Abstract
To explore the mechanism behind the association between HSV-2 and HIV-1 acquisition, we performed in situ analysis of the cellular infiltrate from sequential biopsies of HSV-2 lesions on and off antiviral therapy. CD4+ and CD8+ T cells, and a mixed population of plasmacytoid and myeloid dendritic cells (DCs), including cells expressing DC-SIGN, persisted at sites of HSV-2 reactivation for months after healing, even in the context of daily antiviral therapy. The CD4+ T cells that persisted reacted to HSV-2 antigen, were enriched for CCR5 expression, and were also contiguous to DCs expressing CD123 or DC-SIGN. Ex vivo infection with a CCR5-tropic strain of HIV-1 revealed increased concentrations of integrated HIV-1 DNA in cells derived from healed genital lesion biopsies as compared to control skin biopsies. The persistence and enrichment of HIV-receptor-positive inflammatory cells in the genitalia help explain the failure of anti-HSV-2 therapy to reduce HIV acquisition.
Both incident and prevalent HSV-2 infections are associated with an increased risk of HIV acquisition1,2, presumably due to frequent infectious HSV-2 ulcerations and the associated influx of activated CD4+ T cells3 that provide HIV easier access to high numbers of potential target cells. Antivirals, such as acyclovir, reduce the frequency of both clinical and subclinical herpetic ulcerations by 75–85%4–6, findings that provided the impetus for conducting two large-scale international trials to determine whether daily suppressive anti-HSV-2 therapy reduces HIV acquisition. Despite significant reductions in HSV-2 genital ulcer disease, a reduction in HIV acquisition was not observed in either study7,8. The biological explanation for these findings is unknown.
In previous studies, we demonstrated that HSV-2-specific CD8+ T cells persist in a very localized area at the dermal-epidermal junction after lesion healing, suggesting that localized inflammatory cells could persist in genital skin despite healing of the epithelial surface and the appearance of a clinically normal epithelium9. We undertook a detailed in situ study of sequential biopsies of genital skin to characterize whether HIV-receptor-positive cells could persist in genital skin after an HSV reactivation and whether this would be affected by antiviral therapy for HSV-2.
Result
Localized persistence of CD4+ T cells in genital skin
We studied eight healthy, HIV-negative subjects (seven women, one man) with culture-proven recurrent symptomatic genital HSV-2 infection. Subjects underwent 3-mm punch biopsies at the time of a clinically symptomatic ulcerative lesion; at the time of lesion resolution (7–10 d later); and at 2, 4 and 8 weeks post-healing. Four of the eight subjects then participated in the second phase of the study, in which acyclovir (ACV) 400 mg twice daily was initiated at the start of an acute episode and continued for 20 consecutive weeks. This was the dose used in both of the clinical trials which evaluated if HSV-2 therapy could reduce HIV-1 acquisition7,8. Biopsies in the acyclovir phase were taken from the lesion site during the acute lesion; at lesion healing; and at 2, 4, 8, 12, 16 and 20 weeks post-healing (Table 1)10. In both phases of the study, biopsies from the unaffected contralateral genital area were also collected at these time points. PBMCs were also obtained at enrollment and at 4–8 week intervals.
Table 1.
Demographic characteristics of subjects and study biopsy procedures
| Demographic characteristics: | ||
|---|---|---|
| Subjects information | HSV-2-infected subjects off therapy (n = 8) | HSV-2-infected subjects studied after initiation of daily ACV (n = 4) |
| Median age (yr) | 44 | 49 |
| Median duration (d) of genital lesion that was biopsied | 8 | 6.5 |
| Lesion site | Buttock Labia Thigh Perianal Sacral |
Buttock Labia Thigh |
|
Biopsy procedure: lesional and normal control tissue was obtained at each time point. Untreated herpes recurrence: lesion → healed → 2 wks → 4 wks → 8 wks Acyclovir-treated herpes recurrence: Acyclovir 400 mg twice daily lesion → healed → 2 wks → 4 wks → 8 wks → 12 wks → 16 wks →20 wks | ||
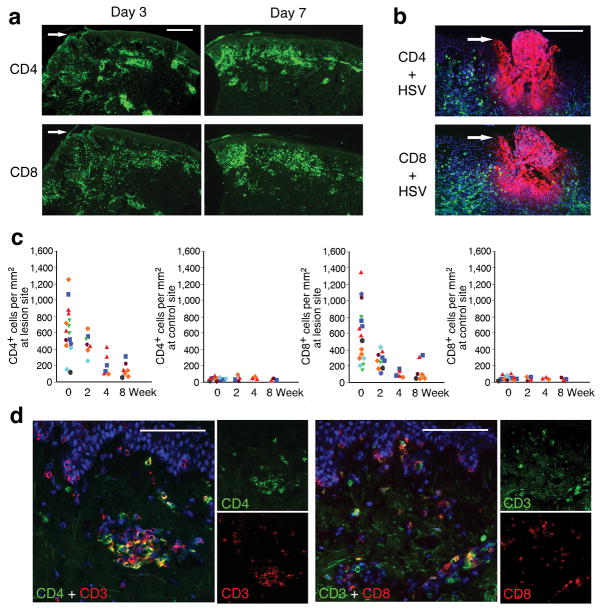
During an acute HSV-2 lesion when HSV-2 antigen was present, CD4+ and CD8+ T cells infiltrated the epidermis and dermis at the ulcer site (Fig. 1a,b). The acute lesion was associated with a massive localized infiltration of cells. The mean number of CD4+ and CD8+ cells/mm2 in HSV-infected skin was 655 and 618, respectively, versus 68 and 55 cells/mm2 in uninvolved genital skin (p≤0.003 for comparison between HSV-2 lesion biopsies and control biopsies) (Fig. 1c,d). Upon healing (d 7), a new epidermal layer replaced the ulcer, and both CD4+ and CD8+ cells were relatively restricted to the upper dermis (Fig. 1a). Follow-up biopsies showed clearance of HSV antigen and a gradual reduction of inflammation. However, localized foci of CD4+ and CD8+ T cells persisted for months (Fig. 1c,d), despite complete healing of lesions, normal skin thickness and appearance, and no clinical evidence of genital herpes. The number of CD4+ and CD8+ cells in lesion-site biopsies obtained at 2–8 weeks post-healing was significantly higher than in skin biopsies from uninvolved genital skin (Fig. 1c). This enrichment in CD4+ T cells ranged from 2- to 37-fold higher than in contralateral uninvolved genital skin (median 8-fold). These higher concentrations of CD4 cells in the skin biopsies from HSV affected versus non-affected areas were statistically significant at all the post healing time points (p=0.01 at week 2 post-healing; p=0.03 at weeks 4–8 post-healing).
