Abstract
Aims/hypothesis
Exercise represents an effective interventional strategy to improve glycaemic control in type 2 diabetes patients. However, the impact of exercise intensity on the benefits of exercise training remains to be established. In the present study, we compared the clinical benefits of 6 months of continuous low- to moderate-intensity exercise training with those of continuous moderate- to high-intensity exercise training, matched for energy expenditure, in obese type 2 diabetes patients.
Methods
Fifty male obese type 2 diabetes patients (age 59 ± 8 years, BMI 32 ± 4 kg/m2) participated in a 6 month continuous endurance-type exercise training programme. All participants performed three supervised exercise sessions per week, either 55 min at 50% of whole body peak oxygen uptake 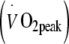 (low to moderate intensity) or 40 min at 75% of
(low to moderate intensity) or 40 min at 75% of 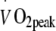 (moderate to high intensity). Oral glucose tolerance, blood glycated haemoglobin, lipid profile, body composition, maximal workload capacity, whole body and skeletal muscle oxidative capacity and skeletal muscle fibre type composition were assessed before and after 2 and 6 months of intervention.
(moderate to high intensity). Oral glucose tolerance, blood glycated haemoglobin, lipid profile, body composition, maximal workload capacity, whole body and skeletal muscle oxidative capacity and skeletal muscle fibre type composition were assessed before and after 2 and 6 months of intervention.
Results
The entire 6 month intervention programme was completed by 37 participants. Continuous endurance-type exercise training reduced blood glycated haemoglobin levels, LDL-cholesterol concentrations, body weight and leg fat mass, and increased 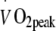 , lean muscle mass and skeletal muscle cytochrome c oxidase and citrate synthase activity (p < 0.05). No differences were observed between the groups training at low to moderate or moderate to high intensity.
, lean muscle mass and skeletal muscle cytochrome c oxidase and citrate synthase activity (p < 0.05). No differences were observed between the groups training at low to moderate or moderate to high intensity.
Conclusions/interpretation
When matched for energy cost, prolonged continuous low- to moderate-intensity endurance-type exercise training is equally effective as continuous moderate- to high-intensity training in lowering blood glycated haemoglobin and increasing whole body and skeletal muscle oxidative capacity in obese type 2 diabetes patients.
Trial registration:
ISRCTN32206301
Funding:
None
Keywords: Diabetes, Glycaemic control, Lifestyle intervention, Training modalities
Introduction
Lifestyle intervention programmes that combine regular exercise, dietary modulation and/or oral blood-glucose-lowering medication have proven an effective therapeutic strategy in type 2 diabetes [1–3]. Continuous endurance-type exercise training has been shown to lower blood HbA1c, increase insulin sensitivity, improve the risk profile for cardiovascular disease and reduce adipose-tissue mass in patients with type 2 diabetes [4, 5]. In addition, exercise training represents the only interventional strategy that has consistently been shown to improve whole body and skeletal muscle oxidative capacity [6]. In accordance, recently published standards of medical care for type 2 diabetes underline the importance of exercise prescription [7].
However, the clinical guidelines do not include detailed information on the preferred exercise modalities that should be implemented to maximise the clinical benefits of exercise intervention in diabetes patients [7]. Exercise intensity has been suggested to represent one of the more important exercise modalities that determine the clinical outcome of exercise interventions [1, 4]. This has been attributed to the inverse relationship between exercise intensity and glycogen use within the muscle [8, 9]. The magnitude of the increase in insulin sensitivity following an acute bout of continuous endurance-type exercise has been associated with the extent of muscle glycogen depletion and the subsequent repletion rate [10, 11]. Whether such differences in the acute glucoregulatory effect of a single bout of exercise also translate into improvements in glycaemic control following more prolonged endurance-type exercise interventions in type 2 diabetes patients remains speculative [12, 13].
Long-term exercise intervention studies investigating the impact of continuous low- to moderate-intensity (LI) vs high-intensity endurance-type exercise training on glycaemic control in healthy and/or glucose-intolerant individuals have reported high intensity exercise training to be, when compared with low-intensity endurance-type exercise training, less [14], equally [15, 16] or more [17] effective. However, long-term exercise intervention studies in type 2 diabetes patients investigating the clinical benefits of either continuous LI or moderate- to high-intensity (HI) exercise training remain lacking.
The preference for implementing either an LI or HI exercise regimen in a continuous endurance-type exercise intervention programme for patients with type 2 diabetes is of great clinical relevance, especially when considering the inverse relationship between exercise intensity and patient compliance [18]. As long-term patient compliance and adherence are required to improve glycaemic control effectively and reduce the prevalence of diabetes-related co-morbidities, it is essential to minimise patient dropout [4].
Therefore, the present study compares the clinical benefits of continuous LI vs HI exercise training, matched for total energy cost, in obese type 2 diabetes patients. Changes in blood HbA1c content, indices of oral glucose tolerance and/or whole body insulin sensitivity, blood lipid profile, body composition, exercise performance and whole body and skeletal muscle oxidative capacity, as well as muscle fibre type composition, were assessed prior to and after 2 and 6 months of either continuous LI or HI endurance-type exercise training.
Methods
Participants Fifty male obese type 2 diabetes patients were selected to participate in this study. Participants had been diagnosed with type 2 diabetes for at least 12 months and were all treated with oral blood-glucose-lowering medication. Participants were sedentary and had not participated in any regular exercise programme for at least 5 years. Participants were informed about the nature and risks of the experimental procedures before their written informed consent was obtained. This study was approved by the medical ethical committee of the Virga Jesse Hospital.
