Abstract
Background
Long term alcohol ingestion may produce severe oxidant stress and lead to skeletal muscle dysfunction. Emerging evidence has suggested that members of the interleukin-6 (IL-6) family of cytokines play diverse roles in the regulation of skeletal muscle mass. Thus, our goals were (1) to minimize the degree of oxidant stress and attenuate atrophy by supplementing the diets of alcohol-fed rats with the glutathione precursor, procysteine, and (2) to identify the roles of IL-6 family members in alcoholic myopathy.
Methods
Age- and gender-matched Sprague-Dawley rats were fed the Lieber-DeCarli liquid diet containing either alcohol or an isocaloric substitution (control diet) for 35 wk. Subgroups of alcohol-fed rats received procysteine (0.35%, w/v) for the final 12 wk. Plantaris morphology was assessed by hematoxylin and eosin staining. Major components of glutathione metabolism were determined by assay kits. Real time PCR was used to determine expression levels of several genes.
Results
Plantaris muscles from alcohol-fed rats displayed extensive atrophy, as well as decreased glutathione levels, decreased activities of glutathione reductase and glutathione peroxidase, decreased superoxide dismutase (SOD)-2 (Mn-SOD2), and increased NADPH oxidase-1 gene expression - each indicative of significant oxidant stress. Alcohol also induced gene expression of catabolic factors including IL-6, oncostatin M, atrogin-1, muscle ring finger protein-1, and IGFBP-1. Procysteine treatment attenuated plantaris atrophy, restored glutathione levels, and increased catalase, Cu/Zn-SOD1, and Mn-SOD2 mRNA expression, but did not reduce other markers of oxidant stress or levels of these catabolic factors. Instead, procysteine stimulated gene expression of anabolic factors such as insulin-like growth factor-1, ciliary neurotrophic factor and cardiotrophin-1.
Conclusions
Procysteine significantly attenuated, but did not completely abrogate, alcohol-induced oxidant stress or catabolic factors. Rather, procysteine minimized the extent of plantaris atrophy by inducing components of several anabolic pathways. Therefore, anti-oxidant treatments such as procysteine supplementation may benefit individuals with alcoholic myopathy.
Keywords: alcoholic myopathy, cardiotrophin-1, ciliary neurotrophic factor, interleukin-6, procysteine
introduction
Long term alcohol ingestion may lead to progressive proximal weakness, muscle soreness and atrophy, altered gait, and impaired mobility (Kiessling et al., 1975). These derangements to skeletal muscle, collectively classified as alcoholic myopathy, are likely caused by severe biochemical, physiological, and structural changes (Preedy et al., 2003), with specific insults documented in muscles rich in type II fibers (anaerobic, white glycolytic) compared to type I fibers (aerobic, red oxidative) (Preedy and Peters, 1988, Lang et al., 2005). More specifically, these derangements due to chronic alcohol ingestion have been attributed to a multitude of factors, including apoptosis (Fernandez-Sola et al., 2003), mitochondrial dysfunction (Cederbaum and Rubin, 1975), acetaldehyde protein adduct formation (Worrall et al., 2001), oxidant stress (Adachi et al., 2000; Fernandez-Sola et al., 2002; Koo-Ng et al., 2000; Otis et al., 2007; Preedy et al., 2001), and altered regulation of catabolic and anabolic factors with resultant effects on protein metabolism (Kumar et al., 2002; Lang et al., 2004; Otis et al., 2007; Preedy et al., 1994; Ronis et al., 2007). We have recently shown that 6 weeks of chronic alcohol ingestion in rats stimulated a pro-atrophy mechanism in plantaris muscle (e.g., glutathione depletion, oxidant stress, atrogin-1 induction) that preceded the overt development of atrophy (Otis et al., 2007). Importantly, these catabolic factors were attenuated in rats that received the anti-oxidant glutathione precursor, procysteine, and strongly suggested that alcohol-induced, pro-atrophic mechanisms may be targeted prior to the clinical onset of alcoholic myopathy. However, glutathione supplementation as an effective therapeutic option to minimize alcohol-induced muscle atrophy required further exploration.
In this report, we analyzed alcohol-induced oxidant stress and the resultant effects on the mRNA levels of several catabolic and anabolic factors in plantaris muscles from rats supplemented with procysteine. Mounting evidence indicates that IL-6 family members, including IL-6, oncostatin M (OSM), leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT1), play diverse roles in the regulation of skeletal muscle mass. For example, overexpression of IL-6 in mice resulted in significant skeletal muscle catabolism (Tsujinaka et al., 1995) potentially by activating intracellular caspases (Ebisu et al., 1995). OSM treatment in cultures of skeletal muscle cells arrested growth at the G1/S checkpoint (Kim et al., 2008) suggesting a potentially significant role of this cytokine in the regulation of muscle mass. With regards to the anabolic effects of IL-6 family members, LIF has been implicated in muscle regeneration (Barnard et al., 1994) and myoblast proliferation (Austin et al., 1992) via a JAK2/STAT3-dependent signaling pathway (Spangenburg and Booth, 2002). Treating rats with rCNTF has been shown to induce soleus muscle hypertrophy (Guillet et al., 1999). Further, the cardiomyocyte anabolic factor, CT1, was increased in overloaded skeletal muscle (Nishikawa et al., 2005) potentially indicative of a similar role in the regulation of skeletal muscle hypertrophy.
Based on this strong evidence, we hypothesized that 35 weeks of chronic alcohol ingestion would induce oxidant stress, stimulate gene expression of several catabolic factors within the IL-6 family of cytokines, and result in overt plantaris atrophy. We further hypothesized that reducing alcohol-induced oxidant stress with the glutathione precursor procysteine would attenuate these catabolic factors and preserve muscle mass.
Materials and Methods
Animals and diet
Male Sprague-Dawley rats (200-250 g, n= 6-7 rats/group) were purchased from Charles River (Wilmington, Massachusetts) and housed in pairs under a 12:12 light-dark cycle. All procedures were approved by Atlanta Veteran Affairs Medical Center Institutional Animal Care and Use Committee.
Rats were fed the Lieber-DeCarli liquid diet (Research Diets, New Brunswick, New Jersey) containing either alcohol or an isocaloric substitution with Maltin-Dextrin (control diet) for 35 wk (Otis et al., 2007; Velasquez et al., 2002). This duration of alcohol abuse is clinically relevant as it represents ∼22% of the Sprague-Dawley rat lifespan, and can be extrapolated to a human ingesting in excess of 100 g/d of alcohol for more than 10 years (Preedy et al., 1999). Alcohol was added gradually to acclimatize the rats to the diet. Alcohol was added as 18% of total calories for 1 wk, then 27% of total calories for 1 wk, and then finally 36% of total calories for 33 wk, respectively. In a subgroup of alcohol-fed rats, the glutathione precursor procysteine (Sigma, St. Louis, Missouri) was added to their diets at a concentration of 0.35% (w/v) for the final 12 wk. At the end of the 35 wk, rats were anesthetized with intraperitoneal injections of sodium pentobarbital (50 mg/kg). All plantaris muscles were removed in the morning, blotted dry, weighed, and mounted in OCT for histochemical analyses or flash frozen in liquid nitrogen for other analyses as described below. Animals were sacrificed by removal of the diaphragm muscle.