Figure 1. Persistence and localization of CD4+ and CD8+ T cells in genital lesions of immunocompetent subjects.
(a) Overview of infiltrating CD4+ and CD8+ cells in skin biopsies of an acute ulcer (d 3) and recently healed lesion (d 7) taken from a single subject who reactivated HSV-2 at two distinct anatomic sites. The arrows illustrate the area of epithelial ulceration. (b) Infiltration of CD4+ (top) and CD8+ (bottom) cells near the site of HSV-2 (red) replication (d 2 lesion) in another patient. (c) Quantitation of CD4+ and CD8+ cells in skin biopsies from the lesion site as compared to uninvolved (control) genital skin biopsies. At least five fields from each biopsy were counted for defining the density of cells 9. Each symbol represents one subject (n = 8). Biopsies from acute herpetic lesion, including the ulcerative (d 1–4) and newly healed (d 6–8) stages, were collated into the week 0 time point. (d) Dual immunofluorescence staining with CD3 and CD4 or CD8 of biopsy after complete healing of the lesion site. Scale bar: 400 μm (a), 200 μm (b), and 100 μm (d).
These persisting CD4+ and CD8+ cells demonstrated distinct spatial distributions (Fig. 1d). CD4+ cells were present in the upper dermis and concentrated perivascularly, while CD8+ cells were localized at the dermal-epidermal junction in close contact with basal keratinocytes (Fig. 1d). Simultaneous CD3 and CD4 or CD3 and CD8 staining demonstrated that 58±6% of the CD4+ cells and 74±6% of the CD8+ cells were CD3+ T cells (Fig. 1d).
Acyclovir therapy does not affect CD4+ T cell persistence
The persistence of CD4+ and CD8+ T cells in genital skin was further evaluated in serial biopsies from the subjects treated with daily acyclovir therapy. Acyclovir therapy reduced the median healing time of the herpetic ulcers (Table 1) and prevented subsequent clinical and subclinical mucosal alternations; none of the post-healing lesion-site biopsies taken while participants were on acyclovir had HSV DNA or HSV antigen detected in them (n = 24). Similarly, all of the control biopsies were negative for HSV DNA11,12.
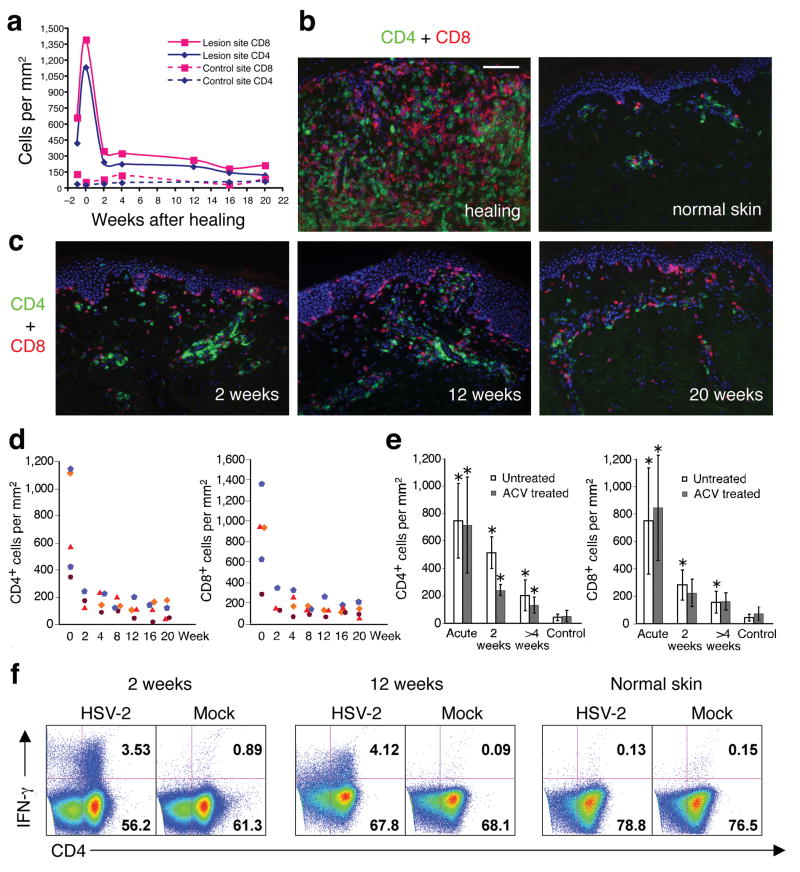
Acyclovir treatment did not significantly alter the spatial distribution or the magnitude of the CD4+ and CD8+ T cell infiltration during either the acute or post-healing biopsies. The time course of CD4+ or CD8+ T cell infiltration in skin tissue, from the onset of genital disease to 20 weeks after the lesion completely healed in a representative subject, showed persistence of CD4+ or CD8+ T cells after HSV-2 reactivation, despite the 20 consecutive weeks of acyclovir treatment (Fig. 2a–c). Persisting CD4+ or CD8+ T cells were seen in all the subjects (Fig. 2d,e). The intensity of the persisting CD4+ T cell infiltration in the dermis was similar at 4–20 weeks post-healing on suppressive acyclovir as compared to that seen with no antiviral therapy (Fig. 2e). Even after 20 continuous weeks of acyclovir therapy, the number of CD4+ T cells in the dermis was significantly higher in lesion-site biopsies than in uninvolved genital skin (p=0.01 at 4–20 weeks post-healing) (Fig. 2e).
