Abstract
The distribution and clearance of macromolecules in ocular delivery are not well understood. It has been hypothesized that iontophoresis can enhance transscleral delivery of macromolecules. The objective of this study was to investigate the ocular distribution of a macromolecule after transscleral iontophoretic delivery and intravitreal injection in vivo using nuclear magnetic resonance imaging (MRI) and to compare these results. Experiments of constant current transscleral iontophoresis of 4 mA or intravitreal injection were performed on New Zealand white rabbits in vivo. Iontophoresis experiments were also performed on rabbits postmortem. Galbumin™ (Gd-labeled albumin) was the model permeant surrogate to clinical therapeutic agents. MRI was used to monitor the distribution of the molecule in the eye after ocular iontophoresis and intravitreal injection. In addition, the conjunctiva, sclera, choroid, and retina were extracted in the transscleral iontophoresis study to determine the amounts of Galbumin™ in these tissues using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). The results show that iontophoresis enhanced the ocular delivery of Galbumin™. The macromolecule was mainly delivered into the conjunctiva and sclera in microgram quantities and then diffused towards the posterior section in the upper hemisphere of the eye in vivo. Both in vivo and postmortem studies show that the iontophoretic delivery of Galbumin™ into the vitreous was below the detection limit. In the intravitreal injection study, the diffusion coefficient of Galbumin™ in the vitreous humor was estimated to be close to that of free aqueous diffusion.
Keywords: ocular, drug delivery, MRI, iontophoresis, intravitreal injection, pharmacokinetics
1. Introduction
Previous MRI studies using manganese ion (Mn2+) and Mn2+ with ethylenediaminetetraacetic acid (EDTA) chelate have revealed the ocular barriers to transscleral drug delivery and contributed to the understanding of the mechanisms of ocular iontophoresis (Li et al., 2004a,b; Molokhia et al., 2007, 2008). There were several advantages of using Mn2+ and EDTA chelate. First, the high paramagnetism of Mn2+ provided good MRI signal enhancement and allowed the use of practically low concentration of MnCl2 solution. Second, the transport pathways of Mn2+ and MnEDTA2− penetrating the eye during iontophoresis could be used to study the electric current pathways in ocular iontophoresis. Third, free Mn2+ (contrary to free gadolinium ion) showed no significant binding to proteins in the vitreous. However, Mn2+ and MnEDTA2− are small ions that can easily penetrate ocular barriers that larger molecules cannot. It is unclear if the Mn2+ and MnEDTA2− data can be extrapolated to macromolecules and therapeutic agents in ocular drug delivery, particularly in ocular iontophoresis.
For macromolecule delivery, it is generally believed that the diffusion and electromigration of macromolecules depend mainly on their molecular sizes and charges. The pharmacokinetics of macromolecules in the eye is likely to be related to these factors unless facilitated/active transport such as endocytosis is involved. Surrogate permeants of therapeutic agents allow scientists to envision what can ultimately be delivered by ocular iontophoresis in clinical studies. It has been shown that the internal membrane of the retina impedes diffusion of linear molecules larger than 40 kDa and globular molecules larger than 70 kDa (Smelser et al., 1965; Peyman and Bok, 1972; Marmor et al., 1985; Kamei et al., 1999), precluding intravitreous delivery of many antiangiogenic macromolecular drugs under development. However, intravitreal injection of bevacizumab (Avastin®) which has molecular weight 3 times that of ranibizumab (Lucentis®) had resulted in a significant decrease in macular edema and improvement in visual acuity (Lang, 1995; Iturralde et al., 2006). The macromolecule selected in the present study Galbumin™ has a molecular weight approximately 1.5 times that of Macugen® (≈ 50 kDa) and Lucentis® (≈48 kDa). This compound could serve as a surrogate adequate for the study and the understanding of the migration and distribution of these macromolecules in the eye during transscleral iontophoresis and intravitreal injection.