Cross-sectional area measurements
Fresh plantaris muscles were removed, embedded in OCT, and immediately frozen in isopentane cooled in liquid nitrogen. Serial sections from the mid-belly of the plantaris muscle were cut at 14 μm and adhered to superfrost slides. Plantaris sections were processed for hematoxylin and eosin staining, dehydrated, mounted and visualized with a Leica microscope as previously described (Otis et al., 2007; Otis et al., 2008a). Approximately 125 fibers per muscle were analyzed and cross-sectional areas determined using ImageJ software (NIH).
Glutathione and glutathione disulfide levels
Levels of glutathione (GSH) and glutathione disulfide (GSSG) were determined using commercially available assay kits (Cayman Chemical, Ann Arbor, Michigan). Briefly, frozen plantaris tissues were thawed and homogenized in 10 volumes of buffer containing: 50 mM HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 100 mM NaF, 10% glycerol, and 1% Nonidet P-40. To avoid potential interference due to particulates and sulfhydryl groups on proteins, samples were deproteinated with equal volumes of metaphosphoric acid, centrifuged at 5000g for 5 minutes, and supernatants mixed with triethanolamine (1:20 dilution). Aliquots of deproteinated samples were derivitized with 1M 2-vinylpyridine (1:100 dilution) for exclusive measurements of GSSG. Sulfhydryl groups were reacted with 5,5′-dithio-bis-2(nitrobenzoic acid) and the provided assay cocktail to produce 5-thio-2-nitrobenzoic acid. The absorbance at 405-414 nm was measured in triplicate at 5 min intervals for 30 min on a Victor3 1420 Multilabel counter (PerkinElmer, Waltham, Massachusetts) and final concentrations of GSH and GSSG (in μM) were calculated according to the manufacturer’s instructions.
Glutathione reductase and glutathione peroxidase activity levels
Activity levels of glutathione reductase and glutathione peroxidase were determined using commercially available assay kits (Cayman Chemical, Ann Arbor, Michigan). Enzyme activity was measured as the oxidation of NADPH to NADP+ and the accompanying decrease in absorbance over time at 340 nm. Thus, glutathione reductase activity was measured directly, whereas glutathione peroxidase activity was measured indirectly via a coupled reaction with glutathione, glutathione reductase and NADPH. Briefly, frozen plantaris tissues were thawed and homogenized in 10 volumes of buffer containing: 50 mM HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 100 mM NaF, 10% glycerol, and 1% Nonidet P-40. Samples were mixed with appropriate kit components in triplicate and absorbance was read once every minute at 340 nm for 5 minutes using a Victor3 1420 Multilabel counter (PerkinElmer, Waltham, Massachusetts). Enzyme activity was calculated using the rate of decreased absorbance according to the manufacturer’s instructions. Protein content in each sample was determined using the Bradford assay (Bradford, 1976). Specific enzyme activity has been reported as the absolute value of pmol/min/ml/μg.
Real time PCR
Plantaris muscles were collected, immediately frozen in liquid nitrogen and stored at -80°C until processed for real time PCR analyses as previously described (Otis et al., 2007; Otis et al., 2008a,b). Trizol was added to the tissues (1 ml/100 mg tissue) that were then homogenized using an electric tissue homogenizer. Total RNA (2.5 μg) was reverse transcribed in a 40 μl final reaction volume using random primers and M-MLV reverse transcriptase (Invitrogen, Carlsbad, California). The reverse transcription reaction was incubated at 65°C for 10 min, 80°C for 3 min, and 42°C for 60 min, respectively. Real time PCR products were analyzed using the iCycler iQ system (Bio-Rad, Hercules, California). cDNA (5 μl of a 1:10 dilution) was amplified in a 25 μl reaction containing 400 nm gene-specific primer pair and iQ Sybr Green Supermix (Bio-Rad, Hercules, California). Primer sequences were designed using Primer3, ordered from Sigma-Genosys (The Woodlands, Texas), and are listed in Table 1. Samples were incubated at 95°C for 15 min, followed by 40 cycles of denaturation, annealing and extension at 95°C, 60°C and 72°C, respectively, with fluorescence recorded at the end of each annealing and extension step. As a control, real time PCR was also performed on 2 μl of each RNA sample to confirm absence of contaminating genomic DNA. All reactions were performed in triplicate and the starting quantities of the genes of interest were normalized to 18S rRNA (primers supplied by Ambion, Austin, Texas). The 2-ΔΔCT method was used to analyze alterations in gene expression and values were expressed as fold changes relative to control (Otis et al., 2007; Otis et al., 2008a, b).
Table 1.
Real-time RT-PCR primer sequences
| Gene | Accession No. | Primer Sequences (5′→3′) |
|---|---|---|
| Atrogin1 | NM_133521 | F: TCCAGACCCTCTACACATCCTT R: CCTCTGCATGATGTTCAGTTGT |
| Catalase | NM_012520 | F: GGACCAGTACAACTCCCAGAAG R: ACTCCATCCAGCGATGATTACT |
| CT1 | NM_017129 | F: CTGGCCTCGAACTCATAGAAAT R: GATTTGCCTACAAAGGAACTGG |
| CNTF | NM_013166 | F: CCTTGACTCAGTGGATGGTGTA R: AGGCAGAAACTTGGAGCATAAG |
| IGF1 | NM_178866 | F: TCTGAGGAGGCTGGAGATGT R: GTTCCGATGTTTTGCAGGTT |
| IGFBP-1 | NM_013144 | F: AAAGTTGTTTCCTCCCTCCTTC R: TACAGCACAAGGACAGGGTAGA |
| IL6 | NM_012589 | F: GGTCTTCTGGAGTTCCGTTTC R: GGTCTTGGTCCTTAGCCACTC |
| LIF | NM_022196 | F: AGTGCCAATGCCCTCTTTATT R: GGGTTGAGGTTTTTCTGATCC |
| MuRF1 | NM_080903 | F: ATCACTCAGGAGCAGGAGGA R: CTTGGCACTCAAGAGGAAGG |
| OSM | NM_001006961 | F: AACATCCAAGGGATCAGGAAC R: GAAGACTCTCCCCACTGAACC |
| NOX1 | NM_053683 | F: GTGGCTTTGGTTCTCATGGT R: TGAGGACTCCTGCAACTCCT |
| NOX4 | NM_053524 | F: GGGCCTAGGATTGTGTTTGA R: CTGAGAAGTTCAGGGCGTTC |
| Cu/Zn-SOD1 | NM_017050 | F: CTTCTGTCGTCTCCTTGCTTTT R: CCTGTAATCTGTCCTGACACCA |
| Mn-SOD2 | NM_017051 | F: TGTGGCTGAGCTGTTGTAATCT R: GATGGCCTTATGATGACAGTGA |
| Cu/Zn-SOD3 | NM_012880 | F: CAGACACCTCTGTATGGCTCAG R: GGGCATAAAGTAGTTCGAGGTG |
| TGFβ1 | NM_021578 | F: CTACTACGCCAAAGAAGTCACC R: CTGTATTCCGTCTCCTTGGTT |
Statistics
One-way analyses of variance were performed followed by Student-Newman-Keuls post-hoc tests using SigmaStat v2.0 software. Significance was accepted at p ≤ 0.05.