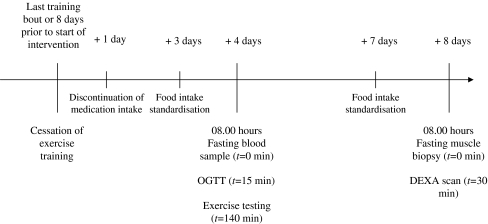
Study design All participants participated in a 6 month exercise intervention programme. Prior to the start of the programme and after 2 and 6 months of intervention blood glycated haemoglobin content, basal plasma glucose and insulin concentrations, plasma lipid profile, oral glucose tolerance, body composition, whole body oxidative capacity, maximal workload capacity, skeletal muscle oxidative enzyme activity and muscle fibre type composition were assessed after an overnight fast. Measurements were performed prior to intervention and 4 and 8 days after the last exercise training session at 2 and 6 months of intervention (see Fig. 1). Three days prior to the oral glucose tolerance test patients abstained from taking oral blood-glucose and/or lipid-lowering medication. All measurements were performed as depicted in Fig. 1, and were executed at the same time of day prior to and after 2 and 6 months of intervention.
Fig. 1.
Study design. These steps were performed for each participant 1 week prior to the intervention and at 2 and 6 months of exercise training. On the experimental day, participants reported to the laboratory at 08:00 hours following an overnight fast. DEXA, dual-energy X-ray absorptiometry
Diet and physical activity prior to testing All participants maintained normal physical activity and dietary patterns, and refrained from exhaustive physical exercise 3 days prior to each experimental day. In addition, participants recorded dietary intake over 2 days prior to the OGTT and copied their diet prior to the 2 and 6 month follow-up assessments. The evening before each test day, all participants received the same standard meal (45 ± 1 kJ/kg, consisting of 33% of energy from carbohydrate, 47% from fat and 20% from protein).
Blood analysis Participants arrived at the hospital by car or public transport and reported at the laboratory at 08:00 hours following an overnight fast. After 20 min of supine rest, a venous blood sample was collected. Thereafter, an OGTT was performed. Blood samples were immediately centrifuged at 1,000 ×g and 4°C for 5 min, after which plasma was frozen in liquid nitrogen and stored at −80°C until analysis. Blood samples were analysed for glucose (Beckman Synchron LX 20 Analyzer, Beckman Coulter, Fullerton, CA, USA), insulin (Advia Centaur Immunoassay System, Bayer Diagnostics, Tarrytown, NY, USA), HbA1c (Hi-Auto A1c Analyzer, Menarini Diagnostics, Florence, Italy), total cholesterol, HDL-cholesterol, LDL-cholesterol and total plasma triacylglycerols (Beckman Synchron LX 20 Analyzer, Beckman Coulter). Levels of NEFA were quantified by an enzymatic method (Wako Chemicals, Neuss, Germany). The HOMA index was calculated as an index of whole body insulin sensitivity.
Whole body oxygen uptake and workload capacity Whole body peak oxygen uptake 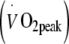 and maximal workload capacity (Wmax) were assessed during an exhaustive incremental exercise test on a cycle ergometer (Ergo 1500, Ergofit, Pirmasens, Germany) using a 3 min work stage protocol.
and maximal workload capacity (Wmax) were assessed during an exhaustive incremental exercise test on a cycle ergometer (Ergo 1500, Ergofit, Pirmasens, Germany) using a 3 min work stage protocol. 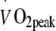 measurements were performed continuously (CS 200, Schiller, Baar, Switzerland) to assess
measurements were performed continuously (CS 200, Schiller, Baar, Switzerland) to assess 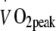 . Cardiac function was monitored using a 12 lead electrocardiogram with heart rate recorded continuously.
. Cardiac function was monitored using a 12 lead electrocardiogram with heart rate recorded continuously.
Muscle-tissue analysis In the morning, following an overnight fast, a muscle biopsy was collected from the vastus lateralis muscle [19, 20]. Muscle samples were dissected and freed from any visible non-muscle material. Thereafter, the muscle samples were rapidly frozen in liquid-nitrogen-cooled isopentane.
Immunohistochemistry From the muscle samples, cryosections of 5 µm thickness were cut. Cryosections from samples collected prior to the intervention period and after 2 and 6 months of intervention from each individual participant were thaw mounted together on uncoated glass slides. After a 5 min fixation step, the cryosections were incubated for 45 min with antibodies against human laminin (polyclonal rabbit antibody, Sigma Diagnostics, Steinheim, Germany), human slow myosin heavy chain (A4.840) and human fast myosin heavy chain (N2.261) in 0.05% Tween 20–PBS, enabling visualisation of individual cell membranes and muscle fibre typing (type 1 and 2a), respectively. Incubation was followed by rinsing in PBS, after which the appropriate conjugated antibodies, i.e. goat anti-rabbit IgG (Alexa488) or goat anti-mouse IgM (Alexa350; Molecular Probes, Leiden, the Netherlands) were applied for 30 min. After the final washes in PBS, the slides were mounted with Mowiol.All images were digitally captured using fluorescence microscopy with a Nikon E800 fluorescence microscope (Nikon Instruments Europe, Badhoevedorp, the Netherlands) coupled to a Basler A113 C progressive scan colour charge coupled device (CCD) camera with a Bayer colour filter. The epifluorescence signal was recorded using a FITC excitation filter (465–495 nm) for laminin, a Texas Red excitation filter (540–580 nm) for slow myosin heavy chain and a DAPI UV excitation filter (340–380 nm) for fast myosin heavy chain. Image processing and quantitative analyses were performed using the Lucia 4.81 software package (Nikon).