Recent interest in drug delivery to the back of the eye has stimulated new interest in ocular iontophoresis. In ocular iontophoresis, a donor electrode containing the drug to be delivered into the eye is placed on the eye. To complete an electrical circuit, another electrode is placed on another body surface. Ocular iontophoresis does not have some of the adverse effects associated with intravitreal and periocular injections such as retinal detachment, endophthalmitis, globe perforation and ptosis (Haupert and Jaffe, 2000; Antcliff et al., 2001). Ocular iontophoresis has also been shown to enhance the delivery of a broad range of drugs into the eye such as antibiotics (Vollmer et al., 2002; Eljarrat-Binstock et al., 2004), corticosteroids (Lam et al., 1989; Eljarrat-Binstock et al., 2008), non-steroidal antiinflammatory agents (Voigt et al., 2002; Kralinger et al., 2003), and immunosuppressants (Eljarrat-Binstock et al., 2007). The existing devices were also shown to be effective in the treatment of certain eye diseases and without known side effects (Voigt et al., 2002; Yoo et al., 2002; Parkinson et al., 2003). Most transscleral iontophoresis studies have been focused on the delivery of small molecules with only a few studies on macromolecules. Particularly, the barriers, distribution, and penetration of macromolecules into the eye are not well understood.
The objectives of the present study were to examine the delivery of a model macromolecule into the eye in transscleral iontophoresis and to determine the distribution of this macromolecule after intravitreal injection in vivo. Postmortem experiments were also conducted to assess the transscleral barrier without the interference of ocular clearance. The outcome of this study was expected to provide insights into the distribution and clearance of macromolecular therapeutic agents for future ocular drug delivery development.
2. Experimental section
2.1. Materials
Galbumin™ (25 mg/ml) was purchased from BioPAL, Inc. (Worcester, MA). Galbumin™ is a bovine albumin conjugated with gadolinium (Gd). It has approximately 10–15 Gd atoms per albumin unit. It is a globular protein with an estimated net negative charge of 27. Spectra/Gel® absorbent (polyacrylate-polyalcohol) and dialysis tubing were both purchased from Spectrum® Labs, Inc. (Rancho Dominguez, CA). Gadolinium atomic absorption standard solution (1005 ppm of Gd in 1.0 wt% HNO3) was purchased from Aldrich (St. Louis, MO).
2.2. Galbumin™ characterization and preparation
Matrix-assisted laser desorption/ionization time of flight mass spectroscopy (MALDI-TOF) was used to determine the molecular weight distribution of Galbumin™. For the purpose of the present study, the free aqueous diffusion coefficient (D) of Galbumin™ was determined under the assumption that Galbumin™ has similar frictional coefficient ratio as bovine serum albumin (BSA). The diffusion coefficient of BSA at 20°C in pure water is 6.1 × 10−7 cm2/s (Cantor and Schimmel, 1980). The free aqueous diffusion coefficient of Galbumin™ at 37°C (DGalbumin) was calculated using the diffusion coefficient of BSA (DBSA) and correcting for the molecular weight difference and the viscosity of water at 37°C.
| (1) |
where η20°C is the viscosity of water at 20 °C, η37°C is the viscosity of water at 37 °C, MBSA is the molecular weight of BSA, and MGalbumin is the molecular weight of Galbumin™.
To concentrate the Galbumin™ solution, Galbumin™ was pipetted into a Spectra/Por® dialysis tubing of 1000 Da molecular weight cut off and covered with spectra/gel absorbent. Galbumin™ was concentrated to approximately 100 mg/ml for the transscleral iontophoresis experiments.
2.3. Iontophoresis device
The iontophoresis device was obtained from Aciont, Inc. (Salt Lake City, UT). The electrode device was made of silicone polymer and has a lens shaped design for application onto a rabbit eye (Miller et al., 2008). The device had three drug delivery chambers (about 2 mm apart). This lens shaped design permitted direct contact of these chambers with the bulbar conjunctiva adjacent to the limbus. In the present study, only the middle chamber which contained a Ag/AgCl electrode was used for Galbumin™ delivery. Instrument Wipe, 2 mm thickness, was purchased from Ivalon® and circular discs (diameter approximately 0.07 cm2) were cut out from the wipe and left to equilibrate in the Galbumin™ solution for at least 24 h.
