Abstract
We investigated the contribution of large conductance calcium-activated potassium (BK) channels to spontaneous activity of cerebellar Purkinje neurons. In Purkinje neurons which fire tonically block of BK channels increased the firing rate and caused the neurons to fire irregularly. In Purkinje neurons which exhibited a trimodal pattern of activity, present primarily in mature animals, block of BK channels had little effect on firing rate or regularity but shortened the single cycle duration of the trimodal pattern. The contribution of BK channels to the action potential waveform was also examined. BK channels contributed a brief afterhyperpolarization (AHP) of approximately 3 mV which followed each action potential, but made little contribution to action potential repolarization. The amplitude of the BK-dependent AHP did not change with age although there was an increase in the total AHP. The difference in the contribution of BK channels to the firing rate among the two populations of Purkinje neurons was the consequence of the decrease in the fractional contribution of BK channels to the AHP. We also found that block of BK channels increases intracellular calcium concentration during spontaneous firing. Thus, although BK channels do not affect action potential repolarization, they nevertheless control calcium entry with each action potential by contributing to the AHP.
Keywords: cerebellum, Purkinje cell, BK channels, calcium, spontaneous firing, action potential
Introduction
Large conductance calcium-activated potassium (BK) channels are present in many excitable cells. Their activation requires membrane depolarization and increases in intracellular calcium concentration. For this reason activation of BK channels is typically time locked to sodium-or calcium-dependent action potentials where BK channels contribute to action potential repolarization and to a brief afterhyperpolarization (AHP) (Lancaster and Nicoll, 1987; Robitaille and Charlton, 1992; Shao et al., 1999; Hu et al., 2001; Edgerton and Reinhart, 2003; Faber and Sah, 2003). Activation of BK channels limits calcium entry by speeding action potential repolarization, an action that serves to regulate transmitter release at nerve terminals (Robitaille and Charlton, 1992). Because the BK-dependent AHP is brief they often contribute little to the rate or pattern of firing although in some cells BK channels can regulate firing rate (Lovell and McCobb, 2001; Smith et al., 2002).
Mice which lack BK channels exhibit cerebellar ataxia (Sausbier et al., 2004) indicating a clear role for these channels in the cerebellum. Other molecules which are important in calcium-dependent signaling are also crucial for normal cerebellar function. Mutations in voltage-gated calcium channels (Pietrobon, 2002), in molecules responsible for intracellular calcium homeostasis (Matsumoto et al., 1996; Street et al., 1997; Airaksinen et al., 1997), and in calcium-activated potassium channels (Sausbier et al., 2004; Shakkottai et al., 2004) cause cerebellar ataxia. Cerebellar Purkinje neurons are central to cerebellar function since they are the only neurons which project out of the cerebellar cortex (Ito, 1984). Purkinje cells are intrinsically active (Hausser and Clark, 1997; Nam and Hockberger, 1997; Raman and Bean, 1999; Jaeger and Bower, 1999; Womack and Khodakhah, 2002a) and alterations in their intrinsic activity is thought to be a contributing factor to cerebellar dysfunction in hereditary ataxia (Walter et al., 2006). BK channels are present in Purkinje neurons (Gruol et al., 1991; Womack and Khodakhah, 2002b; Edgerton and Reinhart, 2003), where they are activated following action potentials and dendritic calcium spikes (Edgerton and Reinhart, 2003; Womack and Khodakhah, 2004; Womack et al., 2004; Walter et al., 2006). However, the exact role of these channels in regulation of Purkinje cell activity is not understood.
We thus investigated the role of BK channels in regulating spontaneous firing of Purkinje neurons. We found that contribution of BK channels to spontaneous firing depended on the preexisting pattern of activity. In neurons which fired tonically, present predominantly in immature animals, block of BK channels increased the spontaneous firing rate and altered the pattern of activity by promoting bursting. In neurons which exhibited a trimodal pattern of activity, comprising most adult Purkinje neurons in slices, BK channels made no significant contribution to the firing rate but when blocked shortened the pattern duration. The difference in the contribution of BK channels to the firing rate among the two populations of neurons was related to an age-dependent decrease in the fractional contribution of BK channels to the AHP. We also found that that BK channels are one of the factors that determine the extent of calcium entry to the soma of Purkinje cells with each action potential.
Experimental Procedures
Preparation of slices
CD1 mice, or Wistar rats, at 11–33 days postnatal were anaesthetized with halothane and sacrificed by decapitation. 300 μm thick sagittal slices were prepared from the vermis of the cerebellum using a vibratome (Campden Instruments, UK). Slices were maintained at room temperature in the recording solution until use (1 to 8 hours)
Recording and analysis
Slices were mounted in a chamber on the stage of an upright Zeiss microscope and visualized using a 40 water immersion objective with infrared optics. Slices were continuously superfused at a rate of 1.5 ml per minute with recording solution (in mM) NaCl 125, KCl 2.5, NaHCO3 26, NaH2PO4 1.25, MgCl2 1, CaCl2 2, glucose 10, pH 7.4 when gassed with 5% CO2:95% O2. Where indicated the solution also contained kynurenic acid (5 mM) a broad spectrum ionotropic glutamate receptor antagonist (Stone, 1993) and the GABA A antagonist, picrotoxin (Yoon et al., 1993) The slice temperature was maintained at 35 °C (±0.5 °C) by adjusting the temperature of the bathing solution. The volume of the chamber was 0≈2 ml, requiring several minutes for complete wash in of the antagonists or blockers. For local perfusion, a glass pipette connected to a reservoir containing perfusate was positioned just above the surface of the slice. Fast green (0.4%) was included in the perfusate in order to monitor the location of the perfusate. At this concentration the dye has no effect on the firing of Purkinje neurons. A suction pipette was placed downstream from the perfusion pipette in order to limit the spread of the perfusate. Previously we have shown that the visible dye front is a reliable measure of the extent of localized perfusion (Womack and Khodakhah, 2002a). Extracellular field potential recordings were made from individual Purkinje neurons using a home-made differential amplifier with glass pipette electrodes (tip size 0.3 to 1 μm) filled with the recording solution. The pipette tip was positioned just above, or lightly touching, the cell body near the axon hillock where the largest potential changes were usually recorded. Action potentials appeared as fast negative deflections of 50–1000 μV. Whole cell recordings were made with borosilicate glass pipettes (3–6 MΩ) filled with (in mM) K methyl sulfate 140, KCl 10, NaCl 5, MgATP 2, EGTA 0.01, HEPES 10, (pH 7.2). For calcium measurements Fluo-4 or Fura-4F (200 μM) was also included in the pipette solution. Fluorescence intensity of the cell soma was measured using a cooled CCD camera (EEV chip, Frame transfer mode, Princeton Instruments), or a photon counting photomultiplier (Hamamatsu Photonics). Fluorescence emitted by the calcium indicators were measured at wavelength >510 nm. Fluo-4 was excited at 480±15 nm whereas Fura-4F at 425±15 nm. The fluorescence emitted by Fura family of indicators when excited at 425 nm is negligible when these dyes are bound with calcium. Consequently, the auto-fluorescence recorded in the cell attached mode was taken as a good estimate of Fmax. The resting fluorescence just prior to the experimental manipulation was background corrected and assumed to reflect fluorescence at 50 nM free calcium concentration (Llano et al., 1991). The Fmin was then estimated from this assumption and used to estimate free calcium concentration using the equation [Ca2+]=Kd(F−Fmin)/(Fmax−F) where F is the measured fluorescence.