Oxidative enzyme activity assays Skeletal muscle citrate synthase, cytochrome c oxidase, succinate dehydrogenase and β-hydroxyacyl-CoA dehydrogenase activity were assessed in 40 mg mixed muscle tissue. For the assessment of succinate dehydrogenase and cytochrome c oxidase, a 5% (wt/vol.) muscle homogenate was prepared in SET buffer (based on sucrose (250 nmol/l), EDTA (2 mmol/l) and TRIS (10 mmol/l), pH 7.4). The homogenate was mixed with the KCl/glutathione/EDTA buffers (potassium chloride [175 nmol/l], glutathione [10 mmol/l] and EDTA [2 mmol/l], pH 7.4; 1:1 ratio) for the analysis of succinate dehydrogenase activity. For cytochrome c oxidase activity, 140 μl phosphate buffer 50 mmol/l (pH 7.4), 10 μl muscle homogenate (5%, wt/vol.) and 100 μl digitonin–BSA were mixed. For the measurement of muscle citrate synthase and β-hydroxyacyl-CoA dehydrogenase activity, a 2.5% (wt/vol.) muscle homogenate was prepared in SET buffer. Citrate synthase activity was measured by monitoring the formation of CoA-DTNB (5,5′-dithio-bis[2-nitrobenzoic acid]) during the citrate synthase reaction spectrophotometrically at 412 nm. Cytochrome c oxidase activity was assessed by monitoring the amount of reduced cytochrome c during oxidation spectrophotometrically at 550 nm. Succinate dehydrogenase activity was determined by monitoring the reduction of Fe3+ in the K3[Fe(CN)6] complex during the oxidation of succinate spectrophotometrically at 420 nm. β-Hydroxyacyl-CoA dehydrogenase activity was measured by monitoring the dehydrogenation of NADH during the reduction of acetoacetyl-CoA spectrophotometrically at 340 nm.
Body composition Body mass was measured using a calibrated analogue weight scale. Segmental and whole body fat mass and fat-free mass were determined using whole body dual X-ray absorptiometry (DEXA; Lunar DPXL, WI, USA).
Exercise training intervention All participants performed three individually supervised, continuous endurance-type exercise sessions per week during a 6 month intervention period within the rehabilitation centre of the hospital. During each training session, walking, cycling and cross-country ski-type exercise was performed. In the LI exercise programme, each exercise session consisted of 55 min exercise at a heart rate corresponding with exercise performed at exactly 50% of baseline 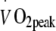 (heart rate 105 ± 3 beats per min). In the HI exercise programme, each exercise session consisted of 40 min exercise at a heart rate corresponding with exercise performed at exactly 75% of baseline
(heart rate 105 ± 3 beats per min). In the HI exercise programme, each exercise session consisted of 40 min exercise at a heart rate corresponding with exercise performed at exactly 75% of baseline 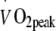 (heart rate 118 ± 3 beats per min). The exercise training intensity was standardised by continuous heart rate monitoring (Polar, Oy, Finland). Total energy expenditure during the exercise training sessions was matched between groups (1.30 ± 0.05 MJ per session). As the target heart rate was maintained throughout the exercise training programme and maximal workload capacity increased, energy expenditure during each exercise session gradually increased in both groups (from 1.08 ± 0.04 MJ per session in week 1 to 1.34 ± 0.05 MJ per session by week 24). After 2 months of exercise training, the relation between heart rate and
(heart rate 118 ± 3 beats per min). The exercise training intensity was standardised by continuous heart rate monitoring (Polar, Oy, Finland). Total energy expenditure during the exercise training sessions was matched between groups (1.30 ± 0.05 MJ per session). As the target heart rate was maintained throughout the exercise training programme and maximal workload capacity increased, energy expenditure during each exercise session gradually increased in both groups (from 1.08 ± 0.04 MJ per session in week 1 to 1.34 ± 0.05 MJ per session by week 24). After 2 months of exercise training, the relation between heart rate and 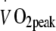 was re-assessed to recalculate the target heart rate (at 50% or 75%
was re-assessed to recalculate the target heart rate (at 50% or 75% 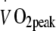 ). This target was maintained for the remaining 4 month intervention period.During the 6 month exercise intervention programme, daily habitual physical activity was assessed over a 14 day period every 2 months using the International Physical Activity Questionnaire. In addition, dietary intake was recorded over the same 14 day period using a dietary intake questionnaire.
). This target was maintained for the remaining 4 month intervention period.During the 6 month exercise intervention programme, daily habitual physical activity was assessed over a 14 day period every 2 months using the International Physical Activity Questionnaire. In addition, dietary intake was recorded over the same 14 day period using a dietary intake questionnaire.
Statistical analysis Data are expressed as means ± SEM. Data were analysed according to the intention-to-treat principle. To minimise type I errors and loss of power, endpoint analyses according to the ‘last observation carried forward’ principle were performed for missing values at 6 months of follow-up. To assess whether differences existed between the application of the HI and LI training regimens, we used two-way ANOVA with treatment (LI and HI) and time as the two factors. For non-time-dependent variables, one-way ANOVA was applied. Relations of changes between variables were examined with Pearson correlation coefficients. Statistical significance was set at p < 0.05. All calculations were performed using the Statistical Package for the Social Sciences, version 13.0.
Results
Participants Participants (n = 50, BMI 32 ± 4 kg/m2, age 59 ± 8 years) had been diagnosed with type 2 diabetes for 5 ± 1 years and were using oral blood-glucose-lowering medication (in the HI and LI groups, respectively: sulfamides, 24% and 40%; biguanides, 72% and 80%; alpha-glucosidase inhibitors, 8% and 8%; thiazolidinediones, 8% and 8%; there were no significant differences between the groups). Prior to the intervention, participants engaged in 74 ± 19 min/week physical exercise (0.8 ± 0.2 days/week for 36 ± 9 min of exercise/day). Thirty-four participants did not engage in any physical activity and two engaged in high intensity exercises.Participants were randomly assigned to either the LI or HI training regimen. Participants’ characteristics are reported in Table 1. All patients completed 2 months of exercise training (and were re-evaluated), whereas 37 participants completed the entire 6 month programme. Eight participants dropped out from the HI training regimen and five from the LI training group before completing the full 6 months of intervention. On average, 64 ± 2 and 64 ± 2 of the 72 exercise sessions were attended by the participants who completed the exercise programme, resulting in an overall compliance of 89 ± 2% and 89 ± 3% in the LI and HI training groups, respectively. The blood-glucose-lowering medication dose had to be reduced in six participants (three in the LI group and three in the HI group).