Results
Plantaris morphology and markers of oxidant stress
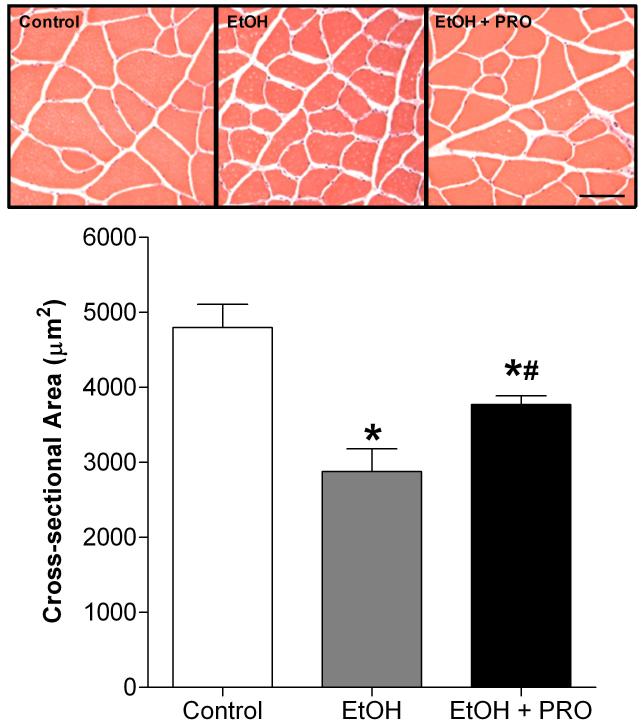
We first identified the effect of 35 wk of chronic alcohol ingestion on plantaris fiber cross-sectional area (CSA). The muscles from alcohol-fed rats displayed a significant reduction in average CSA compared to controls (Figure 1). Interestingly, plantaris muscles from alcohol-fed rats that had their diets supplemented with the glutathione precursor procysteine for 12 wk had increased fiber CSAs compared to the non-supplemented, alcohol-fed group. A panel showing representative images of plantaris sections is presented.
Figure 1. Plantaris fiber areas.
Plantaris muscles from rats chronically fed alcohol (EtOH) for 35 weeks had a 46% reduction in cross-sectional area (CSA) compared to controls. Interestingly, plantaris muscles from a subgroup of EtOH-fed rats that had their diets supplemented with the glutathione precursor procysteine (PRO) for 12 weeks displayed increased CSAs compared to unsupplemented, EtOH-fed rats. Values are expressed as means + SEM (n=6-7 rats/group). Significance was accepted at p ≤ 0.05. *, compared to control group. #, compared to EtOH group.
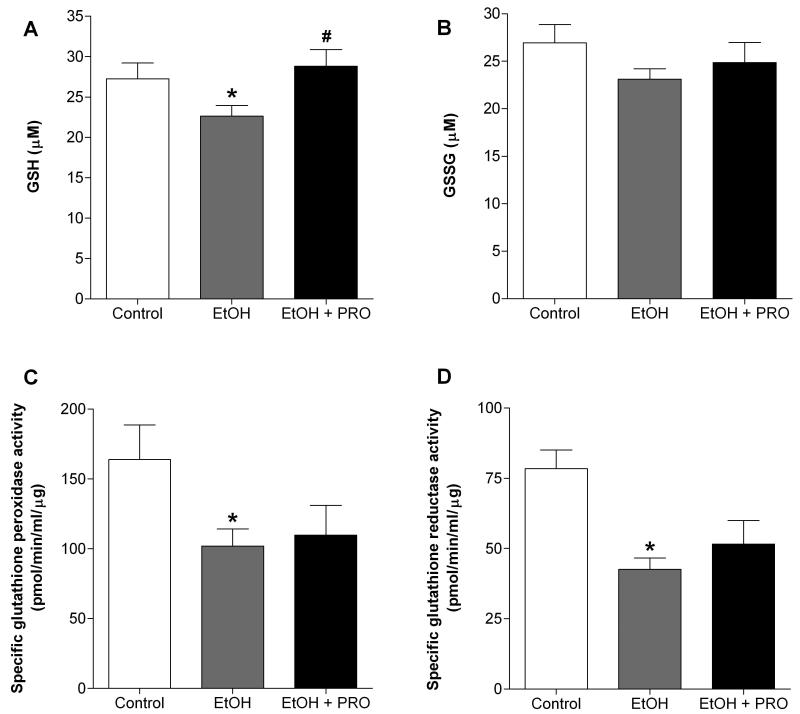
We next determined the levels of glutathione (GSH), glutathione disulfide (GSSG), and the activities of glutathione reductase and glutathione peroxidase. Similar to our previous findings in rats chronically fed alcohol for 6 wk (Otis et al., 2007), continued chronic alcohol ingestion for 35 wk significantly decreased GSH levels, but had no effect on the levels of GSSG compared to controls (Figures 2A and B, respectively). Importantly, procysteine supplementation restored GSH levels (Figure 2A). Alcohol ingestion also significantly decreased the enzyme activities of glutathione reductase and glutathione peroxidase (Figures 2C and D, respectively), an effect that was not reversed by procysteine supplementation.
Figure 2. Glutathione and glutathione disulfide levels and glutathione peroxidase and reductase activities in rat plantaris.
(A) Plantaris muscles from rats chronically fed alcohol (EtOH) for 35 weeks had reduced levels of glutathione (GSH), an effect that was prevented following 12 weeks of procysteine (PRO) supplementation. (B) Glutathione disulfide (GSSG) levels remained unchanged. Specific enzyme activities of both (C) glutathione peroxidase and (D) glutathione reductase were reduced in plantaris muscles from alcohol-fed rats. These effects were not ameliorated by procysteine supplementation. Values are expressed as means + SEM (n=6-7 rats/group). Significance was accepted at p ≤ 0.05. *, compared to control group. #, compared to EtOH group.
