Abstract
Inbred Wistar-Kyoto (WKY) rats have been proposed as a model of anxiety vulnerability as they display behavioral inhibition and a constellation of learning and reactivity abnormalities relative to outbred Sprague-Dawley (SD) rats. Together, the behaviors of the WKY rat suggest a hypervigilant state may contribute to its anxiety vulnerability. To test this hypothesis, open-field behavior, acoustic startle, pre-pulse inhibition and timing behavior were assessed in WKY and Sprague Dawley (SD) rats. Timing behavior was evaluated using a modified version of the peak-interval timing procedure. Training and testing of timing first occurred without audio-visual (AV) interference. Following this initial test, AV interference was included on some trials. Overall, WKY rats took much longer to leave the center of the arena, made fewer line crossings, and reared less, than did SD rats. WKY rats showed much greater startle responses to acoustic stimuli and significantly greater pre-pulse inhibition than did the SD rats. During timing conditions without AV interference, timing accuracy for both strains were similar; peak times for WKY and SD rats were not different. During interference conditions, however, the timing behavior of the two strains was very different. Whereas peak times for SD rats were similar between non-interference and interference conditions, peak times for WKY rats were shorter and response rates higher in interference conditions than in non-interference conditions. The enhanced acoustic startle response, greater prepulse inhibition and altered timing behavior with audio-visual interference supports a characterization of WKY strain as hypervigilant and provides further evidence for the use of the WKY strain as a model of anxiety vulnerability.
Keywords: anxiety, timing, sensorimotor gating, hypervigilance
Anxiety disorders have become a major mental health concern. Although many studies have focused on the events that precipitate the symptoms, it is clear that vulnerability or risk factors are important in the development of the disorders. Risk factors that have been previously identified include individuals' sex, brain structure and neurochemical differences, psychiatric history, personality traits, exposure to previous events, and coping style [16, 19-21, 36, 44, 53, 60]. Some have proposed that the different types of anxiety disorders may be explained by an interaction of common general risk factors with specific types of environmental experiences [36].
Trait behavioral inhibition is one of the risk factors for anxiety disorders [35, 48, 56]. People with behavioral inhibition are reserved or withdrawn in response to novel social and nonsocial situations [22]. This personality trait has been linked to a dysregulation of the hypothalamic pituitary adrenal axis, stress sensitivity, and hyper-responsiveness [52, 57]. Behavioral inhibition is also associated with disturbances of attention, particularly a difficulty in disengaging attention from novel stimuli or stimuli associated with threat or distress [18].
Hypervigilance has also been associated with anxiety disorders. Persons with high trait anxiety are more likely to display hypervigilance, suggesting that this feature may also serve as a vulnerability factor for the development of anxiety disorders [13]. Hypervigilance may take on different forms. For instance, general hypervigilance will make a person more distractible to task irrelevant stimuli, whereas specific hypervigilance is demonstrated by attending more to threatening stimuli with enhanced environmental scanning in search of threats [25, 29]. Hypervigilance also plays a role in the hypervigilance-avoidance theory whereby it is proposed that individuals with anxiety disorders initially attend to threatening stimuli, then avoid these same stimuli [3]. Some of these symptoms of hypervigilance have been observed in persons with trait behavioral inhibition. Thus, hypervigilance may underlie a risk factor associated with behavioral inhibition and the development of anxiety disorders.
Acetylcholine is an important neurotransmitter in the modulation of vigilance and attention. The nucleus basalis magnocellularis (NBM) is the source of cholinergic afferents to the neocortex, and damage or inactivation of cells in the nucleus basalis impairs attention [8, 33, 34, 39, 46, 58]. Damage of the frontal cortex produces similar impairments of attention, suggesting that the NBM projection to the frontal cortex is involved [37]. More selective damage to cholinergic neurons by the immunotoxin 192-IgG saporin impaired divided and sustained attention (vigilance) [9, 26-28, 55]. In contrast to the debilitating effects of reducing cholinergic NBM neurons, enhancing the cholinergic system with prenatal choline improves divided attention [31]. However, an overactive cholinergic NBM system has also been proposed to cause a hyperattentive or hypervigilance state that might be involved in mental illnesses such as schizophrenia [49]. These results suggest that the basalocortical cholinergic system may be involved in the hypervigilance associated with anxiety vulnerability.
The Wistar Kyoto (WKY) rat has been proposed as a model to study anxiety vulnerability [50]. A prominent feature of WKY rats is behavioral inhibition, observed as decreased activity or inactivity in novel social [43] and nonsocial challenges [42]. Moreover, these rats display low activity in the open field [14, 42], sensitivity to stress-induced ulcer formation [41], hyper-responsive peripheral and central stress responses [40], and learning and memory alterations [14]. WKY rats also develop abnormal avoidant behavior compared to outbred Sprague Dawley (SD) rats [50]; this is an important feature because avoidance is a critical feature of all anxiety disorders [4]. Finally, WKY rats exhibit higher acetylcholinesterase activity in the NBM and cortex compared to Sprague Dawley rats, suggesting an abnormal cholinergic system in the WKY rat [2].
Therefore, the present study was performed to determine whether WKY rats express hypervigilance in addition to the previously described behavioral inhibition. Furthermore, the study will assess the cholinergic system to determine whether abnormal transmission in this transmitter system could contribute to hypervigilance. A finding of both hypervigilance and enhanced cholinergic system in the WKY rats would provide further support of the WKY rat as a good model of anxiety vulnerability and implicate the cholinergic system in anxiety vulnerability.
Method
Animals
Sixteen adult, male Wistar Kyoto (WKY) rats and sixteen adult, male Sprague Dawley (SD) rats (Harlan, Indianapolis, IN) were used for this study. Rats were housed individually in a room under controlled conditions (near 22 °C with a 12:12 light/dark cycle, lights on at 7:00 a.m. Eastern time). Each animal received food and water ad libitum during all portions of the study except during peak interval training and testing. Training and testing were performed between 8:30 a.m. and 5:00 p.m. All procedures used in this study followed NIH guidelines for handling and caring of animals and were approved by the Bowling Green State University Institutional Animal Care & Use Committee.
