of concept experiment, mice injected with an AAV vector carrying the IgG1b12 gene produced authentic, biologically active IgG1b12 that was detected in mouse sera for over six months 20.
These data, while encouraging, were generated in mice, which is an artificial model system that is sometimes difficult to translate to higher order primates. Moreover, it was unclear whether the serum levels of neutralizing activity generated would translate to protection from a challenge infection. To more rigorously test the basic concept of antibody gene transfer as an approach to immunization, we turned to the well-characterized SIV model system in monkeys. Herein, we describe the adaptation of the original antibody gene transfer concept to macaques and also detail the derivation of macaque-specific “designer” molecules (immunoadhesins) that neutralize SIV and afford protection from SIV challenge infection.
RESULTS
SIV immunoadhesins
In pilot experiments in mice (J.Z., B.C.S., P.R.J. and K.R.C., unpublished observations), we showed that immunoadhesins (defined as chimeric, antibody-like molecules that combine the functional domain of a binding protein with immunoglobulin constant domains) were superior to single chain (scFv) or whole antibody (IgG) molecules with respect to achievable steady-state serum concentrations. Our constructs followed a formula whereby an anti-viral moiety was fused to an IgG crystalizable fragment (Fc) domain (Fig. 1).
Figure 1.
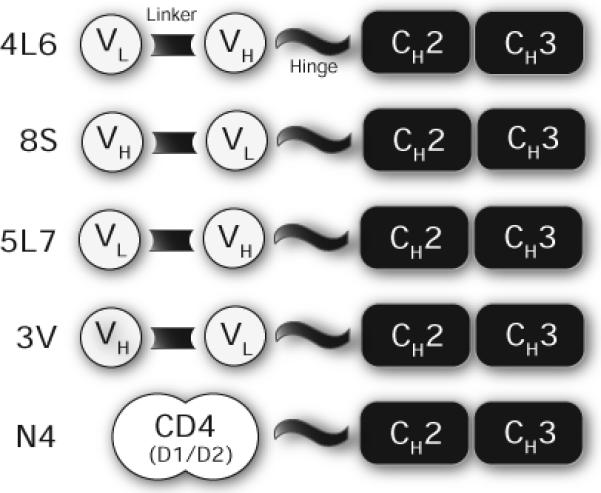
Schematic representation of immunoadhesin constructs. For molecules derived from macaque Fabs (4L6, 8S, 5L7, and 3V), VH and VL domains were joined by a synthetic linker. Rhesus CD4 (domains 1 and 2) was cloned as described in the Methods section. Antigen-binding domains were attached to the Fc fragment of a rhesus IgG2 molecule.
To generate SIV-specific immunoadhesins using native macaque sequences, we obtained previously characterized SIV gp120-specific Fab molecular clones that had been derived directly from SIV-infected macaques 21. These Fab molecules were shown to neutralize in vitro several defined stocks of SIV, thereby affording us the opportunity to test in vivo neutralizing activity after gene transfer to macaques. For these experiments, we chose two Fabs for further modification: 346−16h and 347−23h (see Table 4 in ref 21). Both Fabs had potent in vitro neutralizing activity against SIVmac316, a macrophage tropic derivative of SIVmac239 21-23. Consequently, we chose SIVmac316 as our challenge strain. Although SIVmac316 has not routinely been used in SIV vaccine experiments, it was ideally suited for our purposes since we were able to match antibody reagents with a pathogenic SIV strain. In the end, the SIVmac316 challenge stock was highly infectious and pathogenic for rhesus monkeys (see below).
We joined the variable heavy (VH) and variable light (VL) chains from the Fabs via a linker to create an scFv, and then joined the scFv to a rhesus IgG2 Fc fragment (Fig. 1). Because the linear order of the VH and VL moieties was arbitrary, we made both orientations for each Fab. We also included a construct based on the rhesus CD4 domains 1 and 2, modeled after CD4-gamma fusion proteins 24.
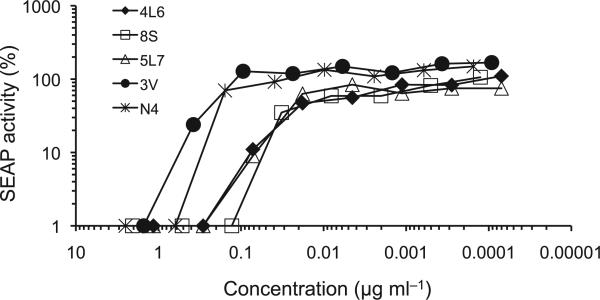
Plasmid was transfected into 293 cells and the expressed proteins were secreted into the cell culture medium as disulfide-linked homodimers (data not shown). They all bound to SIV gp120 in a standard ELISA, and each construct showed significant in vitro neutralizing activity against SIVmac316 (Fig. 2). We subsequently chose constructs 4L6 and 5L7 (representing Fabs 346−16h and 347−23h, respectively) for testing in macaques. Even though N4 did not neutralize as potently as 4L6 or 5L7, we chose to test it because of its unique composition.