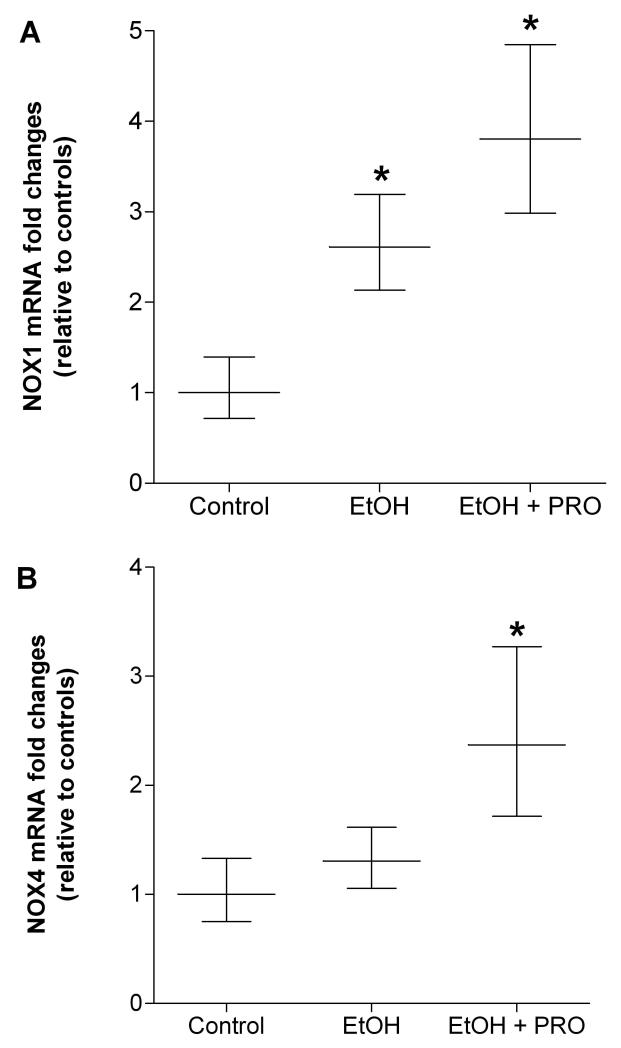
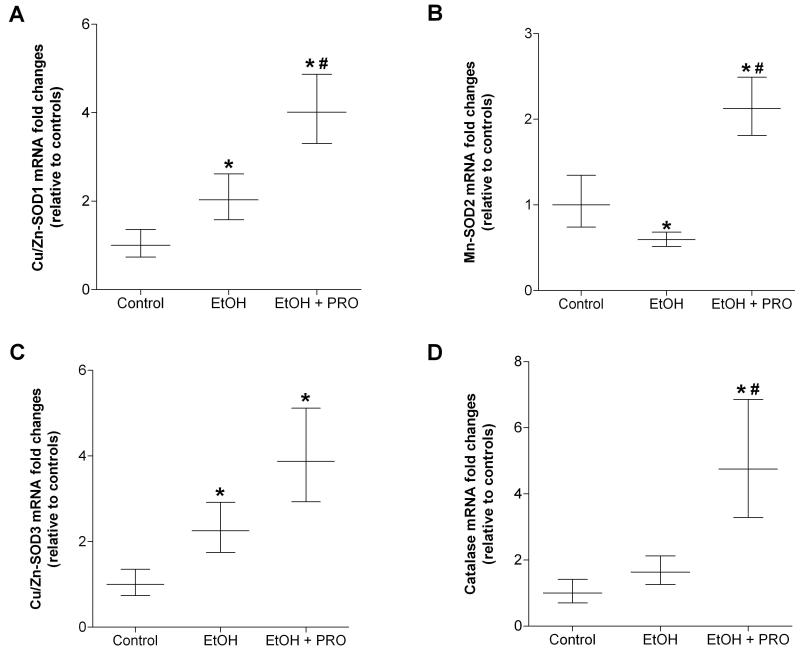
We also determined the mRNA expression levels of enzymes associated with superoxide production and scavenging. Thirty five weeks of alcohol ingestion increased the expression of the superoxide-producing NADPH (NOX)-1 subunit, but had no effect on NOX4 expression levels (Figures 3A and B, respectively). Further, procysteine supplementation for 12 wk did not attenuate alcohol-induced NOX1 mRNA expression. In parallel, plantaris muscles from alcohol-fed rats had significantly lower mRNA levels of the mitochondrial form of the superoxide scavenging enzyme, superoxide dismutase (SOD)-2 (Mn-SOD2) (Figure 4B). In contrast, mRNA levels of the cytoplasmic and extracellular forms of SOD (i.e., Cu/Zn-SOD1 and Cu/Zn-SOD3, respectively) were upregulated in plantaris muscles from alcohol-fed rats (Figures 4A and C). Procysteine increased gene expression of Cu/Zn-SOD1, Mn-SOD2, and the hydrogen peroxide scavenging enzyme, catalase (Figures 4A-B and D, respectively).
Figure 3. NADPH oxidase subunits, NOX1 and NOX4, in rat plantaris.
Real time PCR analyses of the superoxide-generating NADPH oxidase subunits, (A) NOX1 and (B) NOX4, were performed in plantaris muscles from rats chronically fed alcohol (EtOH) for 35 weeks. NOX1, but not NOX4, was increased in the EtOH group, an effect that was refractory to 12 weeks of procysteine (PRO) supplementation. Data are represented as means ± range of potential values based on the 2-ΔΔCT method (Otis et al., 2007; Otis et al., 2008a, b) and expressed as fold changes relative to controls (n=6-7 rats/group). Significance was accepted at p ≤ 0.05. *, compared to control group.
Figure 4. Superoxide dismutase isozymes and catalase in rat plantaris.
Real time PCR analyses of three superoxide dismutase isozymes (i.e., Cu/Zn-SOD1, Mn-SOD2, and Cu/Zn-SOD3) and catalase were performed in plantaris muscles from rats chronically fed alcohol (EtOH) for 35 weeks. Cu/Zn-SOD1 and Cu/Zn-SOD3 were induced in plantaris muscles from alcohol-fed rats while Mn-SOD2 mRNA was decreased (Panels A, C and B, respectively). Procysteine stimulated the production of Cu/Zn-SOD1 and Mn-SOD2 in alcohol-fed rat plantaris muscles (A and B, respectively). (D) Expression of the hydrogen peroxide scavenging enzyme, catalase, was unaffected by chronic alcohol ingestion, however, mRNA levels were induced following 12 weeks of procysteine supplementation. Data are represented as means ± range of potential values based on the 2-ΔΔCT method (Otis et al., 2007; Otis et al., 2008a, b) and expressed as fold changes relative to controls (n=6-7 rats/group). Significance was accepted at p ≤ 0.05. *, compared to control group. #, compared to EtOH group.