Open Field Test
Open field activity was evaluated as described previously [38]. Rats were placed individually in the center of a circular open field (75 cm diameter, 40 cm high) under a bright light (3040 lux at the center of the open field floor). Behavior was recorded for two minutes by a video camera mounted directly above the arena. The open field had two concentric circles (45.7 cm and 15.2 cm diameter) drawn on the floor. In addition, six lines radiating 60° from the center circle divided the field into sixths. The center circle was not divided, for a total of thirteen sectors. A fan in the room produced constant background noise (70 dB). The arena was wiped with a mild soapy solution between each animal testing. Dependent measures were latency to leave the center sector, number of line segments crossed, and number of rearings, fecal boli, and grooming episodes. Any animal that did not leave the center sector was given a score equal to the total duration in the open field arena (120 s).
Acoustic Startle and Pre-Pulse Inhibition
Animals were placed in a loosely fitted plastic restrainer. The restrainer was placed on a movement sensing module located in a dark sound-attenuating chamber (San Diego Instruments, San Diego, CA). Prior to testing, animals were placed in the apparatus for 5 minutes and presented with constant white noise (70 dB) that continued throughout the testing phase. Following the acclimation period, animals were exposed to three types of stimuli: An acoustic startle pulse alone (PA; 20 ms, 118 dB), a pre-pulse alone (PPA; 20 ms, 80 dB), and pre-pulse preceding a startle pulse (PPP; 50 ms inter-stimulus interval). Animals were presented with a pseudorandom sequence of 60 trials with a variable inter-trial-interval (M = 15 s).1 Each subject received 20 PA, 20 PP, and 20 PPP trials in the same pseudorandom order. Following testing, the acoustic startle restrainers were cleaned with a mild soapy solution before the next animal was tested. The startle system was calibrated before each test.
Dependent measures were the raw startle amplitude (measured in mV) for PA, PPA, PPP trials, and a widely used measure of pre-pulse inhibition, (%PPI): [startle to PA - startle to PPP]/startle to PA × 100 [54].
Peak-Interval Procedure
Apparatus
Testing was conducted in sixteen operant boxes (28 × 28 × 37 cm), custom-made of clear acrylic. Located in each box were a lamp, speaker, water bottle, response lever, and food cup. The stimulus lamp (4.8 watts) was positioned near the top of the operant box on the same side of the response lever. The speaker (Mallory Sonalert, Indianapolis, IN), located approximately 21.5 cm from the floor of the operant box and on the wall opposite the food cup and lamp, delivered sound at 4500 Hz between 78 to 89 dB. Sucrose pellets (45mg, PJFSC-0045; Research Diets, Inc., New Brunswick, NJ) were delivered by a pellet dispenser (ENV-203; Med-Associates, St. Albans, VT) into a food cup on one wall. The response lever was located to the right of the food cup. Each operant box was placed in an environmental isolation chamber to minimize external light and sound. Inside the chamber, a house light provided indirect lighting and a solenoid valve provided an audible click upon delivery of reinforcement and behaved as a secondary reinforcer. A fan provided ventilation to each chamber. A Dell (Optiplex GX240) computer with a Med-Associates SmartCtrl system (MED-PC IV; DIG-716; SG-716; Med-Associates, St. Albans, VT) controlled the presentation of stimuli, delivery of reinforcement, and recorded lever response times.
Pre-training
Rats were trained to press on a continuous reinforcement schedule (CRF, ≈ 2-3 sessions). Following CRF, rats received training on a variable response schedule (VR-3 ≈ 2-3 sessions) where a minimum of one and a maximum of six lever presses were required for a sugar pellet reward. Following VR training, rats were assigned to either a 24 s (WKY, n = 8; SD, n = 8) or 36 s (WKY, n = 8; SD, n = 8) fixed interval (FI ≈ 5 sessions) reinforcement schedule. Trials began with the onset of a light stimulus. After the FI target duration had elapsed, the first lever press resulted in delivery of a sucrose pellet and termination of the light stimulus. Any lever presses prior to the target duration were recorded but did not have any effect. For the 24 s and 36 s FIs, the trial was terminated if no lever presses were made within 60s or 90 s after stimulus onset, respectively. Sessions lasted between 2 and 3 hours with 60-80 trials per session.
PI training and testing
Following FI training, rats received 20-22 sessions of training on the peak interval (PI) procedure. This procedure is the same as the FI procedure, except for the addition of unreinforced peak (probe) trials. During peak trials, the light stimulus remained on for the entire trial; the trial duration was 60 s for rats tested with the 24 s target and 90 s for rats tested with the 36 s target. Peak and FI trials were equally and randomly presented in a session. During peak trials, the time of each lever press was recorded. Sessions typically lasted between 2 to 3 hours with animals completing approximately 70 to 90 trials in a session.
PI testing with interference
Rats next performed a modified peak-interval procedure that included auditory and visual interference on 50% of the trials (FI and peak trials). AV interference consisted of random tone bursts and flashing house lights that varied in duration between 0.5 and 3 sec. The interval between tone bursts and light flashes also varied between 0.5 and 3 sec. Six interference sessions were interleaved (on alternate days) with non-interference sessions over a three week period.
PI testing post-interference
Finally, rats completed five sessions of the standard peak-interval procedure without AV interference.
Choline acetyltransferase (ChAT) assay
After completion of behavioral testing, subjects were anesthetized using a mixture of CO2/O2, and then sacrificed. Each brain was removed rapidly, rinsed with ice-cold saline, and relevant brain areas were dissected on a cold plate. Tissue samples (≈2 × 2 mm, ≈ 35mg) were taken from the left and right prefrontal cortex, frontal cortex, and parietal cortex. In addition, the hippocampus was removed from each hemisphere. All samples were transferred to a pre-weighed microcentrifuge tube, weighed again, quickly frozen on dry ice and stored at -80°C until further analysis.
Tissue samples were homogenized with Tris/Triton solution, dilution 1:20, (containing 0.05M Trizma HCl, 0.05M Trizma Base, and 0.02% Triton-X-100 (pH 7.6) with 1.0 mm glass disruption beads and then centrifuged for 10 min at 10,000 g. The resulting supernatant was pipetted into a separate microcentrifuge tube, subjected to a second centrifuge for 10 minutes and supernatant collected.
