Abstract
We combined fluorescence labeling, digital image processing, and micromanipulation to investigate the intracellular events induced by inflicting a mechanical stress on rat basophilic leukaemia cells. Our findings are as follows|: i) Most cells displayed a localized calcium rise in response to micropipette aspiration. This represented an average threefold increase as compared to resting level and it was observed during the first ten seconds following aspiration. A slow return to initial level occured within about 3 minutes. Further, this calcium rise involved a mobilization of intracellular stores since it was not prevented by adding a calcium chelator into the extracellular medium. ii) All micropipette-aspirated cells displayed a local accumulation of microfilaments, with a preferential localization in the cell protrusions or near the pipette tips. iii) No absolute correlation was found between the localization of calcium rise and cytoskeletal accumulation. iv) Cell deformability was decreased when intracellular calcium was maintained at a constant (high or low) level with ionomycin and/or EGTA.
It is concluded that cells have a general ability to respond to mechanical stimulation by a coordinated set of events. More parameters must be studied before the mechanisms of cell shape regulation are fully understood.
Keywords: Animals; Calcium; analysis; Cell Membrane; physiology; Cell Membrane Permeability; Cytoskeleton; ultrastructure; Egtazic Acid; pharmacology; Fluorescent Dyes; Image Processing, Computer-Assisted; Ionomycin; pharmacology; Leukemia, Basophilic, Acute; Microfilaments; chemistry; ultrastructure; Rats; Stress, Mechanical; Tumor Cells, Cultured
Keywords: Cell deformation, calcium, microfilaments, image processing, mechanical stress, rat basophilic leukemia cells, micropipette aspiration
Living cells have a basic capacity to change their shape and move through the surrounding milieu (1). It is an obvious requirement that these processes be regulated. For example, if a cell sends a large lamellipodium towards some region of interest, due to the finiteness of membrane area and constancy of cell volume (2), some negative signal must limit the protrusive activity in order to avoid useless loss of energy. Also, when a cell hits a solid obstacle, this encounter must be perceived in order to initiate a direction change, even if the foreign surface is not recognized by specific membrane receptors. Finally, in multicellular organisms, it is necessary that the components of tissues endowed with a mechanical function be able to respond to exogeneous forces: thus, it is well known that arterial walls can modify their tension in response to variations of the blood pressure.
It is therefore not surprising that many authors reported on the ability of various cells to respond to mechanical stimulation. The migratory activity of isolated corneal cells was enhanced by the application of tension (3). Mechanical loading of chicken chondrocytes increased DNA and glycosaminoglycan synthesis (4). Fluid shear forces were found to induce the formation of stress fibers in human endothelial cells (5). When blood leukocytes were sucked into glass micropipettes, they displayed erratic deformations of the mechanically induced protrusion within several tens of seconds (6). Finally, turbulent but not laminar shear stress increased the turnover of vascular endothelial cells in vitro (7).
However, the mechanisms by which mechanical stimuli generated diverse cell responses remain incompletely understood. Indeed, stretch-activated cationic channels were demonstrated in vascular endothelial cells (8, 9) or epithelial cells (10), but no direct link was demonstrated between ionic fluxes and modifications of cell behavior. In the present work, we took advantage of fluorescent labeling and digital image processing to localize calcium and microfilament changes in rat basophil leukaemia (RBL) cells subjected to mechanical aspiration in glass micropipettes. It is concluded that the imposed stress induced transient and local calcium rises with a participation of intracellular calcium stores, together with cytoskeletal changes. However, both events were not always spatially correlated, which strongly suggested that unidentified biochemical events were involved in cell responses.
MATERIALS AND METHOD
Cells
Rat basophil leukaemia cells (RBL-2H3)) were from Dr. Metzger’s laboratory (NIH, Bethesda, MD (11)). They were kindly provided by Dr. J. Kanellopoulos (Institut Pasteur, Paris). They were cultured in RPMI 1640 medium (Flow laboratories, GB) buffered with 20 mM HEPES, pH 7.2 and supplemented with 10 % fetal calf serum, 2 mM L-glutamin, 100 U/ml penicillin and 100 μg/ml streptomycin. They were detached from culture dishes with 2.5 % trypsin, then washed and maintained in RPMI 1640 medium.