2.4. In vivo transscleral iontophoresis and intravitreal injections
New Zealand white rabbits of 3–4 kg were purchased from Western Oregon Rabbit Co. (Philomath, OR) and were used in the experiments under the approval of the Institutional Animal Care and Use Committee at the University of Utah. After the rabbits were anesthetized with 25–50 mg/kg ketamine IM and 5–10 mg/kg xylazine IM, the lens device was placed on the conjunctiva/sclera in the superior cul-de-sac (under the upper eyelid) near the limbus at the pars plana. The return electrode (approximately 4 cm2 surface area) was placed on a section of gauze pad (approximately 40 cm2 surface area) wetted in saline and clamped to the ear on the side opposite to that of the treated eye. The Galbumin™ solution soaked Ivalon® wipe was placed in the middle chamber of the device. When the device was applied on the eye, this wipe was in direct contact with the bulbar conjunctiva. Cathodal iontophoresis was performed on the eye using constant direct current of 4 mA for 20 min. Rabbits without any treatments were the control. Passive delivery using the Galbumin™-loaded device without the application of an electric field was another control.
Intravitreal injections of Galbumin™ were carried out at concentrations of 0.25,1, and 4 mg/ml using a 29-gauge needle. The volume used in the injection was 0.1 ml. The injection site was the pars plana approximately 3 mm away from the limbus. In the analysis of the intravitreal injection data, a simple model of passive diffusion from a spherical source (Li et al., 2004a) was used to study the early distribution of Galbumin™ from the injection site in the vitreous. Briefly, this analysis assumed that the bolus injection initially created a 0.1 ml sphere of equally distributed Galbumin™ in the vitreous, and that the concentration at radial position r at time t (Cr) from the center of an initially uniformly distributed spherical source of concentration C0 and radius a was related to the diffusion coefficient according to:
| (2) |
The in vivo diffusion coefficient of Galbumin™ could then be determined using these parameters and equation (2).
2.5. Postmortem iontophoresis studies
The purpose of the postmortem studies was to assess the transscleral barrier without ocular clearance (e.g., vasculature and lymphatic clearance). Previous studies have suggested that ocular clearance is a major barrier in transscleral transport and experiments with animals postmortem would allow the identification of the importance of this barrier (Kim et al., 2004; Li et al., 2004b; Molokhia et al., 2008). In the present study, experiments of Gal-bumin™ in postmortem rabbits were conducted approximately 5–10 min after sacrificing the rabbits. The procedure of transscleral iontophoresis on the postmortem rabbits was the same as that described in the in vivo study. After iontophoresis, the animals were left at room temperature for up to 24 h.
2.6. MRI calibration
Experiments were conducted to generate a calibration curve of Galbumin™ enhanced signal intensity versus Galbumin™ concentration as described previously (Li et al., 2004a,b). Briefly, Galbumin™ standard solutions of 0.025–12.5 mg/ml were prepared in saline. The solutions (~ 1 ml) in HPLC vials were imaged with spinecho pulse sequences: TR was 50, 100, 200, 400, 800, 1600, 2400, and 3200 ms with TE fixed at 13 ms for T1 measurement, and TE was 9, 18, 27, 36, 45, 54, 63, 72, and 82 ms with TR fixed at 3000 ms for T2 measurement. The relaxation times T1 and T2 of these standards were determined by curve fitting of the signal intensity of a region of interest (ROI) with respect to the time variables TR and TE using the signal equation of the spin-echo imaging (Li et al., 2004b):
| (3) |
where SI is the signal intensity, So is the intrinsic fully recovered signal intensity, T1 is the spin-lattice relaxation time, and T2 is the spin-spin relaxation time.
2.7. Animal MRI
In the in vivo study, the animals were anesthetized (re-anesthetized) as described in “in vivo transscleral iontophoresis and intravitreal injections” before each MRI scan. In the postmortem study, the animals were scanned directly at the predetermined time points. MRI experiment was conducted in a clinical 3-T MRI system (Trio with Avanto gradient, Siemens Medical Solution, Erlangen, Germany) with a human wrist coil (a transmit/receive volume coil). MR imaging was performed with T1 weighted spin-echo imaging technique. The imaging parameters were 675 ms TR, 13 ms TE, 12 signal averages, FOV of 90 mm and fat suppression. The slice thickness was 1.0 mm with no spacing. The spatial resolution was 0.28 × 0.28 × 1.0 mm3. Each scan provided at least twenty transaxial image slices to cover the whole eye. Imaging time for single time data was approximately 26 min. The MR images were analyzed with MRIcro and Image J (obtained from MRIcro and NIH websites).