The formation of [14C]Acetylcholine from [acetyl-1-14C]-acetyl-coenzyme A was used to measure the activity of the enzyme choline acetyltransferase (ChAT), which synthesizes acetylcholine [17]. A reaction mixture (20 μl) containing 1.34mM 14C-Acetyl-CoA (60 mCi/mmol; MP Biomedicals, Irvine, CA) and 20.6 mM unlabeled compound was added to the tissue supernatant (20 μl) and buffer (70 μl; 300 mM NaCl, 8 mM choline bromide, 20 mM EDTA, 0.1 mM eserine sulfate, 0.5% Triton X-100, and 50 mM dibasic sodium phosphate at pH 7.4). The mixture was quickly vortexed and incubated at 37 °C for 30 minutes. The reaction was terminated by adding 5 ml 0.1 M cold phosphate buffer (pH 7.4). To measure ChAT activity, labeled acetylcholine was extracted by transferring the tube contents to a scintillation vial and adding 10 ml of toluene (containing 15g/l PPO and 1g/l POPOP) and 2 ml acetonitrile (containing 5g/l Na-tetraphenylboron). Vials settled for 12 hours before the organic phase (top layer) was counted with a scintillation counter. Assays were performed in triplicate.
The protein content of each sample was calculated according to the method described previously [5] using a Bio-Rad (Bio-Rad Laboratories, Hercules, CA) protein assay. Supernatant was diluted to 1:200 with Tris/Triton solution. Ten microliters of diluted supernatant was transferred in triplicate to flat-bottom assay plates and 200 μl Bio-Rad dye added to each well. Absorbance was measured at 595 nm. Protein content of the homogenates was calculated from standard curves constructed from known amounts of bovine serum albumin. ChAT activity in the prefrontal, frontal, parietal, and hippocampal sections are reported as nmol/mg protein/hr.
Data Analysis
All statistical analyses were conducted using SPSS for Windows with an alpha level of 0.05 (Version 15.0, SPSS, Inc., Chicago, IL). Descriptive measures reported in the text indicate mean values and standard error (M ± SEM).
For the PI procedure, only data from probe trials were analyzed. The number of lever responses was determined for each successive 1-second interval (bin) for the entire stimulus duration (60 s for the 24 s FI and 90 s for the 36 s FI). The total responses in each bin were divided by the total number of trials to determine the mean number of lever responses per trial for each bin. Mean lever responses for each bin were plotted as a function of time to create a temporal response function. Response rate was calculated by multiplying the number of responses in each bin by 60 so that response rate could be expressed as responses per minute [47].
A Gaussian + linear equation [7] that gave the best fit to the temporal response function was determined by minimizing root mean-square error using the Solver add-in package for Microsoft Excel 2002 (Version 10.65, Microsoft Corporation, Seattle, WA). The following generalized Gaussian + linear model was fit to the temporal response function:
| (1) |
where t is the current time bin and R(t) is the mean response rate at time t. Model fitting determined estimates for the parameters a, b, c, d, and t0. Peak-time was estimated by t0, peak-rate was estimated by a + d, and variability was estimated by dividing b by the peak-time, t0, to obtain a coefficient of variability (CV) score.
To assess the effect of AV interference stimuli (random tone bursts and light flashes) on peak-interval timing, an Interference Ratio (I) was calculated for interference sessions. For this measure, peak-times on trials with AV Interference (PTI) was divided by peak-times on trials without interference (PTNI) and then the value of 1 was subtracted from the ratio: I = (PTI/PTNI) -1. Positive values of I indicate that interference lengthens peak-times on interference trials relative to non-interference trials, whereas negative values of I indicate that interference shortens peak-times on interference trials relative to non-interference trials.
Results
Open Field Test
Wistar-Kyoto (WKY) rats took much longer to leave the center of the arena, t(30) = 2.10, p < 0.05, made fewer line crossings, t(30) = 5.694, p < .001, and reared less, t(30) = 3.05, p = .006, than did Sprague-Dawley (SD) rats (Figure 1). Mean latencies ± SEM (time taken to leave the center of the arena) for the WKY and SD rats were 20.90 ± 7.57 s and 4.90 ± 0.72 s, respectively. The overall greater latency variability for the WKY strain was due partly to one rat that stayed in the center of the arena for the entire duration of the test. Mean number of line crossings for the WKY and SD rats were 6.40 ± 0.57 and 17.70 ± 0.94, respectively. Mean number of rearings for WKY and SD rats were 0.70 ± 0.25 and 2.60 ± 0.56, respectively. Other open field measures, defecation and grooming, rarely occurred; comparisons between the two strains revealed no significant difference in either number of fecal boli (WKY: M = 0.19 ± 0.14; SD: M = 0.19 ± 0.14; t(30) = 0.00, p = 1.00) or number of grooming episodes (WKY: M = 0.06 ± 0.06; SD: M = 0.19 ± 0.14; t(30) = -0.84, p = 0.41; t(30) = 0.84, p = 0.41).
Figure 1.