Figure 2. Persistence of CD4+ and CD8+ T cells in genital skin of subjects on daily acyclovir therapy.
(a) Quantitation of CD4+ and CD8+ cells infiltrating an HSV-2 lesion vs. uninvolved (control) genital skin. Week 0 is the time of complete lesion healing (lesion onset is labeled week -1). (b and c) Dual immunofluorescence staining of CD4+ (green) and CD8+ (red) cells. Panel b: comparison of healing stage at lesion site versus normal uninvolved genital skin. Panel c: shows CD4+ and CD8+ cells in post-healing biopsies taken from the time points indicated in panel a. (d) Quantitation of CD4+ and CD8+ cells in genital lesions of all four subjects on acyclovir therapy from acute lesion to 20 weeks post-healing. Each symbol represents a subject. (e) Comparison of CD4+ and CD8+ T cell infiltration in genital skin from untreated versus acyclovir-treated HSV recurrence. No statistically significant differences were present between acyclovir versus untreated episodes either during or after herpes reactivation (P > 0.5). The asterisk marks biopsies of statistical significance (P < 0.05) in comparison of lesion site versus control site. (f) Persistent CD4+ T cells in post-healing biopsies were HSV-2 antigen specific. After incubation with whole UV-killed HSV-2 antigen, IFN-γ expression was detected in lymphocytes collected from biopsies at 2 and 12 weeks post-HSV-2 reactivation but not in lymphocytes collected from the uninvolved genital skin (12 weeks post healing) of the subject depicted in panels a-c. Scale bar: 100 μm (B and C).
HSV-2 specificity of the persisting CD4+ T cells
To evaluate the specificity of the resident CD4+ T cells in genital skin, we isolated the lymphocytes from the biopsies, expanded the lymphocytes in vitro, and tested their reactivity to HSV antigen10. Representative experiments from two separate biopsies taken at weeks 2 and 12 post-healing, while on acyclovir, showed persisting CD4+ T cells reacted to HSV-2 antigen (Fig. 2c,f). No HSV DNA was detected from these biopsies, and their histological appearance was normal (data not shown). CD4+ T cells derived from lesion-site biopsies on daily suppressive acyclovir secreted gamma interferon (IFN-γ) upon in vitro stimulation with UV-inactivated HSV-2. HSV-2-specific CD4+ T cells were not found in control genital skin biopsies from the same subjects, indicating the persisting CD4+ T cells in areas of prior HSV-2 reactivation were enriched for HSV-2 antigen specificity (Fig. 2f). Similar findings were seen in biopsies taken post-healing in untreated patients (data not shown).
The mean IFN-γ response to inactivated HSV-2 antigen in CD4+ T cells obtained from PBMCs from HSV-2 infected persons was 0.42±0.3% (n = 29), 8–10 fold lower than was demonstrated in these lesional biopsies, indicating HSV-2 specific CD4+ T cells were enriched in genital skin as compared to circulating PBMCs13.