Table 1.
Participant characteristics
| Characteristic | LI group | HI group |
|---|---|---|
| n | 25 | 25 |
| Age (years) | 58 ± 1 | 59 ± 2 |
| Body weight (kg) | 98.7 ± 3.2 | 97.7 ± 3.2 |
| Body mass index (kg/m2) | 32.7 ± 0.8 | 32.1 ± 0.9 |
| Time since diagnosis (years) | 4.0 ± 0.8 | 5.1 ± 0.8 |
| Whole body insulin sensitivity and glycaemic control | ||
| Fasting glucose (mmol/l) a | 9.8 ± 0.6 | 9.7 ± 0.4 |
| Fasting insulin (pmol/l) a | 147 ± 24 | 128 ± 10 |
| HOMA indexa | 8.5 ± 1.3 | 7.7 ± 0.8 |
| HbA1c (%) | 7.4 ± 0.3 | 7.1 ± 0.2 |
| AUC OGTT (mol/l × min) a | 1.76 ± 0.08 | 1.83 ± 0.08 |
| Blood lipid profile | ||
| Triacylglycerols (mmol/l) | 1.7 ± 0.19 | 1.7 ± 0.18 |
| HDL-cholesterol (mmol/l) | 1.1 ± 0.04 | 1.1 ± 0.05 |
| LDL-cholesterol (mmol/l) | 3.1 ± 0.14 | 3.3 ± 0.14 |
| LDL/HDL ratio | 2.9 ± 0.16 | 3.2 ± 0.16 |
| Total cholesterol (mmol/l) | 4.9 ± 0.19 | 5.0 ± 0.13 |
| NEFA (mmol/l) | 425 ± 27 | 444 ± 18 |
| Body composition | ||
| Trunk fat mass (kg) | 22.1 ± 1.3 | 21.6 ± 1.4 |
| Legs fat mass (kg) | 7.7 ± 0.7 | 7.3 ± 0.8 |
| Trunk lean mass (kg) | 31.3 ± 0.7 | 31.4 ± 0.6 |
| Legs lean mass (kg) | 18.8 ± 0.5 | 18.9 ± 0.4 |
| Exercise performance | ||
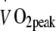 (l/min) (l/min) |
2.05 ± 0.11 | 1.99 ± 0.09 |
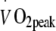 (ml kg−1 min−1) (ml kg−1 min−1) |
20.9 ± 1.0 | 20.8 ± 1.1 |
| Maximal workload capacity (W) | 161 ± 7 | 169 ± 6 |
| Maximal workload capacity (W/kg) | 1.7 ± 0.1 | 1.8 ± 0.1 |
| Maximal heart rate (beats per min) | 139 ± 4 | 139 ± 3 |
Data are means ± SEM
Values for leg lean and fat mass are expressed as averages of measurements of both legs
aFasting glucose, insulin, HOMA index and AUC OGTT were determined from an OGTT performed after 3 days of discontinuation of habitual use of oral blood-glucose and lipid-lowering medication
No significant differences were observed between groups (p > 0.05)
Food intake and physical activity Energy expenditure was similar between groups and averaged 1.3 ± 0.08 and 1.3 ± 0.07 MJ per session, or 85.4 ± 5.0 and 82.4 ± 4.5 MJ for the entire LI and HI exercise training programme, respectively. Prior to intervention, food intake averaged 12.7 ± 1.4 MJ/day, consisting of 41.2 ± 1.5% energy from carbohydrate, 33.4 ± 1.5% from fat and 21.9 ± 0.8% from protein. No differences were observed between groups. Food intake, assessed on a 2 monthly basis, did not change significantly throughout the intervention and averaged 10.9 ± 1.8 MJ and 11.9 ± 1.6 MJ (2 months) and 10.2 ± 1.6 MJ and 13.6 ± 2.2 MJ (6 months) per day in the LI and HI groups, respectively. In accordance, the macronutrient composition of the diet did not change over time and did not differ between groups (data not shown).Total sport and/or recreational physical activity, unrelated to the exercise performed within the intervention programme, did not change throughout the intervention programme (from 74 ± 19 to 52 ± 13 min exercise per week) and did not differ between groups (from 58 ± 20 and 9 ± 6 min to 18 ± 10 and 6 ± 6 min of moderate and vigorous intensity exercise, respectively, in the HI group, and from 81 ± 23 and 0 ± 0 min to 67 ± 15 and 13 ± 6 min of moderate and vigorous intensity exercise, respectively, in the LI group). However, total sport and recreational physical activity (including the exercise implemented in the intervention programme) increased significantly throughout the intervention (from 81 ± 32 and 67 ± 20 min to 244 ± 21 and 144 ± 11 min of exercise per week in the LI and HI groups, respectively, p < 0.05). The total time attributed to sport and recreational physical activity was significantly greater in the LI vs the HI exercise intervention group (p < 0.05), as the supervised exercise sessions were 15 min longer in the LI than in the HI exercise training regimen.
Blood analyses Blood HbA1c content decreased from 7.2 ± 0.2% to 6.9 ± 0.2% following exercise training (p < 0.05; Table 2). No interaction was observed between exercise intensity and the decrease in blood HbA1c content. Basal plasma glucose and insulin levels and indices of glucose tolerance and/or whole body insulin sensitivity, all assessed 3 days after discontinuation of oral blood-glucose-lowering medication, did not change following 2 and 6 months of intervention. Furthermore, plasma LDL-cholesterol levels decreased following exercise training (p < 0.01), with no differences between groups (p > 0.05).
Table 2.