2.8. Traditional ocular pharmacokinetics
A traditional ocular pharmacokinetic study was performed to verify the MRI data. The rabbits were sacrificed at either 30 min or 12 h after transscleral iontophoresis. Both eyes were then enucleated. The eye was dissected using a surgical scalpel. The conjunctiva and sclera composite tissues were removed separately from the upper hemisphere region underneath the electrode. The conjunctiva composite (C/M composite) included the conjunctiva and superior muscle. The sclera composite (S/C/R composite) included the sclera, choroid and retina tissues. After eye excision, the eye tissues were left in 5 ml saline for 24 h at 4 °C. A second extraction of the same eye tissues was performed using 5 ml saline for another 24 h, which was followed by a third extraction using the same procedure. The extraction method was validated by spiking the tissues with a known amount of Galbumin™. The first two extractions retrieved 95% of the total amount while the third extraction retrieved less than 1% of the total.
The concentrations of Galbumin™ in the C/M and S/C/R composites were determined using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) at wavelength 342.247 nm in Gd assay:
| (4) |
To confirm that the observed signal in MRI experiments was from the Gd linked to albumin (from Galbumin™) and not free Gd ions, the extracted solution (from eye tissues) was centrifuged in an Amicon centrifugal tube with molecular weight cut off 10 kDa. Samples from the filtrate were checked for Gd content. The filtrate showed negligible amount of Gd present implying the absence of free Gd3+ ions in the extracted solution. A positive control (i.e., standard Galbumin™) was checked for free Gd3+ and negligible amount of Gd was found in the filtrate. Also, a standard Gd atomic absorption solution (free Gd3+ ions) diluted to 2 ppm in water was filtered with the 10 kDa molecular weight cut off centrifugal tube and showed 91% ± 2% recovery in the filtrate.
3. Results
3.1. Galbumin™ characterization
The MALDI-TOF spectrum of Galbumin™ showed average molecular weight of 80 kDa. Other peaks at 40, 24, and 20 kDa represented the double, triple, and quaternary charge ion dissociation, respectively. The free aqueous diffusion coefficient of Galbumin™ in water at 37°C was estimated to be 8 × 10−7 cm2/s using the diffusion coefficient of BSA, viscosity of water at 20° and 37°C, and Galbumin™ and BSA molecular weights (see equation (1)).
3.2. MRI calibration
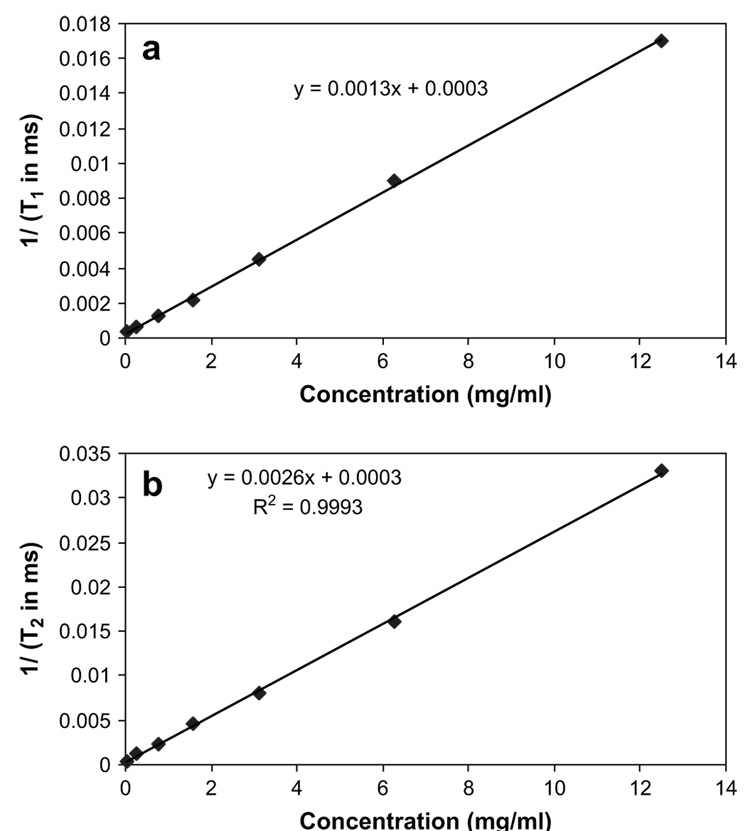
Fig. 1a and b shows the relationships of the relaxation rates 1/T1 and 1/T2 versus Galbumin™ concentration in saline. Linear relationships between the relaxation rates and Galbumin™ concentration were observed. The y-intercept of the 1/T1 versus concentration plot is consistent with T1 of saline/water in the literature (~3–4 s).