Enrichment of CCR5+CD4+ T cells persisting in genital skin
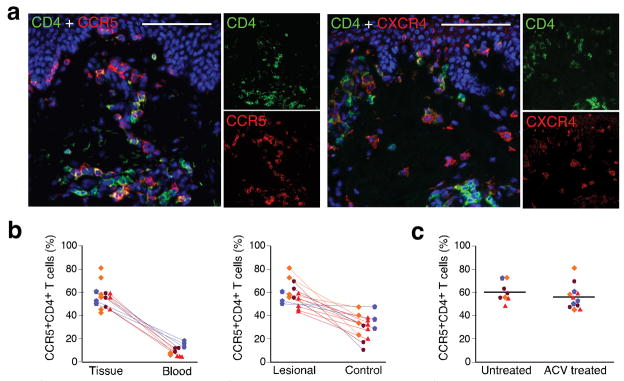
The CD4+ T cells that persisted in the genital skin expressed the HIV co-receptors CCR5 and CXCR4 (Fig. 3a). On average, 55% (range 42–81%) of the CD4+ T cells detected in lesion-site biopsies expressed CCR514, 6-fold higher than those found among circulating CD3+CD4+ lymphocytes in peripheral blood obtained at the same time (p=0.04), and 2-fold higher than those from uninvolved genital skin biopsies obtained at the same time (p=0.04) (Fig. 3b). The percent of CD4+ T cells expressing CCR5 in dermal skin in the acute and post-healing time periods was higher in biopsies taken from HSV-infected versus control skin in 14 of the 15 biopsies evaluated (Fig. 3b). The degree of CCR5 expression in dermal CD4+ T cells that persisted after healing of HSV-2 was similar between those on daily acyclovir (n = 12) and those on no therapy (n = 8) (Fig. 3c).
Figure 3. HIV co-receptor expression on CD4+ T cells in HSV-2 lesions.
(a) CCR5 and CXCR4 expression on CD4+ cells in lesions after healing. A 2 weeks post healing biopsy from an acyclovir treated subject was dual stained with either CCR5 and CD4, or CXCR4 and CD4 antibodies. (b) Comparison of CCR5 expression on CD4+ cells in genital skin after infection (n = 20) and in circulating PBMC (n = 11) (left panel) or in unaffected control skin (n = 15) (right panel). Each symbol represents one subject. Lines connect samples taken from the same subject on the same day (p=0.04 for comparison in number of CCR5-expressing CD4+ cells from genital skin previously infected with HSV-2 versus contralateral non-infected genital skin). (c) Comparison of CCR5 expression in post-healing biopsies during acyclovir-treated (n = 12) versus untreated (n = 8) time periods (P > 0.5). Lines indicate the median percentage of CCR5-expressing CD4+ cells. Scale bar: 100 μm.
Thus, prolonged acyclovir treatment did not alter the persistence, quantity, spatial distribution and degree of CCR5 enrichment, or HSV-2 specificity of dermal CD4+ T cells detected at the site of genital herpes infection after lesion healing.
Persistence of DCs and their interaction with CD4+ T cells
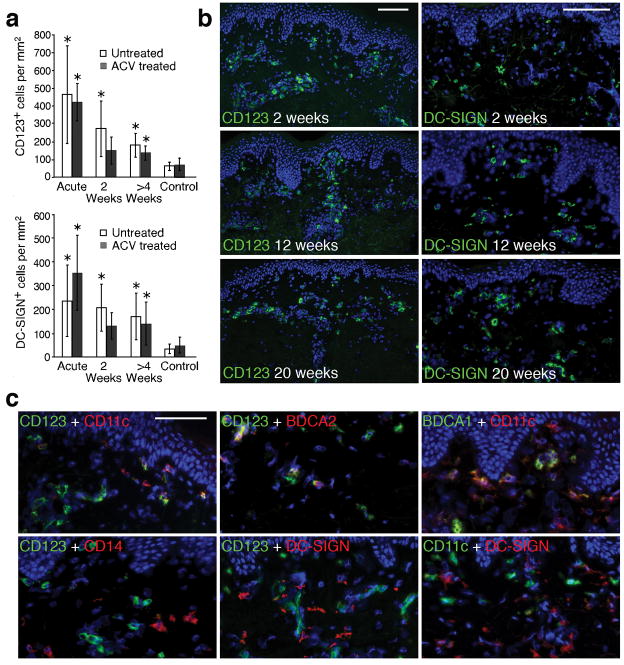
As dendritic cells (DCs) have been detected in HSV lesions of mice and humans15,16, we utilized a variety of cell surface markers to determine the anatomic distribution, quantity, and type of DCs present in human HSV lesions. Genital herpes lesions were associated with a marked infiltration of cells expressing CD123 or DC-SIGN, the receptor for HIV-1 capable of transferring HIV-1 to CD4+ T cells. Both CD123- and DC-SIGN-expressing cells were detected in all skin biopsies from healed genital lesions (Fig. a,b). Importantly, both CD123+ and DC-SIGN+ cells persisted at significantly higher concentrations in the dermis of HSV-2-involved skin at all measured post-healing time points as compared to control skin (average 3.3- and 5.6-fold, respectively; p=0.01 and p=0.035, respectively) (Fig. 4a). The persistent enrichment in CD123+ or DC-SIGN+ DCs in skin biopsies from HSV-2-infected regions compared to normal control skin was seen even during acyclovir therapy (average 2.0- and 2.9-fold, respectively; p=0.013 and p=0.019, respectively) (Fig. 4a,b). Furthermore, acyclovir had no statistically significant effect on the persistence of CD123+ or DC-SIGN+ cells over time (p=0.96 and p=0.64, respectively).
Figure 4. Persistence of dendritic cells in genital lesion biopsies.