Gene expression of Interleukin-6 (IL-6) family members
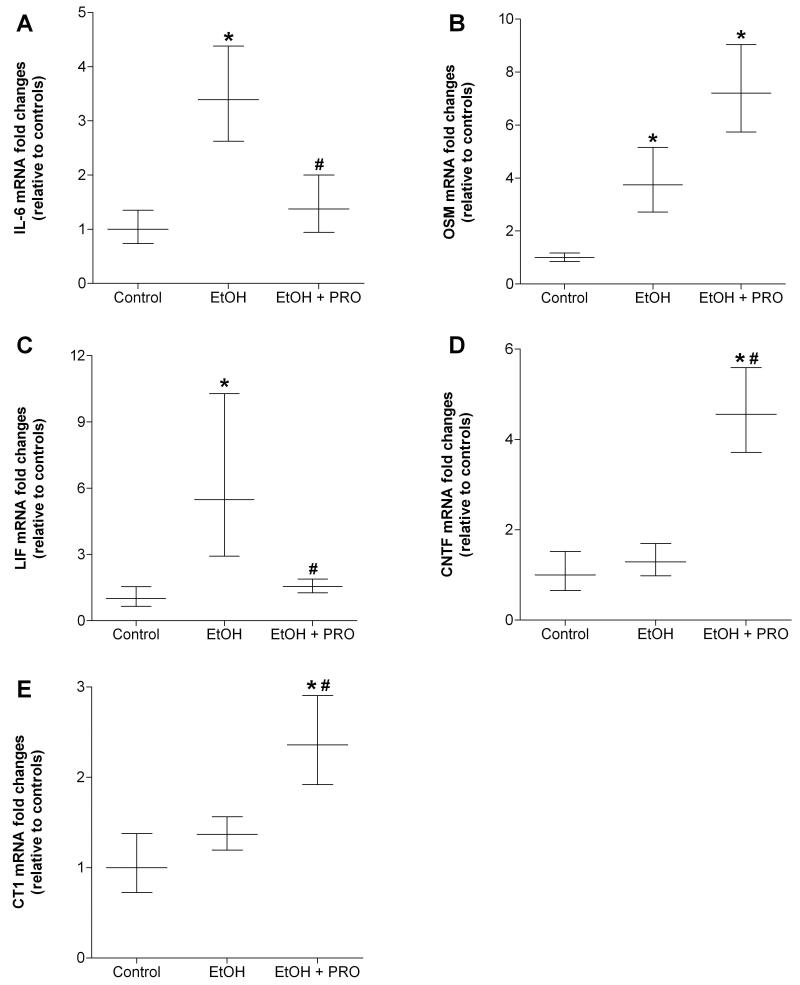
We next analyzed the effect of chronic alcohol ingestion on mRNA levels of several IL-6 family members, including IL-6, oncostatin M (OSM), leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT1). Thirty five weeks of alcohol ingestion increased IL-6, OSM, and LIF mRNA levels in plantaris muscles compared to controls (Figure 5A-C, respectively). Alcohol-induced Il-6 and LIF were sensitive to procysteine supplementation (Figures 5A and C, respectively). In contrast, alcohol had no effect on gene levels of CNTF and CT1, but these anabolic factors were stimulated by 12 wk of procysteine supplementation (Figures 5D and E, respectively).
Figure 5. Gene expressions of IL-6 family members in rat plantaris.
Real time PCR analyses were performed on several members of the IL-6 family of cytokines in plantaris muscles from rats chronically fed alcohol (EtOH) for 35 weeks. IL-6 and OSM were strongly induced in plantaris muscles from EtOH-fed rats (A and B, respectively). Procysteine (PRO) supplementation attenuated IL-6 induction in plantaris muscles from alcohol-fed rats. Gene expression of (C) ciliary neurotrophic factor (CNTF) and (D) cardiotrophin-1 (CT1) were increased in plantaris muscles from the EtOH-PRO group. Data are represented as means ± range of potential values based on the 2-ΔΔCT method (Otis et al., 2007; Otis et al., 2008a, b) and expressed as fold changes relative to controls (n=6-7 rats/group). Significance was accepted at p ≤ 0.05. *, compared to control group. #, compared to EtOH group.
Gene expressions of common catabolic and anabolic factors in skeletal muscle
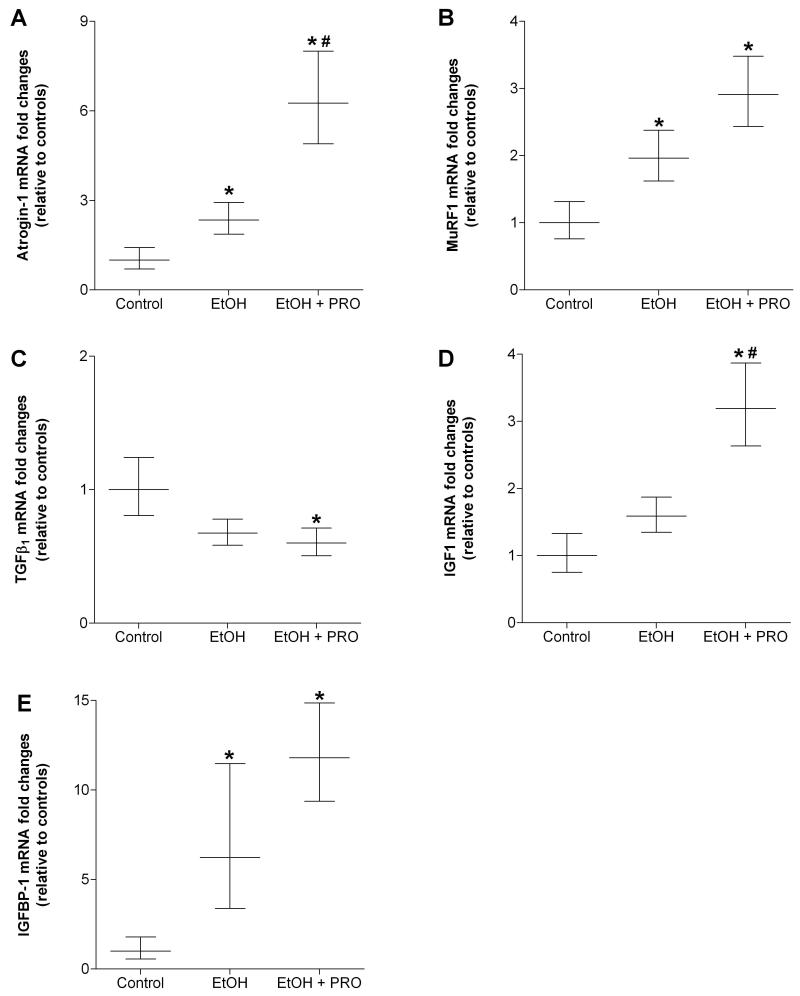
Thirty five weeks of daily alcohol ingestion induced mRNA expressions of the muscle-specific E3 ubiquitin ligases, atrogin-1 and muscle ring finger protein-1 (MuRF1) (Figures 6A and B). Procysteine supplementation did not attenuate atrogin-1 and MuRF1 expressions, but did reduce the expression of the catabolic cytokine, transforming growth factor-β1 (TGFβ1) (Figure 6C), and stimulated expression of insulin-like growth factor-1 (IGF1) in plantaris muscles from alcohol-fed rats (Figure 6D). Gene expression of IGFBP-1 was strongly induced by 35 weeks of chronic alcohol ingestion and did not respond to procysteine treatment (Figure 6E).
Figure 6. Gene expressions of common catabolic and anabolic factors in rat plantaris.
Real time PCR analyses were performed on common catabolic and anabolic factors in plantaris muscles from rats chronically fed alcohol (EtOH) for 35 weeks. Alcohol ingestion increased gene expressions of muscle-specific E3 ubiquitin ligases, (A) atrogin-1 and (B) muscle ring finger protein-1 (MuRF1), however, their expression levels were not reduced in procysteine (PRO)-treated rats. In contrast, PRO reduced the gene expression of transforming growth factor-β (TGFβ) (C). Insulin-like growth factor-1 (IGF1) was increased in plantaris muscles from alcohol-fed rats receiving PRO (D). Insulin-like growth factor binding protein-1 (IGFBP-1) was also induced by alcohol ingestion and was not attenuated by procysteine treatment (E). Data are represented as means ± range of potential values based on the 2-ΔΔCT method (Otis et al., 2007; Otis et al., 2008a, b) and expressed as fold changes relative to controls (n=6-7 rats/group). Significance was accepted at p ≤ 0.05. *, compared to control group. #, compared to EtOH group.
