Abstract
General anesthetics variably enhance inhibitory synaptic transmission that relies on (-aminobutyric acid (GABA) and GABAA receptor function with distinct differences across brain regions. Activation of “extra-synaptic” GABAA receptors produces a tonic current considered the most sensitive targets for general anesthetics, particularly in forebrain neurons. To evaluate the contribution of poor drug access to neurons in slices, we tested the intravenous anesthetic propofol in mechanically isolated neurons from the solitary tract nucleus (NTS). Setting chloride concentrations to ECl = −29 mV made GABA currents inward at holding potentials of −60 mV. Propofol triggered pronounced but slowly developing tonic currents that reversed with 5 min washing. Effective concentrations in isolated cells were lower than in slices and propofol enhanced phasic IPSCs more potently than tonic currents (1 µM increased phasic decay time constant vs. >3 µM tonic currents). Propofol increased IPSC frequency (>3 µM), a presynaptic action. Bicuculline blocked all propofol actions. Gabazine blocked only phasic IPSCs. IPSCs persisted in TTX and/or cadmium but these agents prevented propofol-induced increases in IPSC frequency. Furosemide (>1 mM) reversibly blocked propofol-evoked IPSC frequency changes without altering waveforms. We conclude that presynaptic actions of propofol depend on a depolarizing chloride gradient across presynaptic inhibitory terminals. Our results in isolated neurons indicate that propofol pharmacokinetics intrinsically trigger the tonic currents slowly and the time course is not related to slow permeation or delivery. Unlike forebrain, phasic NTS GABAA receptors are more sensitive to propofol than tonic receptors but that presynaptic GABAA receptor mechanisms regulate GABA release.
Keywords: brainstem, anesthetic, autonomic, GABAA
1. Introduction
Inhibitory transmission is a critical part of brain function and general anesthesia. GABAA receptors located at the postsynaptic cell membrane are responsible for transient inhibition during inhibitory postsynaptic currents (IPSCs). These transient or phasic IPSCs are a well established target for general anesthetics which enhance or prolong the activation of GABAA receptors. Recent evidence indicates that a second form of “extra-synaptic” GABAA receptors mediates a longer-lasting, low-amplitude synaptic current and these pharmacologically distinct, tonic GABAA receptors are more sensitive to general anesthetics than the receptors responsible for phasic IPSCs (Farrant and Nusser, 2005;Orser, Canning, and MacDonald, 2002). Phasic GABAA inhibitory currents are blocked by low concentrations of the GABAA antagonist, gabazine, but leave tonic inhibitory currents intact whereas bicuculline blocks both the phasic and tonic currents (Semyanov, Walker, and Kullmann, 2003). Recent work in our lab suggests that both phasic and tonic GABAA receptors are present in brainstem neurons in the nucleus of the solitary tract (NTS) (McDougall, Bailey, Mendelowitz, and Andresen, 2008). However, unlike forebrain neurons (Bieda and MacIver, 2004; Hemmings, Jr., Akabas, Goldstein, Trudell, Orser, and Harrison, 2005), GABAA receptor mediated tonic currents in NTS second order neurons are less sensitive to the general anesthetic propofol than are phasic GABAA receptor mediated currents.
Limitations of slice work include potential concerns about drug access and reversibility of lipophilic substances. Previous work in hippocampal slices (Gredell, Turnquist, MacIver, and Pearce, 2004) has suggested that diffusion of propofol is quite slow and this likely affects effective drug concentrations at the neurons. Limited reversal of propofol responses also fundamentally limits testing protocol such as repeated trials within neurons. To improve drug access to these neurons, we studied NTS neurons isolated from medial portions of NTS (Jin, Bailey, Li, Schild, and Andresen, 2004). We harvested mechanically isolated neurons with intact native synaptic terminals using a vibrating stylus using no enzymes. This approach allowed harvest of neurons from carefully delimited sub regions. A fast perfusion system optimized propofol access to these neurons and allowed repeated testing on single neurons. Throughout these experiments, we recorded from neurons voltage clamped to −60 mV and chloride concentrations were set to yield inward IPSCs at this holding potential (calculated ECl = −29 mV). Studying isolated neurons allowed us to address three critical aspects of propofol actions in NTS neurons. First, does the slow time course of propofol actions in brain slices reflect poor access or is it a property of propofol action itself? Second, does direct access alter the effective concentrations of propofol? Third, does propofol act presynaptically on GABA release frequency?
2. Results
Non-enzymatic, mechanical dispersion yielded single, isolated NTS neurons that retained functioning synaptic boutons whose synaptic responses to spontaneous neurotransmitter release could be studied (Jin, Bailey, and Andresen, 2004). Both glutamate- and GABA-releasing boutons were present and these classes of synaptic events could be distinguished by their characteristic event kinetics as well as their pharmacological responses. Glutamatergic EPSCs had rapid decay phases and smaller amplitudes than GABAergic IPSCs which had slower decay time constants and larger amplitudes. These synaptic events in isolated neurons pharmacologically closely resemble in all respects (see below) those recorded in second order, medial NTS neurons recorded in situ in brainstem slices (McDougall, Bailey, Mendelowitz, and Andresen, 2008). For the present studies, spontaneously occurring IPSC events (sIPSCs, Figure 1A) were pharmacologically isolated by recording in the continuous presence of ionotropic glutamate receptor blockers (20 µM NBQX and 100 µM AP-5). In order to record these IPSCs and GABAA receptor-dependent currents at near resting potentials, we used ionic conditions with chloride gradients (calculated ECl = −29 mV) across the recorded NTS neuron membrane that yielded net inward currents at −60 mV holding potentials.
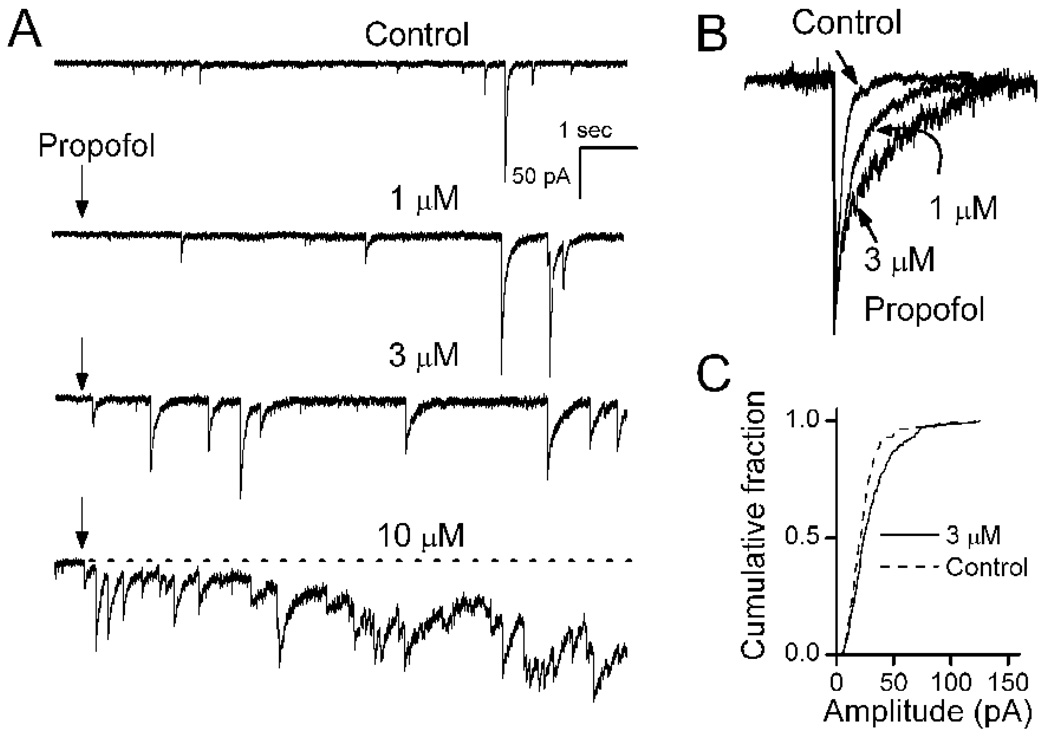
Figure 1.