The clinical benefits of exercise intervention
| Variable | Assessment | Effect of training (p value) | Interaction between training and intensity (p value) | |||||
|---|---|---|---|---|---|---|---|---|
| LI group | HI group | |||||||
| Baseline | 2 months | 6 months | Baseline | 2 months | 6 months | |||
| Whole body insulin sensitivity and glycaemic control | ||||||||
| Fasting glucose (mmol/l)a | 9.8 ± 0.6 | 9.6 ± 0.6 | 9.7 ± 0.6 | 9.7 ± 0.4 | 9.4 ± 0.4 | 9.2 ± 0.4 | ||
| Fasting insulin (pmol/l)a | 147 ± 24 | 174 ± 29 | 170 ± 25 | 128 ± 10 | 123 ± 9 | 119 ± 9 | ||
| HOMA indexa | 8.4 ± 1.3 | 9.9 ± 1.6 | 10.1 ± 1.5 | 7.7 ± 0.8 | 7.1 ± 0.7 | 6.5 ± 0.6 | ||
| HbA1c (%) | 7.4 ± 0.3 | 7.3 ± 0.3 | 7.2 ± 0.3 | 7.1 ± 0.2 | 6.9 ± 0.2 | 6.6 ± 0.18 | <0.01 | |
| AUC OGTT (mol/min/l)a | 1.76 ± 0.08 | 1.68 ± 0.08 | 1.75 ± 0.08 | 1.83 ± 0.08 | 1.78 ± 0.07 | 1.71 ± 0.08 | ||
| Blood lipid profile | ||||||||
| Triacylglycerols (mmol/l) | 1.7 ± 0.19 | 1.7 ± 0.19 | 1.6 ± 0.18 | 1.7 ± 0.18 | 1.8 ± 0.18 | 1.6 ± 0.17 | ||
| HDL-cholesterol (mmol/l) | 1.1 ± 0.04 | 1.0 ± 0.03 | 1.0 ± 0.04 | 1.1 ± 0.05 | 1.1 ± 0.04 | 1.1 ± 0.05 | ||
| LDL-cholesterol (mmol/l) | 3.1 ± 0.14 | 3.0 ± 0.15 | 2.8 ± 0.14 | 3.3 ± 0.14 | 3.2 ± 0.17 | 3.1 ± 0.13 | <0.01 | |
| LDL/HDL ratio | 2.9 ± 0.16 | 2.9 ± 0.18 | 2.8 ± 0.20 | 3.2 ± 0.16 | 3.1 ± 0.17 | 2.9 ± 0.16 | ||
| Total cholesterol (mmol/l) | 4.9 ± 0.19 | 4.8 ± 0.28 | 4.7 ± 0.26 | 5.0 ± 0.13 | 4.9 ± 0.17 | 4.7 ± 0.15 | ||
| NEFA (mmol/l) | 425 ± 27 | 427 ± 26 | 428 ± 31 | 444 ± 18 | 491 ± 33 | 461 ± 33 | ||
| Body composition | ||||||||
| Body weight (kg) | 98.7 ± 3.2 | 98.0 ± 3.3 | 97.6 ± 3.4 | 97.7 ± 3.2 | 96.7 ± 3.1 | 95.9 ± 3.2 | <0.01 | |
| Body mass index (kg/m2) | 32.7 ± 0.8 | 32.4 ± 0.9 | 32.3 ± 0.9 | 32.1 ± 0.9 | 31.7 ± 0.9 | 31.4 ± 0.9 | <0.01 | |
| Trunk fat mass (kg) | 22.1 ± 1.3 | 21.3 ± 1.3 | 21.1 ± 1.4 | 21.6 ± 1.4 | 20.4 ± 1.2 | 19.6 ± 1.2 | <0.01 | <0.05 |
| Legs fat mass (kg) | 7.7 ± 0.7 | 7.7 ± 0.7 | 7.5 ± 0.8 | 7.3 ± 0.8 | 7.1 ± 0.7 | 6.9 ± 0.7 | <0.01 | |
| Trunk lean mass (kg) | 31.3 ± 0.7 | 31.9 ± 0.7 | 32.2 ± 0.7 | 31.4 ± 0.6 | 32.1 ± 0.8 | 32.5 ± 0.8 | <0.01 | |
| Legs lean mass (kg) | 18.8 ± 0.5 | 19.1 ± 0.6 | 19.4 ± 0.6 | 18.9 ± 0.4 | 19.4 ± 0.5 | 19.6 ± 0.5 | <0.01 | |
| Exercise performance | ||||||||
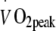 (l/min) (l/min) |
2.05 ± 0.11 | 2.21 ± 0.12 | 2.26 ± 0.10 | 1.99 ± 0.09 | 2.31 ± 0.11 | 2.32 ± 0.11 | <0.001 | |
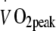 (ml kg−1 min−1) (ml kg−1 min−1) |
20.9 ± 1.0 | 22.7 ± 1.1 | 23.5 ± 1.1 | 20.8 ± 1.1 | 24.3 ± 1.2 | 24.7 ± 1.2 | <0.001 | |
| Maximal workload capacity (W) | 161 ± 7 | 173 ± 8 | 184 ± 8 | 169 ± 6 | 180 ± 7 | 185 ± 7 | <0.001 | |
| Maximal workload capacity (W/kg) | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | <0.001 | |
| Maximal heart rate (beats per min) | 139 ± 4 | 138 ± 3 | 141 ± 3 | 139 ± 3 | 140 ± 3 | 139 ± 3 | ||
Data are means ± SEM. Values for leg lean and fat mass are expressed as averages of measurements of both legs
aFasting glucose, insulin, HOMA index and AUC OGTT were determined from an OGTT performed after 3 days of discontinuation of habitual use of oral blood-glucose and lipid-lowering medication
Maximal workload and whole body oxygen uptake capacity Maximal workload capacity and whole body peak oxygen uptake increased significantly following exercise training (p < 0.001; Table 2), with no differences between groups after 6 months of intervention. However, there was a significant interaction effect (exercise training × intensity) for the changes in VO2peak after 2 months of exercise training. 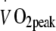 increased to a greater extent in the HI-training group than in the group following the LI programme (16 ± 2% vs 9 ± 2%, respectively; p < 0.05). No changes were observed in maximal heart rate. No correlations were found between changes in
increased to a greater extent in the HI-training group than in the group following the LI programme (16 ± 2% vs 9 ± 2%, respectively; p < 0.05). No changes were observed in maximal heart rate. No correlations were found between changes in 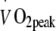 and blood HbA1c levels (p > 0.05).
and blood HbA1c levels (p > 0.05).