Figure 2.
Neutralization of SIV in vitro. The five immunoadhesins shown in Fig. 1 were tested for in vitro neutralizing activity against SVmac316. All five showed significant 50% neutralization at < 1 μg ml−1. The IC50 (ug ml−1) for each was 4L6 (0.01), 8S (0.01), 5L7 (0.02), 3V (0.20), and N4 (0.25).
AAV gene transfer vectors
Expression cassettes for 4L6 and 5L7 were individually cloned between AAV inverted terminal repeats (ITR) designed to produce self-complementary genomes 25. Such genomes have been shown to direct more efficient in vitro and in vivo transduction when compared to traditional single-stranded DNA AAV vectors 26. N4 was a traditional single-stranded DNA genome because this construct was made before the self-complementary vector was available to us. All three recombinant genomes were packaged into AAV serotype 1 (AAV1) capsids using standard techniques 27.
Immunization by in vivo gene transfer
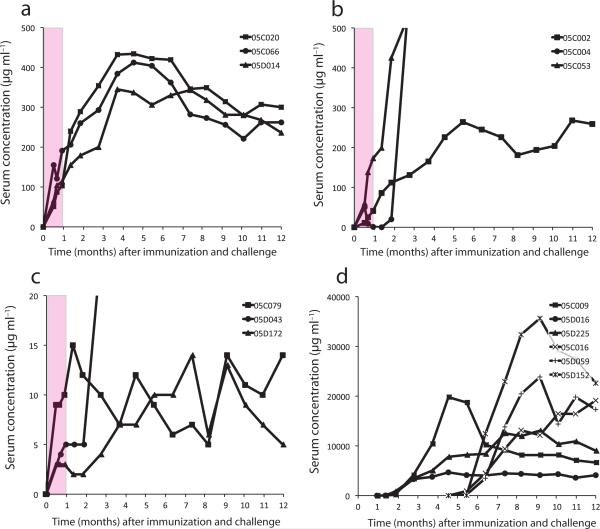
Nine rhesus macaques were immunized with AAV vectors carrying immunoadhesin genes: three received 4L6, three received 5L7, and three received N4. Each vector was administered at time 0 by intramuscular injection (2 × 1013 vector genomes), and serum from each animal was periodically collected and tested for protein level and biologic activity. Levels in the 4L6 vector recipients reached 100 − 190 μg ml−1 by four weeks after immunization, which was the time of challenge (Fig. 3a). These concentrations corresponded well with measured serum neutralization titers on the day of challenge ranging from 1:2,560 to 1:5,120 (Figure 2b).
Figure 3.
Serum concentration of immunoadhesins or antibodies. Sera from unimmunized and immunized animals were tested over time by a SIV gp120 ELISA for immunoadhesins (or antibodies in infected animals) after immunization and challenge infection (see Methods). The pink shaded areas in panels (a) – (c) represent the time period after immunization and before challenge. Day of challenge was one month after immunization. (a) 4L6 immunized animals. (b) 5L7 immunized animals. Levels of reactivity to gp120 for monkeys 05C004 and 05C053 were off scale (due to SIV infection), and peaked at 5,023 and 1,191 μg ml−1, respectively. (c) N4 immunized animals. Note scale of the y-axis. The level of reactivity to gp120 for monkey 05D043 was off scale (due to SIV infection), and peaked at 3,953 μg ml−1. (d) Unimmunized controls were infected at 2 different times (at one month and 4.5 months). Note scale of the y-axis.
Two 5L7-immunized monkeys (05C002 and 05C053) were similar to the 4L6-immunized monkeys. Serum levels reached 40 and 175 μg ml−1, respectively, by the day of challenge (Fig. 3a), which corresponded to measured serum neutralization titers of 1:1,280 and 1:20,480 (Table 1). The third 5L7 recipient (05C004) was different. The 5L7 immunoadhesin was present at two weeks after immunization (50 μg ml−1), but returned to baseline by four weeks (Fig. 3b). This rise and fall was reflected in serum neutralization titers that were 1:3,840 at two weeks and <1:32 at four weeks (Table 1). This decline reflected the appearance of anti-5L7 antibodies in this animal (see below).