(a) Quantitation of CD123+ and DC-SIGN+ cells in lesion-site biopsies during acyclovir-treated and untreated time periods. An asterisk above a bar denotes that the number of CD123+ or DC-SIGN+ cells in a lesion-site skin biopsy was significantly higher than in control skin (P < 0.05). Acyclovir had no statistically significant effect on the persistence of CD123+ or DC-SIGN+ DCs over time (p=0.96 and p=0.64, respectively). (b) Representative skin biopsy sections demonstrating the persistence of DCs positive for either CD123 or DC-SIGN in the upper dermis and near blood vessels in post-healing biopsies obtained at 2, 12 and 20 weeks while on daily acyclovir. (c) Detection of a diverse DC population in post-healing biopsies by co-staining of CD123 or DC-SIGN with CD11c, CD14, BDCA1 and BDCA2 in post-healing biopsies (week 16–20) during acyclovir treatment. The top first panel shows that three populations of DCs: CD123+CD11c−, CD123+CD11c+, and CD123−CD11c+. The top second panel shows co-staining of CD123 and BDCA2 and the top third panel shows co-staining of the CD11c+ cells with BDCA1. The bottom first panel shows the lack of CD14 expression on CD123+ cells. The bottom second and third panel demonstrate that DC-SIGN expressing cells do not co-express CD123 or CD11c. Scale bar: 100 μm (b), 50 μm (c).
The persisting CD123+ cells were both CD11c+ and CD11c−and did not express CD14 (Fig. 4c). The CD11c+ cells largely co-stained with BDCA1 (Fig. 4c). A portion of the CD123+ cells also co-stained with BDCA-2 (Fig. 4c). These data indicate that both plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs) persisted in the skin after HSV-2 reactivation17–20. DC-SIGN+ cells tended not to express CD123 or CD11c (Fig. 4c)20,21. The above data indicate that a large and diverse population of DCs, including mDCs, pDCs and DC-SIGN expressing cells, persist in the localized areas of previously HSV-2-infected genital skin for weeks after lesion healing, and that this enrichment is not mitigated by treatment with acyclovir.
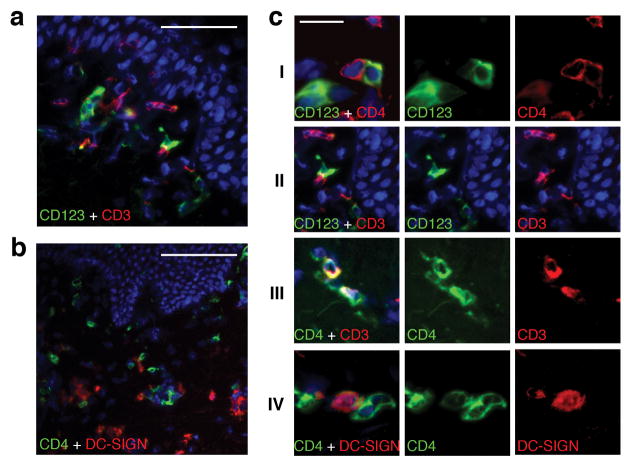
Because the anatomic distribution of dermal DCs appeared similar to that seen for CD4+ T cells (Fig. 2c, Fig. 3a and Fig. 4b), we more closely investigated the association between CD123+ or DC-SIGN+ DCs with dermal CD4+ T cells (Fig. 5a–c). Dual staining for CD4 or CD3 with CD123 showed direct contact between CD123+ DCs and T cells (Fig. 5a,cI,II). Contiguous appearance of DCs (CD3-CD4low) and CD4+ T cells (CD3+CD4high) in the upper dermis of post-healing skin (Fig. 5cIII) were frequently observed. We consistently found evidence of interaction between DC-SIGN+ cells and CD4+ T cells in the upper dermis of post-healing lesion biopsies (Fig. 5b,cIV). These interactions were present whether the biopsy was taken on or off acyclovir.
Figure 5. Interactions of DCs and CD4+ T cells in post-healing biopsies during acyclovir treatment.
(a) Dual immunofluorescence staining of CD123 with CD3 showing the interaction of CD123+ cells (green) and CD3+ T cells (red) in a week 12 post-healing biopsy while on acyclovir therapy. (b) Associations of CD4+ cells (green) with cells expressing DC-SIGN (red). Biopsy was taken after 20 weeks of acyclovir therapy. (c) Detailed micrographs illustrate direct interaction between CD123+ DCs and CD4+ T cells, and DC-SIGN+ DCs and CD4+ T cells at genital lesion site post healing. Panel I shows CD123+ DC are contiguous to CD4+ T cells. Panel II shows interactions between CD123+ DC cells and T cells. Panel III illustrates clusters of CD3+CD4high T cells and CD3-CD4low DCs. Panel IV shows a CD4+ T cell contiguous to a DC-SIGN expressing cell scale bar:50μ(a), 100μ(b), 20μ(c).
Increased HIV-1 infectivity in healed genital herpes lesion
To determine if the persistence of the increased target cell environment for CCR5-tropic HIV-1, which we observed in healed herpes lesions, results in an enhanced susceptibility to HIV-1 infection, we performed ex vivo HIV infection of skin biopsies. Biopsies from healed genital herpes lesions were placed into organ culture and challenged ex vivo with the CCR5-tropic HIV-1JRCSF. Similarly, biopsies from unaffected control sites were obtained on the same day and challenged in the same experiment. After two days in culture, the cells were lysed, DNA extracted, and the amount of integrated HIV-1 DNA measured by quantitative PCR (normalized to β-actin DNA) determined22,23. This measurement allowed us to evaluate the replicative portion of the HIV-1 life cycle, which could reflect increased local replication from a larger number of targets or increased infectious synapse activity in cells contained in the lesion biopsies.