Fluorescent labeling
Calcium monitoring was achieved by loading cells with 1 μM Fura2/AM (12) supplied by Calbiochem (La Jolla, CA) for 30 minutes at 37°C in a solution of 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 5 mM KCl, 10 mM glucose, 135 mM NaCl,1 % bovine albumin, pH 7.4 (labeling buffer). They were then washed and used immediately in the same buffer.
Polymerized actin was revealed as described by Barak et al. (13) by fixing cells for 20 minutes with 3.7 % paraformaldehyde in phosphate buffer at room temperature, then labeling samples by another 20 minute incubation in phosphate buffer containing 10 U/ml 7-nitro-benz 2-oxa-1,3 diazoyl phallacidin (NBD-phallacidin, Molecular Probes, Eugene, OR) and 100μg/ml lysophosphatidylcholine (Sigma).
Microscopical observation
Cells (106/ml) were deposited as 50 μl aliquots on glass coverslips (22×32 mm2) on the stage of an inverted IMT2 Olympus microscope equipped with a Lhesa 1036 video-amplified camera (Lhesa, Cergy Pontoise, France) with dual output. The sensitivity was 0.0015 lux. The first output was connected to a monochrome monitor (Zenith model ZVM 1230-EA) through a videotape recorder (Toshiba DV |90F) and a video timer/character generator (CG-C7S, JVC, Japan) allowing time reading with 0.1 second accuracy. The second output was connected to a PCVision+ digitizer (Imaging Technology, Woburn, MA) mounted on a PC20-III (IBM compatible) computer (Commodore business inc.). This performed real time digitization with 512×512 pixel resolution and 8-bit accuracy. The digitizer output was connected to a CDD-1402E multisync color monitor (Sony, Japan), allowing coded display with 256×256×256 colors. Images were stored on a floppy disk or a hard disk for delayed analysis. The linearity of light intensity determinations was checked as previously described (14). The microscope was modified by removing excitation filters from the blocks provided by the manufacturer and mounting combinations of excitation and neutral filters on a custom-made bar replacing the shutter used to switch off fluorescence illumination. It was thus possible to switch the excitation wavelength manually within about one second, allowing pH (15) and calcium monitoring by determination of the ratio R between fluorescence intensities obtained with two different illumination frequency domains. A laboratory-written software allowed superposition of two cell images with optimum correlation (in order to correct for a possible cell displacement during filter change), background subtraction and pixel-per-pixel intensity ratio calculation. Grynciewicz’s formula (12) was used to calculate local calcium concentrations as follows:
where Kd is the affinity constant for association between fura-2 and calcium under intracellular ionic conditions (this was taken as 224 nM (12)). Rmin and Rmax are the ratios between fluorescence intensities at low ( 340 nm) and high (380 nm) excitation wavelength, these parameters were equal to 1.11 and 7.74 respectively under standard observation conditions (50 W mercury burner, 40X DPlanapo-UV objective). Parameter L is the ratio between fluorescence intensities measured at high wavelength excitation (380 nm) at high and low calcium concentration respectively and was equal to 0.21. Parameters Rmin, Rmax and L were obtained by studying cells incubated in buffer containing calcium and 1μM calcium ionophore ionomycin (Calbiochem), with or without an excess of EGTA to chelate free calcium.