Table 1.
Serum neutralizing activity against SVmac316 before and after immunization and challenge.
| |
|
Time (weeks) after immunization |
||||
|---|---|---|---|---|---|---|
| Vaccine | Animal | 0 | 2 | 4 (doc) | 6 | 12 |
| 05C009 | < 32 | < 32 | < 32 | < 32 | 20480 | |
| None | 05D016 | < 32 | < 32 | < 32 | < 32 | 20480 |
| |
05D225 |
< 32 |
< 32 |
< 32 |
< 32 |
81920 |
| 05C020 | < 32 | 640 | 2560 | 2560 | 5120 | |
| 4L6 | 05C066 | < 32 | 5120 | 5120 | 2560 | 5120 |
| |
05D014 |
< 32 |
640 |
2560 |
2560 |
5120 |
| 05C002 | < 32 | 480 | 1280 | 2560 | 5120 | |
| 5L7 | 05C004 | < 32 | 3840 | < 32 | < 32 | 40960 |
| |
05C053 |
< 32 |
1920 |
20480 |
30720 |
40960 |
| 05C079 | < 32 | 128 | 128 | 256 | 128 | |
| N4 | 05D043 | < 32 | 64 | 128 | 64 | 81920 |
| 05D172 | < 32 | 64 | 64 | 64 | 64 | |
Titers shown are reciprocal serum dilutions representing 50% neutralization. The day of challenge (doc) was at week 4.
The three animals immunized with N4 had 20 − 30 fold lower levels of immunoadhesin in serum on the day of challenge (3 − 10 μg ml−1; Fig. 3c) when compared to 4L6-immunized monkeys. Serum neutralizing titers were likewise lower in these animals, ranging from 1:64 to 1:128 (Table 1). The lower levels are likely due to the fact that N4 was a single-stranded DNA vector that resulted is less efficient vector transduction26.
SIV challenge
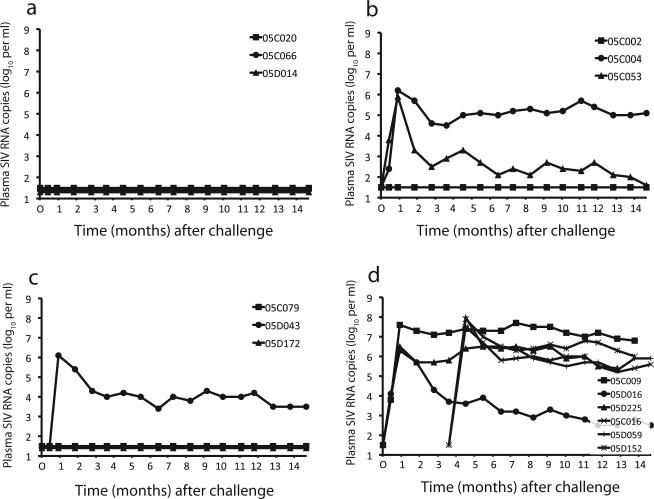
Four weeks after immunization, all nine animals were given an intravenous injection of SIVmac316. In addition, three unimmunized control animals were injected at that time with the same dose of the same challenge stock. Sixteen weeks later, three more unimmunized control animals were inoculated with the same dose of the same challenge stock (for a total of six naïve control animals). Viral loads after challenge are shown in Fig. 4.
Figure 4.
SIV viral loads after challenge infection. Plasma samples from unimmunized and immunized animals were tested after challenge infection for SIV RNA genomes (viral load). Day of challenge was 0 months (and 3.5 months for the second set of unimmunized control animals). (a) 4L6 immunized animals. (b) 5L7 immunized animals. (c) N4 immunized animals. (d) Unimmunized controls. Monkeys C009, D225, D059, and D152 from the unimmunized control group were euthanized between 57 and 60 weeks after infection due to AIDS-related complications.
The challenge virus infected all six unimmunized control animals, with peak plasma viral loads ranging between 106.5 − 108 RNA copies ml−1 (Fig. 4d). Only a single control animal (05D016) suppressed viral replication below 105 copies ml−1 in the post-acute phase of infection. In fact, four of the six macaques were euthanized between 57 and 60 weeks after infection due to AIDS-related complications. With death as an endpoint, the immunized monkeys were significantly protected (zero of nine died) relative to the unimmunized controls (four of six died; p = 0.01).