Whole cell data was recorded using an Optopatch amplifier (Cairn Research Ltd., U.K.). Data was sampled at 10 kHz for extracellular recordings and 20 kHz for whole cell recordings using a National Instruments D/A-A/D card (MIO-16XE-10; Austin, TX) and an IBM-compatible computer. Data acquisition and analyses were done with software written in-house using LabView (National Instruments, Austin, TX). To analyze firing rate, a threshold level for spike detection was set by eye during the experiment. The number of spikes crossing the threshold was counted every 500 ms and is reported as the firing rate in terms of spikes per second. Kynurenic acid, picrotoxin, apamin, and fast green, were obtained from Sigma Chemical (St. Louis, MO). Potassium methyl sulfate was purchased from Pfaltz and Bauer Inc. (Waterbuy, CT). Fluo-4 and Fura-4F were obtained from Molecular Probes (Eugene, OR), and iberiotoxin was purchased from Tocris (Ellisville, MO). All other chemicals were of reagent grade.
Results
In the absence of synaptic blockers, block of BK channels increases firing rate and promotes bursting in Purkinje neurons
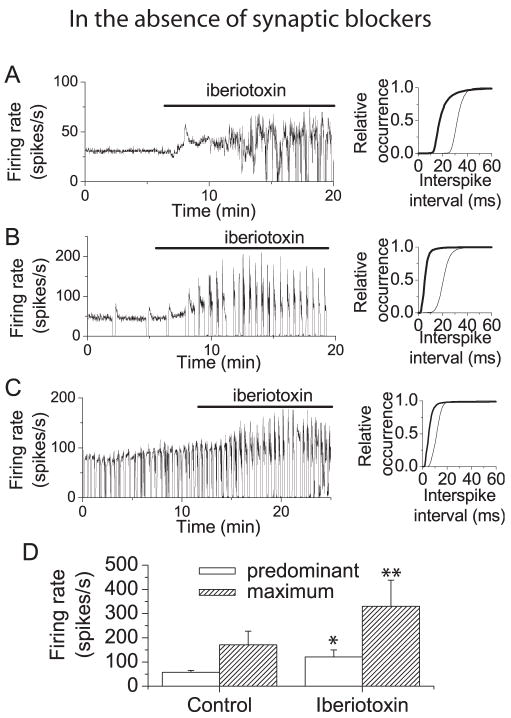
The contribution of BK channels to spontaneous activity of Purkinje neurons was assessed by recording firing in the presence and absence of iberiotoxin, a specific BK channel blocker (Giangiacomo et al., 1992). To avoid washout of cytoplasmic constituents, particularly calcium buffers, which might alter spontaneous activity, extracellular recording was used to monitor spontaneous firing of individual Purkinje neurons. The first set of experiments were conducted in the presence of intact synaptic transmission to obtain an overall idea of the consequences of dysfunction of BK channels in the cerebellar cortex. As previously described (Womack and Khodakhah, 2003), in the absence of synaptic blockers, Purkinje neurons exhibited one of three modes of spontaneous firing. While most neurons fired tonically, a second population exhibited a trimodal pattern of activity in which they cycled regularly through periods of tonic firing, bursting, and silence, while a third population alternated between periods of bursting and silence. The left panels in Figure 1 (A–C) show recordings of average firing rate obtained from each population of neurons. Application of iberiotoxin (100 nM) increased the average firing rate in all three populations of neurons (Figure 1A–C). This is shown clearly in the right panels of Figure 1 (A–C) where iberiotoxin produces a shift to shorter interspike intervals (increased firing rate). For each cell the predominant firing rate was defined as the inverse of the most prevalent interspike interval. Under control conditions the mean predominant firing rate for all neurons tested was 58 spikes/s (± 8 spikes/s, S.E.M., n=5). Block of BK channels increased the mean predominant firing rate to 121 (± 29 spikes per second, S.E.M., n=5) (Figure 1D).
Figure 1. Block of BK channels increases firing rate and promotes bursting in cerebellar Purkinje neurons.
A, Left panel: Average firing rate (calculated every 500 ms) vs. time recorded from a Purkinje neuron from a 14 day old rat which fired tonically. Superfusion with iberiotoxin (100 nM) increased the firing rate and caused the cell to fire irregularly. The cumulative distribution of interspike intervals shows a shift to shorter intervals (higher firing rate) in iberiotoxin (right panel). Thin trace, control distribution; thick trace, distribution in iberiotoxin. B, Left panel: Average firing rate vs. time from a Purkinje neuron from a 21 day old rat which exhibited the trimodal pattern of activity. Application of iberiotoxin increased the firing rate and increased the fraction of time that the cell was in the bursting mode. Right panel: Cumulative probability distribution s of interspike intervals under control conditions (thin traces) and in iberiotoxin (thick traces). C, Left panel: Average firing rate vs. time from a Purkinje neuron from a 18 day old rat which burst at random. Application of iberiotoxin increased the firing rate. Right panel: Cumulative probability distribution s of interspike intervals under control conditions (thin traces) and in iberiotoxin (thick traces). D, Mean predominant and maximum firing rates under control conditions and in the presence of iberiotoxin. Predominant and maximum firing rates for each cell were determined from histograms of interspike intervals. The predominant firing rate was defined as the firing rate most often observed (peak of the histogram). The maximum firing rate was the fastest firing rate observed at 5% of the frequency of the predominant rate. Error bars are ± SEM (n=5). Under control conditions the mean predominant and maximum firing rates were 58 ± 8 and 171 ± 56 spikes per second, respectively. In the presence of iberiotoxin the mean predominant firing rate increased to 121 ± 29 spikes per second while the maximum firing rate was 330 ±108 spikes per second. Error bars are mean ± S.E.M. (n=5). Control values were significantly different from those in iberiotoxin (*, p< = 0.07; ** p< = 0.05 by Oneway ANOVA).
In tonic firing neurons in the absence of synaptic input, block of BK channels increases firing rate and promotes bursting
BK channels are present on presynaptic terminals of some neurons where they contribute to action potential repolarization and limit neurotransmitter release (Robitaille and Charlton, 1992). In some neurons, BK channels also control the rate of spontaneous activity and membrane excitability. It is therefore possible that the effects of iberiotoxin reported in Figure 1 are due to increased release of excitatory or inhibitory transmitters onto the Purkinje neurons following block of presynaptic BK channels rather than alterations in the intrinsic conductances of Purkinje cells. To examine the direct contribution of BK channels to spontaneous activity of Purkinje neurons, spontaneous firing was monitored in the presence of kynurenic acid (5 mM) and picrotoxin (100 μM) to block fast excitatory and inhibitory synaptic input, respectively. As reported previously (Womack and Khodakhah, 2002a), in the presence of synaptic blockers Purkinje neurons either fired tonically or exhibited the trimodal pattern of activity. In the absence of synaptic input, the two types of Purkinje neurons respond differently to BK channel block.
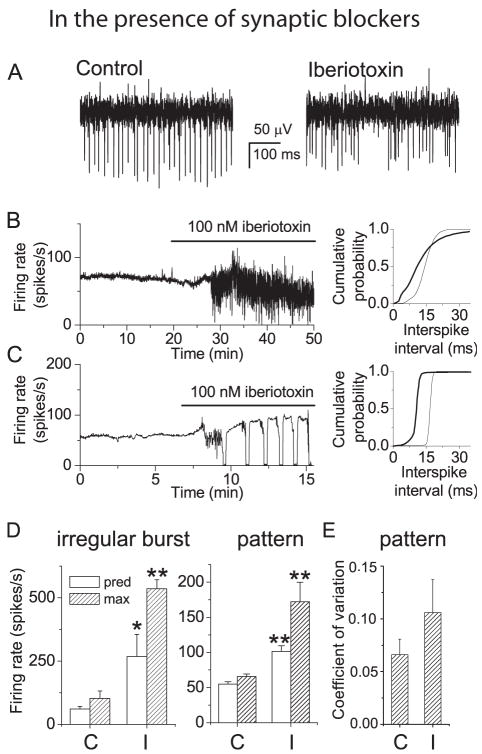
In tonically firing neurons BK channel block increased the firing rate and caused the neurons to fire in bursts. Figure 2 (A, B, C) shows the responses of two tonic firing neurons to bath application of iberiotoxin. Half the tonically firing neurons tested (5/10) responded to BK channel block by bursting irregularly (Figure 2B) while in the other half application of iberiotoxin induced a trimodal pattern of activity (Figure 2C). In all cases BK channel block increased the firing rate, as illustrated by the shifts in the interspike interval distributions in the right-hand panels of Figures 2B and C. In neurons where block of BK channels caused irregular bursting the predominant firing rate increased from 61 ± 10 to 268 ± 87 spikes per second (mean ± S.E.M, n=5) (Figure 2D, left panel) while in neurons where BK channel block induced a trimodal pattern the predominant firing rate increased from 55 ± 3 to 102 ± 8 spikes per second (mean ± S.E.M, n=5) (Figure 2D, right panel). The fraction of Purkinje cells that have the trimodal pattern of activity increases with age. We wondered whether there was a correlation between the age of the animals and whether iberiotoxin induced the trimodal pattern of activity or irregular firing. We find that such a correlation does exist with the tonic Purkinje cells that showed the trimodal pattern of activity in iberiotxin were taken from older animals (21.2 ± 1.11 days old; mean ± S.E.M, n=5) compared with those that burst at random when iberiotoxin was applied (14.4 ± 1.05 days old; mean ± S.E.M, n=5). Clearly, the effect of iberiotoxin on the rate of activity of Purkinje cells is age dependent.