Spontaneously released GABA produces sIPSCs recorded in mechanically isolated NTS neurons. A. Propofol (arrow shows onset) produced three distinct effects indicated in this single representative NTS neuron recorded at a holding potential of −60 mV (VH). Under our ionic conditions, chloride gradients (calculated ECl = −29 mV) produced inward currents at this VH. Application of 1 µM propofol rapidly prolonged the duration of each sIPSC. B. Propofol prolongs the decay phase of sIPSCs in peak normalized traces on an expanded scale from control, 1 and 3 µM propofol at approximately 5 s exposure time (all events extracted from longer records to the left). At higher concentrations, propofol increased the frequency of sIPSCs and evoked a tonic current indicated as an inward shift from the baseline holding current (broken line) at constant VH. C. Cumulative amplitudes distribution for this neuron shows that sIPSCs were unaltered by 3 µM propofol (K-S test, p<0.05, 114 control events and 231 propofol events). Overlapping compound sIPSCs prevented meaningful analysis of amplitudes in 10 µM propofol although event onsets could generally still be discriminated. Each propofol concentration was tested for 3–4 min (left traces show only the initial ten seconds). Propofol was washed out with normal ACSF (minimum of 5 min, not shown) before application of the next concentration. Control ACSF contained NBQX and AP5 to block glutamatergic synaptic currents and isolate IPSCs for study.
Propofol enhances phasic and tonic GABAA currents
Under control conditions in isolated NTS neurons, sIPSCs varied widely in amplitude and generally occurred at frequencies of about 0.5 Hz. Application of propofol rapidly evoked increases in the duration of sIPSCs that incremented with concentration (Figure 1, inset). We rapidly delivered drugs directly to the neurons using a Y-tube placed within 100 µm of the cell surface (Murase, Ryu, and Randic, 1989). In each case, control solution perfused the cells and responses were initiated by quickly switching to a new drug containing solution. This method delivers concentration changes that are complete within 10 ms. Propofol responses began in less than 1 s from the onset of drug, the frequency and duration of sIPSCs was incremented. However, at concentrations >3 µM, propofol evoked a slowly-developing increasing inward shift in the holding or baseline current that required many seconds to reach a maximum (Figure 1). The tonic current evoked by propofol began within 1 s from the onset of drug. The propofol-induced prolongation of sIPSCs (Figure 1B) and the changes in the tonic level of the holding current correspond to actions attributed to postsynaptic sites of action on “phasic” and “tonic” GABAA receptors, respectively, as observed in other neurons (Farrant and Nusser, 2005;Mody and Pearce, 2004). Amplitudes of sIPSCs were not altered by propofol (Figure 1C). At the high concentrations, propofol often increased sIPSC frequency, evidence of a presynaptic action on GABA release.
Bicuculline rapidly blocks all propofol actions
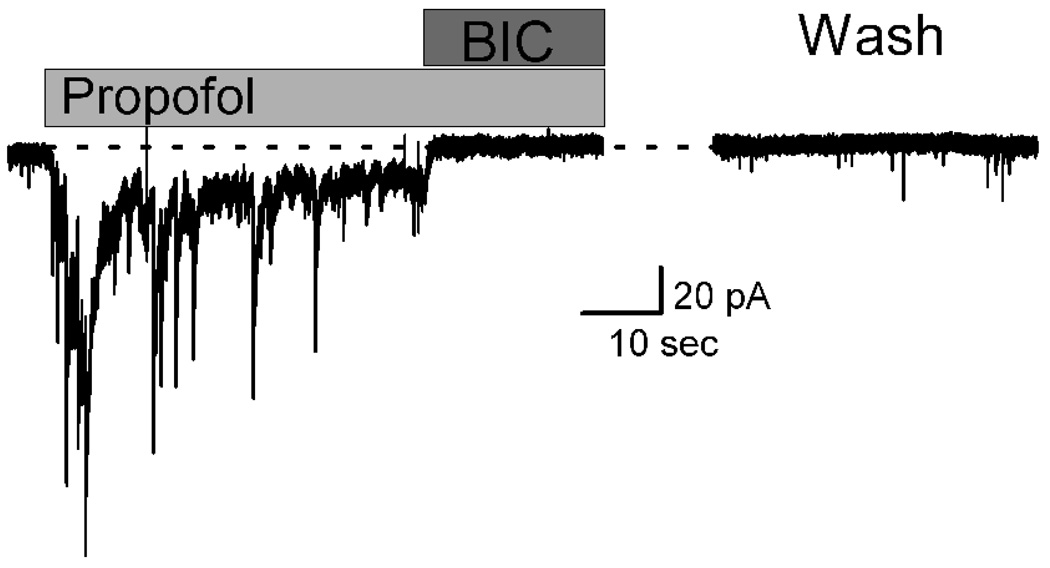
Propofol actions to slow decay kinetics, increase sIPSC frequency and evoke a steady tonic current were eliminated by a high concentration of bicuculline (Figure 2) and thus both pre- and postsynaptic actions of propofol depend on GABAA receptor function. Despite a rapid arrival (<10 ms) of propofol using the y-tube, IPSC decay kinetics were apparent within 1 s (Figure 1) but the peak tonic current developed over 5–10 s (Figure 2). Bicuculline (100 µM) rapidly and completely blocked both the phasic IPSCs as well as the tonic current within <1 s (Figure 2). The holding current level in bicuculline was equal to the pre-propofol level of current. This suggests that the GABAA receptors responsible for the tonic current response were not contributing to the resting holding current during the control period. Consistent with this conclusion, application of bicuculline alone had no effect on baseline holding current in these neurons (results not shown). Returning to the normal perfusion solution (Wash) reversed these effects within 4–5 min and sIPSCs returned (Figure 2).
Figure 2.
Bicuculline (BIC, 100 µM) blocked all actions of propofol including the evoked tonic currents. Propofol (10 µM) evoked a shift in holding current (i.e. tonic current) and this tonic current peaked within 10 sec before subsiding to a lower sustained level. Simultaneous with the increase in tonic current, propofol induced prolongation of phasic IPSCs. BIC rapidly eliminated both the intermittent sIPSCs as well as the sustained propofol-induced tonic current. Dotted line shows the control holding current level. Three minutes following return to the control ACSF that contained NBQX and AP5, sIPSCs and holding current returned to basal levels. Traces were from a single representative neuron.