Micropipette aspiration
We used the same methodology as was selected in a previous study made on macrophage deformability (16). Briefly, glass micropipettes were drawn out of capillary tubes (0.85 mm inner diameter; Terumo, Tokyo, Japan) with a vertical pipette puller (David Kopff instruments, Tujunga, Ca) and enlarged to about 4 μm diameter by frontal shock against a smooth glass surface. They were then connected to a water-filled U tube manometer allowing pressure stabilization and reading with a few mm H20 accuracy. Pipettes were held with a de Fonbrune type C pneumatic micromanipulator (Beaudouin, Paris) stuck on the microscope stage with a two-sided adhesive tape. The equilibrium pressure was determined by observation of the movements of free particles near the pipette mouth. Calcium monitoring was performed by subjecting Fura-2-loaded cells to a constant sucking pressure of 5 cm H2O for 4 minutes at 37°C (using a heating stage). The cytosolic calcium concentration was measured at regular intervals of about 15 seconds. Cells were then fixed and stained with phallacidin without being removed from the pipette. In other experiments, cells were subjected to sequential steps of aspiration with pressures of 2, 5 and 10 cm H2O (i.e. 196, 490 and 980 Pa) for 30 seconds each under continuous recording. The protrusion length was measured subsequently.
RESULTS
Mechanical stress induces a transient calcium rise in RBL cells
Thirty-two cells were loaded with fura|2 and sucked into micropipettes with a negative pressure of 980 Pa. This induced the formation of a cylindrical protrusion of about 5–10μm length within a few seconds (figure 1). Intracellular calcium distributions were measured immediately before aspiration, and at regular intervals of 20–50 seconds for the following four minutes. Data were stored on floppy disks, and delayed analysis was performed with coded color display (figure 1C) or digital representation (figure 2). In most cells, aspiration caused a rapid calcium rise: indeed, whereas the maximum local concentration of free calcium was 75 nM±5.8 nM Standard Error (range 1–323) on resting cells, this rose to 269 nM±49.5 nM S.E. (range 74–1088) ten seconds after aspiration and returned to the initial value within about 3 minutes (figure 3). Further, 21 cells displayed a relative increase of the maximum local calcium concentration higher than 30 % ten seconds after the beginning of aspiration, whereas no such increase was found on ten control cells that were observed for 4 minutes. Hence, what we observed was a consequence of the inflicted stress, not spontaneous calcium fluctuations.
Fig. 1. Calcium and cytoskeletal changes induced by mechanical aspiration of RBL cells.
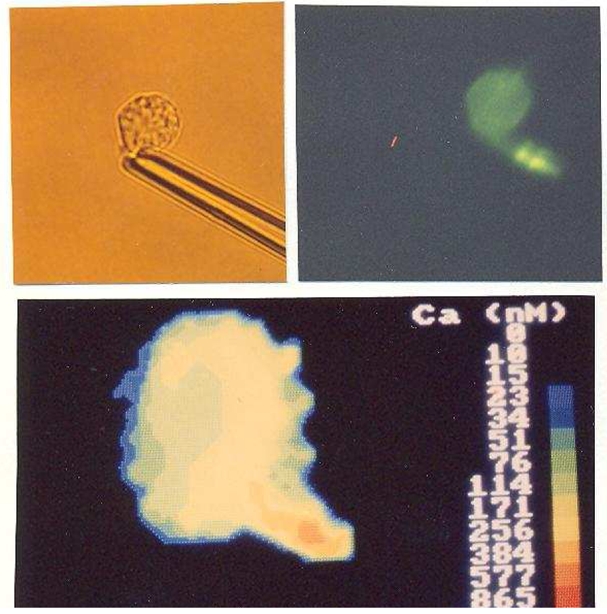
A single cell was sucked into a micropipet, thus inducing the formation of a visible protrusion (A). Calcium was measured repeatedly, and the cell was fixed 4 min later and stained with NBD-phallacidin to reveal microfilaments. Microscopical examination revealed a local concentration of polymerized actin in the protrusion (B). A cytosolic calcium rise was visible in the same cell region 40–60 s after the onset of aspiration with a maximum level higher than 800 nM in the protrusion (C). Calcium concentrations were displayed with a color-coded scale.
Fig. 2. Calcium distribution in a mechanically aspirated cell.

A micropipet-aspirated cell was studied for fluorescence distribution after formation of a small protrusion (arrow). Using the ratio between fluorescence intensities measured with two different excitatory conditions, the local calcium concentration was calculated for each pixel and expressed as nanomoles.
Fig. 3. Kinetics of cytosolic calcium rise in mechanically stressed cells.