Body composition Exercise training significantly reduced body weight, body mass index and trunk fat mass (p < 0.05; Table 2). The change in trunk fat mass after 6 months of intervention significantly differed between groups (p < 0.05), with decreases of 2.0 ± 0.4 kg and 1.0 ± 0.4 kg in the HI and LI training groups, respectively. No interaction effect was observed after 2 months of intervention (p > 0.05). Trunk and leg muscle mass increased significantly following the exercise programme (p < 0.05), with no differences between groups (Table 2).
Muscle tissue analysis The oxidative enzyme activity of the skeletal muscle and the skeletal muscle fibre type composition before and after 2 and 6 months of exercise training are shown in Table 3. The distribution of skeletal muscle fibre types and the activities of succinate dehydrogenase and β-hydroxyacyl-CoA dehydrogenase did not change after 2 or 6 months of intervention (p > 0.05). In contrast, cytochrome c oxidase and citrate synthase activity increased by 56 ± 16% and 46 ± 22% following 6 months of exercise training, respectively (p < 0.05), with no differences between groups. No significant correlations were observed between changes in skeletal-muscle oxidative enzyme activity and blood HbA1c levels (p > 0.05).
Table 3.
Distribution of skeletal muscle fibre type and oxidative capacity
| Variable | Assessment | Effect of training | |||||
|---|---|---|---|---|---|---|---|
| LI group | HI group | ||||||
| Baseline | 2 months | 6 months | Baseline | 2 months | 6 months | ||
| Skeletal muscle fibre distribution (% CSA) | |||||||
| Type 1 muscle fibre | 49.3 ± 1.9 | 50.2 ± 2.1 | 52.2 ± 2.6 | 49.3 ± 1.6 | 49.7 ± 1.9 | 49.7 ± 1.9 | |
| Type 2a muscle fibre | 44.5 ± 1.7 | 42.9 ± 1.9 | 41.8 ± 2.2 | 44.0 ± 1.4 | 42.9 ± 1.9 | 41.8 ± 2.2 | |
| Type 2x muscle fibre | 6.2 ± 0.9 | 6.9 ± 0.8 | 6.0 ± 0.7 | 6.7 ± 1.0 | 7.9 ± 1.0 | 8.5 ± 1.1 | |
| Oxidative enzyme activity (µmol [g wet weight]−1 min−1) | |||||||
| Succinate dehydrogenase | 13.3 ± 0.4 | 12.7 ± 0.3 | 12.8 ± 0.3 | 13.7 ± 0.4 | 12.9 ± 0.4 | 14.3 ± 0.5 | |
| Cytochrome c oxidase | 5.2 ± 0.6 | 6.0 ± 0.7 | 7.4 ± 0.9 | 4.5 ± 0.4 | 5.8 ± 0.6 | 6.5 ± 0.7 | <0.01 |
| Citrate synthase | 9.5 ± 0.9 | 11.1 ± 1.3 | 12.9 ± 1.1 | 10.2 ± 0.9 | 9.9 ± 0.9 | 11.9 ± 0.9 | <0.01 |
| β-Hydroxyacyl-CoA dehydrogenase | 4.8 ± 0.7 | 5.7 ± 0.8 | 6.4 ± 0.7 | 5.6 ± 0.6 | 4.9 ± 0.6 | 6.1 ± 0.7 | |
Data are means ± SEM
CSA, fibre cross-sectional area
Discussion
In this study, the clinical benefits of 6 months of continuous LI vs HI endurance-type exercise training were assessed in obese type 2 diabetes patients. After 6 months of intervention, blood HbA1c content, body composition and whole body and skeletal muscle oxidative capacity had improved significantly, with no differences between the two groups.
It has been firmly established that regular exercise effectively lowers blood HbA1c content in type 2 diabetes patients [1–5]. In accordance, we observed a significant reduction in blood HbA1c level following 2 and 6 months of exercise intervention in the obese type 2 diabetes patients (Table 2). It has been well established that the magnitude of the reduction in HbA1c content following prolonged exercise intervention strongly depends on pre-intervention HbA1c levels [4]. In the present study, we observed a 0.3% reduction in HbA1c level from the pre-intervention level of 7.2 ± 0.2%. This represents a clinically relevant decline and translates into a 6% reduction in the risk of premature death and an 11% reduction in the risk of microvascular disease [21]. Furthermore, none of the participants had to increase oral blood-glucose-lowering medication within the 6 month intervention period. In contrast, six participants had to lower their medication dose. Though we confirm the benefits of exercise intervention to lower blood HbA1c in type 2 diabetes patients, it still remains to be established which exercise training modalities should be applied to maximise the clinical benefits of exercise intervention [1, 5]. Some suggest that (relatively) high intensity exercise activities are required to effectively improve glycaemic control [8, 9]. However, others have failed to observe differences in the acute effects of a low-to-moderate vs a high intensity exercise bout on subsequent glycaemic control in type 2 diabetes patients, when exercise bouts were matched for total energy cost [12, 13].