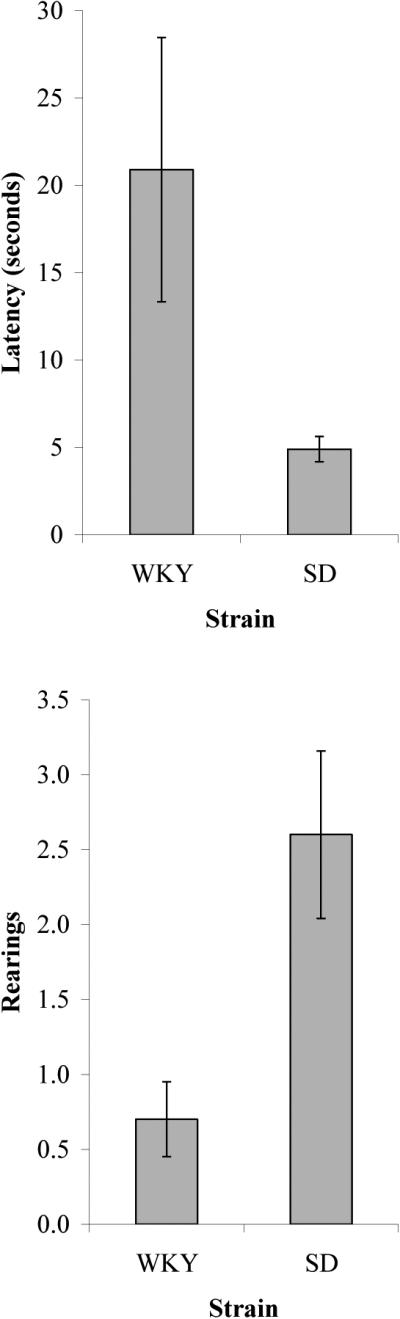
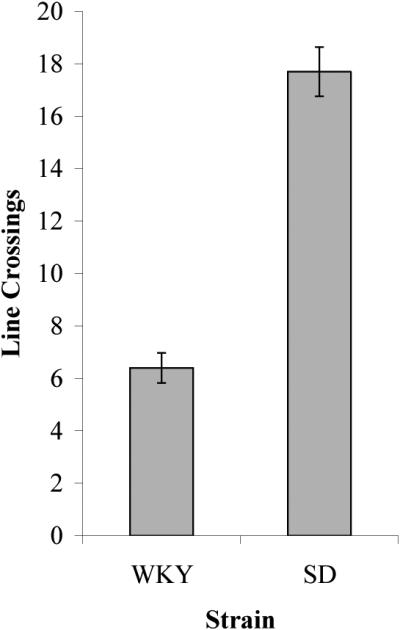
Comparison of open field behavior for the WKY and SD strains.
Pre-Pulse Inhibition
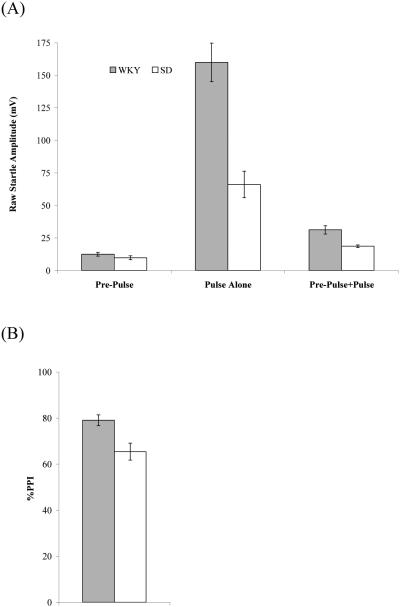
Acoustic startle was elevated for WKY rats in comparison to SD rats (Figure 2). Startle was higher for WKY strain compared to SD strain to PA, t(30) = 4.27, p < 0.001, and PPP, t(30) = 3.07, p < 0.01, but not to PPA, t(30) = 1.06, p = 0.30. With respect to PPI, the WKY strain showed much greater inhibition than the SD strain (WKY, M = 79.1% ± 2.3%; SD, M = 65.4% ± 3.7%), t(30) = 3.16, p < 0.01.
Figure 2.
(A) Acoustic startle responses for Pulse Alone, Pre-Pulse Alone, and Pre-Pulse+Pulse conditions for the WKY and SD strains. (B) %PPI for the WKY and SD strains.
Peak-Interval Procedure
Peak-interval training
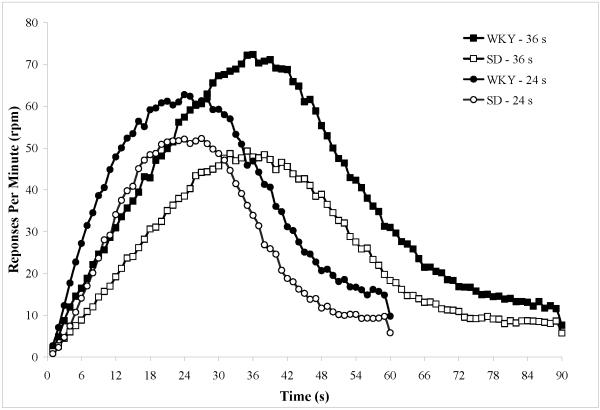
Timing accuracy was similar between WKY and SD rats for both 24 s and 36 s target FIs (Figure 3). In general, peak-times were slightly under-estimated for both the 24 s target FI (WKY, M = 22.3 ± 0.51 s; SD, M = 22.8 ± 0.39 s) and the 36 s target FI (WKY, M = 33.5 ± 0.8 s; SD, M = 33.0 ± 0.8 s). A 2 (Strain) × 2 (Target FI) ANOVA on peak-times revealed the expected main effect of Target FI, F(1,28) = 284, MSE = 3.23, p < 0.001, but no main effect of Strain, F(1,28) = 0.001, MSE = 3.23, p = 0.98, or interaction between the two factors, F(1,28) = 0.58, MSE = 3.23, p = 0.46. Peak rates were higher for the WKY strain than for SD rats for the 24 s target FI (WKY, M = 64.3 ± 6.9 responses/minute, rpm; SD, M = 54.4 ± 6.5 rpm) and the 36 s target FI (WKY, M = 73.1 ± 10.1 rpm; SD, M = 49.2 ± 6.4 rpm). A 2 (Strain) × 2 (Target FI) ANOVA on peak rates revealed a main effect of Strain, F(1,28) = 4.92, MSE = 464.1, p = 0.035, but no main effect of Target FI, F(1,28) = 0.056, MSE = 464.1, p = 0.81, or interaction, F(1,28) = 0.84, MSE = 0.84, p = 0.37. With respect to variability, coefficient of variability (CV) scores were similar across target fixed intervals (FI = 24 s: M = 0.58 ± 0.02; FI = 36 s: M = 0.57 ± 0.03). There was little evidence of a strain difference in CV scores, although WKY rats (M = .61 ± 0.03) were slightly more variable overall than SD rats (M = .54 ± 0.02). For the CV data, an ANOVA revealed no main effect of Strain, F(1,28) = 2.61, MSE = 0.012, p = 0.12, no main effect of Target FI, F(1,28) = 0.16, MSE = 0.012, p = 0.69, and no interaction, F(1,28) = 1.57, MSE = 0.012, p = 0.22).