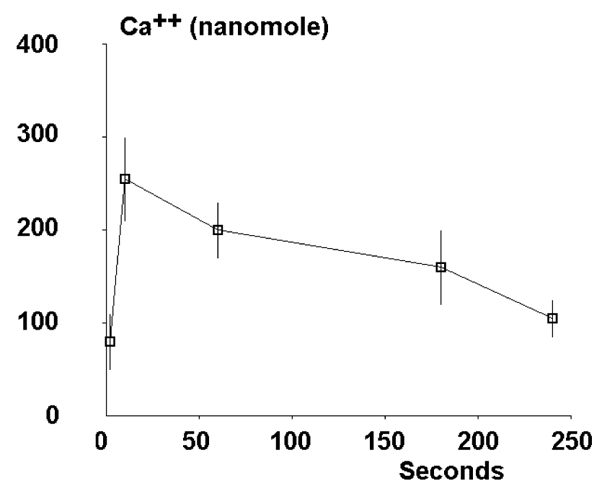
Twenty-two individual cells were sucked into a micropipet at time zero, and the maximum local calcium concentration was measured at regular intervals. mean values are shown. vertical bar length is twice the SE.
Cytosolic calcium increase cannot be solely ascribed to an increase of membrane permeability
The simplest interpretation of our findings might be that membrane stretch resulted in substantial permeability increase, with subsequent calcium influx due to the high difference between extracellular and intracellular electrochemical potential of calcium ions. In order to test this possibility, we compared the calcium changes displayed by cells subjected to mechanical stress in calcium-containing and calcium-deprived media. As shown in table 1, similar calcium increases were observed under both experimental conditions. However, calcium rises were somewhat delayed in presence of an extracellular calcium chelator. Hence, some release of intracellular calcium stores must contribute the observed rise of cytosolic calcium.
Table 1.
Influence of extracellular calcium on cytosolic calcium rise in stressed cells
| Maximum calcium level (nM) at time | ||||
|---|---|---|---|---|
| EGTA concentration | 0 second | 10 second | 60 second | Number of studied cells |
| 0 | 81±16 | 257±48 | 200±30 | 26 |
| 5mM | 52±15 | 115±29 | 216±58 | 10 |
Cells were monitored for intracellular calcium distribution before and ten or sixty seconds after being sucked into micropipettes. The maximum local calcium concentration was then recorded. Experiments were conducted in calcium-containing and calcium-deprived medium. Mean values are shown ± standard error.
Local calcium increase is often but not always located in regions of highest membrane tension
If cell calcium increases were initiated by local membrane stimulation, they might be expected to occur primarily at sites of maximum stress, i.e. near the pipette mouth or the protrusion tip (where the radius of curvature was highest). As shown on table 2, this occured in nearly two thirds of observed cells, but some cells displayed definite calcium rise only at distance from the pipette tip. The most usual patterns of calcium distribution are exemplified in figure 4.
Table 2.
Localization of calcium rise in stressed cells
| Number of cells with calcium rise | |||||
|---|---|---|---|---|---|
| Time (second) | in protrusion | near pipette tip | far from pipette | No calcium rise | Total cell number |
| 10 | 3 | 14 | 9 | 6 | 32 |
| 60 | 7 | 9 | 7 | 9 | 32 |
Thirty two cells were sucked into micropipettes and monitored for intracytoplasmic calcium distribution. Ten and sixty seconds after the onset of aspiration, most cells (26/32 and 23/32 respectively) displayed a local cytoplasmic rise as shown.
Fig. 4. Localization of calcium rise in mechanically stressed cells.