In contrast to the control monkeys, six of the nine immunized monkeys remained uninfected after challenge as judged by a lack of SIV RNA in plasma (Fig. 4a–c) and lack of seroconverison to Gag (see Supplementary Figure). All three of the 4L6 animals were protected from infection (compared to unimmunized controls: p = 0.01), while two of three were protected in the N4 group (p = 0.08), and one of three in the 5L7 group (p = 0.33).
Humoral responses after SIV challenge
As expected, all six naïve control monkeys had SIV-specific antibody responses after infection. All seroconverted to gp120 (Fig. 3d) and Gag (see Supplementary Figure). There was also a concomitant rise in serum neutralizing activity. By eight weeks after challenge, neutralization titers in three naïve controls ranged from 1:20,480 to 1:81,920 (Table 1). The three control animals (05C016, 05D059, and 05D152) infected at 16 weeks after the first group were not tested for pre- or post-challenge neutralizing antibodies.
Likewise, the three immunized animals that became infected after challenge (05C053, 05C004, and 05D043) also seroconverted to gp120 and Gag (see Supplementary Figure). Shortly after challenge, each had sizable increases in anti-gp120 ELISA levels that were clearly distinguishable from levels generated by AAV vector mediated gene transfer (Fig. 3b,c). These higher levels also correlated with increases in serum neutralization titers. By eight weeks after challenge, titers in these monkeys ranged from 1:40,960 to 1:81,920 (Table 1).
The six immunized animals that remained uninfected after challenge had a distinctly different profile. All three monkeys that received 4L6 were protected from challenge infection and had serum neutralizing activity on the day of challenge that was maintained through eight weeks after challenge (Table 1). Serum 4L6 levels gradually rose to a peak at about four months after immunization and ultimately plataued between eight and 12 months at between 200 and 300 μg ml−1 (Fig. 3a). 5L7 recipient 05C002 was also protected from infection and had a pattern of immunoadhesin levels (Fig. 3b) and neutralizing activity similar (Table 1) to the 4L6 monkeys. This general pattern of expression was entirely consistent with the biology of AAV vectors and has been demonstrated for other transgene products in mice, dogs, and monkeys28-30. Moreover, unlike antibodies that arise in response to SIV infection, but like the authentic immunoadhesins, the gp120-binding and neutralizing activity present in the sera of these monkeys at nine months after immunization was completely sensitive to heat treatment at for 30 minutes (data not shown)31.
Two N4 monkeys were also protected (05C079 and 05D172), even though serum neutralization titers on the day of challenge were 20 − 40 fold lower than in the other four protected monkeys (Table 1). As with the other protected monkeys, serum levels of N4 were maintained through 12 months, albeit at much lower levels (5 − 15 μg ml−1).
Correlates of protection
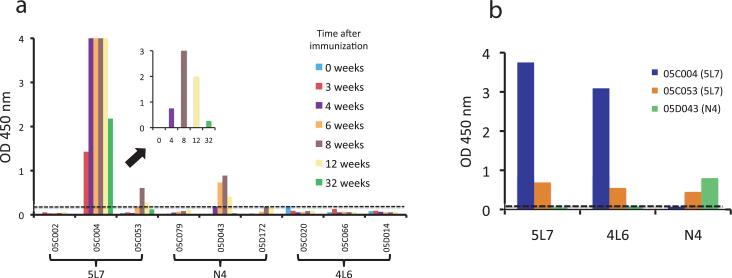
Variations in protection among immunized animals allowed us the opportunity to search for potential correlates of protection. A clue came from animal 05C004, who had an early peak and then a rapid decline in serum 5L7, such that by the day of challenge, neutralizing activity in serum was below the threshold of detection (Table 1). This scenario was similar to expression patterns observed in mice with anti-transgene antibody responses. To explore this possibility, we used purified 5L7 protein as the solid phase antigen and tested 05C004 serum in a standard ELISA (Fig. 5a). The reactivity pattern showed a high-titered anti-5L7 antibody response that was detected by three weeks after immunization, peaked at eight weeks, and declined thereafter (Fig. 5a, inset). These data were consistent with the peak and rapid decline of the 5L7 immunoadhesin in the serum of animal 05C004, and also explained why the animal became infected.
Figure 5.