Figure 2. In tonic firing neurons block of BK channels increases firing rate and promotes bursting.
A, Extracellular recordings from a Purkinje neuron which fired tonically under control conditions in the presence of synaptic blockers (left trace). Superfusion with iberiotoxin (100 nM) caused the cell to fire irregularly (right trace). B, Left panel: Average firing rate vs. time from the neuron discussed in A. Right panel: Cumulative probability distributions of interspike intervals in the presence of iberiotoxin (thick traces) and in control conditions (thin traces) show that iberiotoxin shifted the distribution to shorter intervals (higher firing rate). C, Left panel: Average firing rate vs. time from another tonically firing Purkinje neuron. Superfusion with iberiotoxin increased the firing rate and induced the trimodal pattern of activity. Right panel: Cumulative probability distributions of interspike intervals in the presence of iberiotoxin (thick traces) and in control conditions (thin traces). D, Mean predominant and maximum firing rates for cells in which iberiotoxin caused irregular bursting (left panel) and for cells in which iberiotoxin caused the trimodal pattern of activity (middle panel). In irregular bursting cells block of BK channels significantly increased the predominant firing rate from 61 (± 10) spikes/s in control conditions (C) to 268 (± 87) in iberiotoxin (I) and the maximum firing rate from 102 (± 29) spikes/s to 536 (± 36) spikes/s. In Neurons which acquired the trimodal pattern, block of BK channels significantly increased the predominant firing rate from 55 (± 3) spikes/s to 102 (± 29) and the maximum firing rate from 66 (± 4) spikes/s to 173 (± 28) spikes/s. Values in iberiotoxin were significantly different from control for (*) p<=0.05 and (**) p<= 0.005 (One-way ANOVA). Error bars are mean ± S.E.M. (n=5 for each type of neuron). E, For cells in which iberiotoxin induced a trimodal pattern of firing, the coefficient of variation (CV) for interspike intervals was measured under control conditions and in the presence of iberiotoxin during a period of the tonic phase of the trimodal pattern. Block of BK channels increased the CV from 0.066 (± 0.01) to 0.11 (± 0.03). Error bars are ± S.E.M. (n=5).
In all neurons the firing rate also became more irregular in iberiotoxin. This is shown clearly in the raw data traces and average firing rate of Figures 2A, B. The firing rate was also less regular in neurons which acquired the trimodal pattern, in part because BK channel block promoted bursting as part of the trimodal pattern of activity. However, even during the tonic firing periods of the trimodal pattern, spontaneous firing was more irregular than under control conditions. To quantify this difference, the coefficient of variation was determined for a 5 s period of firing under control conditions and in iberiotoxin during a period of tonic firing where the average firing rate was not changing. The histogram in Figure 2E shows that the CV increases in the presence of iberiotoxin. Thus BK channels serve to slow the firing rate and maintain regular firing in tonically firing Purkinje neurons.
In neurons with the trimodal pattern block of BK channels decreases the pattern cycle period with no effect on mean firing rate
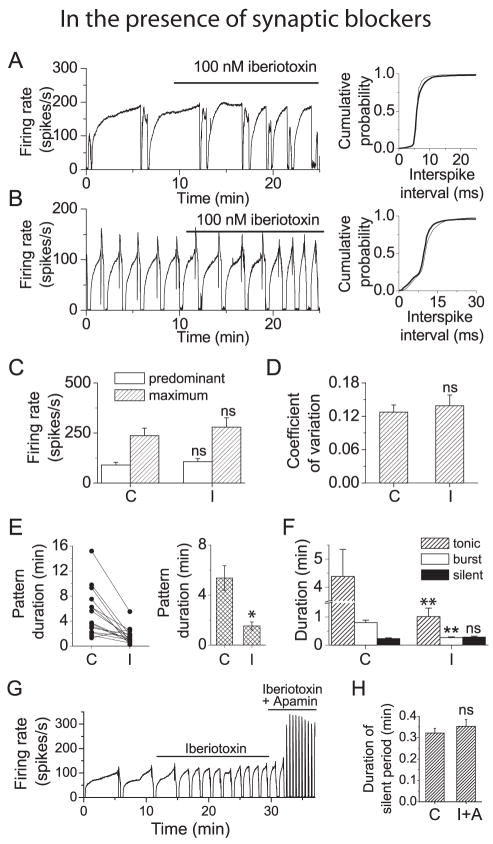
Records of average firing rate from two neurons which exhibited the trimodal pattern of activity are shown in Figure 3 (A, B). In contrast to its effects on tonically firing neurons, application of iberiotoxin (100 nM) had little effect on average firing rate (left panels). The lack of change in firing rate is illustrated more clearly by the interspike interval distributions (Figure 3A, B, right panels). The average predominant firing rate for all cells tested is shown in figure 3C. The averages were 90 ± 14 spikes/s in control conditions and 107 ± 15 spikes/s in iberiotoxin (mean ± S.E.M, n=15) (Figure 3C). Comparison of coefficients of variation during the tonic phase of firing shows that BK channel block also had no effect on regularity of firing (Figure 3D). These results are in contrast to those obtained for neurons with the trimodal pattern of activity in the absence of synaptic blockers and suggest that BK channels also play a role in regulating the release of glutamate or GABA from presynaptic neurons or glia.
Figure 3. In Purkinje neurons with the trimodal pattern of activity block of BK channels shortens the pattern period but does not increase the average firing rate.