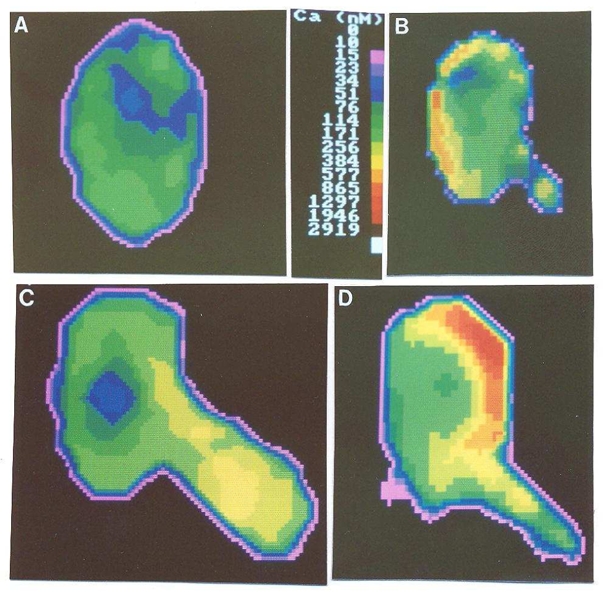
the cytosolic calcium concentration was uniformly lower than 200 nM in resting cells (A). After aspiration, local calcium rises could be detected at distance from the pipet tip (B), in the cell protrusion (C), or near the pipet tip (D), with maximum values higher than 500 – 1000 nM.
Sucking cells into micropipettes resulted in cytoskeletal reorganization
It was of interest to know whether the calcium rise we detected in stretched cells was associated to some structural or functional change. Since cytoskeletal elements are expected to play a role in cell deformation, we compared the distribution of polymerized actin in resting and deformed cells. Fourteen cells were stained with NBD-phallacidin after micropipette aspiration. All of them displayed a clearcut localized fluorescence accumulation (as exemplified in figure 1). Comparison between control and stressed cells (table 3) strongly suggested that i) no substantial actin polymerization was triggered by micropipette aspiration, since both groups of cells displayed similar labeling intensity, and ii) the apparent fluorescence concentration we reported was indeed a consequence of cell deformation, since the highest fluorescence intensity was substantially (50 %) and significantly (P<0.001 according to Student’s t test) higher in these cells than in controls (table 3).
Table 3.
Mechanical stress induces a local concentration of polymerized actin in mechanically stressed cells
| Cell treatment | Mean cell fluorescence | Maximum local fluorescence | Number of studied cells |
|---|---|---|---|
| None (control) | 51.4 ± 2.7 | 81.6 ±5.6 | 11 |
| Pipette aspiration | 55.7±2.6 | 128±8.5 (P<0.001) | 14 |
Eleven control cells and fourteen cells that had been subjected for 4 minutes to micropipette aspiration were fixed and microfilaments were stained with NBD-phallaciding. Mean cell fluorescence and maximum pixel fluorescence were then recorded under the same conditions. Mean values are shown (using arbitrary units) ± Standard Error.
Calcium rise and cytoskeletal accumulation do not always occur in the same cell region
An absolute correlation between the localization of calcium rise and cytoskeletal accumulation in individual cells would be strongly suggestive of the existence of a direct link between both events. However, as shown on table 4, there was no strict correlation between both phenomena. Hence, calcium changes migth not be the only parameter involved in cytoskeletal reorganization.
Table 4.
Colocalization of cytosolic calcium rise and local microfilament accumulation in micropipette-aspirated cells.
| Localization of calcium rise | |||||
|---|---|---|---|---|---|
| Protrusion | Near pipette tip | Elsewhere | No calcium rise | ||
| Localization of microfilament accumulation | Protrusion | 1 | 0 | 0 | 1 |
| Near pipette tip | 1 | 5 | 2 | 3 | |
| Elsewhere | 1 | 0 | 0 | 0 | |
Fourteen individual RBL cells were subjected to micropipette aspiration for 4 minutes under calcium monitoring, then fixed and stained with NBD-phallacidin. All cells displayed local microfilament accumulation, and ten of them exhibited a local calcium rise. The localization of both events is shown.