Discussion
In this study, we showed that long term alcohol ingestion for 35 weeks induced oxidant stress and created an overall catabolic state in skeletal muscle. Chronic alcohol ingestion depleted glutathione and potentially dampened the ability of muscle to sequester alcohol-induced superoxide radicals as evidenced by increased expression of the superoxide-producing NADPH oxidase (NOX)-1 subunit and decreased superoxide dismutase (SOD)-2 (Mn-SOD2). Further, mRNA expression of several catabolic factors, including the E3 ubiquitin ligases, atrogin-1 and muscle ring finger protein-1 (MuRF1), and two interleukin-6 family members, IL-6 and oncostatin M (OSM), were induced in atrophied plantaris muscles from alcohol-fed rats. Importantly, supplementing the diets with the glutathione precursor procysteine for 12 weeks minimized the degree of plantaris fiber atrophy even though it did not completely abrogate alcohol-mediated oxidant stress and did not decrease the alcohol-induced gene expression of these catabolic factors. In parallel, procysteine stimulated mRNA production of several anabolic factors such as insulin-like growth factor-1 (IGF1), ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT1). Together, these results suggest that procysteine supplementation lessens alcohol-induced oxidant stress and in parallel stimulates components of several anabolic pathways, thereby mitigating alcoholic myopathy. If these experimental findings prove to translate to the clinical setting, then glutathione replacement therapies with precursors such as procysteine, N-acetylcysteine, or S-adenosyl methionine may provide significant benefits to alcoholics suffering from myopathy.
The negative effects of chronic alcohol ingestion on skeletal muscle redox state have been well-documented (Adachi et al., 2000; Fernandez-Sola et al., 2002; Koo-Ng et al., 2000; Otis et al., 2007; Preedy et al., 2001). However, most alcohol studies that attempted to attenuate alcohol-induced oxidant stress with anti-oxidant or anti-oxidant cofactor supplementation have reported limited successes (Duran Castellon et al., 2005; Koll et al., 2003; Reilly et al., 2000). In contrast, we have previously shown that procysteine restored muscle glutathione levels in rats fed alcohol for 6 weeks while reducing oxidant stress and mRNA expression of two catabolic factors, atrogin-1 and TGFβ1 (Otis et al., 2007) - each occurring despite the absence of clinical alcoholic myopathy (e.g., atrophy). Based on these intriguing findings, the current study was designed to analyze the potentially beneficial effects of procysteine in atrophied plantaris muscles from rats fed alcohol for 35 weeks. Similar to our previous results and in agreement with other published reports (Fernandez-Sola et al., 2002; Wang et al., 2007); chronic alcohol ingestion depleted glutathione pools, and reduced enzyme activities of glutathione peroxidase (GPx) and glutathione reductase (GR). While procysteine normalized glutathione levels, GPx and GR activities remained reduced. One possible explanation for the inability of glutathione to restore these enzyme activity levels is that chronic alcohol ingestion also decreases levels of vital enzyme cofactors such as zinc and selenium (Duran Castellon et al., 2005; Ward and Peters, 1992). Therefore, solely restoring glutathione substrate may be insufficient to normalize GPx and GR activities. While zinc supplementation alone failed to improve alcoholic myopathy (Fernandez-Sola et al., 2002), a more robust therapeutic approach using cofactors in combination with glutathione precursors such as procysteine may normalize glutathione cycling and improve overall anti-oxidant capacity.
Chronic alcohol ingestion increased mRNA expression of NOX1 and reduced mRNA expression of the Mn-SOD2 suggesting a potential imbalance between superoxide anion production and muscle’s ability to sequester this alcohol-induced influx. In response, skeletal muscle may upregulate the expression of other SOD enzymes such as Cu/Zn-SOD1 and Cu/Zn-SOD3. In support of this notion, total superoxide dismutase activity was increased in deltoid muscles from alcoholics (Fernandez-Sola et al., 2002). Despite the insufficiency of glutathione replacement to attenuate alcohol-induced NOX4 gene expression, procysteine did upregulate the gene expression of catalase, the central enzyme that plays a complementary and synergistic role with SOD to decompose hydrogen peroxide (Kono and Fridovich, 1982). Further, because alcohol dehydrogenase activity is low in skeletal muscle, oxidation of ethanol is predominantly metabolized via catalase activity (Garcia-Bunel, 1984). Thus, our data may suggest that procysteine supplementation improved the capacity of skeletal muscle to function as an ethanol metabolizing organ. However, the levels of acetaldehyde in skeletal muscle and their downstream effects on morphology and function in our model remain to be quantified.
Members of the IL-6 family of cytokines play diverse roles in the regulation of skeletal muscle mass (Barnard et al., 1994; Tsujinaka et al., 1995; Guillet et al., 1999; Nishikawa et al., 2005; Kim et al., 2008), and thus provide an intriguing signaling system to investigate in our model of alcohol-induced atrophy. Gene expressions of catabolic family members such as IL-6 and OSM were induced by chronic alcohol ingestion; and only the former responsive to glutathione replacement as has been previously described (Pena et al., 1996). Interestingly, IL-6 has also been implicated in the early inflammatory processes of muscle regeneration (Bunn et al., 2004). Because IL-6 is induced in alcoholic muscle the cytokine may be an important target to minimize the degree of fibrosis and disrepair following muscle injury in alcoholics. Paradoxically, gene expression of LIF, an IL-6 family member implicated in skeletal muscle hypertrophy (Gregorevic et al., 2002; Spangenburg and Booth, 2002, 2006), was increased in atrophied plantaris in alcohol-fed rats. Similarly, LIF expression was also induced in chronic human disuse muscle atrophy (Reardon et al., 2001) and may reflect a proactive response of this anabolic cytokine to catabolic signals. Despite the inability of procysteine to completely attenuate gene expression of catabolic IL-6 family member, it did increase mRNA expression of CNTF and CT1, two predominantly anabolic cytokines (Guillet et al., 1999; Nishikawa et al., 2005). Together, these data suggest that the minimization of alcohol-induced plantaris atrophy in procysteine supplemented rats may be due to a surge in anabolic factors and not a reduction in catabolic factors.