Tonic currents induced by propofol are chloride selective
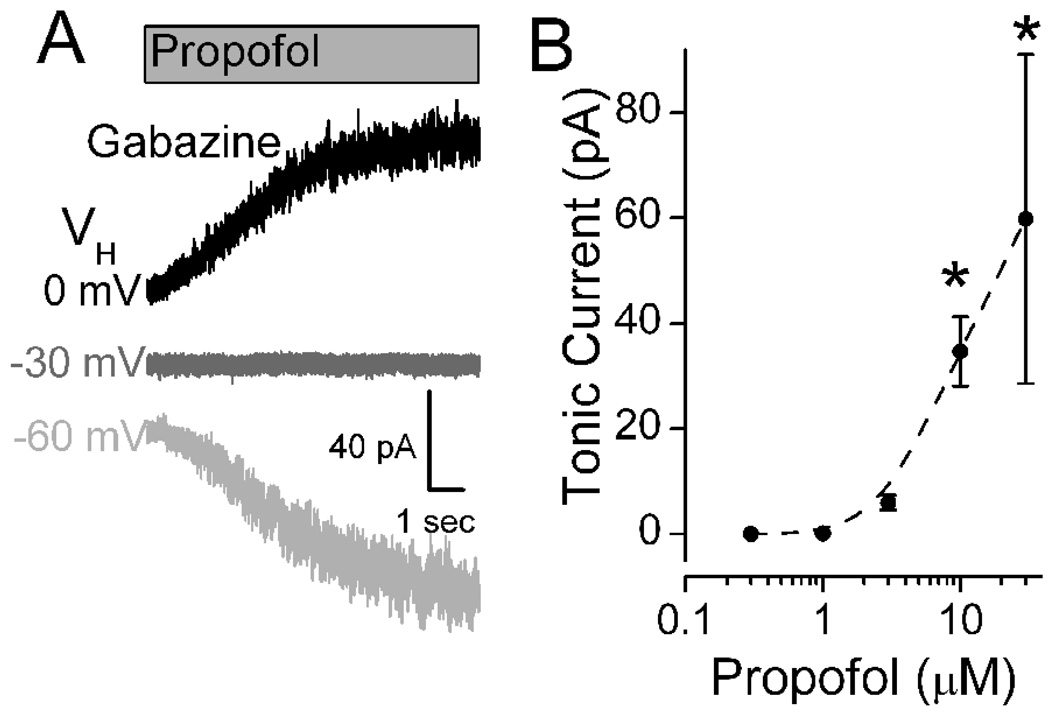
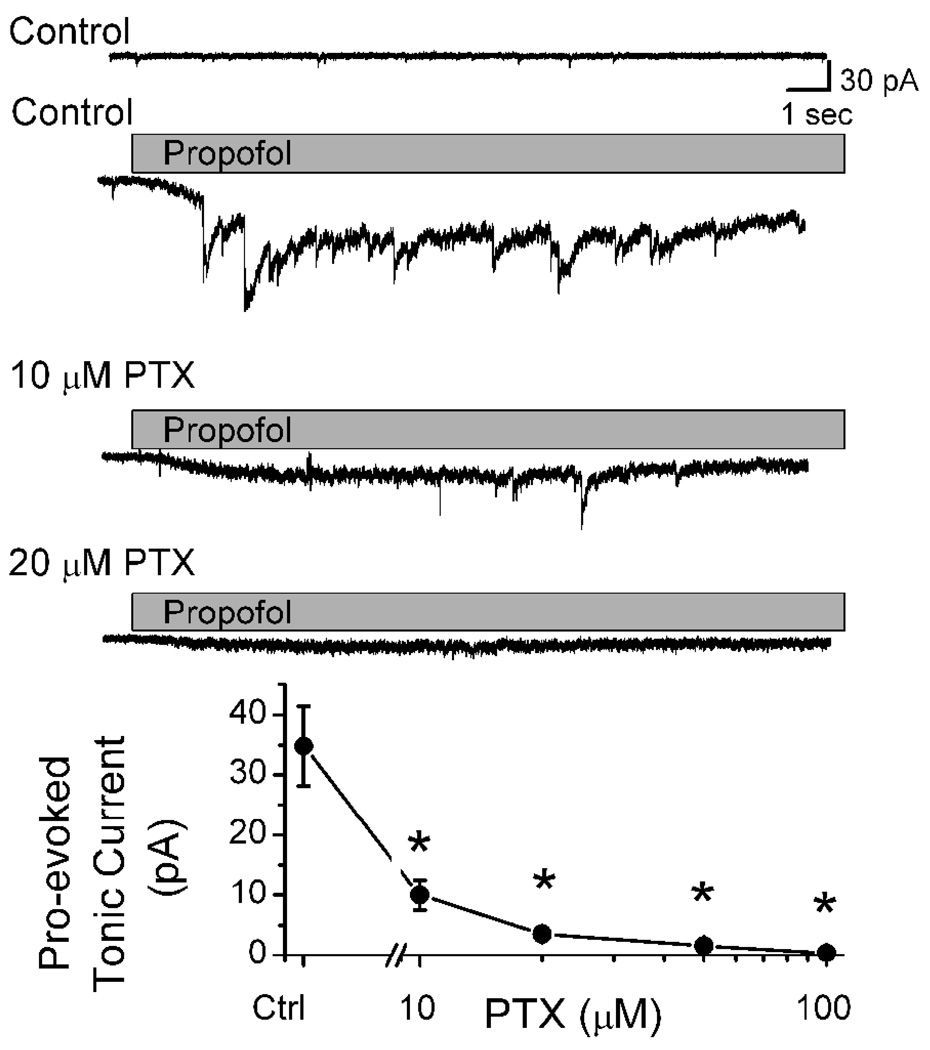
Low concentrations of gabazine (6 µM) blocked phasic sIPSCs, but addition of propofol (10 µM) evoked the characteristic, slowly developing tonic current even in the absence of phasic IPSCs (Figure 3A). The propofol-induced tonic current was associated with a substantial increase in small current fluctuations (“noise”) about the mean tonic current. To test the reversal potential of the propofol-evoked tonic current, the holding potential VH was changed and the propofol test repeated. Shifts in VH to more depolarized levels reduced and then reversed the propofol-evoked tonic current to outward (Figure 3A). The reversal potential for propofol-evoked tonic current was −30 mV and equaled ECl for the recording conditions (Figure 3A). Even under identical conditions, however, the magnitude of the propofol-evoked tonic current was quite variable across neurons. Although highly variable across individuals, propofol ≥10 µM evoked a significant tonic current (Figure 3B, p < 0.001). At 10 µM, propofol induced tonic currents in gabazine that averaged 34.7±6.64 pA (n=15) and were not different from those without gabazine (31.9±14.6, n=6, p=0.84). Picrotoxin, an allosteric Cl− channel pore blocker (Newland and Cull-Candy, 1992)), attenuated both the phasic and tonic postsynaptic current responses to propofol (Figure 4).
Figure 3.
Propofol evoked a shift in the mean level of tonic current following block of phasic currents. A. Voltage dependence of propofol-evoked tonic current. All traces recorded from same cell in the presence of NBQX, AP5 and gabazine (6 µM), a combination that blocked all phasic synaptic events including sIPSCs. At VH = −60 mV, propofol (10 µM) evoked a slowly increasing tonic inward current, but that current was outward at VH = 0 mV. No current was observed at VH = −30 mV. Levels of VH are indicated by shading: black, gray and light gray as 0, −30 and −60 mV respectively. Under these experimental recording conditions, ECl was −30 mV. Traces measured in a single representative NTS neuron. Indicated potentials are corrected for the liquid junction potential.
B. Propofol increased the tonic inward current at VH = −60 mV in a concentration dependent manner. Data points represent the peak current means ± SEM for 4–19 different neurons. Asterisks mark significant differences in mean responses from Control (Repeated Measures ANOVA with Holm-Sidak method of post hoc pairwise comparisons at p<0.05).
Figure 4.
Picrotoxin (PTX) attenuated propofol-induced changes in both phasic and tonic GABAA currents in a concentration-dependent fashion. In a single representative neuron, 10 µM propofol induced both a tonic inward current and prolonged sIPSCs in Control (compare upper two original traces). Note that sIPSCs were typically quite brief and infrequent in Control. Propofol evoked changes were attenuated by 10 or 20 µM PTX and sIPSCs became less frequent. Traces show both pre- and postsynaptic actions of propofol within a single representative neuron (VH = −60 mV). On average (lower graph), PTX inhibited propofol induced tonic currents to near baseline levels at or above 10 µM (n= 4 to 8 different neurons). Points indicate mean and SEM.