In two subjects studied, the skin biopsies from healed genital herpes lesions (2 weeks post healing) supported a significantly greater amount of HIV replication than skin biopsies from the unaffected control area. The amount of integrated HIV-1 DNA per cell was 2.7 and 4.8-fold difference, respectively, in the ex vivo infected cells obtained from each of the two genital herpes skin biopsies as compared to levels from uninvolved skin tissue (1.53 and 2.87 HIV-1 DNA copies per cell vs. 0.56 and 0.60 copies per cell, respectively). These data provide experimental evidence that tissue contained within healed genital herpes lesions are more susceptible to HIV-1 infection than in unaffected genital skin.
Discussion
Our data provide several novel insights about the immunobiology of HSV-2 reactivation and offer a possible explanation for the observation that, while HSV-2 increases the likelihood of HIV acquisition1, suppression of HSV-2 reactivation with systemic antivirals does not result in reduction of HIV infection7,8. Surprisingly, HSV-2 infection results in a profound persistent localized inflammatory response in the dermis below the healed lesion. This inflammatory focus, which is detected in clinically normal-appearing skin, consists of HSV-2-specific CD4+ T cells that express CCR5 or CXCR4 as well as a mixed population of DCs, including inflammatory cells that express DC-SIGN. These cells persist in spatial closeness at a density that range from 2- to 37-fold higher than in genital skin not previously infected by HSV-2. Unfortunately, even prolonged antiviral therapy for HSV-2 does not significantly influence this dense localized inflammatory infiltrate. Ex vivo infection of skin biopsies containing this dense inflammatory infiltrate support 3- to 5- fold higher levels of localized HIV-1 replication than skin biopsies from control areas that do not contain these inflammatory infiltrates.
The mechanism behind this persistent inflammatory infiltrate requires further study. While it has been known for decades that acyclovir is not completely effective in abolishing neuronal release of virus into the periphery4–6, our recent studies are the first to show that lymphocytes taken from the site of prior HSV-2 reactivation that is devoid of detectable HSV-2 antigen or DNA contains a high percentage of resident HSV-specific CD4+ and CD8+ T cells9. Compared to the lack of such cells in non-HSV-involved genital skin, our data suggest that the localized dermal inflammation that persists in genital skin post healing of the ulcer is related either to long-term immunologic memory or to continuously renewed immune responses due to the intermittent expression of HSV antigen into these localized areas of skin13,24. T cell-DC interactions in the peripheral tissue has been reported to be an important mechanism for orchestrating a rapid adaptive immune response and in maintaining persistent immunologic memory to HSV-2 antigen in the genital skin and mucosa15. However, these CD4+ T cell-DC interactions also provide an increased opportunity for HIV to infect additional CD4+ T cells25.
The wide anatomical distribution of HSV-2 in the male and female genital tracts underscores the likely importance these localized reservoirs of inflammatory cells play in HIV acquisition26,27. The persistence and spatial closeness of CD4+ T cells with the dendritic cells that could either support or enhance HIV-1 infection likely increase the potential for HIV-1 to initiate a focus of infection and increase the speed and likelihood of dissemination when deposited onto genital skin25–28. While antiviral therapy for HSV-2 is effective in reducing breaches in the epithelial barrier due to HSV-25, non-infectious ulcerations in the epithelium can initiate lentivirus infection29. Coitus is associated with a high number of breaks in the epithelial barrier which would provide HIV access to this persistent nidus of CCR5-expressing CD4+ T cells and DC-SIGN-expressing DCs in the dermis and genital mucosa17,20. Thus, HSV-2 infection provides a wide surface area and long duration of time, due to its lifelong infection and reactivation frequency, for allowing HIV access to more target cells26,30, providing a greater chance for the initial “spark” of infection. Additionally, the close proximity with DC-SIGN-expressing DCs likely fuels these embers and provides a mechanism for more efficient localized spread of initial infection. Our ex vivo experiments in which we show greater integration of HIV-1 into organ cultures taken from genital lesions containing these inflammatory cells versus control skin supports this potential sequence of events. Keeping the anatomic and spatial characteristics of the inflammatory cells that we found in vivo in an ex vivo environment is difficult and makes further mechanistic studies difficult. Similarly, the complexities of organ culture limit the ability to determine the role of increased CCR5 expression contributes to the CCR5 tropism of HIV-1 strains associated with acute infection. Unfortunately, there is no well-developed nonhuman primate model of HSV-2 reactivation to study the detailed interaction between reactivating HSV-2 and a replicating lentivirus in vivo.