Anti-immunoadhesin antibodies in immunized monkeys. (a) Serum (1:100 dilution) collected over time from each immunized animal was tested by ELISA for reactivity against the homologous transgene. For example, sera from 5L7 recipients 05C002, 05C004, and 05C053 were reacted against purified 5L7 protein (as indicated on the x-axis). The dashed line indicates the limit of sensitivity. Animals with positive homologous reactivity were 5L7 recipients 05C004 and 05C053, and N4 recipient 05D043. The inset shows sera from animal 05C004 at a 1:1000 dilution, demonstrating a peak of activity at eight weeks, and a significant waning of the response by week 32. (b) Sera from the three animals with homologous reactivity were tested against all three purified proteins (as indicated on the x-axis). As expected, each animal serum reacted with the homologous transgene. 05C004 reacted against 5L7 (homologous) and 4L6 (heterologous). 05C053 reacted against 5L7 (homologous), 4L6, and N4 (both heterologous). 05D043 reacted only with the homologous N4 protein. The dashed line indicates the limit of sensitivity.
We next tested serum from the remainder of the immunized animals in the same fashion (Fig. 5a). It was readily apparent that the other two immunized monkeys that became infected (05C053 and 05D043) also had anti-immunoadhesin antibody responses, albeit at much lower levels than 05C004. None of the protected monkeys showed anti-immunoadhesin responses. In contrast to 05C004, both 05C053 and 05D043 possessed in vitro serum neutralizing activity on the day of challenge. In fact, 05C053 had the highest serum neutralization titer (1:20,480) on the day of challenge (Table 1).
These data prompted us to examine the specificity of the anti-immunoadhesin responses. As shown in Fig. 5b, antibodies from each animal had a distinct specificity. Serum from 05C004 (5L7 recipient) reacted with purified 5L7 and 4L6, but not N4, suggesting that these antibodies were raised against the framework sequence in the scFv domain of 5L7 and 4L6. Serum from 05C053 (5L7 recipient) reacted with all three immunoadhesins, suggesting that these antibodies were raised against the Fc fragment that each chimeric protein had in common. Finally, serum from 05D043 (N4 recipient) reacted only with N4, demonstrating specificity for the CD4 moiety that was unique to N4.
DISCUSSION
In the experiments described herein, the adaptive immune system was effectively bypassed by the use of gene transfer technology to generate long-lived, protective anti-SIV biologic activity in the sera of otherwise naïve monkeys. Six of nine immunized monkeys were protected against infection by the SIV challenge, and all nine were protected from AIDS, whereas all six of the controls became infected and two-thirds (four of six) died over the course of the experiment.
Much of the success in these experiments can be attributed to AAV gene transfer technology. AAV vectors have an established record of high efficiency gene transfer in a variety of model systems 32,33. When delivered to post-mitotic organs like muscle, brain, or liver, AAV vector genomes take on the form of intranuclear high molecular weight episomal concatamers that direct transgene expression for extended periods of time 34-39. Our previous work demonstrated the feasibility of this approach in mice for in vivo HIV neutralization 20, and other investigators have adapted AAV (and other vector systems) for antibody gene delivery for a variety of purposes 40-50. In this experiment, we showed that in monkeys, a single intramuscular injection of an AAV vector could direct long-term (> 1 year) continuous expression of a biologically active protein. In addition, serum immunoadhesin levels achieved using the self-complementary (double-stranded DNA) AAV vectors (4L6 and 5L7) were far superior to those observed with the traditional single-stranded DNA vector (N4).
Three important caveats emerged from our data. First, transgene immunogenicity appeared to be an important correlate of protection that must be better understood. We tested three different immunoadhesins and observed anti-immunoadhesin responses that ran the gamut from undetectable (4L6) to almost completely incapacitating (5L7). It is tempting to speculate, but remains unproven, whether immunoadhesins expressed by in vivo transduction might behave like exogenously administered recombinant DNA-derived proteins (e.g., monoclonal antibodies or chimeric molecules like etanercept) whose safety profiles can be readily established. Moreover, it should be possible to reduce the potential for immunogenicity by specific residue modifications 51,52. Notably, none of the three animals that displayed anti-transgene antibody responses have shown clinical signs or symptoms of disease.
A second caveat was that traditional in vitro neutralization assays did not appear to faithfully represent in vivo activity. While animals without measurable neutralizing activity on the day of challenge were all infected, the opposite was not true; the presence of in vitro neutralizing activity, even at high titer, did not guarantee protection. Animal 05C053 exhibited an anti-immunoadhesin antibody response that was directed at the Fc domain of the chimeric protein that did not inhibit in vitro neutralizing activity. In fact, this animal had the highest neutralizing titer of all the immunized animals on the day of challenge (Table 1). These data suggested that immunoglobulin effector functions not measured in standard in vitro assays (like Fc activity) might be very important for in vivo neutralizing activity 53. Interestingly, our data appear to confirm the recent work of Hessell et al., where the Fc effector function of a neutralizing monoclonal antibody was inactivated by mutation and the in vivo protective effect of the antibody was blunted54. These observations suggest that optimizing antibody functions, over and above antigen binding, might be of benefit in future iterations of antiviral transgenes.