Propofol potently prolongs phasic GABAA currents
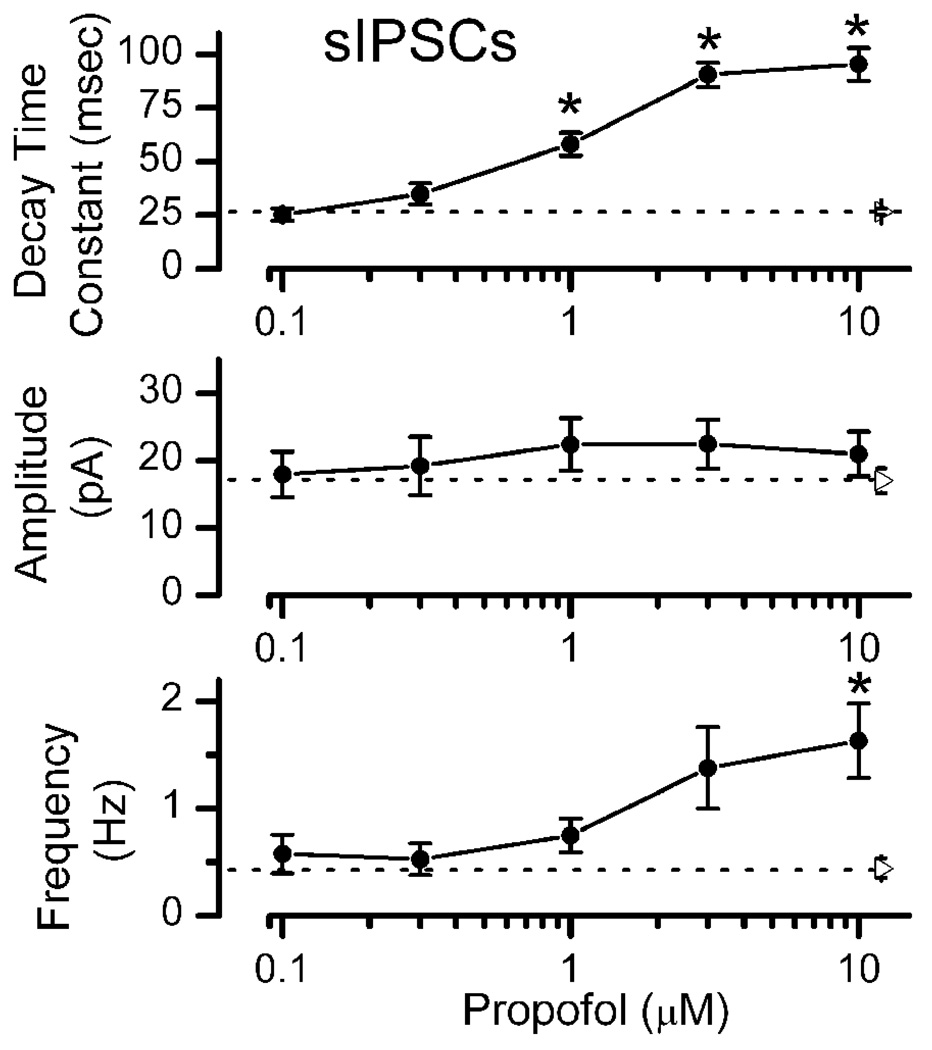
Clearly, the tonic GABAA current activation requires very high concentrations of propofol. In contrast, on average (n=17), 1 µM propofol increased the mean decay time constant of sIPSCs concentration dependently (propofol ANOVA main effects p<0.001, Figure 5) by a greater than two-fold change compared to control. Threshold effects on IPSC kinetics lie between 0.3 and 1 µM and correspond to the calculated clinical range for general anesthesia (Franks and Lieb, 1994). Propofol failed to alter sIPSC amplitude (propofol ANOVA main effects p = 0.69). On average, relatively high concentrations (10 µM) of propofol were required to significantly increase the mean sIPSC frequency (propofol ANOVA main effects p<0.001, Figure 5). It should be noted that sIPSC frequency effects of propofol were quite variable and significant frequency effects were observed in some neurons at 3 µM (K-S test on amplitude distributions comparing control to propofol within individual neurons were not significant, results not shown).
Figure 5.
Summary of propofol concentration-response relationships for sIPSC event characteristics. Decay time constant of sIPSC increased at ≥1 µM propofol. Amplitudes of sIPSC were unaltered by propofol (p>0.7) but sIPSC frequency increased in 10 µM propofol. Filled circles are means ± SEM. Right pointing triangles and broken lines indicate the Control mean value ± SEM for each parameter (n=17). Means in each condition were based on 5–17NTS neurons and significant differences from Control are noted with asterisks (Repeated Measures ANOVA with Holm-Sidak method of post hoc pairwise comparisons at p<0.05).
Propofol-induced increases in sIPSC frequency require calcium and sodium channel signaling
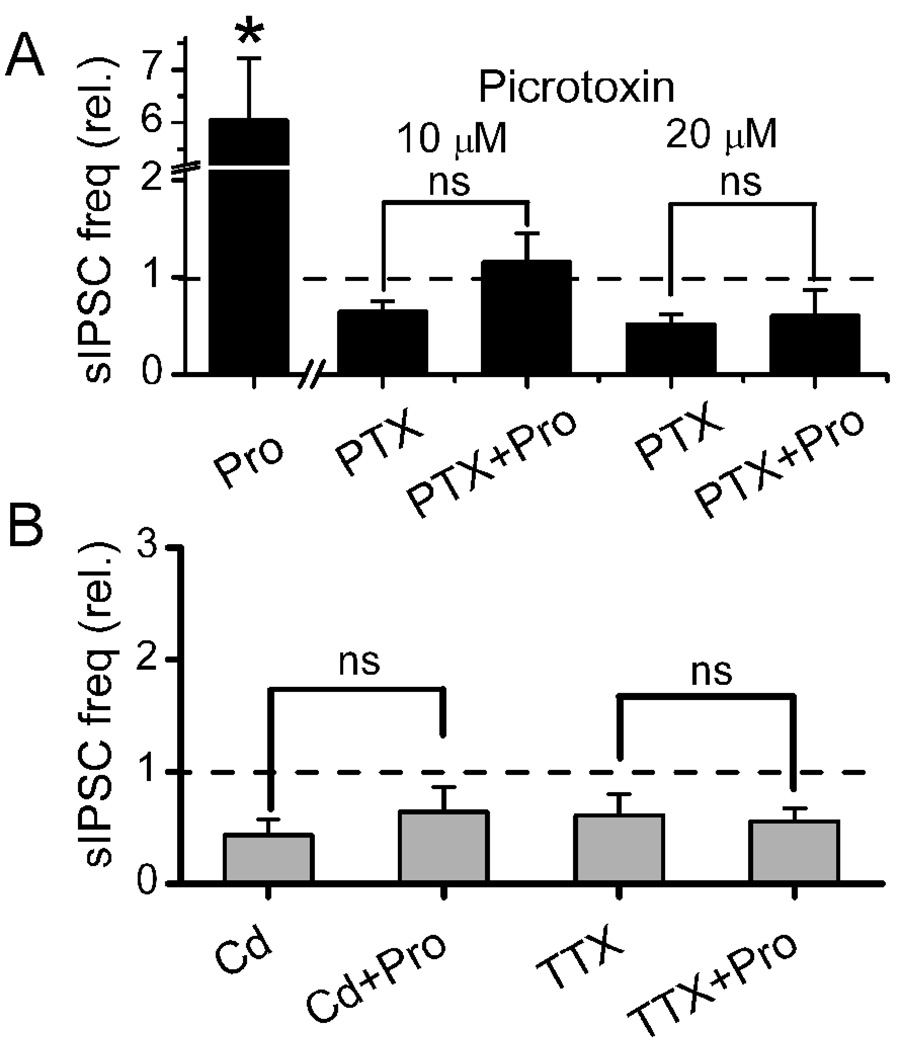
Changes in the frequency of sIPSCs reflect presynaptic changes that govern GABA release and although our protocol conditions fixed the chloride gradients across the postsynaptic membrane, the presynaptic chloride gradient was unknown. Propofol (10 µM) increased sIPSC frequency by an average of nearly six fold consistent with a presynaptic facilitation of GABA release. Such a result is consistent with propofol triggering an inward or depolarizing current within GABAergic terminals. As with bicuculline (Figure 2), this propofol-evoked sIPSC frequency increase was blocked by 10 µM picrotoxin (Figure 6A), a finding consistent with propofol-actions on presynaptic GABAA receptors on GABA terminals. To better understand the mechanism of presynaptic sites of propofol action, we tested whether voltage-dependent processes such as action potential driven mechanisms might be critical. As voltage-dependent Ca2+ channels likely contribute to GABA release by action potentials, we blocked these channels with Cd2+ and found that not only was basal release of GABA inhibited but the propofol-evoked increases in IPSC frequency were eliminated (Figure 6B, left). Consistent with the Cd2+ finding, block of voltage-dependent Na+ channels with TTX reduced the basal frequency of sIPSCs and addition of propofol failed to augment GABA release (Figure 6B, right). Thus, presynaptic actions by propofol appear to rely on contributions of two key presynaptic ion channels, calcium channels and sodium channels to increase the frequency of sIPSCs. These results are consistent with a response in which propofol induces a presynaptic depolarization and that this depolarization leads to GABA release.