We recognize that we studied only a limited number of individuals. However, the demographic and clinical characteristics of the participants were similar to those with recurrent genital herpes studied in our clinic for the last two decades4–6,26,30,31. The detailed clinical evaluations, biopsies, and laboratory studies required preclude this protocol’s widespread applicability to large patient cohorts. However, the consistency of our findings between individuals and the typical appearance of histological and clinical findings from acute lesions make us feel that our central finding—that HSV reactivation leaves a residual inflammatory response not appreciated clinically—is typical of HSV-2 genital lesions. There is a large body of epidemiological data to indicate that ulcerations in the skin are an important point of entry for HIV-1, especially in the heterosexual male6,32,33. Recent data indicating that circumcision reduces HIV acquisition provides direct experimental support of this concept34–36. It remains to be studied whether the persistent T cell infiltration we described in genital skin also occurs on mucosal surfaces such as the cervix. While sequential sampling of the cervix is possible, clinically visible ulcerations and subclinical viral shedding at the cervix due to HSV-2 reactivation are infrequent in established HSV-2 infection26. In addition, the ability to anatomically define an area of the cervix that is unaffected by HSV-2 as the “control” region is problematic.
Interventions designed to interrupt the association between HSV-2 and HIV must be directed at diminishing the chronic inflammatory milieu caused by HSV-2 infection in the genital tract through a reduction of HSV-2 reactivation from sacral ganglia or, perhaps more importantly, at addressing the need to prevent the acquisition of HSV-2 infection through the development of an effective HSV-2 vaccine.
Methods
Study population and biopsy procedures
The protocol, biopsy procedures and informed consent were approved by the University of Washington Institutional Review Board. All subjects had clinically symptomatic genital herpes30,31. Each 3 mm punch biopsy of an acute lesion included 50% of the vesicle area and 50% of the immediately adjacent erythematous skin area9. We obtained post-healing biopsies from the predominant area of the lesion, usually contiguous to the prior biopsy, and control skin biopsies from the opposite anatomic site of the HSV reactivation.
Immunofluorescence
We sectioned the frozen tissue into 7 μm slices before fixing and permeabilizing in acetone for 20 m at −20°C. We applied primary antibodies overnight at 4°C, washed in PBS, and added appropriate fluorescence-labeled secondary antibody at room temperature for 1 h, counterstained with DAPI (Fluka) and mounted in Mowiol 40-88 containing 2.5% DABCO (Sigma). Primary monoclonal antibodies were specific for human CD3, CD8, CD11c, CD14, CD123 (BD Biosciences), CD4 (Dako), DC-SIGN (e-Bioscience), BDCA1 (Miltenyi Biotec), CCR514. Polyclonal antibodies were specific for HSV-2 (rabbit, Dako), CXCR4 (rabbit, Abcam), BDCA2/DLEC (goat, R&D System). Secondary antibodies were donkey anti-mouse Alexa Fluor 488, donkey anti-mouse Alexa Fluor 647, donkey anti-rabbit Alexa Fluor 594, donkey anti-rat Alexa Fluor 594, donkey anti-goat Alexa Fluor 633 and goat anti-mouse HRP coupled with Alexa Fluor-labeled tyramide (Invitrogen). We used antibody CD4 (Abcam), CD8-AF647 (BD Biosciences), CD11c-AF647 (BioLegend), CD14-APC (BD Biosciences) and DC-SIGN-AF647 (e-Bioscience) in the dual fluorescence staining.
T cell function assay
We expanded lymphocytes in bulk from skin biopsies using mitogenic stimulation37. After 14–16 d, we labeled autologous peripheral blood mononuclear cells (PBMC) with CFSE and cultured in T cell medium at a 1:1 ratio with bulk responder skin lymphocytes38. Stimuli were media, mock Vero cell lysate, HSV-2 strain 186 UV-irradiated, or positive control ionomycin/phorbol ester38. We added Brefeldin A at 1 h and processed for IFN-γ intracellular flow cytometry at 6 h38. We performed analysis on FacsCanto flow cytometer (Becton Dickenson) and FlowJo software (Tree Star) gated on lymphocyte forward/size scatter and then CFSE-negative cells to exclude PBMC.
Detection of HSV-2 antigen and DNA
We detected HSV-2 antigen by immunofluorescence staining using rabbit HSV-2 antibody (Dako). We used a sensitive PCR assay to detect HSV-2 DNA from eight sections of each biopsy. We considered one copy per reaction well as positive11,12.
Ex vivo HIV infection of skin biopsies
We blinded and processed biopsies within 1 h of removal from donors. Using a Zeiss KL1500 stereoscope, we removed deep dermis and visible microvasculature, and used as uninfected control tissue in the PCR assay. We cut the remaining tissues into small pieces in a 6-well plate, infected with 50 ng/ml Gag p24 of CCR5-tropic HIV-1JRCSF by spinoculation for 2 h at 1,200 × g22,23, and cultured in HEPES-buffered RPMI containing 10% fetal calf serum for 40 h at 37°C. We isolated DNA from infected skin biopsies using the QiaAmp Blood Mini Kit (Qiagen) and performed an Alu-LTR-based nested PCR assay, which specifically amplifies chromosomally integrated viral DNA23,39. We modified the previously published method using Platinum Taq SuperMix (Invitrogen) for the first round amplification, started with denaturation at 96°C for 2 min followed by 12 cycles of amplification (96°C for 15 s, 55°C for 60 s, and 70°C for 60 s). Using one-tenth the volume of the first round amplicons, we carried out the second round of amplification in the 1X ABsolute Blue QPCR ROX Mix (ABgene), started with denaturation at 96°C for 15 min, followed by 40 cycles of amplification (96°C for 15 s and 60°C for 60 s). We placed the multiplex quantitative PCR amplified Alu-LTR sequences and β-actin gene in the same wells, for normalization purposes. For the β-actin gene, we used the primer set (82 mM) from the TaqMan B-actin Detection Reagent (Applied Biosystems) in the first round of PCR, and inner primer set (Forward: 5′-ACCCACACTGTGCCCATCTACGA-3′ and Reverse: 5′-CGGAACCGCTCATTGCCAATGG-3′) in the second round. We selected the 6-FAM-labeled LTR probe (200 nM) and the JOE labeled β-actin probe (200 nM) to detect product in the second round of PCR. We quantified viral DNA by generating a standard curve using DNA isolated from serially diluted ACH-2 cells that are latently infected with HIV-1 LAV (NIH AIDS Research and Reference Reagent Program). We ran quadruplicate PCR reactions for each sample. Detection limit for integrated HIV-1 DNA was five copies.