A, Left panel: Average firing rate vs. time from a Purkinje neuron with the trimodal pattern of activity. Superfusion with iberiotoxin decreased the single cycle duration of the pattern period but did not increase the average firing rate. Right panel: Cumulative probability distributions of interspike intervals in the presence of iberiotoxin (thick traces) and in control conditions (thin traces) show that there was no change in firing rate in iberiotoxin. B, Left panel: Average firing rate vs. time from another Purkinje neuron with the trimodal pattern. Right panel: Cumulative probability distributions of interspike intervals. C, Histogram showing average predominant and maximum firing rates for all Purkinje neurons with the trimodal pattern. The mean value of the predominant firing rate was 90 (± 14) spikes/s in control conditions (C) and 107 (± 15) spikes/s in iberiotoxin (I). Maximum firing rates were 237 (± 37) spikes/s in control conditions, and 279 (± 47) spikes/s in iberiotoxin, respectively. Error bars are ± S.E.M., n=15. ns, values were not significantly different from control. D, The coefficient of variation was determined for a 5 s interval where there was no increase in the average firing rate during the tonic phase of firing for each neuron. Block of BK channels did not change the coefficient of variation. mean values were o.13 (± 0.01) in control conditions and 0.14 (± 0.02) in iberiotoxin. ns, values were not significantly different from control. E, Left panel: The average single cycle duration for each cell tested under control conditions (C) and in the presence of iberiotoxin (I). Right panel: Average single cycle duration for all cells tested. The mean pattern duration was 5.36 (± 1.0) min under control conditions and 1.53 (± 0.3) min in iberiotoxin. Error bars are ± S.E.M (n = 15). Values in iberiotoxin were significantly different from control (*, p = 0.001). F, Histogram showing the average duration of the tonic, bursting, and silent periods in control conditions and in iberiotoxin. Block of BK channels decreased the average duration of the tonic firing mode from 4.37 (± 1.0) min to 1.0 (± 0.3) min and the duration of the bursting mode from 0.79 (± 0.09) to 0.26 (± 0.03) min. (**, differences were significant at p<=0.005). There was no significant change in the duration of the silent period (0.23 (± 0.03) min in control vs. 0.28 (± 0.03) min in iberiotoxin (ns). Error bars are ± S.E.M. (n = 15) G, Average firing rate vs. time following application of iberiotoxin (100 nM) and simultaneous application of iberiotoxin and the specific SK channel blocker apamin (100 nM) to a neurons with the trimodal pattern. In the presence of both blockers the neuron continues to fire with the trimodal pattern. H, Histogram comparing the average duration of the silent period in control conditions (C) and in the presence of iberiotoxin and apamin (I+A). Simultaneous block of both BK and SK channels did not alter the duration of the silent period (0.32 (±0.02) min in control vs. 0.35 (±0.03) min. in iberiotoxin + apamin). ns, values not significantly different from control. Error bars are ± S.E.M. (n = 5).
Further examination of the data records from the two cells discussed in figure 3 shows that BK channel block decreased the single cycle duration of the trimodal pattern of activity and that the decrease is greater for the neuron with the longer single cycle duration (Figure 3A). Comparison of single cycle pattern duration in control conditions and in iberiotoxin for all neurons tested (Figure 3E, left panel) supports this conclusion. Block of BK channels decreased the average single cycle duration from 5.4 ± 0.99 min to 1.5 ± 0.31 min (mean ± S.E.M, n=15) (Figure 3E, right panel). In the presence of synaptic blockers, block of BK channels does not increase the firing rate of Purkinje neurons with the trimodal pattern of activity, but significantly decreases the single cycle duration of the pattern.
Calcium-activated potassium channels do not contribute to the silent phase of the trimodal pattern of activity
Each cycle of the trimodal pattern of activity consists of a phase of tonic firing, a period of bursting, followed by a silent period lasting approximately 20 seconds. During the silent period the membrane potential hyperpolarizes well below threshold (Womack and Khodakhah, 2003). It is conceivable that the silent period results from activation of calcium-activated potassium channels activated following buildup of intracellular calcium during the bursting phase. We have previously shown, however, that the duration of the silent phase is not altered by block of SK channels (Womack and Khodakhah, 2003). We asked whether BK channels contributed to the silent phase by analyzing the effect of iberiotoxin on each phase of the trimodal pattern. While block of BK channels significantly decreased the duration of the tonic and bursting phases of the pattern, there was no effect on the silent phase (Figure 3F). Simultaneous block of BK and SK channels did not abolish the trimodal pattern of activity (Figure 3G) or alter the duration of the silent period (Figure 3H). These results show that neither BK nor SK channels contribute to the silent periods.
Spontaneous firing is controlled by both dendritic and somatic BK channels
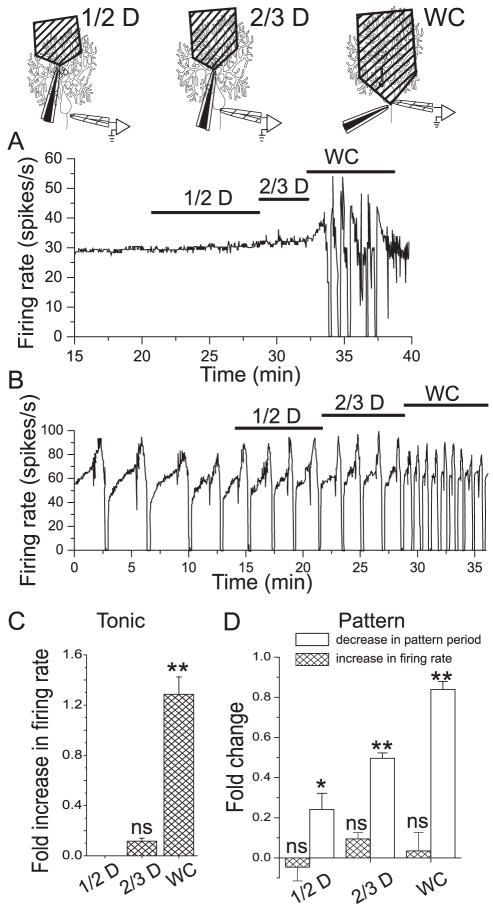
Ion channels present in the dendrites of Purkinje neurons contribute significantly to spontaneous firing and are particularly critical for maintaining spontaneous activity in Purkinje neurons with the trimodal pattern of activity (Womack and Khodakhah, 2002a; Womack and Khodakhah, 2003). To examine the contribution of dendritic BK channels to spontaneous firing, iberiotoxin was applied selectively to the dendrites of Purkinje neurons by local perfusion. The effect of blocking BK channels in the dendrites of a tonically firing Purkinje neuron is shown in Figure 4A. Application of iberiotoxin to the distal half of the dendritic tree had no effect on spontaneous firing while application to the distal two thirds produced only a slight increase in firing rate. Application of iberiotoxin to the whole cell caused the neuron to burst irregularly. Similar results obtained from six additional Purkinje neurons are summarized in Figure 4C. In tonic firing Purkinje neurons dendritic BK channels make no contribution to spontaneous firing. Figure 4B shows the effect of dendritic BK channel block on spontaneous firing in a Purkinje neuron with the trimodal pattern of activity. Application of iberiotoxin to the distal half of the dendrites decreased the pattern period from 3.2 to 2.1 minutes. A further decrease to 1.7 minutes was observed upon application to the distal two thirds of the dendrites. Block of BK channels throughout the cell decreased the pattern duration to 0.63 minutes. No effect on firing rate was observed. Similar results obtained from four additional Purkinje neurons are summarized in Figure 4D. In Purkinje neurons which exhibit the trimodal pattern of activity, both dendritic and somatic BK channels contribute to the pattern of spontaneous activity.
Figure 4. BK channels which control firing rate are localized to the soma of Purkinje neurons while BK channels which regulate the duration of the trimodal pattern are localized to both soma and dendrites.
Iberiotoxin (100 nM) was applied selectively via local perfusion to the distal half (1/2 D) or distal two thirds (2/3 D) of the dendrites or to the whole cell (WC). A, Average firing rate of a tonic firing neuron in response to local application of iberiotoxin. Application to the dendrites had no effect. B, Average firing rate of a neuron with the trimodal pattern of activity in response to local application of iberiotoxin. C, Average fold-increase in firing rate of tonic firing neurons following local block of BK channels. Block of CK channels to the distal ½ (no effect) or distal 2/3 of the dendrites (0.11 (± 0.02)-fold increase) did not significantly change the firing rate. Block of BK channels I the whole cell increased the firing rate by and average of 1.28 (± 0.14)-fold. Error bars are ± S.E.M., n=4. ns, value not significantly different from control. **, significantly different from 0 (p< = 0.001, by Oneway ANOVA). D, Average fold-increase in firing rate (hatched bars) and fold- decrease in pattern duration (open bars) in Purkinje neurons with the trimodal pattern of activity. Block of BK channels did not significantly increase firing rate. The mean fold-increases in firing rate were (1/2 D,) −0.05 (± 0.07), (2/3 D), 0.09 (± 0.03), and (WC), 0.04 (± 0.09). None of these values was significantly different from 0 (ns). Block of BK channels in the dendrites decreased the pattern period. Mean fold- decreases were 0.24 (± 0.08) (1/2 D), and 0.5 (± 0.03) (2/3 D). Application to the whole cell decreased the period 0.84 (± 0.04)-fold. Error bars are ± S.E.M., n=7. The changes were significant (*, p = 0.02, **, p = 0.001 by Oneway ANOVA).