Figure 6.
Propofol induced changes in the frequency of sIPSCs suggest presynaptic actions. Propofol at 10 µM evoked increases in the frequency of sIPSCs (Panel A). Picrotoxin (PTX) at both 10 µM and 20 µM blocked the propofol evoked increases. Blockade of voltage-dependent Ca++ or Na+ channels by Cd2+ (200 µM) or TTX (300 nM), respectively, prevented propofol induced increases (brackets show no difference in propofol, p>0.05, Panel B). Thus, the presynaptic actions of propofol appear to depend on depolarization. TTX or Cd2+ alone reduced IPSC frequency to 61.4±16.8% and 43.4±14.0% of the control, respectively, and suggest that voltage dependent processes contributed to basal sIPSC activity. All data have been normalized to the control frequency of sIPSC before propofol. Broken line indicates control levels of activity.
Blocking chloride transport prevents propofol-induced increases in sIPSC frequency
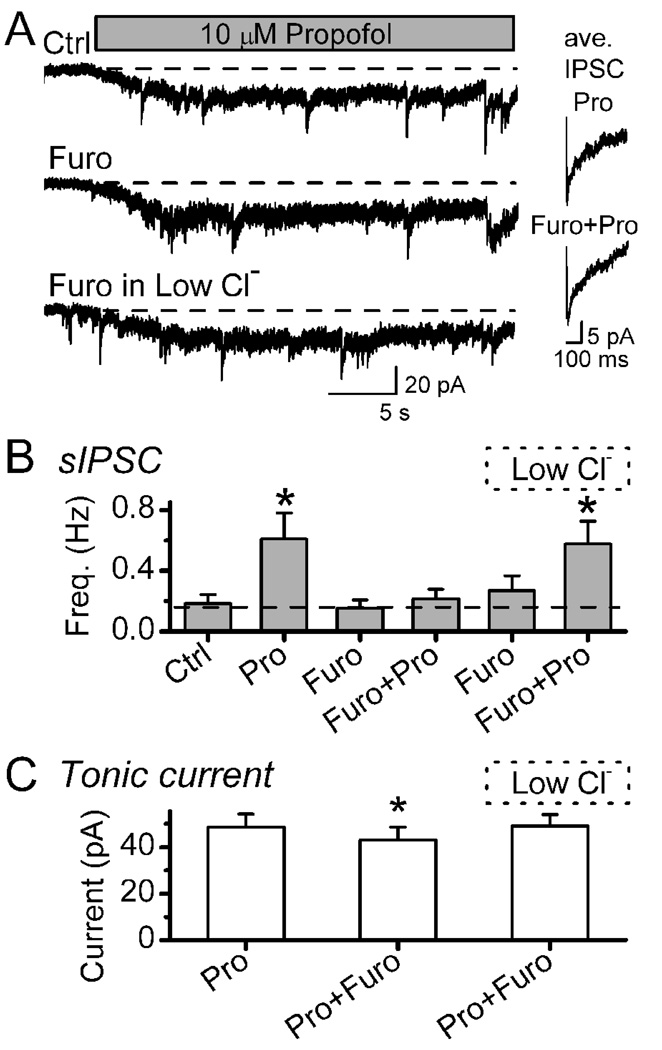
Our results suggest that propofol acts on presynaptic terminals via GABAA receptors. Like the postsynaptic responses at GABAA receptors, presynaptic depolarization via GABAA receptors should depend on the electrochemical gradient of Cl− in this case across the terminal membrane. Since GABAA receptor blockade prevented IPSCs, we tested whether interrupting the Cl−gradient might alter the increase in GABA release evoked by propofol. Pretreatment for 5 min with furosemide (1 mM) did not significantly alter sIPSC basal frequency (Figure 7A,B) and prevented the propofol-evoked increase in sIPSCs (Figure 7B, p=0.92, n=6). The amplitudes of sIPSCs were unaltered (p>0.05) by furosemide presumably due to a concentration gradient for these postsynaptically localized receptors that was fixed by the pipette and bath conditions. While the frequency of sIPSCs was not increased by propofol during furosemide (Figure7B), propofol slowed the relaxation kinetics of the sIPSCs, a postsynaptic property that was preserved in furosemide (Figure 7A insets). Thus, furosemide did not alter GABAA receptor function in NTS neurons, a finding unlike reports of antagonist properties in limited brain regions such as the cerebellum (Korpi, Kuner, Seeburg, and Luddens, 1995;Korpi and Luddens, 1997). Furosemide effects were readily reversed by washing and only very modestly reduced the propofol-evoked tonic currents in such neurons (Figure 7C, n=5). To test whether the terminal chloride gradient was responsible, we repeated the propofol exposure after furosemide treatment after rapidly changing to a new external solution containing a lower Cl− concentration (40 mM NaCl was replaced with 40 mM Na-methanesulfonate). Although we cannot know what the chloride gradient across the terminal is precisely, this maneuver should restore an inward gradient for Cl− and, as predicted, this restored the propofol-evoked increase in sIPSCs (Figure 7 A–C). Thus, short exposures to furosemide concentrations sufficient to block Cl− transport differentially blocked pre- but not postsynaptic mechanisms and suggest that both actions were mediated by GABAA receptors.
Figure 7.
Furosemide (Furo) selectively blocked propofol-evoked increases in sIPSC frequency without altering sIPSC kinetics. In a representative neuron (Panel A), propofol (Pro, 10 µM) increased the sIPSC frequency and evoked a tonic current in Control (Ctrl), but following 5 min incubation in furosemide (1 mM, middle trace), the propofol-induced increase in sIPSC frequency was eliminated. To provide a chloride gradient across the terminal membrane following Furo treatment, rapidly perfusing with a low chloride solution (replacement of 40mM NaCl with 40mM Na-methansulfonate) restored the increased sIPSC response to propofol (lower trace). Insets (right) show that sIPSC average waveforms were unaltered by Furo. Monoexponential fits using a least squares fitting routine yielded average descriptions of sIPSCs as follows: In control propofol, sIPSC trace is an average of 17 events with a rise time (10%–90%) of 2.94 ms and decay time (90%–37%) of 109.0 ms (R2 = 0.93). In Furo+Pro, sIPSC trace showed similar kinetics with an average of 27 events with a rise time (10%–90%) of 1.97 ms and decay time (90%–37%) of 112 ms. (R2 = 0.99). Low chloride solution did not alter sIPSC kinetics (not shown). Wash between trials with ACSF reversed these changes to control levels within 6–7 min. Traces taken from same neuron and recorded at VH −60 mV. Broken line indicates control levels of activity. On average across neurons (n=6, Panel B left), propofol increased mean sIPSC frequency substantially compared to Ctrl, Furo and Furo+Pro conditions and thus but furosemide blocked the normal increase to propofol (Pairwise Multiple Comparison Procedures using Student-Newman-Keuls Method). In the low chloride condition, Furo did not alter sIPSC rate but Pro now significantly increased the sIPSC rate. Panel C displays the mean changes in tonic current evoked by Pro in each condition. Asterisks mark significant differences from Control (Repeated Measures ANOVA, *p<0.005). Points are means ±SEM.