Figure 3.
Temporal response functions for probe trials from the last 10 sessions of peak-interval training for the WKY and SD strains for the 24 s and 36 s fixed-interval targets.
Peak-interval testing with AV interference
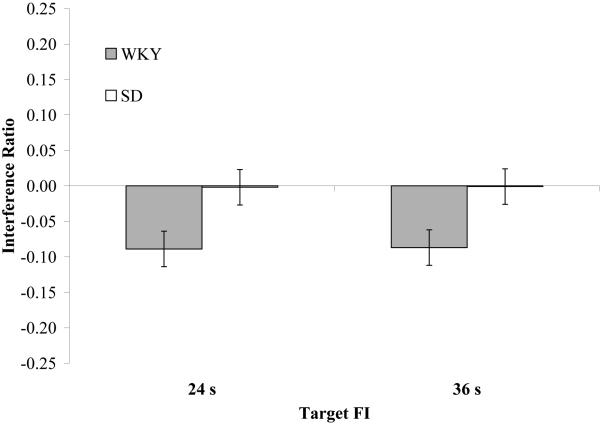
Timing in WKY rats but not SD rats was adversely affected by the presentation of random tone bursts and light flashes (Table 1). No strain difference was observed for peak-time on trials without AV interference (non-interference trials during interference sessions and interleaved non-interference sessions). However, in the presence of AV interference, WKY rats under-produced the target duration (Figure 4). This influence of AV interference was not observed for SD rats. Values of I were negative (distraction shortened peak-times) for the WKY strain in both 24 s FI (M = -0.09 ± 0.02) and 36 s FI (M = -0.09 ± 0.02), and the effect of distraction was proportional to the target FI. In contrast, no effect of AV interference was observed for peak-times of SD rats (24 s, M = 0.00 ± 0.01; 36 s, M = 0.00 ± 0.04). A 2 (Strain) × 2 (Target FI) ANOVA on the interference ratio, I, revealed a main effect of Strain, F(1,28) = 11.96, MSE = 0.005, p < 0.01, but no reliable effect of Target FI, F(1,28) = 0.004, MSE = 0.005, p = 0.95, or Strain × Target FI interaction, F(1,28) = 0.0, MSE = 0.005, p > 0.99.
Figure 4.
Effect of the presentation of random tone bursts and light flashes (AV interference) on peak-interval timing for the WKY and SD strains for the 24 s and 36 s fixed-interval targets.
For peak rates, AV interference increased responding in WKY rats but not SD rats for both 24 s FI (Interference Trials: WKY, M = 73.7 ± 10.1 rpm; SD, M = 67.3 ± 8.4 rpm; Non-Interference Trials: WKY, M = 66.2 ± 9.5 rpm; SD, M = 65.7 ± 9.1 rpm) and 36 s FI (Interference Trials: WKY, M = 95.1 ± 12.1 rpm; SD, M = 70.8 ± 6.6 rpm; Non-Interference Trials: WKY, M = 78.3 ± 9.3 rpm; SD, M = 65.2 ± 7.1 rpm). This conclusion was supported statistically by a 2 (Strain) × 2 (Target FI) × 2 (Type of Trial: Interference versus Non-Interference) mixed-measures ANOVA on peak rates, which revealed no main effect of Strain F(1,28) = 1.48, MSE = 1319.697, p = .234, or Target FI, F(1,28) = .998, MSE = 1319.697, p = .326, but critically a main effect of Type of Trial, F(1,28) = 39.526, MSE = 25.05, p < .001, and a significant Type of Trial × Strain interaction, F(1,28) = 11.59, MSE = 25.05, p < .01.
An examination of variability (CV) scores with and without AV interference shows that the WKY strain was marginally more variable than the SD strain (WKY, M = 0.54 ± 0.02; SD, M = 0.48 ± 0.02), F(1,28) = 3.15, MSE = 0.019, p = 0.087. There was no main effect of Target FI, Type of Trial, or significant interactions.
Peak-interval testing post-interference
Finally, peak-interval performance was determined following testing with interference. Similar to performance prior to the introduction of random tone bursts and light flashes, peak-times were slightly under-estimated for both target FIs and there were no difference between strains. A 2 (Strain) × 2 (Target FI) ANOVA on peak-times revealed a main effect of FI, F(1,28) = 323.7, MSE = 3.86, p < 0.001, but no main effect of strain, F(1,28) = 0.006, MSE = 3.86, p = 0.94, or interaction between the two factors, F(1,28) = 1.98, MSE = 3.23, p = 0.17. Unlike initial peak-interval training, peak rates did not differ between strains. A 2 (Strain) × 2 (Target FI) ANOVA on peak rates revealed no main effect of strain, F(1,28) = 0.11, MSE = 625.3, p = 0.74 or target FI, F(1,28) = 0.90, MSE = 625.3, p = 0.35, and no interaction between the two factors, F(1,28) = 1.07, MSE = 625.3, p = 0.31. The ANOVA of CV scores post-interference revealed a slightly different pattern compared to pre-interference performance. CV scores were higher for the 24 s FI (M = 0.56 ± .02) than for the 36 s FI (M = 0.48 ± .03), F(1,28) = 5.02, MSE = 0.011, p < 0.05, and were marginally significantly higher for the WKY strain (M = 0.55 ± .03) than for the SD strain (M = 0.49 ± .03), F(1,28) = 2.92, MSE = 0.011, p = 0.10); there was no interaction, F(1,28) = 0.043, MSE = 0.011, p = 0.84.
ChAT results
ChAT activity in WKY and SD rats were compared for prefrontal cortex, frontal cortex, parietal cortex and hippocampus (see Table 2). Levels of ChAT activity in prefrontal cortex were marginally higher for WKY rats (M = 1.02 ± .31) compared to SD rats (M = .69 ± .12),t(30) = 1.86, p = 0.072. No appreciable differences between strains were found for the other brain regions (all p's > 0.1).