Contribution of BK channels to spontaneous activity of Purkinje cells is age-dependent
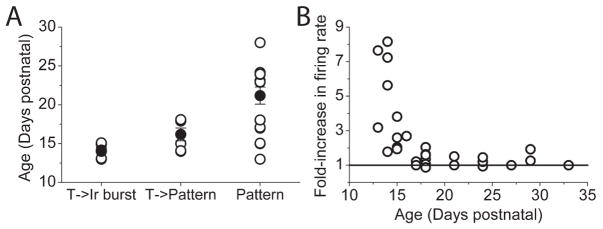
Block of BK channels markedly increased the firing rate of tonic cells which converted to irregular bursting cells, somewhat increased the firing rate of tonic cells that demonstrated the trimodal pattern of activity in iberiotoxin, and had no effect on the cells which showed the trimodal pattern of activity. Edgerton & Reinhart (2003) report a small but statistically insignificant decrease in the contribution of BK channels to the AHP in older animals. To examine whether the diversity of effects of iberiotoxin was age related, we examined the age of the animals for each of the three groups. As shown in Figure 5A, Purkinje cells in which tonic firing was converted to irregular bursting by iberiotoxin were, on average, recorded in cerebella of the youngest animals examined (14.2±0.3 days postnatal, mean±S.E.M., n=7). The tonic cells whose activity converted to the trimodal pattern by iberiotoxin came from animals which were slightly older from the first group (16.1±0.8 days postnatal, mean±S.E.M., n=6). The difference between the age of these two groups while small, was statistically significant (p<0.05, Oneway ANOVA). Lastly, the Purkinje cells with the trimodal pattern of activity were from animals with an average age of 21.1±1.1 days postnatal (mean±S.E.M., n=15). The average age of this group was also significantly greater than that of the other two groups (p<0.01, compared with each of the two tonic groups, Oneway ANOVA). Further, there is a clear relationship between the effectiveness of iberiotoxin in increasing the firing rate of a Purkinje cell and the age of the animal studied (Figure 6B).
Figure 5. Age-dependence of effects of Iberiotoxin on spontaneoeuos firing of Purkinje cells.
A,Open symbols show the age of animals in which iberiotoxin altered tonic firing to irregular burst firing (T->Ir Burst; n=7), tonic firing to the trimodal pattern of activity (T->Pattern; n=6), and cells that in the absence of iberiotoxin exhibited the trimodal modal pattern of activity (Pattern; n=15). Filled symbols show the average age (±S.E.M.). The average ages of the three groups were significantly different from each other. B, The relationship between the age of animals and the extent to which iberiotoxin increased their firing rate.
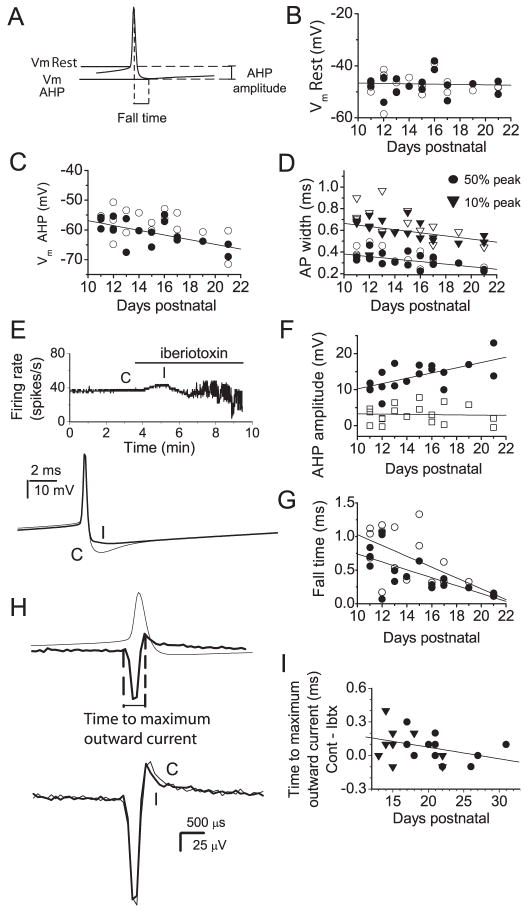
Figure 6. Contribution of BK channels to action potential waveform.
A–G, Whole cell current clamp recordings were obtained from spontaneously firing Purkinje neurons. Trains of 100–1500 action potentials were averaged and action potential waveforms analyzed from the average traces. A, The diagram shows parameters measured from the average action potentials. Vm Rest, membrane potential 500 μs prior to the peak of the action potential; Vm AHP, minimum membrane potential following action potential; AHP amplitude, Vm Rest - Vm AHP; fall time, time from peak of action potential to Vm AHP. The action potential width at 10 %and 50 % of the difference between the peak and Vm Rest was also measured. B, Vm Rest vs. age for individual Purkinje neurons under control conditions (filled symbols) and in iberiotoxin (open symbols). Linear regression fit to the control data shows no correlation of Vm Rest with age of the animal (R2=0.053, p=0.83). C, Vm AHP vs. age for all neurons tested. A linear regression fit to the control values shows a clear correlation between the AHP and age (R2=0.69, p=0.008). D, Action potential width at 10% and 50 % of peak value vs. age. Closed symbols show values under control conditions and open symbols values for the same neurons in iberiotoxin. Linear regression fits to control data show a clear correlation between age and action potential width (R2=0.65, p=0.04 at 10%; R2=0.68, p=0.008 at 50%). E, Effect of BK channel block on a spontaneously active Purkinje neuron. Top panel: Average firing rate vs. time. Application of iberiotoxin caused an initial increase in firing rate followed by irregular firing. Bottom panel: Average action potentials under control conditions (C) and in the presence of 100 nM iberiotoxin (I) taken at the times indicated in the top trace. BK channels contributed to an AHP which persisted for several ms following each action potential. F, AHP amplitude vs. age. Values for individual cells are shown for the total AHP (circles) and for the iberiotoxin-sensitive component of the AHP (squares). Linear regression fits show a clear correlation between total AHP amplitude and age (R2=0.67, p=0.006) but no correlation between the iberiotoxin-sensitive component of the AHP and age (R2=0.043, p=0.87). G, Fall time vs. age. Values for individual cells under control conditions (filled symbols) and in iberiotoxin (open symbols) are shown. Linear regression fits show a correlation between fall time and age for both sets of data. (R2=0.69, p=0.005 for control; R2= 0.63, p= 0.005 in iberiotoxin). The slope increases from −0.06 ms/day in control conditions to −0.08 ms/day in iberiotoxin indicating that BK channels make a larger contribution to fall time at younger ages. H, I, Extracellularly recorded spike waveforms were analyzed. Averages of 100–1500 action potentials were generated. H, Upper panel: Action potential recorded in whole cell mode (thin trace) and an extracellular spike waveform (thick trace) which shows the temporal correlation between potential changes recorded with an extracellular electrode and the action potential waveform. The positive peak of the extracellular recording corresponds to the time at which the rate of membrane repolarization, hence the net outward current, is maximal. Lower panel: Extracellular spike waveforms recorded under control conditions (thin trace) and in iberiotoxin (thick trace) shows that block of BK channels causes a decrease in the time to maximum outward current. I, The difference between the time to maximal outward current in control conditions and the time to maximal outward current in iberiotoxin vs. age. Values for individual cells which fired tonically (triangles) and those with the trimodal pattern of activity (circles) are shown. A linear regression fit shows a negative correlation between the contribution of BK channels to the maximum outward current and age (R2=0.36. p=0.11).
