Abstract
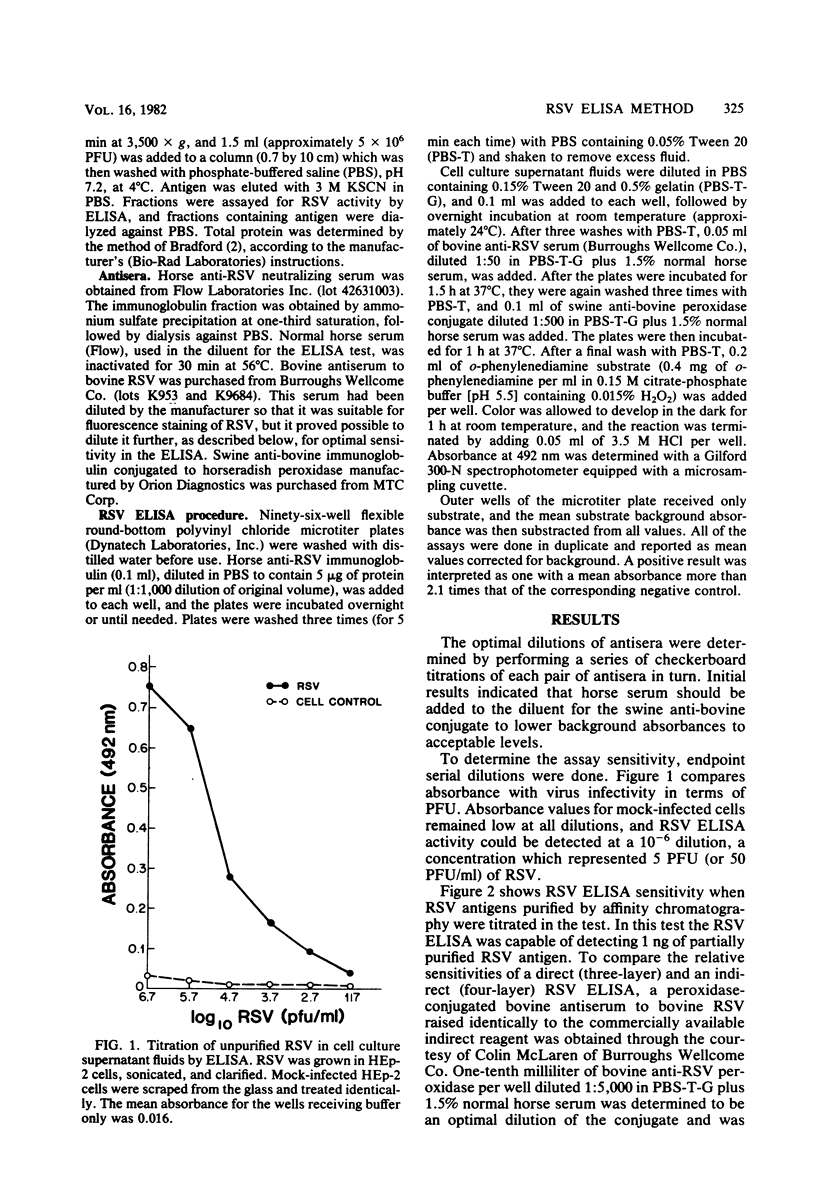
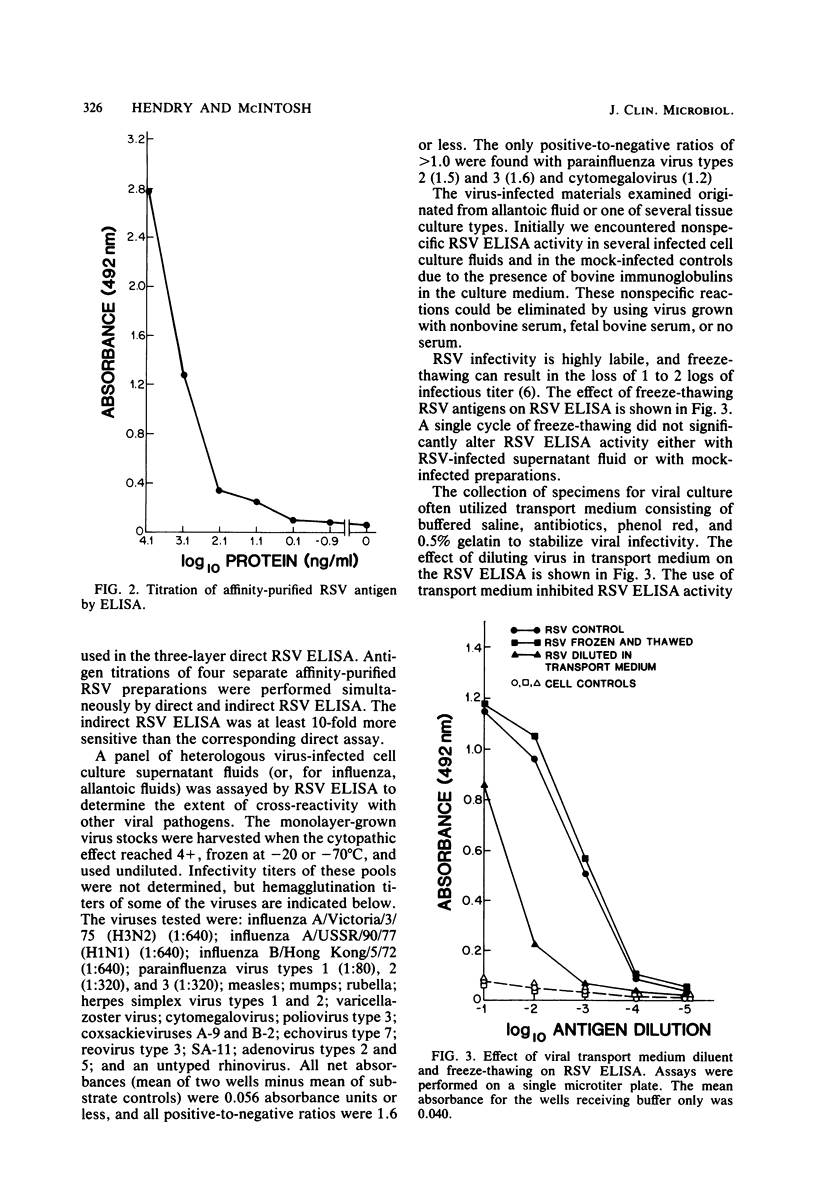
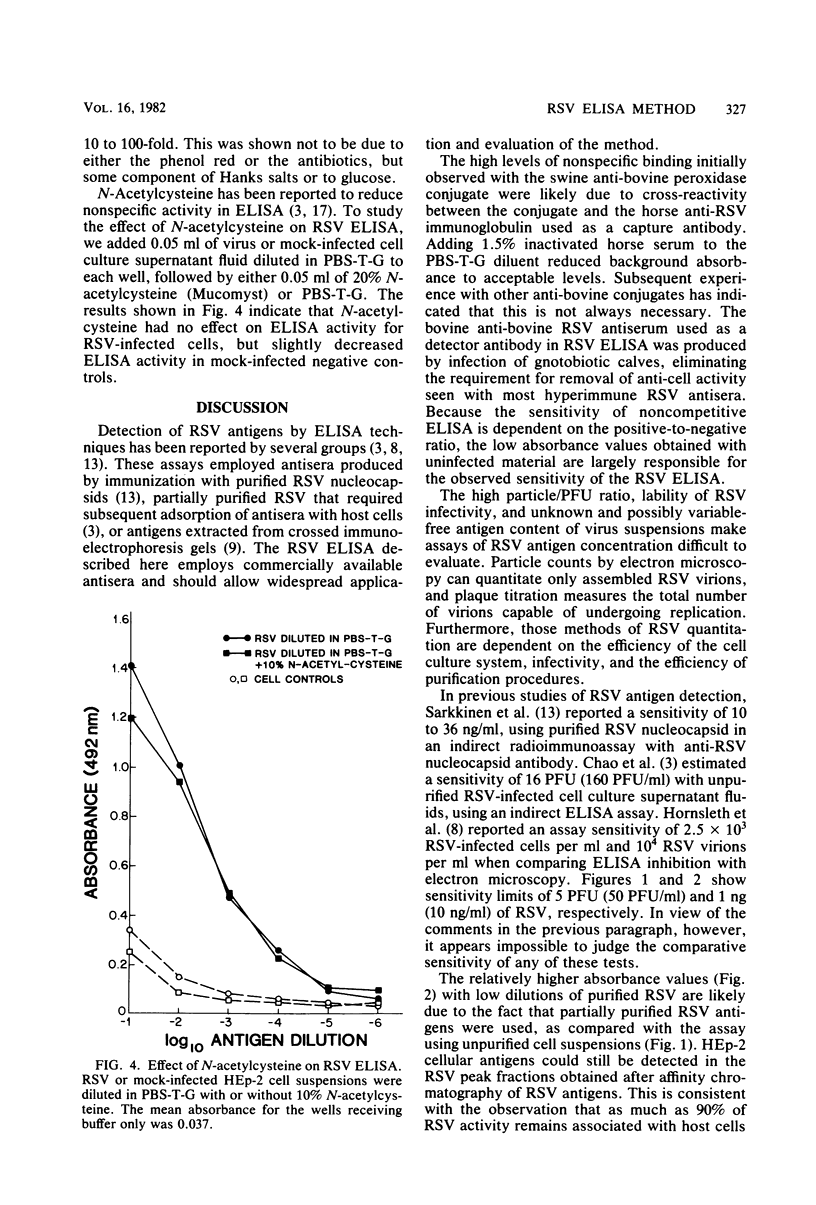
An indirect enzyme-linked immunosorbent assay for detection of respiratory syncytial virus (RSV) antigens was developed, using commercially available antisera. Horse anti-RSV and calf antiserum to bovine RSV were used as capture and detector antibodies, respectively. The assay could detect as few as 50 PFU of unpurified RSV per ml in infected cell culture supernatant fluids and as little as 10 ng of affinity-purified RSV antigen per ml. No cross-reactions were observed with heterologous virus types. Freeze-thaw treatment had no effect on RSV enzyme-linked immunosorbent assay titers, but viral transport medium inhibited RSV enzyme-linked immunosorbent assay titers from 10- to 100-fold. The assay can be easily performed in 24 h and is a sensitive and specific method for the detection of RSV antigens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Yolken R. H., Rennard S. I., Dolin R., Murphy B. R., Straus S. E. New enzyme immunoassays for measurement of influenza A/Victoria/3/75 virus in nasal washes. Lancet. 1980 Apr 19;1(8173):851–853. doi: 10.1016/s0140-6736(80)91356-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chao R. K., Fishaut M., Schwartzman J. D., McIntosh K. Detection of respiratory syncytial virus in nasal secretions from infants by enzyme-linked immunosorbent assay. J Infect Dis. 1979 Apr;139(4):483–486. doi: 10.1093/infdis/139.4.483. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Stott E. J., Nagington J., Coombs R. R. A reverse passive haemagglutination test for the detection of respiratory syncytial virus in nasal secretions from infants. J Med Virol. 1981;8(3):153–160. doi: 10.1002/jmv.1890080301. [DOI] [PubMed] [Google Scholar]
- HAMBLING M. H. SURVIVAL OF THE RESPIRATORY SYNCYTIAL VIRUS DURING STORAGE UNDER VARIOUS CONDITIONS. Br J Exp Pathol. 1964 Dec;45:647–655. [PMC free article] [PubMed] [Google Scholar]
- Harmon M. W., Drake S., Kasel J. A. Detection of adenovirus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979 Mar;9(3):342–346. doi: 10.1128/jcm.9.3.342-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsleth A., Brenøe E., Friis B., Knudsen F. U., Uldall P. Detection of respiratory syncytial virus in nasopharyngeal secretions by inhibition of enzyme-linked immunosorbent assay. J Clin Microbiol. 1981 Nov;14(5):510–515. doi: 10.1128/jcm.14.5.510-515.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsleth A., Grauballe P. C., Friis B., Genner J., Pedersen I. R. Production of antiserum to respiratory syncytial virus polypeptides: application in enzyme-linked immunosorbent assay. J Clin Microbiol. 1981 Nov;14(5):501–509. doi: 10.1128/jcm.14.5.501-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Hamilton R. Kinetics of the respiratory syncytial virus growth cycle in HeLa cells. Arch Gesamte Virusforsch. 