Surprisingly, long-term intervention studies investigating the impact of exercise intensity on the clinical benefits of exercise training in type 2 diabetes patients are lacking. Therefore, in the present study, we compared the impact of continuous LI with HI endurance-type exercise training, matched for total energy expenditure, in type 2 diabetes patients. Even though 6 months of endurance-type exercise training significantly lowered blood HbA1c, this reduction did not differ between the groups undertaking the LI and HI exercise regimens (Table 2). Furthermore, no changes were observed in basal blood glucose and insulin concentrations and/or in the glucose response in the oral glucose tolerance tests. The present findings tend to be in line with previous observations of the acute effects of a low vs a high intensity exercise bout on glucose tolerance [12, 13], and indicate that the implementation of continuous high intensity endurance-type exercise is not required to lower blood HbA1c during more prolonged exercise intervention in obese type 2 diabetes patients. However, it should be noted that there is recent evidence showing that more intense interval-type exercise training is more effective than continuous-type exercise training in various metabolically and/or functionally compromised populations [22–24]. However, the clinical benefits and feasibility of high intensity interval training remain to be established in type 2 diabetes patients.
The present findings suggest that prolonged continuous low-to-moderate-intensity exercise training is equally effective when compared with more intense exercise training as a means to lower blood HbA1c content, when exercise bouts are being matched for total energy expenditure. This is of important clinical relevance, as patients with long-standing type 2 diabetes generally suffer from muscle weakness, cardiovascular co-morbidities and reduced exercise tolerance. Consequently, it has proven difficult to engage sedentary patients with type 2 diabetes in more intense exercise intervention programmes [25]. As an inverse relationship exists between exercise training intensity and patients’ adherence to an exercise intervention programme [18], it is not surprising that the implementation of a relative high intensity exercise regimen is generally associated with low patient adherence and higher dropout rates [18]. In accordance, we observed a greater dropout rate from the HI group (n = 8) than from the LI group (n = 5), though this did not reach statistical significance (p > 0.05). From the present findings, we conclude that the implementation of a continuous high intensity exercise programme is not required to lower blood HbA1c content in obese type 2 diabetes patients. Consequently, a less intense continuous endurance-type exercise regimen can be equally effective when longer duration of exercise compensates for the lower exercise intensity.
Endurance-type exercise is generally implemented as the main type of exercise in diabetes intervention programmes to effectively reduce whole body fat mass. Historically, low-to-moderate intensity endurance-type exercise has been prescribed to maximise oxidation of skeletal muscle fat [26] and thus maximise loss of fat mass. In the present study, we observed improvements in body composition, including an increase in skeletal muscle mass and a decline in body fat mass following 2 and 6 months of exercise intervention (Table 2). We observed a significantly greater reduction in trunk fat mass in the group undergoing HI exercise than in those undergoing LI training (Table 2). Although this may reflect a greater lipolytic response in adipose tissue during post-exercise recovery from more intense exercise [27], the greater loss of trunk fat mass in the HI group was not accompanied by a measurable increase in oral glucose tolerance and/or whole body insulin sensitivity.
A low oxidative capacity of skeletal muscle has been associated with reduced fat oxidative capacity and greater risk of developing obesity, insulin resistance and/or type 2 diabetes. This has raised the question as to whether impaired mitochondrial function might represent an important factor in the aetiology of insulin resistance [28]. In line with observations of differences in the morphological structure of skeletal muscle mitochondria in patients with type 2 diabetes [28], reduced rates of expression of multiple nuclear respiratory factor-1-dependent genes have been reported in muscle tissue obtained from type 2 diabetes patients [29]. Furthermore, studies applying 31P-magnetic resonance spectroscopy (MRS) with the intention to assess in vivo muscle mitochondrial function suggest the presence of impairments in mitochondrial function in insulin-resistant elderly men and the insulin-resistant offspring of people with type 2 diabetes [30]. However, a significant limitation of these studies is that during the 31P-MRS measurements, O2 flux was not normalised for mitochondrial content. When normalised, both oxidative phosphorylation and electron-transport capacity do not seem to be impaired in type 2 diabetes patients [31]. Therefore, mitochondrial dysfunction does not represent a cause but rather a consequence of the obese insulin-resistant and/or type 2 diabetic state [31, 32]. Prospective long-term training studies aiming to prevent or treat insulin resistance and type 2 diabetes are warranted to determine to what extent the proposed impairments in mitochondrial function can be remedied. In the present study, we observed increases in whole body oxygen uptake capacity (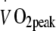 : +16 ± 4%) and skeletal muscle oxidative enzyme activity (cytochrome c oxidase: +56 ± 16% and citrate synthase: +46 ± 22%; Table 3) following 6 months of exercise training. As no interactions were observed within this timeframe, we conclude that continuous moderate-to-high intensity exercise training does not further augment
: +16 ± 4%) and skeletal muscle oxidative enzyme activity (cytochrome c oxidase: +56 ± 16% and citrate synthase: +46 ± 22%; Table 3) following 6 months of exercise training. As no interactions were observed within this timeframe, we conclude that continuous moderate-to-high intensity exercise training does not further augment 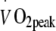 or skeletal muscle oxidative capacity when compared with a less intense exercise regimen. Moreover, changes in
or skeletal muscle oxidative capacity when compared with a less intense exercise regimen. Moreover, changes in 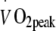 and skeletal muscle oxidative enzyme activity did not correlate with the decline in blood HbA1c levels. Interestingly, the continuous HI exercise regimen tended to result in a faster increase in
and skeletal muscle oxidative enzyme activity did not correlate with the decline in blood HbA1c levels. Interestingly, the continuous HI exercise regimen tended to result in a faster increase in 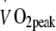 during the first 2 months of the intervention programme. However, this was no longer apparent after 6 months of exercise training (Table 2).
during the first 2 months of the intervention programme. However, this was no longer apparent after 6 months of exercise training (Table 2).