A final caveat was that we used the intravenous route for the SIV challenge. Since most new HIV infections occur across a mucosal surface, it will be important to show protection against a mucosal challenge. However, there is cause for optimism. Several studies have shown that traditional systemic passive immunization with antibody preparations can protect against an SIV or SHIV mucosal challenge 16-18. Moreover, there is ample evidence to suggest that systemic immunization with a non-replicating immunogen can protect from natural infection at a mucosal surface. A recent example is the vaccine against human papillomavirus, which is a non-replicating virus-like particle that when given intramuscularly elicits antibodies that protect against infection of the female genital tract 55. Importantly, the animals immunized by gene transfer maintained high levels of circulating antibodies that likely permeated most body tissues and mucosal surfaces.
Although significant hurdles remain, the HIV immunization approach outlined here appears to be a viable alternative to more traditional strategies. To ultimately succeed, more and better neutralizing monoclonal antibodies against primary HIV isolates that can be tested in gene transfer experiments will be needed. Also, more non-antibody inhibitors like CD4 and its derivatives should be developed in anticipation of combinatorial approaches that target multiple steps along the HIV entry pathway. And finally, as we have shown here, the SIV-monkey model can be used to investigate a variety of variations on this theme.
METHODS
Anti-SIV immunoadhesin gene constructs
DNA was synthesized (Geneart) using optimized codons. SIV Fab 21 346−16h was used to derive 4L6 and 8S, and Fab 346−16h was used to derive 5L7 and 3V. Variable domains were joined by a 15 amino acid glycineserine (G4S)3 linker, and a synthetic signal peptide for optimized secretion 56 was placed at the 5” end. The Fc fragment was rhesus IgG2 cloned from lymphocyte RNA. Each construct was placed between a CMV promoter (similar to Ostedgaard et al. 57) and a synthetic polyadenylation signal 58. N4 contained the rhesus CD4 signal sequence followed by the D1/D2 domains of rhesus CD4.
Recombinant proteins
HeLa cells were transfected (Superfect, Qiagen) with plasmid and proteins were purified from the medium using protein-A (Nunc International). Purified proteins were quantified by ELISA using purified rhesus IgG as a standard (Bethyl Laboratories).
AAV vectors
All vectors were produced and purified as described 25,27,59. Titers ranged between 2 − 10 ×1012 vg ml−1.
Monkeys and immunization
Rhesus macaques of Indian origin were purchased from Covance (Alice, TX) and housed in the vivarium at the The Research Institute at Nationwide Children's Hospital in accordance with standards set forth by the American Association for Accreditation of Laboratory Animal Care. All animals were negative for antibodies to SIV, simian type D retrovirus, and simian T-cell lymphotropic virus type 1. Weights at the time of immunization ranged from 2.7 to 4.2 kg. Each immunized animal received 2 × 1013 vg divided into 4 equal portions (0.75 ml each), delivered by 4 separate (2 in each quadriceps) deep intramuscular injections.
SIV neutralization assay
Purified proteins or macaque sera were tested for in vitro neutralization activity using the SEAP assay described by Means et al22.
Immunoadhesin concentrations
4L6, 5L7, and N4 levels in serum were measured by using SIV gp120 to coat ELISA plates (100 ng per well) in PBS buffer at 4°C overnight. Antigen was decanted and wells blocked with 3% BSA in PBST (PBS + 0.1% Tween-20) for 2 h with constant shaking at 25° C. After washing with PBST, wells were incubated with 100 μl of diluted serum (or 4L6, 5L7, or N4 standards) for 1 h at 25° C. After washing, goat anti-human IgG-Fc HRP conjugated secondary antibody (1:1000) was added to each well (100 μl) (Bethyl Laboratories) for 30 min at 25° C. After four more washes, TMB substrate (100 μl) was added (Pierce) for 10 min. at 25° C. After he reaction was terminated with 1N H2SO4, plates were read at 450 nm on a Molecular Devices microplate spectrophotometer. Quantification was achieved by extrapolation from the standard curve using software from Molecular Devices (coefficient of linearity, ≥ 0.99). Assay sensitivity was 0.4 ng ml−1 for 5L7 and 4L6, and 4 ng ml−1 for N4.