To determine if alcohol-induced gene expression changes in the plantaris were limited to IL-6 family members or represented a more robust phenomenon, we also quantified the gene levels of several common catabolic and anabolic factors. For example, atrogin-1 and MuRF-1, two muscle-specific E3 ligases, have been implicated in skeletal muscle atrophy (Bodine et al., 2001; Gomes et al., 2001) and are elevated in a variety of chronic diseases states, including burn injury (Lang et al., 2007), cancer cachexia (Mastrocola et al., 2008), HIV-1 (Otis et al., 2008a; Pruznak et al., 2008), and sepsis (Wray et al., 2003). We have previously shown that atrogin-1 mRNA expression was induced by 6 weeks of chronic alcohol ingestion, a time that precedes atrophy, implying that this ligase may represent an early biological marker of muscle atrophy. Atrogin-1 expression returned to baseline levels after 28 weeks, when overt atrophy was apparent. In fact, several reports have suggested muscle proteolysis or atrophy may occur independently of changes in atrogin-1 or MuRF1 mRNA levels (Cai et al., 2004; de Boer et al., 2007; Fareed et al., 2006; Vary et al., 2008). We now show a modest induction of atrogin-1 gene expression that is unresponsive to glutathione replacement. Together, these data may reveal important temporal associations between alcohol-induced oxidant stress, induction of catabolic factors and resultant muscle atrophy. Nevertheless, the influence of atrogin-1 on alcohol-induced atrophy and responsiveness of the ligase to anti-oxidant therapy requires further exploration.
One possible explanation as to why these catabolic factors have become refractory to procysteine is that long term alcohol abuse established a biochemically distinct and more severe phase of atrophy that could not be manipulated by glutathione alone. For example, continued alcohol ingestion from 28 to 35 weeks resulted in an additional 14% reduction in plantaris fiber area (Otis et al., 2007). This longer duration of abuse may have established a strongly catabolic environment, which may also explain in part the re-recruitment of atrogin-1 and MuRF gene expression. In parallel, gene expression of IGFBP-1, a negative regulator of IGF1 bioactivity (Lang et al., 1998), was upregulated in plantaris muscle from alcohol-fed rats. Similarly, low levels of IGFBP-1 mRNA have been detected in skeletal muscles that are then strongly induced due to thermal injury (Balasubramaniam et al., 2009). These data would support our current work that IGFBP-1 is induced in response to catabolic conditions. While muscle IGF1 levels were unaffected by chronic alcohol ingestion as has been previously reported (Lang et al., 1998; Lang et al., 2004), the general misbalance between IGF1 and IGFBP-1 would suggest an impaired signaling mechanism. Importantly, plantaris muscles from alcohol-fed rats receiving procysteine had increased IGF1 mRNA levels that may ultimately serve to normalize the IGF1:IGFBP-1 relationship.
In conclusion, we showed that long term alcohol ingestion created an overall catabolic state in atrophied rat plantaris muscles, as evidenced by oxidant stress and the production of catabolic members of the IL-6 family (i.e., IL-6 and OSM mRNA), as well as components of the ubiquitin proteasome system (i.e., atrogin-1 and MuRF1 mRNA). Procysteine treatment significantly attenuated, but did not completely abrogate alcohol-induced oxidant stress in the plantaris. However, there were salutary effects on the overall redox environment as reflected by normalized pools of glutathione, increased SOD1 and SOD2 gene expressions, and increased catalase gene expression. In parallel, these improvements in anti-oxidant defenses were accompanied by a significant induction of anabolic factor mRNAs, including CNTF, CT1, and IGF1. Ultimately, procysteine led to increased plantaris fiber area in alcohol-fed rats suggesting that it may be an effective treatment option to combat alcoholic myopathy.
Acknowledgments
Support: This work was supported by grant K01 AA017190-02 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (to JSO) and by grant P-50 AA013757 from the NIAAA and a VA Merit Review (to DMG).
References
- 1.Adachi J, Asano M, Ueno Y, Reilly M, Mantle P, Peters TJ, Preedy VR. 7-α and 7 β-Hydrocycholesterol-5-en 3β-ol in muscle as indices of oxidative stress: response to ethanol dosage in rats. Alcohol Clin Exp Res. 2000;24:675–681. [PubMed] [Google Scholar]
- 2.Austin L, Bower J, Kurek J, Vakakis N. Effects of leukaemia inhibitory factor and other cytokines on murine and human myoblast proliferation. J Neurol Sci. 1992;112:185–191. doi: 10.1016/0022-510x(92)90149-f. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramaniam A, Joshi R, Su C, Friend LA, Sheriff S, Kagan RJ, James JH. Ghrelin inhibits skeletal muscle protein breakdown in rats with thermal injury through normalizing elevated expression of E3 ligases, MrRF1 and MAFbx. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.00015.2008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Barnard W, Bower J, Brown MA, Murphy M, Austin L. Leukemia inhibitory factor (LIF) infusion stimulates skeletal muscle regeneration after injury: injured muscle expresses lif mRNA. J Neurol Sci. 1994;123:108–113. doi: 10.1016/0022-510x(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 5.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Bunn JR, Canning J, Burke G, Mushipe M, Marsh DR, Li G. Production of consistent crush lesions in murine quadriceps muscle--a biomechanical, histomorphological and immunohistochemical study. J Orthop Res. 2004;22:1336–1344. doi: 10.1016/j.orthres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Cai DS, Frantz JD, Tawa NE, Melendez PA, Oh BC, Lidov HGW, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKK beta/NF-kappa B activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 9.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signaling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durán Castellón MC, González-Reimersa E, López-Lirola A, Martín Olivera R, Santolaria-Fernández F, Galindo-Martín L, Abreu-González P, González-Hernández T. Alcoholic myopathy: Lack of effect of zinc supplementation. Food Chem Toxicol. 2005;43:1333–1343. doi: 10.1016/j.fct.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Ebisu C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Konimani E, Tanaka K, Mondaen M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathespins L and B, proteasome) in C2C12. Clin Sci. 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- 12.Fareed M, Evenson A, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren P. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1589–R1597. doi: 10.1152/ajpregu.00668.2005. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Sola J, Garcia G, Elena M, Tobias E, Sacanella E, Estruch R, Nicolas JM. Muscle anti-oxidant status in chronic alcoholism. Alcohol Clin Exp Res. 2002;26:1858–1862. [PubMed] [Google Scholar]
- 14.Fernández-Solà J, Nicolás JM, Fatjó F, García G, Sacanella E, Estruch R, Tobías E, Badia E, Urbano-Márquez A. Evidence of apoptosis in chronic alcoholic skeletal myopathy. Hum Pathol. 2003;34:1247–1252. doi: 10.1016/j.humpath.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregorevic P, Williams DA, Lynch GS. Effects of leukemia inhibitory factor on rat skeletal muscles are modulated by clenbuterol. Muscle Nerve. 2002;25:194–201. doi: 10.1002/mus.10015. [DOI] [PubMed] [Google Scholar]