Statistical Analyses
Paired T test and linear random effects models were used to test the quantitative differences in inflammatory cells from biopsies taken at lesion sites versus uninfected genital skin (control sites). Random effects models allow one to account for the correlation of repeated measures on the same subjects over time40. We used the data from 4 weeks or longer past lesion healing as the primary time point for defining a persistent inflammatory infiltrate.
Acknowledgments
We thank M. Mack (University Hospital Regensburg, Germany) for the CCR5 antibody and C. McClurkan, L. Ballweber and H. Xie for technical assistance. We also thank our dedicated study participants. This work was supported by National Institutes of Health (R37AI042528, P01AI030731, AI50132, HD51455) and the Tietze Foundation.
Footnotes
Author Contributions
J.Z. designed the study, developed the technologies, conducted the experiments, prepared the figures, and wrote the draft. Am.W. and A.K. performed the staining and quantification. T.P. developed the first data suggesting DCs were part of the inflammatory milieu in the healing biopsy stage. F.H. performed the ex vivo organ culture studies. C.J. and M.R. enrolled subjects and performed the biopsies; An.W. supervised the clinic. D.K. designed and analyzed experiments on HSV-2-specificity of skin-derived T-cells and HIV co-receptor flow cytometry. A.M. performed the statistical analyses. L.C. and An.W. formulated the hypothesis and designed the study. L.C. provided funding for the study and led the writing of the paper. All authors contributed to critical revisions of the paper.
References
- 1.Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 2.Wald A, Link K. Risk of Human Immunodeficiency Virus (HIV) Infection in Herpes Simplex Virus Type-2 (HSV-2) Seropositive Persons: a Meta-Analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 3.Koelle D, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virolo. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald A, et al. Frequent genital HSV-2 shedding in immunocompetent women. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta R, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–1381. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 6.Corey L, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 7.Watson-Jones D, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celum C, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barcy S, Huang ML, Corey L, Koelle DM. Longitudinal analysis of herpes simplex virus-specific CD4+ cell clonotypes in infected tissues and blood. J Infect Dis. 2005;191:2012–2021. doi: 10.1086/430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark KE, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 14.Segerer S, Mac KM, Regele H, Kerjaschki D, Schlondorff D. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int. 1999;56:52–64. doi: 10.1046/j.1523-1755.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 15.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 16.Donaghy H, et al. A role for Plasmacytoid Dendritic Cells in the immune control of human recurrent herpes simplex. J Virol. 2008 doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pope M, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 18.Masten BJ, et al. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J Immunol. 2006;177:7784–7793. doi: 10.4049/jimmunol.177.11.7784. [DOI] [PubMed] [Google Scholar]
- 19.Dzionek A, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 20.Granelli-Piperno A, Shimeliovich I, Pack M, Trumpfheller C, Steinman RM. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J Immunol. 2006;176:991–998. doi: 10.4049/jimmunol.176.2.991. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brussel A, Sonigo P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J Virol. 2003;77:10119–10124. doi: 10.1128/JVI.77.18.10119-10124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Posavad CM, Koelle DM, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 25.Johnston C, et al. Impact of HIV infection and kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS ONE. 2009;4:e4222. doi: 10.1371/journal.pone.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 27.Rebbapragada A, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. Aids. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 28.Estes JD, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 29.Weiler AM, et al. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated simian immunodeficiency virus SIVmac239. J Virol. 2008;82:4154–4158. doi: 10.1128/JVI.01947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Castro KG, et al. Transmission of HIV in Belle Glade, Florida: lessons for other communities in the United States. Science. 1988;239:193–197. doi: 10.1126/science.3336781. [DOI] [PubMed] [Google Scholar]
- 33.Hook EW, 3rd, et al. Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection in heterosexuals. J Infect Dis. 1992;165:251–255. doi: 10.1093/infdis/165.2.251. [DOI] [PubMed] [Google Scholar]
- 34.Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 35.Auvert B, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 37.Koelle DM, et al. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koelle DM, Huang J, Hensel MT, McClurkan CL. Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes. J Virol. 2006;80:2863–2872. doi: 10.1128/JVI.80.6.2863-2872.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hladik F, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford University Press; Oxford: 2002. [Google Scholar]