Anti-transgene antibody responses
Plates were coated with purified N4, 5L7, or 4L6 purified proteins in PBS buffer (100 ng per well) overnight at 4°C and processed as described above. Mouse anti-human IgG1 (1:500 dilution; Sigma-Aldrich) in blocking buffer was added to the wells and incubated at 25° C for 30 min. An IgG1 isotype specific secondary antibody was chosen to avoid cross-reactivity with the IgG2 immunoadhesins. After washing, wells were incubated with 100 μl (1:1000 dilution) of an anti-mouse IgG-Fc HRP conjugate (Sigma-Aldrich) for 30 min. TMB substrate development was performed as above. To detect cross-reactivity in specific monkey serum samples, alternative coating antigens were processed in an identical manner as that done for the cognate coating antigen.
SIV challenge
Infectious SIVmac316 was generated by transfecting full-length proviral DNA into 293T cells using the calcium phosphate method (Promega). Virus concentrations in supernatant were quantified by determining the concentration of p27 capsid protein using an antigen capture assay according to the manufacturer's instructions (Advanced Bioscience Laboratories, Inc.). The stock used in this study contained 357 ng ml−1 of p27. Virus stock was diluted 1:2500 in sterile PBS, and 1 ml was injected intravenously. One ml of the diluted stock was estimated to contain approximately 40 macaque infectious doses by comparison of its infectivity in cell culture to that of other SIV stocks. Viral loads in plasma were determined using a quantitative real-time reverse transcriptase PCR assay as previously described60.
Statistical analyses
Because of small sample sizes, Fisher's Exact Probability Test (two-tailed) was used to examine the difference between groups.
Supplementary Material
ACKNOWLEDGEMENTS
We thank A. Hessell and D. Burton (The Scripps Research Institute, La Jolla, CA) for providing the SIV Fab molecular clones, D. McCarty (The Research Institute at Nationwide Children's Hospital, Columbus, OH) for the self-complementary AAV vector genome, R. Doms (University of Pennsylvania, Philadelphia, PA) for purified SIVmac gp120, J. Bixby and E. Mackenizie for technical assistance, and M. Piatek and J. Lifson for SIV viral load data. The authors also thank J. Hoxie and S. Douglas for helpful comments on the manuscript. Funding for this work was provided by grants from NIH/NIAID/DAIDS (P.R.J. and R.C.D.), NIH/NCRR (R.C.D.), and support from The Children's Hospital of Philadelphia.
Footnotes
AUTHORCONTRIBUTIONS
Project planning was performed by P.R.J., B.C.S., and K.R.C.; experimental work by B.C.S., J.Z., M.J.C., S.M.G. and E.Y.; data analysis by P.R.J., K.R.C., R.C.D., B.C.S., J.Z., M.J.C., S.M.G., and; and manuscript composition by P.R.J., R.C.D., B.C.S., and K.R.C.
REFERENCES
- 1.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn NM, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 3.McElrath MJ, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitisuttithum P, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 5.Desrosiers R. Prospects for an AIDS vaccine. Nat Med. 2004;10:221–223. doi: 10.1038/nm0304-221. [DOI] [PubMed] [Google Scholar]
- 6.Fauci AS, et al. HIV vaccine research: the way forward. Science. 2008;321:530–532. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 7.Morgan C, et al. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 2008;5:e173. doi: 10.1371/journal.pmed.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker B, Burton D. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 9.Watkins DI. Basic HIV Vaccine Development. Top HIV Med. 2008;16:7–8. [PubMed] [Google Scholar]
- 10.Binley JM, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton DR, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 13.Muster T, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwick MB, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 17.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 18.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanhadji K, et al. Gene transfer of anti-gp41 antibody and CD4 immunoadhesin strongly reduces the HIV-1 load in humanized severe combined immunodeficient mice. AIDS. 2000;22:2813–2822. doi: 10.1097/00002030-200012220-00002. [DOI] [PubMed] [Google Scholar]
- 20.Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 2002;76:8769–8775. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson WE, et al. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J Virol. 2003;77:9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Means RE, et al. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol. 2001;75:3903–3915. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori K, Ringler DJ, Kodama T, Desrosiers R. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allaway GP, Ryder AM, Beaudry GA, Maddon PJ. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res Hum Retroviruses. 1993;9:581–587. doi: 10.1089/aid.1993.9.581. [DOI] [PubMed] [Google Scholar]