Table 2.
ChAT levels in prefrontal, frontal, and parietal cortex, and hippocampus
| Strain | Area | |||
|---|---|---|---|---|
| Prefrontal Cortex | Frontal Cortex | Parietal Cortex | Hippocampus | |
| WKY | 1.02 ± 0.31 | 0.74 ± 0.08 | 0.85 ± 0.07 | 0.80 ± 0.09 |
| SD | 0.69 ± 0.12 | 0.88 ± 0.17 | 0.92 ± 0.16 | 0.96 ± 0.23 |
Note. Values represent M ± SEM. All values are reported in nmol/mg/hr.
Discussion
Behavioral inhibition is a vulnerability factor for the development of anxiety disorders [48]. WKY rats demonstrate behavioral inhibition and have been suggested to be a good animal model of anxiety vulnerability [50]. Hypervigilance is associated with high trait anxiety and may be related to vulnerability for the development of anxiety disorders (Eysenck, 1992). In the present study, an animal model demonstrating behavioral inhibition, the WKY rats, were examined for hypervigilance. In order to test the proposed hypervigilance hypothesis, WKY and SD rats were given a battery of behavioral tests that included an open field test, assessments of acoustic startle response and pre-pulse inhibition, and peak-interval timing with and without AV interference. Additionally, the cholinergic system was assessed in WKY and SD rats because of the previously identified role of acetylcholine in attentional processes [6, 9, 26-28, 33, 34, 55].
Six main findings were obtained. First, consistent with previous research [42], WKY rats showed dramatically less open activity than the SD rats. Second, WKY rats demonstrated an enhanced acoustic startle response and greater prepulse inhibition than SD rats. Third, WKY rats were slightly more variable in timing (less timing precision) than SD rats. Fourth, timing accuracy was similar in both strains, as measured by the times of peak response, and timing was scalar. Fifth, random presentations of auditory and visual stimuli (i.e., AV interference) disrupted timing accuracy for WKY rats but not SD rats. Finally, the activity of choline acetyltransferase (ChAT) was greater in the prefrontal cortex of WKY rats than in SD rats.
The finding that WKY rats have a longer latency to move from the center of the open field is consistent with an assessment of behavior inhibition. Alternatively, one might use the longer latency to move as a sign that WKY rats were less anxious than SD rats [45]. However if WKY rats were less anxious, they would be expected to show more overall locomotion in the open field and more vertical exploration (rearings). WKY rats do not show these other behaviors, and in fact, the pattern is in the opposite direction than one would expect of a less anxious rat. Together, the direction of these measures is more consistent with a view of a behavioral inhibited animal rather than an animal that is less anxious (and therefore more likely to explore the environment). Behavioral inhibition is defined as a reserved response or withdrawal from novel social and non-social situations [22]. In this respect, it is further noteworthy that in addition to the low activity in open field, WKY rats also demonstrate withdrawal from other novel social and interactions [42, 43]. These results suggest that WKY have the manifestations of behavioral inhibition in humans. Humans with trait behavioral inhibition are more vulnerable to developing anxiety disorders [35, 48, 56], supporting the idea that WKY rats may be a good animal model of anxiety vulnerability.
The present study was designed in part to assess whether trait behavioral inhibition in WKY rats was associated with hypervigilance. In this respect, WKY rats demonstrated increased acoustic startle responses and enhanced prepulse inhibition compared to SD rats. Only a few studies have compared acoustic startle in WKY and SD rats. Startle responses in WKY have varied from lower, higher and no difference compared to SD rats [14, 24, 40, 51]. Methodological differences may have contributed to the conflicting results. Besides the present study, the other study that reported enhanced acoustic startle also assessed the startle response in the context of investigating prepulse inhibition [24].
Prepulse inhibition in the present study was enhanced in WKY rats compared to SD rats. In a previous study, prepulse inhibition in WKY and SD rats did not differ [24]. Procedural differences may account for discrepancies in the results, as the administration of saline occurred in the study by Martin et al. but not in the present study. Decreased prepulse inhibition has been associated with some anxiety disorders, such as panic disorder [23]. In the study by Ludewig et al., reduced PPI was mildly associated with high trait anxiety measures. Although trait anxiety measures have high common loadings with behavioral inhibition in factor analyses, these anxiety measures are only moderately correlated with measures of behavioral inhibition. Studies on the neurobiology of trait anxiety and behavioral inhibition provide evidence for a distinction between anxiety and behavioral inhibition (anxiety vulnerability) [32]. Thus, the enhanced PPI in WKY rats with behavioral inhibition may distinguish this vulnerability from the actual anxiety disorder and high trait anxiety which are both associated with reduced PPI.
Consistent with the present study and a hypervigilance hypothesis, other studies have demonstrated that prepulse inhibition is increased when attention is directed to the pre-pulse stimulus [1, 15], and attention may be driven by potential or imminent threat [10]. As with humans, the behavioral inhibition features of WKY rats may cause attention to be focused on novel, unexpected stimuli such as the prepulse and startle stimuli resulting in an increased pre-pulse inhibition. Thus, the enhanced acoustic startle response and the increased pre-pulse inhibition compared to SD rats are consistent with the idea that WKY rats are hypervigilant.
In the absence of AV interference, interval timing was only marginally different in WKY rats than in SD rats. Accuracy of timing and the scalar properties of interval timing were similar in both strains, while the precision of timing was slightly more variable in WKY compared to SD rats [30]. However, presentation of random tone bursts and light flashes on some trials produced large differences in the timing behavior of the two strains. SD rats were able to ignore task-irrelevant stimuli (i.e, they were unaffected by the AV interference) with timing accuracy and precision similar in both interference and non-interference conditions. In contrast, AV interference clearly altered the timing behavior of the WKY rats. With AV interference, peak times of WKY rats were left-shifted (corresponding to a shortening of temporal productions), peak rates increased, and variability increased. This pattern of alterations in the WKY rats following AV interference is consistent with an arousal explanation. That is, random presentations of stimuli appeared to increase the arousal of the WKY rats, but not the SD rats, possibly due to increased sensory stimulation or presentation of “novel” stimuli. It is also important to note that the arousing effects of the stimuli were confined to individual trials with AV interference; performance on non-interference trials in the same session was similar to performance in sessions without AV interference. Thus, the greater influence of AV interference in WKY but not SD rats provides further evidence for an association between hypervigilance and behavioral inhibition. Hypervigilance has also been proposed by Eysenck and colleagues to be present in individuals with trait anxiety [11, 12].