3. Discussion
Inhibitory transmission within the NTS critically shapes normal visceral afferent signal processing (Andresen and Kunze, 1994;Andresen and Mendelowitz, 1996;Bailey, Appleyard, Jin, and Andresen, 2008). General anesthetics enhance GABAA receptor function in NTS without affecting glutamatergic transmission (McDougall, Bailey, Mendelowitz, and Andresen, 2008;McDougall, Peters, LaBrant, Wang, Koop, and Andresen, 2008;Peters, McDougall, Mendelowitz, Koop, and Andresen, 2008). In earlier work on propofol, we found the onset and duration of propofol actions to be quite slow whereas isoflurane was more rapid (McDougall, Bailey, Mendelowitz, and Andresen, 2008;McDougall, Peters, LaBrant, Wang, Koop, and Andresen, 2008;Peters, McDougall, Mendelowitz, Koop, and Andresen, 2008). One explanation relates to differences in diffusion and slow access of propofol to neurons in slices suggested in earlier work (Gredell, Turnquist, MacIver, and Pearce, 2004).
With the improved drug access of isolated NTS neurons and focal drug delivery, the major findings of the present work indicate that propofol responses were readily reversible and phasic GABAA responses had more rapid onsets than effects on tonic GABAA currents (compare Fig. 1 of the present work with Fig. 2 of the slice recordings (McDougall, Bailey, Mendelowitz, and Andresen, 2008). The reversibility of propofol effects by washing in this system permitted multiple tests with recovery within single neurons. Thus, propofol rapidly (1–2 s) enhanced GABAA receptor-mediated IPSCs by slowing their decay time constants at concentrations ≤1 µM – a level consistent with propofol general anesthesia (Franks and Lieb, 1994) and one-tenth of the level required to discriminate similar change in sIPSC kinetics at similar neurons in NTS slices (McDougall, Bailey, Mendelowitz, and Andresen, 2008). Interestingly, propofol activated a sustained, tonic current only after a considerable delay and required higher concentrations (10 µM) despite the improved local perfusion of isolated NTS neurons. This temporal difference (phasic vs. tonic currents) indicates that the delay is unlikely to arise from poor drug access and must represent an intrinsic characteristic of propofol actions at the tonic GABAA receptors. In addition, propofol enhanced the rate of spontaneous release of GABA as a result of a presynaptic GABAA receptor action - a finding not observed in situ. Both presynaptic and postsynaptic actions of propofol depended on the Cl− gradient and were blocked by the GABAA receptor antagonist, bicuculline. Thus, this new report identifies three sites of propofol action: two distinct postsynaptic GABAA receptor actions on separate phasic and tonic populations that could be discriminated pharmacologically and kinetically plus a presynaptic site of action at GABAA receptors that affected GABA release frequency.
Slow propofol gating of tonic GABAA currents
Our rapid delivery system coupled with isolated NTS neurons clearly indicates that the delayed (minutes) propofol actions in observed in slices (both phasic and tonic GABAA responses) was due to slow delivery of propofol as document by others (Gredell, Turnquist, MacIver, and Pearce, 2004). The development of the propofol-evoked tonic current in isolated neurons required many seconds even at very high concentrations of propofol. Note that in isolated neurons antagonists such as bicuculline or gabazine acted quite rapidly on both phasic and/or tonic currents so that drug access to the neurons did not contribute to the timing differences in these responses. However, the slow development of the tonic current at extrasynaptic GABAA receptors observed in our isolated neurons must reflect a characteristically different mechanism to evoke the sustained tonic Cl− current compared to the rapid mechanism which changes phasic sIPSC decay kinetics. These gabazine-insensitive, propofol-activated tonic currents in NTS neurons may be similar to GABAA receptors in hippocampal pyramidal neurons in which direct channel gating by propofol in the absence of GABA has been suggested (Adodra and Hales, 1995;McCartney, Deeb, Henderson, and Hales, 2007).
Tonic GABAA currents are less sensitive to propofol than phasic currents
At least 16 different genes encode GABAA subunits that assemble into diverse GABAA receptors and these receptors vary characteristically across brain regions (Costa, 1998;Mody and Pearce, 2004;Rudolph and Antkowiak, 2004). In medial NTS neurons, propofol revealed a pharmacological profile of potency order for tonic (“extrasynaptic”) and phasic GABAA currents that was distinctly different than common forebrain patterns. Propofol increased decay time constants for sIPSCs at ≤1 µM whereas the tonic current required 10 µM or more in our NTS neurons. This order of potency for propofol is the reverse of observations in hippocampal and neocortical neurons in which tonic currents are considered more sensitive to anesthetics than phasic IPSCs (Bai, Zhu, Pennefather, Jackson, MacDonald, and Orser, 2001;Belelli, Peden, Rosahl, Wafford, and Lambert, 2005;Caraiscos, Newell, You, Elliott, Rosahl, Wafford, MacDonald, and Orser, 2004;Drasbek, Hoestgaard-Jensen, and Jensen, 2007;Hemmings, Jr., Akabas, Goldstein, Trudell, Orser, and Harrison, 2005). It should be noted, however, that our nominal cited concentrations of propofol may over-estimate the actual concentrations of propofol exposure by two fold as suggested by direct measurements (Murugaiah and Hemmings, Jr., 1998). The tonic current evoked by propofol in our NTS neurons was similar in the presence or absence of phasic IPSCs that were selectively eliminated by 6 µM gabazine. The propofol-evoked tonic current reversed at the equilibrium potential for our Cl− conditions. Together the results suggest that propofol acts at both synaptic and extra-synaptic GABAA receptors but with distinctly different concentration dependence which likely reflect subunit differences for the two classes of GABAA receptors within NTS neurons. Presumably, the rank order switch with tonic currents less sensitive in NTS neurons also reflects a different subunit composition compared to forebrain regions (Kasparov, Davies, Patel, Boscan, Garret, and Paton, 2001;Milligan, Buckley, Garret, Deuchars, and Deuchars, 2004).
Presynaptic GABAA receptors mediate propofol-increased GABA release
We observed increases in the frequency of sIPSCs during application of propofol, a response consistent with a presynaptic action to facilitate GABA release. To test whether an inward Cl− current might be responsible for this propofol action, we tested the transport blocker furosemide. We reasoned that if presynaptic propofol actions depolarized GABAergic terminals by enhancing GABAA receptor function then this process might depend on the Cl− gradient, development and transporter expression (DeFazio, Keros, Quick, and Hablitz, 2000;Rivera, Voipio, Payne, Ruusuvuori, Lahtinen, Lamsa, Pirvola, Saarma, and Kaila, 1999). The Cl− gradient across the terminal membrane of our NTS boutons is unknown especially at room temperature but the gradient likely depends on a Na-K-2Cl symporter that can be blocked by high concentrations of furosemide (Jarolimek, Lewen, and Misgeld, 1999). Furosemide incubation selectively and reversibly eliminated the propofol-evoked increase in sIPSC frequency without altering the propofol evoked tonic current and the propofol-evoked increase in sIPSC frequency was restored by quickly stepping to an external solution containing low Cl−. Note that the presence of sIPSCs with unaltered decay kinetics in high concentrations of furosemide indicates this compound does not alter postsynaptic GABAA receptors in medial NTS neurons (Huang and Dillon, 2001;Korpi and Luddens, 1997). Responses of NTS neurons to exogenous GABA are not altered by furosemide (Huang and Dillon, 2001). The increase in sIPSC rate during propofol was surprising as it was not observed in our previous slice work in medial NTS neurons (McDougall, Bailey, Mendelowitz, and Andresen, 2008). One possible explanation is that the Cl− gradient and reversal potential might be near the resting potential across the GABA terminals at physiological temperatures in those developmentally mature animals. In our isolated neurons from immature animals at room temperature, the Cl− gradient appears to be depolarizing. Presynaptic actions of anesthetics at GABAA receptors to enhance GABA release have been observed in isolated cortical synaptosomes (Murugaiah and Hemmings, Jr., 1998) and in reticular thalamic neurons (Ying and Goldstein, 2005).