1969;28(2):122–132. doi: 10.1007/BF01249378. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Hendry R. M., Fahnestock M. L., Pierik L. T. Enzyme-linked immunosorbent assay for detection of respiratory syncytial virus infection: application to clinical samples. J Clin Microbiol. 1982 Aug;16(2):329–333. doi: 10.1128/jcm.16.2.329-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Wilfert C., Chernesky M., Plotkin S., Mattheis M. J. From the National Institute of Allergy and Infectious Diseases. Summary of a workshop on new and useful techniques in rapid viral diagnosis. J Infect Dis. 1980 Nov;142(5):793–802. doi: 10.1093/infdis/142.5.793. [DOI] [PubMed] [Google Scholar]
- Oellerich M. Enzyme immunoassays in clinical chemistry: present status and trends. J Clin Chem Clin Biochem. 1980 Apr;18(4):197–208. doi: 10.1515/cclm.1980.18.4.197. [DOI] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Arstila P. P., Salmi A. A. Detection of respiratory syncytial, parainfluenza type 2, and adenovirus antigens by radioimmunoassay and enzyme immunoassay on nasopharyngeal specimens from children with acute respiratory disease. J Clin Microbiol. 1981 Feb;13(2):258–265. doi: 10.1128/jcm.13.2.258-265.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Salmi A. A. Detection of influenza A virus by radioimmunoassay and enzyme-immunoassay from nasopharyngeal specimens. J Med Virol. 