Intracellular calcium changes do not substantially affect cell deformability
Despite the aforementioned conclusions, in view of previous evidence on the regulation of cell cytoskeleton (17, 18), it was reasonable to expect a direct relationship between intracellular calcium changes and alterations of cell deformability. This point was addressed by comparing the deformability of cells with high and low intracytoplasmic calcium. RBL cells were thus subjected to micropipette aspiration i) in control medium, ii) in presence of 10−6M ionomycin and iii) in presence of 10−6M ionomycin and 5 mM EGTA. The latter two procedures resulted in rapid rise and lowering of intracytoplasmic calcium level, as demonstrated with Fura 2 monitoring (not shown). However, as shown on table 5, both procedures induced only moderate although significant (P<0.01) decrease of cell deformability.
Table 5.
Influence of calcium rise on the deformability of RBL cells
| Length of the protrusion induced by a pressure of | ||||
|---|---|---|---|---|
| Extracellular medium | 196 Pa | 490 Pa | 980 Pa | Number of tested cells |
| Control | 5.2±0.48 | 8.6±0.50 | 12.6±0.53 | 16 |
| Ionomycin 1 μM | 4.4±0.61 (NS) | 6.8±0.72 (P<0.05) | 9.2±0.82 (P<0.01) | 15 |
| Ionomycin 1 μM + EGTA 5 mM | 3.8±0.4 (P<0.05) | 6.1±0.81 (P<0.05) | 9.4±1.37 (NS) | 21 |
Cells were sucked into micropipettes with increasing pressure for steps of 30 seconds each, and the length of the protrusion obtained after each step was measured. Mean values are shown ± Standard Error. The significance of the influence of ionomycin or ionomycin and EGTA was calculated with Student’s t test and indicated in brackets. NS means: not significant at the 0.05 confidence level.
DISCUSSION
The goal of this work was to analyze cell responses to exogeneous stress. The first and quite unambiguous conclusion was that mechanical stress induced a complex biochemical response in rat basophilic leukaemia cells. Indeed, whereas the increase of membrane permeability induced by membrane stretchning (8, 9, 10) might be viewed as some kind of transient leakage, our finding that stress induced a cytosolic calcium rise in a medium containing a chelator of this cation implies that intracellular calcium stores were mobilized, suggesting the occurrence of a cascade of intracellular events (19). A possible mechanism would be the formation of inositol trisphosphate as a consequence of phospholipase activation, with subsequent release of nonmitochondrial calcium (20). Note that it is not inconceivable that mechanical tension per se might activate membrane-embedded phospholipases, since a remarkable activation of these enzymes could be triggered by a reduction of about one dyne/cm of the surface pressure of a lipid layer (21), which is the same order of magnitude as the mechanical tension induced on the cylindrical wall of a protrusion of 2 cm radius by a sucking pressure of 1000 Pa.
Another point of importance concerning calcium measurements must be emphasized: Two-dimensional fluorescence images represent a convolution between three-dimensional fluorescence distributions and the point spread function of the optical system. Hence, what we called “local calcium concentration” was in fact the mean of calcium concentrations in an extended region of space. It is therefore possible that very localized and moderate calcium rises were missed in some cells.œüIt must also be pointed out that the results we presented are in line with the previous finding by Maxfield and colleagues (22) that spreading of human neutrophils was immediately preceded by a calcium spike. Our data support the view that this might be related to a change of the plasma membrane tension, a possibility that was indeed considered by the authors (22).
A second conclusion is that mechanical stress induced a cytoskeletal response in RBL cells. Indeed, all micropipette-aspirated cells displayed a topological reorganization of polymerized actin as visualized with NBD-phallacidin. Since in thirteen cells out of fourteen a local actin concentration was found in the protrusion or near the pipette tip, an attractive hypothesis would be that there was a call for polymerized actin in the region of aspiration (i.e. near the pipette tip), resulting in a flow of blocks of gelled actin filaments that were sometimes stopped near the pipette mouth, resulting in local concentration increase. This view is consistent with qualitative observations made on the deformation of human melanoma cells (23). The local calcium increases we described might result in separation of these blocks following local disruption of the gelled microfilament network, possibly due to gelsolin activation (18, 24). This view would be consistent with the lack of absolute correlation we reported between the localization of calcium spikes and cytoskeletal concentration.