In conclusion, despite a smaller decline in trunk fat mass, prolonged continuous moderate-to-high intensity endurance type exercise is equally as effective as low-to-moderate intensity exercise training at lowering blood HbA1c, elevating muscle mass and increasing whole body and skeletal muscle oxidative capacity in obese type 2 diabetes patients.
Acknowledgements
We are grateful for the technical assistance from J. Senden and A. Zorenc during this study, and for the participation of the patients with type 2 diabetes.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- HI
Moderate to high intensity
- LI
Low to moderate intensity
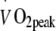
Whole body peak oxygen uptake
References
- 1.Praet SFE, van Loon LJC (2007) Optimizing the therapeutic benefits of exercise in type 2 diabetes. J Appl Physiol 103:1113–1120 [DOI] [PubMed]
- 2.Praet SFE, van Rooij ESJ, Wijtvliet A et al (2008) Brisk walking compared with an individual medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia 51:736–746 [DOI] [PMC free article] [PubMed]
- 3.De Feyter HMD, Praet SF, van den Broek NM et al (2007) Exercise training improves glycemic control in long-standing insulin-treated type 2 diabetic patients. Diabetes Care 30:2511–2513 [DOI] [PubMed]
- 4.Snowling NJ, Hopkins WG (2006) Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients. Diabetes Care 29:2518–2527 [DOI] [PubMed]
- 5.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ (2001) Effect of exercise on glycemic control and body mass in type 2 diabetes mellitus. A meta-analysis of controlled clinical trials. JAMA 286:1218–1227 [DOI] [PubMed]
- 6.Toledo FG, Menshikova EV, Azuma K et al (2008) Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57:987–994 [DOI] [PubMed]
- 7.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD (2006) Physical activity/exercise and type 2 diabetes. Diabetes Care 29:1433–1438 [DOI] [PubMed]
- 8.Kang J, Robertson RJ, Hagberg JM et al (1996) Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care 19:341–349 [DOI] [PubMed]
- 9.Kang J, Kelley DE, Robertson RJ et al (1999) Substrate utilization and glucose turnover during exercise of varying intensities in individuals with NIDDM. Med Sci Sports Exerc 31:82–89 [DOI] [PubMed]
- 10.Garcia-Roves PM, Han DH, Song Z, Jones TE, Hucker KA, Holloszy JO (2003) Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am J Physiol 285:E729–E736 [DOI] [PubMed]
- 11.van Loon LJ, Thomason-Hughes M, Constantin-Teodosiu D et al (2005) Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Am J Physiol 289:E482–E493 [DOI] [PubMed]
- 12.Larsen JJS, Dela F, Kjær M, Galbo H (1997) The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia 40:447–453 [DOI] [PubMed]
- 13.Larsen JJS, Dela F, Madsbad S, Galbo H (1999) The effect of intense exercise on postprandial glucose homeostasis in Type II diabetic patients. Diabetologia 42:1282–1292 [DOI] [PubMed]
- 14.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE (2004) Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 96:101–106 [DOI] [PubMed]
- 15.Hughes VA, Fiatarone MA, Fielding RA et al (1993) Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol 264:E855–E862 [DOI] [PubMed]
- 16.O’Donovan G, Kearney EM, Nevill AM, Woolf-May K, Bird SR (2005) The effects of 24 weeks of moderate or high intensity exercise on insulin resistance. Eur J Appl Physiol 95:522–528 [DOI] [PubMed]
- 17.DiPietro L, Dziura J, Yeckel CW, Neufer PD (2006) Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 100:142–149 [DOI] [PubMed]
- 18.Perri MG, Anton SD, Durning PE et al (2002) Adherence to exercise prescriptions: effects of prescribing moderate vs higher levels of intensity and frequency. Health Psychol 21:452–458 [DOI] [PubMed]
- 19.Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ (2006) Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol 290:E1245–E1252 [DOI] [PubMed]
- 20.Praet SF, De Feyter HM, Jonkers RA et al (2006) 31P MR spectroscopy and in vitro markers of oxidative capacity in type 2 diabetes patients. MAGMA 19:321–331 [DOI] [PubMed]
- 21.Manley S (2003) Haemoglobin A1c-A marker for complications of type 2 diabetes: the experience from the UK Prospective Diabetes Study (UKPDS). Clin Chem Lab Med 41:1182–1190 [DOI] [PubMed]
- 22.Warburton DER, McKenzie DC, Haykomsky MJ et al (2005) Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol 95:1080–1084 [DOI] [PubMed]
- 23.Wisloff U, Stoylen A, Loennechen JP et al (2007) Superior cardiovascular effect of aerobic interval training vs moderate continuous training in heart failure patients: a randomized study. Circulation 115:3086–3094 [DOI] [PubMed]
- 24.Rognmo O, Hetland E, Helgerud J, Hoff J, Slordahl SA (2004) High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 11:216–222 [DOI] [PubMed]
- 25.Dunstan DW, Daly RM, Owen N et al (2005) Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care 28:3–9 [DOI] [PubMed]
- 26.Friedlander AL, Jacobs KA, Fattor JA et al (2007) Contributions of working muscle to whole body lipid metabolism are altered by exercise training and intensity. Am J Physiol 292:E107–E112 [DOI] [PubMed]
- 27.Mulla NAL, Simonsen L, Büllow J (2000) Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: the effects of exercise intensity. J Physiol 524:919–928 [DOI] [PMC free article] [PubMed]
- 28.Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950 [DOI] [PubMed]
- 29.Patti ME, Butte AJ, Crunkhorn S et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed]
- 30.Petersen KF, Befroy D, Dufour S et al (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed]
- 31.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Krausnøe R, Dela F (2007) Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796 [DOI] [PMC free article] [PubMed]
- 32.Bonnard C, Durand A, Peyrol S et al (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800 [DOI] [PMC free article] [PubMed]