1981;7(3):213–220. doi: 10.1002/jmv.1890070305. [DOI] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Salmi A. A. Type-specific detection of parainfluenza viruses by enzyme-immunoassay and radioimmunoassay in nasopharyngeal specimens of patients with acute respiratory disease. J Gen Virol. 1981 Sep;56(Pt 1):49–57. doi: 10.1099/0022-1317-56-1-49. [DOI] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme-linked immunosorbent assay (ELISA): a practical tool for rapid diagnosis of viruses and other infectious agents. Yale J Biol Med. 1980 Jan-Feb;53(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Analysis of nonspecific reactions in enzyme-linked immunosorbent assay testing for human rotavirus. J Clin Microbiol. 1979 Nov;10(5):703–707. doi: 10.1128/jcm.10.5.703-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Stopa P. J. Comparison of seven enzyme immunoassay systems for measurement of cytomegalovirus. J Clin Microbiol. 1980 Jun;11(6):546–551. doi: 10.1128/jcm.11.6.546-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Torsch V. M., Berg R., Murphy B. R., Lee Y. C. Fluorometric assay for measurement of viral neuraminidase--application to the rapid detection of influenza virus in nasal wash specimens. J Infect Dis. 1980 Oct;142(4):516–523. doi: 10.1093/infdis/142.4.516. [DOI] [PubMed] [Google Scholar]