The current study also lends some support to the idea that an enhanced cortical cholinergic system may underlie the hyperattentive or hypervigilant state associated with behavioral inhibition. Levels of ChAT activity were found to be marginally higher in pre-frontal areas in WKY rats compared with SD rats. The cortical cholinergic system is involved in vigilance (sustained attention) [26-28]. Lesions of the basalocortical system or the frontal cortex impair the ability to simultaneously time multiple stimuli [37], remain vigilant in sustained attention procedures [26-28], and discriminate target stimuli from distracting ones [33, 34, 58]. Evidence for the converse is limited. Rats treated prenatally with choline supplements are able to attend and time more stimuli than rats without prenatal choline supplements [31]; moreover, prenatal choline supplements enhance the cholinergic system [59]. Sarter [49] has suggested that the inability to filter out stimuli, such as in schizophrenia, may be due to excess cortical acetylcholine. Results found in WKY rats in the present study are consistent with this view, but we propose that this is a signature of anxiety vulnerability rather than schizophrenia.
In conclusion, the present study demonstrates enhanced acoustic startle, greater pre-pulse inhibition, susceptibility to AV interference in a peak-interval timing task, and up-regulation of the cholinergic synthetic enzyme in WKY rats. These findings support the view that WKY rats demonstrate hypervigilance in addition to trait behavioral inhibition. The presence of hypervigilance and behavioral inhibition, two features of anxiety vulnerability in humans, provide further evidence for the WKY rat as an animal model of anxiety vulnerability [50].
Table 1A.
PI Performance on 24 s target FI during interference (I) trials, non-interference (NI) trials, and interleaved non-interference sessions.
| 24 s Target FI | Peak Time | Peak Rate | CV | |||
|---|---|---|---|---|---|---|
| WKY | SD | WKY | SD | WKY | SD | |
| Interleaved NI Sessions | 23.2 | 22.3 | 64.8 | 64.8 | 0.57 | 0.49 |
| I Sessions (NI Trials) | 24.1 | 22.6 | 66.2 | 65.7 | 0.56 | 0.49 |
| I Sessions (I Trials) | 21.9 | 22.5 | 73.7 | 67.3 | 0.57 | 0.46 |
Table 2B.
PI Performance on 36 s target FI during interference (I) trials, non-interference (NI) trials, and interleaved non-interference sessions.
| 36 s Target FI | Peak Time | Peak Rate | CV | |||
|---|---|---|---|---|---|---|
| WKY | SD | WKY | SD | WKY | SD | |
| Interleaved NI Sessions | 33.6 | 33.6 | 77.1 | 70.9 | 0.53 | 0.47 |
| I Sessions (NI Trials) | 33.9 | 34.3 | 78.2 | 65.2 | 0.52 | 0.49 |
| I Sessions (I Trials) | 30.9 | 34.1 | 95.1 | 70.8 | 0.53 | 0.49 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashare RL, Hawk LW, Mazzullo RJ. Motivated attention: Incentive effects on attentional modification of prepulse inhibition. 2007;44:839–45. doi: 10.1111/j.1469-8986.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck KD, Zhu G, Beldowicz D, Brennan FX, Ottenweller JE, Moldow RL, et al. Central nervous system effects from a peripherally acting cholinesterase inhibiting agent: Interaction with stress or genetics. 2001;933:310–4. doi: 10.1111/j.1749-6632.2001.tb05833.x. [DOI] [PubMed] [Google Scholar]
- 3.Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: Hypervigilance, avoidance and self-focused attention. 2004;24:827–56. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Borkovec TD, Alcaine O, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, Borkovec TD, Alcaine O, Behar E, editors. Generalized anxiety disorder: Advances in research and practice. Guilford Press; New York, NY: 2004. pp. 77–108. [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. 1998;18:8038–46. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhusi CV, Sasaki A, Meck WH. Temporal integration as a function of signal and gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia) 2002;116:381–90. doi: 10.1037/0735-7036.116.4.381. [DOI] [PubMed] [Google Scholar]
- 8.Burk JA, Sarter M. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. 2001;105:899–909. doi: 10.1016/s0306-4522(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 9.Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. 1995;15:7315–22. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornwell BR, Echiverri AM, Covington MF, Grillon C. Modality-specific attention under imminent but not remote threat of shock: Evidence from differential prepulse inhibition of startle. Psychol Sci. 2008;19:615–22. doi: 10.1111/j.1467-9280.2008.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eysenck MW. Anxiety and attention. 1988;1:9–15. [Google Scholar]
- 12.Eysenck MW, Byrne A. Anxiety and susceptibility to distraction. 1992;13:793–8. [Google Scholar]
- 13.Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. 1992;6:409–34. [Google Scholar]
- 14.Ferguson SA, Cada AM. Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacology, biochemistry, and behavior. 2004;77:583–94. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: A tool for investigating early and late attentional processes. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- 16.Foa EB, Stein DJ, McFarlane AC. Symptomatology and psychopathology of mental health problems after disaster. 2006;67:15–25. [PubMed] [Google Scholar]
- 17.Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. 1975;24:407–9. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- 18.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. 2005;56:235–62. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 19.Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. 2005;111 doi: 10.1111/j.1600-0447.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hapke U, Schumann A, Rumpf HJ, John U, Meyer C. Post-traumatic stress disorder: The role of trauma, pre-existing psychiatric disorders, and gender. 2006;256:299–306. doi: 10.1007/s00406-006-0654-6. [DOI] [PubMed] [Google Scholar]
- 22.Kagan J, Reznick JS, Gibbons J. Inhibited and uninhibited types of children. 1989;60:838. [PubMed] [Google Scholar]