Thirdly, our results strongly suggest that cells responded to exogeneous stress by active mechanical yield. Indeed, deeply perturbing cell cytoskeleton regulation by maintaining high or low value of cytoplasmic calcium resulted in moderate but significant decrease of cell deformability (table 5). It must be pointed out that increased cell stiffness was expected after calcium lowering (25, 26), not calcium increase (24), supporting the view that cytoskeletal regulation is not solely dependent on calcium ions. Further work is therefore required to correlate cell response to mechanical stress with other local events such as pH changes or protein phosphorylations. Clearly, only a detailed analysis of local cellular phenomena can allow a precise understanding of the mechanism of cell shape regulation.
Acknowledgments
This work was supported by the INSERM (CJF 89-07).
References
- 1.Allen RD. J Cell Biol. 1981;91:148s–155s. doi: 10.1083/jcb.91.3.148s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson CA, Trinkaus JP. Exp Cell Res. 1976;99:375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi S. Developmental Biol. 1979;70:232–240. doi: 10.1016/0012-1606(79)90019-8. [DOI] [PubMed] [Google Scholar]
- 4.de Witt MT, Handley CJ, Woakes B, Lowther DA. Connective tissue res. 1984;12:97–109. doi: 10.3109/03008208408992775. [DOI] [PubMed] [Google Scholar]
- 5.Franke RP, Grafe M, Schnittler H, Seiffe D, Mittermayer C. Nature. 1984;307:648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 6.Evans EA, Kukan B. Blood. 1984;64:1028–1035. [PubMed] [Google Scholar]
- 7.Davies PF, Remuzzi A, Gordon EJ, Forbes Dewey C, Gimbrone MA. Proc Natl Acad Sci (USA) 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansman JB, Hallam TJ, Rink TJ. Nature. 1987;325:811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 10.Christensen O. Nature. 1987;330:66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- 11.Kulczycki A, Isersky C, Metzger H. J Exp Med. 1974;139:600–616. doi: 10.1084/jem.139.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 13.Barak LS, Yocum RR, Nothnagel EA, Webb WW. Proc Natl Acad Sci (USA) 1980;77:980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.André P, Capo C, Benoliel AM, Buferne M, Bongrand P. Cell Biophys. 1990;16:13–14. doi: 10.1007/BF02989690. [DOI] [PubMed] [Google Scholar]
- 15.Antoine JC, Prina E, Jouanne C, Bongrand P. Infection and Immunity. 1990;58:779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mège JL, Capo C, Benoliel AM, Bongrand P. Biophys J. 1987;52:177–186. doi: 10.1016/S0006-3495(87)83205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitchcock SE. J Cell Biol. 1977;74:1–15. doi: 10.1083/jcb.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lind SE, Janmey PA, Chaponnier C, Herbert TJ, Stossel TP. J Cell Biol. 1987;105:833–842. doi: 10.1083/jcb.105.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carafoli E. Ann Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 20.Muallem S, Schoeffield M, Pandol C, Sachs G. Proc Natl Acad Sci (USA) 1985;82:4433– 4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demel RA, Geurts van Kessel WSM, Zwaal RFA, van Deenen LLM. Biochim et Biophys Acta. 1975;406:97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- 22.Kruskal BA, Shak S, Maxfield FR. Proc Natl Acad Sci (USA) 1986;83:2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.André P, Capo C, Benoliel AM, Bongrand P, Rougé F, Aubert C. Cell Biophys. 1990;17:163–180. doi: 10.1007/BF02990495. [DOI] [PubMed] [Google Scholar]
- 24.Hartvig JH, Zaner KS, Janmey PA. In: Cell physiology of blood. Gunn RB, Parker JC, editors. Rockefeller University Press; New York: 1988. pp. 125–140. [Google Scholar]
- 25.Miller ME, Myer KA. J Reticuloendothelial Soc. 1975;18:337–345. [PubMed] [Google Scholar]
- 26.Mège JL, Capo C, Benoliel AM, Foa C, Bongrand P. J Immunol Methods. 1985;82:3–15. doi: 10.1016/0022-1759(85)90219-4. [DOI] [PubMed] [Google Scholar]