Contribution of BK channels to action potential waveform
We examined the age-dependence of the action of iberiotoxin more closely in order to determine whether differences in the contribution of BK channels to firing rate could be explained by age-dependent changes in the contribution of BK channels to the action potential waveform. Spontaneous firing was recorded in whole cell current-clamp mode and action potentials analyzed before and after application of iberiotoxin. Figure 6A shows the parameters which were analyzed. The resting membrane potential (Vm Rest) was defined as the potential 0.5 ms prior to the action potential peak. The membrane potential at the minimum of the AHP (Vm AHP) and the difference between Vm AHP and Vm Rest (AHP amplitude) were also measured. To estimate the contribution of BK channels to action potential repolarization, the action potential width, measured at 10% and 50% of the difference between Vm Rest and the action potential peak, was determined. Another estimate of the rate of repolarization of the action potential was obtained by measuring the time from the peak of the action potential to the minimum of the AHP (fall time). Under control conditions Vm Rest was −47 mV (± 0.9, S.E.M., n=18) and showed no correlation with age (Figure 6B). In contrast, there was a significant increase in the AHP and decrease in the width of the action potential. The average values for Vm AHP and AHP amplitude were −60.7 mV (±1.0) and 13.7 mV (± 0.9), respectively. Linear regression fits to the control data showed a decrease in Vm AHP of 0.8 mV per day from postnatal day 11 to 22 (Figure 6C) and an increase in AHP amplitude of 0.73 mV per day over the same age range (Figure 5F). The action potential width also changed with age under control conditions. Average values were 0.33 (± 0.02) ms and 0.60 (± 0.02) ms at 50 and 10 %, respectively. Linear regression fits show a significant decrease of approximately 0.01 ms per day for each parameter (Figure 6D). Under control conditions the average fall time was 0.46 (± 0.07) ms. The fall time also showed a clear correlation with age, decreasing by 0.06 (± 0.07) ms per day (Figure 6G).
Figure 6E shows average firing rate of a neuron during application of iberiotoxin. Iberiotoxin caused an initial increase in firing rate which preceded bursting. Measurements of action potential parameters were made under control conditions (C) and in iberiotoxin before the neuron began to burst (I). Average action potentials from this neuron (Figure 6E, bottom panel) show a clear decrease in AHP in the presence of iberiotoxin. In the presence of iberiotoxin the average Vm AHP was −57.8 (± 1.1) mV significantly different from the control value of −60.7 (± 1.3) mV (p<=0.02, by Oneway ANOVA). The average AHP amplitude decreased from 13.7 (± 0.91) mV to 10.6 (± 0.9) mV. The iberotoxin-sensitive AHP amplitude found here was similar to the mean amplitude of the iberiotoxin-sensitive AHP in Purkinje neurons reported earlier (Womack and Khodakhah, 2002b). Block of BK channels did not affect Vm Rest (−47 ± 0.09 mV in control vs.−47 mV (± 1.0, S.E.M., n=18) in iberiotoxin) and, as in control conditions, Vm Rest did not change with age (Figure 6B). There was a small, although statistically insignificant increase in the mean action potential width at 10 % of the peak, from 0.60 (± 0.02) ms in control to 0.64 ms (± 0.04, S.E.M., n=18). The fall time increased from 0.46 (± 0.07) to 0.64 ms (± 0.1, S.E.M., n=18). There was little change in the width at 50 % of peak with average values of 0.33 (± 0.02) ms in control conditions and 0.35 ms (± 0.02, S.E.M., n=18) in iberiotoxin.
Figure 6F shows the amplitude of the iberiotoxin–sensitive component of the AHP as a function of age. While the total AHP amplitude shows a clear increase with age, the iberiotoxin-sensitive component of the AHP remains the same across all ages (Figure 6F). Likewise, the contribution of BK channels to the fall time is greater at younger ages (Figure 6E). Taken together, these results indicate that BK channels make a larger contribution to the AHP and to action potential repolarization in younger animals and provides an explanation for the difference in the effects of BK channel block on firing rate between tonic firing Purkinje neurons and those with the trimodal pattern. In younger, tonic firing neurons BK channels make a large enough contribution to the AHP to alter the voltage trajectory during the interspike interval and slow the rate at which the membrane potential approaches threshold. In older Purkinje neurons (most of which have the trimodal pattern) control of the firing rate is determined by other channels. It is curious, however, that the firing rate of cells with the trimodal pattern of activity from younger animals is affected little by iberiotoxin. BK channels might have contributed less to the AHP in these cells compared that expected purely on the basis of age. In fact, a change in the amplitude of AHP might be a contributing factor to conversion of tonic firing cells to those with the trimodal pattern of activity.
We also analyzed the effects of BK channel block on the waveforms of action potentials recorded extracellularly. This technique has the advantage that cytoplasmic constituents which may be required to maintain normal spontaneous firing are not washed out. Potential changes recorded in this configuration correspond to the derivative of the membrane potential. The peak positive deflection occurs at the time at which the rate of repolarization of the membrane potential is greatest, that is at the time of maximum outward current (Figure 6H, top panel). Trains of extracellular spike waveforms (100–2000) were averaged in control conditions and in the presence of iberiotoxin. The time from the start of the negative voltage deflection (equivalent to the start of the rising phase of the action potential; determined by the time of the first data point whose amplitude was more than two standard deviations away from the mean of the baseline) to the peak outward current was measured to assess the magnitude of the BK channel contribution (Figure 6H, top panel). Average action potentials recorded in control conditions and in the presence of iberiotoxin are shown in Figure 6H (bottom traces). Block of BK channels slightly decreased the time to peak outward current (Figure 6I). These results indicate that, even though they make only a small contribution to action potential repolarization, BK channels are active during the repolarizing phase of the action potential and are activated slightly later than other channels. Figure 6I shows the difference between the time to maximal current in control conditions and in the presence of iberiotoxin vs. age. Linear fits to the data show a decrease in this value of 0.01 ms per day over the ages tested and indicate that the contribution of BK channels to the total outward current is less at older ages. These findings are consistent with the results obtained from whole cell recordings in which the relative contribution of BK channels to action potential waveform diminishes with age. Collectively, the results in figure 6 suggest that the contribution of BK channels to firing rate in tonic firing neurons, which predominate at younger ages, arises because BK channels make a larger contribution to the AHP in these neurons than in neurons with the trimodal pattern of activity.