- 25.McCarty DM, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 26.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 27.Rabinowitz JE, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog RW, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 29.Davidoff AM, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Fang J, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 31.Honegger A. Engineering antibodies for stability and efficient folding. Handb Exp Pharmacol. 2008:47–68. doi: 10.1007/978-3-540-73259-4_3. [DOI] [PubMed] [Google Scholar]
- 32.Coura Rdos S, Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chenuaud P, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Penaud-Budloo M, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera VM, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 37.Toromanoff A, et al. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- 38.Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003;77:3495–3504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnepp BC, Jensen RL, Clark KR, Johnson PR. Infectious molecular clones of adeno-associated virus isolated directly from human tissues. J Virol. 2009;83:1456–1464. doi: 10.1128/JVI.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang J, et al. An antibody delivery system for regulated expression of therapeutic levels of monoclonal antibodies in vivo. Molecular Therapy. 2007;15:1153–1159. doi: 10.1038/sj.mt.6300142. [DOI] [PubMed] [Google Scholar]
- 41.Frade R, Rousselet N, Jean D. Intratumoral gene delivery of anti-cathepsin L single-chain variable fragment by lentiviral vector inhibits tumor progression induced by human melanoma cells. Cancer Gene Ther. 2008;15:591–604. doi: 10.1038/cgt.2008.51. [DOI] [PubMed] [Google Scholar]
- 42.He J, et al. Construction and delivery of gene therapy vector containing soluble TNFalpha receptor-IgGFc fusion gene for the treatment of allergic rhinitis. Cytokine. 2006;36:296–304. doi: 10.1016/j.cyto.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Jiang M, et al. Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin Cancer Res. 2006;12:6179–6185. doi: 10.1158/1078-0432.CCR-06-0746. [DOI] [PubMed] [Google Scholar]
- 44.Kasuya K, et al. Passive immunotherapy for anthrax toxin mediated by an adenovirus expressing an anti-protective antigen single-chain antibody. Mol Ther. 2005;11:237–244. doi: 10.1016/j.ymthe.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Pereboev A, et al. Genetically delivered antibody protects against West Nile virus. Antiviral Res. 2008;77:6–13. doi: 10.1016/j.antiviral.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandalon Z, et al. Secretion of a TNFR:Fc fusion protein following pulmonary administration of pseudotyped adeno-associated virus vectors. J Virol. 2004;78:12355–12365. doi: 10.1128/JVI.78.22.12355-12365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skaricic D, et al. Genetic delivery of an anti-RSV antibody to protect against pulmonary infection with RSV. Virology. 2008 doi: 10.1016/j.virol.2008.04.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Vigna E, et al. “Active” cancer immunotherapy by anti-Met antibody gene transfer. Cancer Res. 2008;68:9176–9183. doi: 10.1158/0008-5472.CAN-08-1688. [DOI] [PubMed] [Google Scholar]
- 49.Yuvaraj S, et al. Human scFv SIgA expressed on Lactococcus lactis as a vector for the treatment of mucosal disease. Mol Nutr Food Res. 2008;52:913–920. doi: 10.1002/mnfr.200700132. [DOI] [PubMed] [Google Scholar]
- 50.Zuber C, et al. Delivery of single-chain antibodies (scFvs) directed against the 37/67 kDa laminin receptor into mice via recombinant adeno-associated viral vectors for prion disease gene therapy. J Gen Virol. 2008;89:2055–2061. doi: 10.1099/vir.0.83670-0. [DOI] [PubMed] [Google Scholar]
- 51.Jones TD, et al. The development of a modified human IFN-alpha2b linked to the Fc portion of human IgG1 as a novel potential therapeutic for the treatment of hepatitis C virus infection. J Interferon Cytokine Res. 2004;24:560–572. doi: 10.1089/jir.2004.24.560. [DOI] [PubMed] [Google Scholar]
- 52.Swann PG, et al. Considerations for the development of therapeutic monoclonal antibodies. Curr Opin Immunol. 2008;20:493–499. doi: 10.1016/j.coi.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Cadogan M, Dalgleish AG. HIV immunopathogenesis and strategies for intervention. Lancet Infect Dis. 2008;8:675–684. doi: 10.1016/S1473-3099(08)70205-6. [DOI] [PubMed] [Google Scholar]
- 54.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 55.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26(Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barash S, Wang W, Shi Y. Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem Biophys Res Commun. 2002;294:835–842. doi: 10.1016/S0006-291X(02)00566-1. [DOI] [PubMed] [Google Scholar]
- 57.Ostedgaard LS, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A. 2005;102:2952–2957. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levitt N, Briggs D, Gil A, Proudfoot NJ. Definition of an efficient synthetic poly(A) site. Genes Dev. 1989;3:1019–1025. doi: 10.1101/gad.3.7.1019. [DOI] [PubMed] [Google Scholar]
- 59.Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 60.Lifson JD, et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75:10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.