Our results in isolated NTS neurons suggest that propofol rapidly increased the sIPSC frequency and this appears to depend on an inward Cl− current in terminals which initiates depolarization to trigger GABA release. Consistent with this idea, exposure to low concentrations of Cd+2 which blocks voltage-dependent Ca+2 channels (Sher, Biancardi, Passafaro, and Clementi, 1991) prevented propofol-induced increases in sIPSCs. Similarly TTX block of voltage-dependent Na+ channels prevented propofol-induced increases in the frequency of sIPSCs. These interventions in GABA release are similar to the roles that voltage-dependent channels play in the release of glutamate in these neurons during pharmacological activation of non-selective cation channels including TRPV1 or P2X3 receptors (Jin, Bailey, Li, Schild, and Andresen, 2004). Thus, GABA release during propofol was eliminated by TTX indicating that terminal depolarization likely generated action potentials and was required for propofol to increase GABA release. Likewise, the absence of propofol actions at glutamatergic terminals (unpublished results and (McDougall, Bailey, Mendelowitz, and Andresen, 2008) suggests that the presynaptic site of action is specific to GABA terminals.
Consistent with our previous studies in cardiac nucleus ambiguus neurons (Wang, Huang, Gold, Bouairi, Evans, Andresen, and Mendelowitz, 2004), the prolongation of phasic IPSCs in NTS neurons may result from a decrease in GABAA receptor desensitization and deactivation. Tonic activation of GABAA receptors, however, will depress intrinsic neuronal excitability by suppressing the activation of voltage dependent processes. The modulation of tonic inhibition and its pharmacological specificity depend on the subunit composition of GABAA receptors and their subcellular distribution so that for example the γ2 and ρ subunits are expressed in brainstem and associated with extrasynaptic localization (Fritschy and Brunig, 2003;Kasparov, Davies, Patel, Boscan, Garret, and Paton, 2001;Milligan, Buckley, Garret, Deuchars, and Deuchars, 2004). Our studies, however, cannot identify subunit composition.
In conclusion, the present results suggest that NTS neurons express GABAA receptors postsynaptically that produce both phasic IPSCs as well as tonic currents with pharmacological / kinetic profiles that differ from the tonic / phasic GABAA profile typical of forebrain neurons. Presynaptically on GABA terminals in NTS, GABAA receptors regulate release that is altered by general anesthetics such as propofol. The interactions with propofol suggest that unlike in forebrain neurons, clinically relevant concentrations of propofol act predominantly to enhance phasic IPSCs through multiple mechanisms and thus will impact temporal interactions with phasic excitatory inputs rather than globally suppressing neuron activity through tonic hyperpolarization. The differential pharmacological profiles are consistent with at least three functionally distinct groups of GABAA receptors in NTS. Neurons within the medial sub nucleus of NTS are involved in cardiorespiratory regulation (Andresen and Kunze, 1994). Thus, our studies suggest that multiple targets likely participate in the reflex depression documented in healthy patients in whom sedative doses of propofol diminish the normal reflex tachycardia to hypotension and significant decreases mean blood pressure with enhanced orthostatic hypotension (Ebert, 2005;Reves, Glass, and Lubarsky, 2000).
4. Experimental Procedure
NTS slices
Hindbrains of male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were prepared from 2- to 3-week-old rats as described previously (Jin, Bailey, and Andresen, 2004). All of the animal procedures were conducted with the approval of the University Animal Care and Use Committee in accordance with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and National Institutes of Health Guide for the Care and Use of Laboratory Animals. The hindbrain was removed and placed in ice-cold artificial CSF (ACSF) composed of the following (in mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 dextrose, and 2 CaCl2, and bubbled with 95% O2-5% CO2. The medulla was trimmed to a 1 cm block (rostral-caudal) centered on the obex. A wedge of tissue was removed from the ventral surface to align the ST within a cutting plane that contained >1 mm of ST in the same plane as the NTS (Doyle, Bailey, Jin, Appleyard, Low, and Andresen, 2004) when mounted in a vibrating microtome (VT-1000S; Leica, Nussloch, Germany). Slices (150–170 µm thick) were cut with sapphire blades (Delaware Diamond Knives, Wilmington, DE). The preparation of these slices was identical to that for in situ recordings of such neurons from slices without dissociation (Jin, Bailey, and Andresen, 2004;Jin, Bailey, Li, Schild, and Andresen, 2004).
Mechanical dissociation
Horizontal brainstem slices were then preincubated (1–3 hr at 31°C) in well-bubbled ACSF before mechanical dispersion. For dispersion, brainstem slices were transferred to custom-made glass bottom perfusion chamber filled with standard external solution containing the following (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH was adjusted to 7.4 with Tris-base). A glass pipette was pulled to a fine tip and fire-polished to a final tip size of 100–120 µm (outer diameter). The polished pipette was mounted in a custom-made vibrator held by a micromanipulator (Jin, Bailey, Li, Schild, and Andresen, 2004). Subpostremal portions of NTS medial to the visible ST were identified for dispersion using a stereomicroscope and the oscillating tip lowered to the surface within this sub region of the nucleus. The pipette oscillated at 30 Hz horizontally, with excursions of 100–300 µm. With the aid of a micromanipulator, the pipette tip was moved slowly to circumscribe and target for cell collection a defined area of the sub nucleus. Generally this area of cell collection was limited to a region bordered by the most caudal end of the fourth ventricle rostrally up to 500 µm and medial from the ST to within 50 µm of the edge of the fourth ventricle. Neurons were dissociated from the upper 100 µm of the dorsal surface of these slices. After removing the slice, dispersed neurons were allowed to settle and adhere to the bottom of the chamber – a process that generally was complete within 20 min. All experiments were conducted at room temperature (21–22° C).
For voltage-clamp recording, dissociated neurons were visualized using an infrared differential interference contrast microscope (TE2000S; Nikon, Tokyo, Japan) with a 100x oil objective (1.4 NA) and 10x ocular lens. Recordings utilized a MultiClamp 700B (Molecular Devices, CA) and pClamp 9 software. Electrical measurements used perforated (nystatin) patch recordings at room temperature (Horn and Marty, 1988). Recording electrodes were filled with a solution composed of the following (in mM): 50 KCl, 100 K gluconate, and 10 HEPES; the pH of this solution was adjusted to 7.2 with Tris-OH. Throughout these experiments, the calculated ECl was −29 mV. The final concentration of nystatin was 450 µg/ml. Neurons dispersed in this manner have intact presynaptic boutons as indicated by the presence of spontaneous synaptic events: IPSCs and EPSCs (Jin, Bailey, Li, Schild, and Andresen, 2004). Neurons were voltage clamped to −60 mV, and currents were sampled every 50 µs and saved to computer. Data were analyzed off-line using pClamp 9 software and Mini Analysis Program (Synaptosoft, Decatur, GA). All spontaneous and miniature synaptic events were detected and analyzed from digitized waveforms using MiniAnalysis (Synaptosoft, Decatur, GA). Except for determination of frequency rates, events < 3 pA and, those with multiple peaks were excluded from waveform analyses. Baseline currents were measured over a 2 ms section of the recorded traces prior to every detected event. Decay-time constants represent decay kinetics independent of amplitude and were calculated by least squares fitting routine for a single decay exponential between the 10% and 90% peak amplitude. For statistical comparison of synaptic events, waveform characteristic values (decay-time constant and amplitude) and baseline values across each group were averaged over a minimum of one min. at each concentration. These waveform parameters and baseline currents were compared with the Friedman repeated measures ANOVA on ranks (frequency, decay-time constant and amplitude) or a one-way, repeated measures ANOVA (baseline values, amplitude and latency) with post hoc pairwise multiple comparisons (Holm-Sidak method, SigmaStat, San Jose, CA). All data are represented as mean ± SEM and p<0.05 was considered statistically significant.