BK channels regulate calcium entry during spontaneous firing
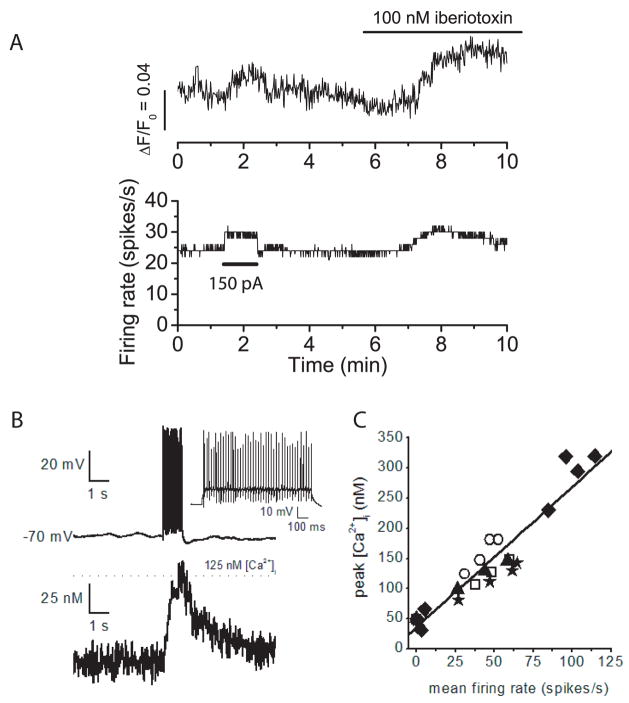
BK channels contribute briefly to an AHP following each action potential, but not to resting membrane potential. These results are consistent with previous findings showing that in Purkinje neurons BK channels require both large calcium increases and membrane depolarization for activation (Womack and Khodakhah, 2002b). In Purkinje neurons with the trimodal pattern of activity the relative contribution of BK channels to the AHP is not great enough to influence the rate or regularity of firing. However, block of BK channels in the neurons with the trimodal pattern of activity produces a change in the pattern duration of spontaneous firing, indicating that BK channels are present on these neurons and have a function. A similar decrease in single cycle period of the trimodal pattern of activity has been observed when the calcium influx is increased by increasing the extracellular calcium concentration from 2 to 5 mM (Chevez and Khodakhah, unpublished observations). Calcium enters Purkinje neurons with each action potential following activation of voltage-gated calcium channels. The calcium channels are activated during the depolarization and deactivate rapidly upon repolarization (Raman and Bean, 1999). Block of BK channels could lead to increased calcium entry during spontaneous firing by slowing action potential repolarization leading to longer activation of calcium channels and, by decreasing the AHP, BK channel block could slow deactivation of the calcium channels. To investigate the effects of BK channel block on intracellular calcium concentration, whole-cell current clamp recordings were made from spontaneously firing Purkinje neurons. Fluo-4, a calcium-sensitive fluorescent dye, was included in the recording pipette and somatic fluorescence levels were monitored using a cooled CCD camera. Figure 7 shows the results of one such experiment. The neuron fired tonically at a rate of approximately 25 spikes/s and showed a steady fluorescence level. Injection of depolarizing current increased firing rate and fluorescence intensity indicating that a significant portion of the fluorescence signal is the result of calcium entry per action potential. Block of BK channels caused an increase in the firing rate and corresponding increase in intracellular calcium concentration as indicated by the fluorescence increase. The increase in fluorescence in iberiotoxin was 23% greater than in control conditions when measurements made at the same average firing rate. The fluorescence increase seen in iberiotoxin was the result of increased calcium entry per action potential as well as increased firing rate. After correction for the increase in firing rate, block of BK channels caused the fluorescence to increase an average of 26% above control levels (± 4%, S.E.M., n=6). It should be noted that this corresponds to an overall increase in the average somatic calcium concentration and thus suggests that there is a significantly larger increase in local calcium concentration close to the plasma membrane when BK channels are blocked.
Figure 7. Block of BK channels increases intracellular calcium concentration during spontaneous firing.
A) Whole cell current clamp recordings were made from a spontaneously firing Purkinje neuron. The pipette solution included Fluo-4, a fluorescent calcium indicator. Fluorescence intensity was measured with a cooled-CCD camera. The top trace shows the increase in fluorescence intensity (ΔF) divided by the fluorescence when the cell was not firing (F0). The bottom trace is the average firing rate. At the time indicated injection of positive current (150 pA) increased both the firing rate and fluorescence intensity. At t=9 minutes, fluorescence levels in iberiotoxin were 23% greater than those in control conditions even though the average firing rate was the same. B) Tope panel. Whole cell current clamp of a Purkinje cell with a patch pipette containing Fura-4F. Injection of 200 pA of current for 1 s from a membrane potential of −70 mV evoked an average firing rate of 47 spikes/s. Bottom Panel. Simultaneous recording of intracellular free calcium concentration showed that calcium increased to about 125 nM. D) Plot of free calcium concentration versus the average firing rate. Data were collected from five cells and each symbol represents a different cell.
In a separate series of experiments we used the calcium dye Fura-4F (that we could easily calibrate; see methods) to measure the actual changes in the free calcium concentration in the soma of Purkinje cells during spontaneous activity (Figure 7B and C). As can be seen, average spontaneous firing rates of 25–115 spikes per second corresponded with linear increases of somatic calcium concentration in the range of 80–320 nM. The increase in the somatic calcium concentration was mainly the consequence of firing of action potential rather than the small steady depolarization; when Purkinje cells were depolarized by 5 mV (the average increase in membrane potential when Purkinje cells fire at 100 Hz) for 1 s in the presence of 100 nM TTX, in all six cells examined the somatic calcium concentration increased by less than 30 nM. Thus the effect of BK channel blockade on somatic calcium is mainly by changing calcium influx per action potential than by changing the membrane potential.
The calcium imaging experiments invariably necessitated the use of whole-cell recordings to introduce the calcium indicator into the cytosol. While clear changes before and after blockade of BK channels were detectable, we caution that the technique suffers from the caveat that it alters the intracellular composition and particularly may impact calcium buffering and homeostasis.
Discussion
Regulation of firing rate by BK channels
Here we show a clear role for BK channels in controlling the rate, regularity, and pattern of spontaneous activity of cerebellar Purkinje neurons. Regulation of regularity and rate of firing by BK channels, however, is limited mainly to neurons that fire tonically and which are present predominantly at young ages. In contrast SK channels regulate firing rate at all ages. Edgerton & Reinhart (2003) examined the spontaneous firing of Purkinje neurons using whole-cell current clamp recordings and reported no statistically significant increase in firing rate following block of BK channels. However, upon closer examination, our results are not inconsistent with theirs. Edgerton & Reinhart (2003) show clear examples of increases in firing rate after BK channel block in individual neurons and their averages may have been biased towards neurons from older animals. An additional consideration may be that Edgerton & Reinhart used solely whole-cell recordings and this might have affected calcium buffering and homeostasis and thus the extent of activation of BK channels.
The BK channels which control firing rate are confined to the soma of tonically firing neurons. This is consistent with the finding that block of dendritic calcium channels also has no effect on tonic firing neurons (Womack and Khodakhah, 2002a). In contrast, block of SK channels, even in the distal half of the dendrites in tonic firing neurons, produces a significant increase in firing rate (Womack and Khodakhah, 2003).
The decrease in the percentage of neurons in which BK channels contribute to firing rate is concurrent with a decrease in the relative contribution of BK current to the total AHP and concurrent with an increase in the total AHP and rate of repolarization of the action potential. An increase in the rate of action potential repolarization during development is a common feature of many neurons (Ribera and Spitzer, 1992). In Purkinje neurons, an increase in spontaneous firing rate occurs over the same developmental window as the increase in AHP described here (Womack and Khodakhah, 2002a). A developmental increase in rate of action potential repolarization and in spontaneous firing rate has also been described in cerebellar slice cultures (Yool et al., 1988) and has been attributed to an increase in BK channel density which occurs over the same window of time (Yool et al., 1988; Gruol et al., 1992). However our results indicate that the increase in firing rate and action potential repolarization is the result of increased contribution of some other potassium conductance. A decrease in the open probability or deactivation rate of P/Q-type calcium channels, which are functionally linked to activation of BK channels in Purkinje neurons (Womack et al., 2004) is also possible.
Regulation of firing pattern by BK channels
Block of BK channels alters the pattern of spontaneous firing in all neurons. In neurons with the trimodal pattern of activity BK channel block shortens the single cycle pattern while in tonic neurons BK channel block induces irregular bursting, or induces the trimodal pattern. Regular bursting which appears as part of the trimodal pattern is associated with generation of dendritic calcium spikes (Womack and Khodakhah, 2004). BK channels are activated during dendritic calcium spikes where they contribute to the peak repolarizing phase (Edgerton and Reinhart, 2003). It is possible that BK channels shorten the duration of the trimodal pattern of spontaneous firing by regulating dendritic excitability, specifically by regulating the probability of generating dendritic calcium spikes. However, this is unlikely to be the sole mechanism of action of BK channels in Purkinje neurons since block of BK channels throughout the whole cell has a significantly greater effect than block of BK channels in even the distal two thirds of the dendrites (Figure 4B).