- 23.Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. 2002;15:55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- 24.Martin S, Lawrence AJ, van den Buuse M. Prepulse inhibition in fawn-hooded rats: Increased sensitivity to 5-HT1a receptor stimulation. 2004;14:373–9. doi: 10.1016/j.euroneuro.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Mathews A. Cognitive approaches to emotion and emotional disorders. 1994;45:25. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- 26.McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: Selectivity of the behavioral impairment and relation to cortical ache-positive fiber density. 1996;110:247–65. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- 27.McGaughy J, Everitt BJ, Robbins TW, Sarter M. The role of cortical cholinergic afferent projections in cognition: Impact of new selective immunotoxins. 2000;115:251–63. doi: 10.1016/s0166-4328(00)00262-x. [DOI] [PubMed] [Google Scholar]
- 28.McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. 2002;22:1905. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally RJ. Cognitive bias in the anxiety disorders. In: Hope DA, Izard CE, McNally RJ, editors. Perspectives on anxiety, panic, & fear. University of Nebraska Press; 1996. pp. 211–50. [PubMed] [Google Scholar]
- 30.Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Res. 1996;3:227–42. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- 31.Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. 1997;8:3045. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 32.Morgan BE. Behavioral inhibition: A neurobiological perspective. 2006;8:270–8. doi: 10.1007/s11920-006-0062-7. [DOI] [PubMed] [Google Scholar]
- 33.Muir JL, Robbins TW, Everitt BJ. Disruptive effects of muscimol infused into the basal forebrain on conditional discrimination and visual attention: Differential interactions with cholinergic mechanisms. 1992;107:541–50. doi: 10.1007/BF02245269. [DOI] [PubMed] [Google Scholar]
- 34.Muir JL, Everitt BJ, Robbins TW. Ampa-induced excitotoxic lesions of the basal forebrain: A significant role for the cortical cholinergic system in attentional function. 1994;14:2313–26. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neal JA, Edelmann RJ, Glachan M. Behavioural inhibition and symptoms of anxiety and depression: Is there a specific relationship with social phobia? 2002;41:361–74. doi: 10.1348/014466502760387489. [DOI] [PubMed] [Google Scholar]
- 36.Ollendick TH, Hirshfeld-Becker DR. The developmental psychopathology of social anxiety disorder. 2002;51:44–58. doi: 10.1016/s0006-3223(01)01305-1. [DOI] [PubMed] [Google Scholar]
- 37.Olton DS, Wenk GL, Church RM, Meck WH. Attention and the frontal cortex as examined by simultaneous temporal processing. Neuropsychologia. 1988;26:307–18. doi: 10.1016/0028-3932(88)90083-8. [DOI] [PubMed] [Google Scholar]
- 38.Ottenweller JE, Servatius RJ, Tapp WN, Drastal SD, Bergen MT, Natelson BH. A chronic stress state in rats: Effects of repeated stress on basal corticosterone and behavior. Physiol Behav. 1992;51:689–98. doi: 10.1016/0031-9384(92)90104-a. [DOI] [PubMed] [Google Scholar]
- 39.Pang K, Williams MJ, Egeth H, Olton DS. Nucleus basalis magnocellularis and attention: Effects of muscimol infusions. 1993;107:1031. doi: 10.1037//0735-7044.107.6.1031. [DOI] [PubMed] [Google Scholar]
- 40.Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: Implications for susceptibility to stress-related neuropsychiatric disorders. 2002;115:229–42. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 41.Pare WP. Stress ulcer and open-field behavior of spontaneously hypertensive, normotensive, and wistar rats. 1989;24:54–7. doi: 10.1007/BF02964537. [DOI] [PubMed] [Google Scholar]
- 42.Paré WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in wky rats. 1994;55:433–9. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 43.Paré WP. Investigatory behavior of a novel conspecific by Wistar Kyoto, Wistar and Sprague-Dawley rats. 2000;53:759–65. doi: 10.1016/s0361-9230(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 44.Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, et al. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. 2006;1071:242–54. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 46.Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ. Comparative effects of ibotenic acid-and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: Further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. 1989;35:221–40. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- 47.Roberts S. Isolation of an internal clock. 1981;7:242–68. [PubMed] [Google Scholar]
- 48.Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, et al. Behavioral inhibition in childhood: A risk factor for anxiety disorders. 1993;1:2–16. doi: 10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- 49.Sarter M. Neuronal mechanisms of the attentional dysfunctions in senile dementia and schizophrenia: Two sides of the same coin? Psychopharmacol. 1994;114:539–50. doi: 10.1007/BF02244983. [DOI] [PubMed] [Google Scholar]
- 50.Servatius RJ, Jiao X, Beck KD, Pang KCH, Minor TR. Rapid avoidance acquisition in Wistarsr Kyoto rats. 2008;192:191–7. doi: 10.1016/j.bbr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Servatius RJ, Ottenweller JE, Beldowicz D, Guo W, Zhu G, Natelson BH. Persistently exaggerated startle responses in rats treated with pyridostigmine bromide. 1998;287:1020–8. [PubMed] [Google Scholar]
- 52.Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. 2005;57:1485–92. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: A twin study. 2002;159:1675–81. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 54.Swerdlow NR, Talledo J, Shoemaker JM, Codon K, Goins J, Auerbach PP. Weak prepulses inhibit but do not elicit startle in rats and humans. 2004;55:1195–8. doi: 10.1016/j.biopsych.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 55.Turchi J, Sarter M. Cortical acetylcholine and processing capacity: Effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. 1997;6:147–58. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- 56.Turner SM, Beidel DC, Wolff PL. Is behavioral inhibition related to the anxiety disorders? 1996;16:157–72. [Google Scholar]
- 57.Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, et al. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. 2006;31:1036–45. doi: 10.1016/j.psyneuen.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. 1994;14:167. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong-Goodrich SJE, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008;30:255–69. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zvolensky MJ, Bernstein A, Marshall EC, Feldner MT. Panic attacks, panic disorder, and agoraphobia: Associations with substance use, abuse, and dependence. 2006;8:279–85. doi: 10.1007/s11920-006-0063-6. [DOI] [PubMed] [Google Scholar]