Drugs
NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione), AP-5 (D-2-amino-5-phosphonopentanoate), bicuculline methylbromide, gabazine (SR-95531, GABAA antagonist, 4-[6-imino-3-(4-methoxyphenyl)pyridazin-1-yl] butanoic acid hydrobromide), picrotoxin, and strychnine were obtained from Sigma-RBI (Natick, MA). Propofol (2,6-diisopropylphenol) was purchased from Sigma-Aldrich (St. Louis, MO) and solutions made to final concentrations of 0.1 to 30 µM using DMSO (dimethyl sulfoxide) and dilution with ACSF. The maximum final concentration of DMSO was 0.03%, a concentration which had no effect alone on the cells. All of the drugs were applied via a rapid application Y-tube system that provided complete solution changes surrounding the recorded neurons within 20 msec (Murase, Ryu, and Randic, 1989).
Acknowledgments
This work was supported by a grant from the National Institutes of Health: HL-58760 (DM, MCA) and the Kyung Hee University Research Fund, KHU-20070720 (YHJ).
Abbreviations
- GABA
aminobutyric acid
- NTS
solitary tract nucleus
- IPSC
inhibitory postsynaptic current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Young-Ho Jin, Department of Physiology, Kyung Hee University, Seoul 130-701, Korea.
Zhenxiong Zhang, Department of Physiology & Pharmacology, Oregon Health & Science University, Portland, OR 97239.
David Mendelowitz, Department of Pharmacology & Physiology, George Washington University, Washington, DC 20037.
Michael C. Andresen, Department of Physiology & Pharmacology, Oregon Health & Science University, Portland, OR 97239
Reference List
- Adodra S, Hales TG. Potentiation, activation and blockade of GABAA receptors of clonal murine hypothalamic GT1-7 neurones by propofol. Br. J. Pharmacol. 1995;115:953–960. doi: 10.1111/j.1476-5381.1995.tb15903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius: gateway to neural circulatory control. Annu. Rev. Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius - common denominators. Chemical Senses. 1996;21:387–395. doi: 10.1093/chemse/21.3.387. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieda MC, MacIver MB. Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol. 2004;92:1658–1667. doi: 10.1152/jn.00223.2004. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Newell JG, You T, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E. From GABAA, receptor diversity emerges a unified vision of GABAergic inhibition. Annu. Rev. Pharmacol. Toxicol. 1998;38:321–350. doi: 10.1146/annurev.pharmtox.38.1.321. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J. Neurosci. Methods. 2004;37:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J. Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology. 2005;103:20–24. doi: 10.1097/00000542-200507000-00007. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol. Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Gredell JA, Turnquist PA, MacIver MB, Pearce RA. Determination of diffusion and partition coefficients of propofol in rat brain tissue: implications for studies of drug action in vitro. Br. J Anaesth. 2004;93:810–817. doi: 10.1093/bja/aeh272. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Functional analysis of GABA(A) receptors in nucleus tractus solitarius neurons from neonatal rats. Brain Res. 2001;921:183–194. doi: 10.1016/s0006-8993(01)03117-1. [DOI] [PubMed] [Google Scholar]
- Jarolimek W, Lewen A, Misgeld U. A furosemide-sensitive K+-Cl− cotransporter counteracts intracellular Cl− accumulation and depletion in cultured rat midbrain neurons. J Neurosci. 1999;19:4695–4704. doi: 10.1523/JNEUROSCI.19-12-04695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Andresen MC. Cranial afferent glutamate heterosynaptically modulates GABA release onto second order neurons via distinctly segregated mGluRs. J. Neurosci. 2004;24:9332–9340. doi: 10.1523/JNEUROSCI.1991-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals. J. Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparov S, Davies KA, Patel UA, Boscan P, Garret M, Paton JFR. GABA(A) receptor epsilon-subunit may confer benzodiazepine insensitivity to the caudal aspect of the nucleus tractus solitarii of the rat. J. Physiol. 2001;536:785–796. doi: 10.1111/j.1469-7793.2001.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Luddens H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol. Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Korpi ER, Luddens H. Furosemide interactions with brain GABAA receptors. Br. J. Pharmacol. 1997;120:741–748. doi: 10.1038/sj.bjp.0700922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol. Pharmacol. 2007;71:539–548. doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Bailey TW, Mendelowitz D, Andresen MC. Propofol enhances both tonic and phasic inhibitory currents in second-order neurons of the solitary tract nucleus (NTS) Neuropharmacology. 2008;54:552–563. doi: 10.1016/j.neuropharm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Peters JH, LaBrant L, Wang X, Koop DR, Andresen MC. Paired assessment of volatile anesthetic concentrations with synaptic actions recorded in vitro. PLoS. ONE. 2008;3:e3372. doi: 10.1371/journal.pone.0003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan CJ, Buckley NJ, Garret M, Deuchars J, Deuchars SA. Evidence for inhibition mediated by coassembly of GABAA and GABAC receptor subunits in native central neurons. J. Neurosci. 2004;24:9241–9250. doi: 10.1523/JNEUROSCI.1979-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci. Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Murugaiah KD, Hemmings HC., Jr Effects of intravenous general anesthetics on [3H]GABA release from rat cortical synaptosomes. Anesthesiology. 1998;89:919–928. doi: 10.1097/00000542-199810000-00017. [DOI] [PubMed] [Google Scholar]
- Newland CF, Cull-Candy SG. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser BA, Canning KJ, MacDonald JF. Mechanisms of general anesthesia. Curr. Opin. Anaesthesiol. 2002;15:427–433. doi: 10.1097/00001503-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Mendelowitz D, Koop DR, Andresen MC. Isoflurane differentially modulates inhibitory and excitatory synaptic transmission to the solitary tract nucleus. Anesthesiology. 2008;108:675–683. doi: 10.1097/ALN.0b013e318167af9a. [DOI] [PubMed] [Google Scholar]
- Reves JG, Glass PSA, Lubarsky DA. Nonbarbiturate intravenous anesthetics. In: Miller RD, editor. Anesthesia. Vol. 1. New York: Churchill Livingstone; 2000. pp. 228–272. [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat. Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Sher E, Biancardi E, Passafaro M, Clementi F. Physiopathology of neuronal voltage-operated calcium channels. FASEB J. 1991;5:2677–2683. doi: 10.1096/fasebj.5.12.1655547. [DOI] [PubMed] [Google Scholar]
- Wang X, Huang ZG, Gold A, Bouairi E, Evans C, Andresen MC, Mendelowitz D. Propofol modulates gamma-aminobutyric acid-mediated inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Anesthesiology. 2004;100:1198–1205. doi: 10.1097/00000542-200405000-00023. [DOI] [PubMed] [Google Scholar]
- Ying SW, Goldstein PA. Propofol-Block of SK Channels in Reticular Thalamic Neurons Enhances GABAergic Inhibition in Relay Neurons. J Neurophysiol. 2005;93:1935–1948. doi: 10.1152/jn.01058.2004. [DOI] [PubMed] [Google Scholar]