- 17.Guillet C, Auguste P, Mayo W, Kreher P, Gascan H. Ciliary neurotrophic factor is a regulator of muscular strength in aging. J Neurosci. 1992;19:1257–1262. doi: 10.1523/JNEUROSCI.19-04-01257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hitomi Y, Watanabe S, Kizaki T, Sakurai T, Takemasa T, Haga S, Ookawara T, Suzuki K, Ohno H. Acute exercise increases expression of extracellular superoxide dismutase in skeletal muscle and the aorta. Redox Rep. 2008;13:213–216. doi: 10.1179/135100008X308894. [DOI] [PubMed] [Google Scholar]
- 19.Kiessling KH, Pilstrom L, Bylund AC, Piehl K, Saltin B. Effects of chronic ethanol abuse on structure and enzyme activities of skeletal muscle in man. Scand J Clin Lab Invest. 1975;35:601–607. doi: 10.1080/00365517509095786. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Jo C, Jang BG, Oh U, Jo SA. Oncostatin M induces growth arrest of skeletal muscle cells in G1 phase by regulating cyclin D1 protein level. Cell Signal. 2008;20:120–129. doi: 10.1016/j.cellsig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Koll M, Beeso JA, Kelly FJ, Simanowski UA, Seitz HK, Peters TJ, Preedy VR. Chronic alpha-tocopherol supplementation in rats does not ameliorate either chronic or acute alcohol-induced changes in muscle protein metabolism. Clin Sci (Colch) 2003;104:287–294. doi: 10.1042/CS20020312. [DOI] [PubMed] [Google Scholar]
- 22.Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- 23.Koo-Ng R, Falkous G, Reilly M, Peters TJ, Mantle D, Preedy VR. Carbonyl levels in type I and II fiber-rich muscles and their response to chronic ethanol feeding in vivo and hydroxyl and superoxide radicals in vitro. Alcohol Clin Exp Res. 2000;24:1862–1868. [PubMed] [Google Scholar]
- 24.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-1 stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol. 2004;283:E917–E928. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- 25.Lang CH, Fan J, Lipton BP, Potter BJ, McDonough KH. Modulation of the insulin-like growth factor system by chronic alcohol feeding. Alcohol Clin Exp Res. 1998;22:823–829. [PubMed] [Google Scholar]
- 26.Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37:2180–2195. doi: 10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-1/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol. 2004;286:E916–E926. doi: 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol. 2007;292:R328–R336. doi: 10.1152/ajpregu.00561.2006. [DOI] [PubMed] [Google Scholar]
- 29.Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med. 2008;44:584–593. doi: 10.1016/j.freeradbiomed.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa J, Sakuma K, Sorimachi Y, Yoshimoto K, Yasuhara M. Increase of Cardiotrophin-1 immunoreactivity in regenerating and overloaded but not denervated muscles of rats. Neuropathology. 2005;25:54–65. doi: 10.1111/j.1440-1789.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 31.Otis JS, Brown LA, Guidot DM. Oxidant-induced atrogin-1 and transforming growth factor-beta1 precede alcohol-related myopathy in rats. Muscle Nerve. 2007;36:842–848. doi: 10.1002/mus.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otis JS, Ashikhmin YI, Brown LA, Guidot DM. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res Ther. 2008a;5:8. doi: 10.1186/1742-6405-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otis JS, Mitchell PO, Kershaw CD, Joshi PC, Guidot DM. Na,K-ATPase expression is increased in the lungs of alcohol-fed rats. Alcohol Clin Exp Res. 2008b;32:699–705. doi: 10.1111/j.1530-0277.2008.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pena LR, Hill DB, McClain CJ. Treatment with glutathione precursor decreases cytokine activity. JPEN J Parenter Enteral Nutr. 1996;23:1–6. doi: 10.1177/014860719902300101. [DOI] [PubMed] [Google Scholar]
- 35.Preedy VR, Peters TJ. The effect of chronic ethanol ingestion on protein metabolism in type-I- and type-II-fibre-rich skeletal muscles of the rat. Biochem J. 1988;254:631–639. doi: 10.1042/bj2540631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preedy VR, Peters TJ, Patel VB, Miell JP. Chronic alcoholic myopathy: transcription and translational alterations. FASEB J. 1994;8:1146–1151. doi: 10.1096/fasebj.8.14.7958620. [DOI] [PubMed] [Google Scholar]
- 37.Preedy VR, Reilly ME, Patel VB, Richardson PJ, Peters TJ. Protein metabolism in alcoholism: effects on specific tissues and the whole body. Nutrition. 1999;15:604–608. doi: 10.1016/s0899-9007(99)00096-9. [DOI] [PubMed] [Google Scholar]
- 38.Preedy VR, Adachi J, Asano M, Koll M, Mantle D, Niemela O, Parkkila S, Paice AG, Peters T, Rajendran R, Seitz H, Ueno Y, Worrall S. Free radicals in alcoholic myopathy: indices and preventive study. Free Radic Biol Med. 2001;32:683–687. doi: 10.1016/s0891-5849(01)00794-8. [DOI] [PubMed] [Google Scholar]
- 39.Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, Rajendram R, Mantle D, Peters TJ. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil. 2003;24:55–63. doi: 10.1023/a:1024842817060. [DOI] [PubMed] [Google Scholar]
- 40.Pruznak AM, Hong-Brown L, Lantry R, She P, Frost RA, Vary TC, Lang CH. Skeletal and cardiac myopathy in HIV-1 transgenic rats. Am J Physiol Endocrinol Metab. 2008;295:E964–E973. doi: 10.1152/ajpendo.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reardon KA, Davis J, Kapsa RM, Choong P, Byrne E. Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve. 2001;24:893–899. doi: 10.1002/mus.1086. [DOI] [PubMed] [Google Scholar]
- 42.Reilly ME, Patel VB, Peters TJ, Preedy VR. In vivo rates of skeletal muscle protein synthesis in rats are decreased by acute ethanol treatment but are not ameliorated by supplemental alpha-tocopherol. J Nutr. 2000;130:3045–3049. doi: 10.1093/jn/130.12.3045. [DOI] [PubMed] [Google Scholar]
- 43.Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 44.Spangenburg EE, Booth FW. Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol. 2002;283:C204–C211. doi: 10.1152/ajpcell.00574.2001. [DOI] [PubMed] [Google Scholar]
- 45.Spangenburg EE, Booth FW. Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF(-/-) mouse. Cytokine. 2006;34:125–130. doi: 10.1016/j.cyto.2006.05.001. 2006. [DOI] [PubMed] [Google Scholar]
- 46.Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun. 1995;207:168–174. doi: 10.1006/bbrc.1995.1168. [DOI] [PubMed] [Google Scholar]
- 47.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1777–R1789. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol -fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Chu H, Zhao H, Cheng X, Liu Y, Jin W, Zhao J, Liu B, Ding Y, Ma H. Nitricoxide synthase-induced oxidative stress in prolonged alcoholic myopathies of rats. Mol Cell Biochem. 2007;304:135–142. doi: 10.1007/s11010-007-9494-6. [DOI] [PubMed] [Google Scholar]
- 50.Ward RJ, Peters TJ. The anti-oxidant status of patients with either alcohol-induced liver damage or myopathy. Alcohol Alcohol. 1992;27:359–365. [PubMed] [Google Scholar]
- 51.Worrall S, Niemela O, Parkkila S, Peters TJ, Preedy VR. Protein adducts in type I and type II fibre predominant muscles of the ethanol -fed rat: preferential localization in the sarcolemmal and subsarcolemmal region. Eur J Clin Invest. 2001;31:723–730. doi: 10.1046/j.1365-2362.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- 52.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol. 2003;35:698–705. doi: 10.1016/s1357-2725(02)00341-2. [DOI] [PubMed] [Google Scholar]