BK channels may also control dendritic excitability by modulating the coupling efficiency between soma and dendrites. It has been suggested that soma and dendrites of neurons can interact electrically via a push-pull mechanism (Rall, 1962; Jaeger et al., 1997; Womack and Khodakhah, 2002a). In this model, some of the charge entering the soma during sodium action potentials enters the dendrites and charges up the dendritic capacitance. In Purkinje neurons, calcium spikes will be generated when the dendritic capacitance is sufficiently charged. The amount of charge transferred to the dendrites depends on the input resistance of the proximal dendrite. Action potential-generated increases in somatic calcium free calcium concentration back propagate into the proximal dendrites (K. Khodakhah, unpublished observations) and could provide calcium for activation of BK channels located there. By decreasing the membrane resistance of the proximal dendrite, BK channels could decrease the coupling efficiency between soma and dendrites. Since the intracellular calcium concentration increases with the firing rate this mechanism would provide a mechanism for control of dendro-somatic coupling, and possibly synaptic integration that depends on the previous firing history. Such a mechanism has been proposed for regulation of spontaneous activity by SK channels (Womack and Khodakhah, 2003).
Regulation of intracellular calcium concentration by BK channels
While neither the mechanism driving the spontaneous pattern of activity nor its functional significance are yet understood, the finding that BK channels regulate the pattern period led us to two important observations. First, functional BK channels are present on Purkinje neurons at all ages tested. Second, the similarity between the effects of BK channel block on the pattern period and those following an increase in calcium influx (C. Chevez and K. Khodakhah, unpublished observations) prompted us to investigate whether BK channels could regulate calcium influx in the soma of Purkinje neurons. Regulation of calcium influx by BK channels is important for controlling transmitter release at nerve terminals (Robitaille and Charlton, 1992; Hu et al., 2001). It has been proposed that by controlling the rate of action potential repolarization, BK channels also regulate calcium influx at the soma in some central neurons (Shao et al., 1999; Faber and Sah, 2003). However, to our knowledge, our results provide the first direct demonstration that BK channels regulate calcium entry in a cellular compartment other than the terminal.
Regulation of calcium entry by BK channels has typically been attributed to their contribution to action potential duration (Robitaille and Charlton, 1992; Hu et al., 2001). By decreasing the duration of the action potential BK channels decrease the time during which voltage-gated calcium channels are activated. Surprisingly, BK channels make little contribution to action potential duration in Purkinje neurons. In Purkinje neurons most of the calcium entering during each action potential is contributed by T- and P/Q-type calcium channels (Raman and Bean, 1999; Swensen A.M. and Bean, 2003). BK channels are unlikely to control calcium entry during spontaneous firing by regulation of T-type calcium channels since increasing calcium entry through these channels does not affect the rate or pattern of spontaneous firing (Womack and Khodakhah, 2004; Womack et al., 2004). It is most likely that BK channels regulate calcium entry by modulating the gating of P/Q-type calcium channels. The Vm AHP ranges form approximately −55 mV at postnatal day 11 to approximately −65 mV at postnatal day 22. Over this voltage range, P/Q-type calcium channels in Purkinje neurons deactivate with a time constant of less than 1 ms (Regan, 1991), a time less than the duration of the iberiotoxin-sensitive AHP. Block of BK channels decreases the average AHP by approximately 3 mV at all ages. Over the range of −55 to −65 mV, a 3 mV decrease in the AHP can slow the rate of deactivation of P/Q channels by approximately 25% (Regan, 1991) and could account for the effect of BK channel block on intracellular calcium concentration.
Changes in intracellular calcium concentration regulate many cellular processes including protein phosphorylation, development and gene expression. Calcium entry during firing could control one or more of these processes in a manner that is sensitive to the level of activity. The activity of BK channels in Purkinje neurons is regulated by phosphorylation via several classes of kinases and phosphatases (Levitan, 1999; Widmer et al., 2003). An exciting possibility is that BK channels represent a site at which calcium entry during firing can be regulated independent of the firing rate and that the sensitivity of calcium-regulated cellular process to ongoing activity could be modulated by modulating the open probability of the channels.
Acknowledgments
This project was supported by the New York City Speakers Fund for Biomedical Research and the NIH. We thank David Alevi and Felipe Castillo for the data shown in Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci U S A. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J Physiol. 2003;552:483–497. doi: 10.1113/jphysiol.2003.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangiacomo KM, Garcia ML, McManus OB. Mechanism of iberiotoxin block of the large-conductance calcium- activated potassium channel from bovine aortic smooth muscle. Biochemistry. 1992;31:6719–6727. doi: 10.1021/bi00144a011. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Deal CR, Yool AJ. Developmental changes in calcium conductances contribute to the physiological maturation of cerebellar Purkinje neurons in culture. J Neurosci. 1992;12:2838–2848. doi: 10.1523/JNEUROSCI.12-07-02838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Jacquin T, Yool AJ. Single-channel K+ currents recorded from the somatic and dendritic regions of cerebellar Purkinje neurons in culture. J Neurosci. 1991;11:1002–1015. doi: 10.1523/JNEUROSCI.11-04-01002.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The cerebellum and neural control. New York: Raven Press; 1984. [Google Scholar]
- Jaeger D, Bower JM. Synaptic control of spiking in cerebellar Purkinje cells: dynamic current clamp based on model conductances. J Neurosci. 1999;19:6090–6101. doi: 10.1523/JNEUROSCI.19-14-06090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, De Schutter E, Bower JM. The role of synaptic and voltage-gated currents in the control of Purkinje cell spiking: a modeling study. J Neurosci. 1997;17:91–106. doi: 10.1523/JNEUROSCI.17-01-00091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol (Lond) 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation. How the brain works. Adv Second Messenger Phosphoprotein Res. 1999;33:3–22. doi: 10.1016/s1040-7952(99)80003-2. [DOI] [PubMed] [Google Scholar]
- Llano I, Dreessen J, Kano M, Konnerth A. Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron. 1991;7:577–583. doi: 10.1016/0896-6273(91)90370-f. [DOI] [PubMed] [Google Scholar]
- Lovell PV, McCobb DP. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J Neurosci. 2001;21:3429–3442. doi: 10.1523/JNEUROSCI.21-10-03429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K, Noda T. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- Nam SC, Hockberger PE. Analysis of spontaneous electrical activity in cerebellar Purkinje cells acutely isolated from postnatal rats. J Neurobiol. 1997;33:18–32. doi: 10.1002/(sici)1097-4695(199707)33:1<18::aid-neu3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- Rall W. Electrophysiology of a dendritic neuron model. Biophys J. 1962;2:145–167. doi: 10.1016/s0006-3495(62)86953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991;11:2259–2269. doi: 10.1523/JNEUROSCI.11-07-02259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera AB, Spitzer NC. Developmental regulation of potassium channels and the impact on neuronal differentiation. Ion Channels. 1992;3:1–38. doi: 10.1007/978-1-4615-3328-3_1. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakkottai VG, Chou CH, Oddo S, Sailer CA, Knaus HG, Gutman GA, Barish ME, LaFerla FM, Chandy KG. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest. 2004;113:582–590. doi: 10.1172/JCI20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521(Pt 1):135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du LS. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Street VA, Bosma MM, Demas VP, Regan MR, Lin DD, Robinson LC, Agnew WS, Tempel BL. The type 1 inositol 1,4,5-trisphosphate receptor gene is altered in the opisthotonos mouse. J Neurosci. 1997;17:635–645. doi: 10.1523/JNEUROSCI.17-02-00635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in Purkinje neurons. J Neurosci. 2003 doi: 10.1523/JNEUROSCI.23-29-09650.2003. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Widmer HA, Rowe IC, Shipston MJ. Conditional protein phosphorylation regulates BK channel activity in rat cerebellar Purkinje neurons. J Physiol. 2003;552:379–391. doi: 10.1113/jphysiol.2003.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar purkinje neurons. J Neurosci. 2002a;22:10603–10612. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci. 2002b;16:1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar purkinje neurons. J Neurosci. 2003;23:2600–2607. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24:3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool AJ, Dionne VE, Gruol DL. Developmental changes in K+-selective channel activity during differentiation of the Purkinje neuron in culture. J Neurosci. 1988;8:1971–1980. doi: 10.1523/JNEUROSCI.08-06-01971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Covey DF, Rothman SM. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]