Fig. 1.
(a) Relationship between the inverse of relaxation time (T1) and Galbumin™ concentration. (b) Relationship between the inverse of relaxation time (T2) and Galbumin™ concentration. The relaxation times T1 and T2 were determined by curve fitting of the signal intensity with respect to the time variables TR and TE using equation (3). For T1 measurement, MRI images were acquired at 50, 100, 200, 400, 800, 1600, 2400, and 3200 ms TR with TE fixed at 13 ms. For T2 measurement, TE was 9, 18, 27, 36, 45, 54, 63, 72, and 82 ms with TR fixed at 3000 ms.
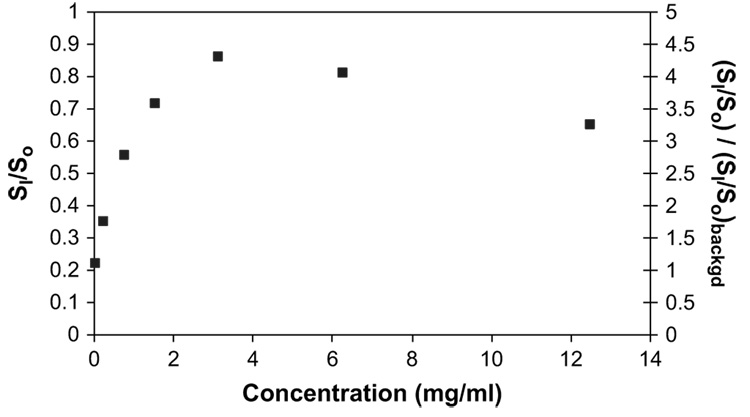
Fig. 2 shows the ratio of SI/So versus Galbumin™ concentrations in saline. The SI/So ratio was calculated using the experimental T1 and T2 measured at each concentration, 675 ms TR, 13 ms TE, and equation (3). The SI/So reached a maximum of approximately 0.86 at around 4 mg/ml Galbumin™ and was relatively linear at lower concentration. The concentration of Galbumin™ used for intravitreal injection was therefore chosen to be 0.25 and 1 mg/ml. 4 mg/ml Galbumin™ was also used but the data were not included in the pharmacokinetic analysis. Fig. 2 also shows the SI/So value divided by the SI/So background versus Galbumin™ concentration. The SI/So background value was approximately 0.2 in the MR images.
Fig. 2.
Relationship between signal intensity ratio SI/So and Galbumin™ concentration in saline (primary y-axis). Relationship between normalized signal intensity ratio of Galbumin™ (SI/So) divided by background (SI/So) and Galbumin™ concentration in saline (secondary y-axis). (SI/So) is the signal SI divided by So. The intensity ratios were calculated using the relaxation times T1 and T2 at varying Galbumin™ concentration, TR of 675 ms, TE of 13 ms, and equation (3).
3.3. In vivo MRI
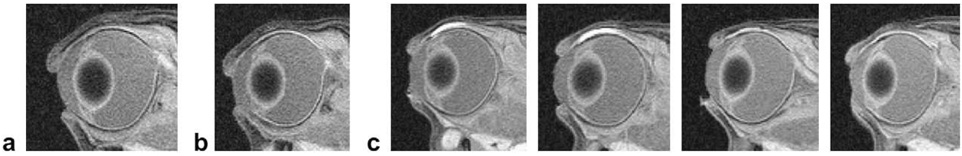
Iontophoresis significantly enhanced the delivery of Galbumin™ as there was no ocular tissue penetration observed in the passive delivery control experiment (without the application of iontophoresis, Fig. 3). Fig. 3 also presents the MR images in the 20-min 4-mA transscleral iontophoresis study. As observed in the images, Galbumin™ was mainly delivered to the conjunctiva and nearby tissues. No Galbumin™ was detected in the anterior chamber or vitreous over the duration of the study. The site of delivery was mainly located below the electrode.
Fig. 3.
Representative MR images of (a) control (no eye treatment), (b) at 50 min after passive delivery (passive control), and (c) at 45 min, 2.5 h, 12.5 h, and 14.5 h, from left to right, respectively, after 20-min 4-mA transscleral iontophoresis of Galbumin™ (concentration in the device ≈ 100 mg/ml) in vivo.
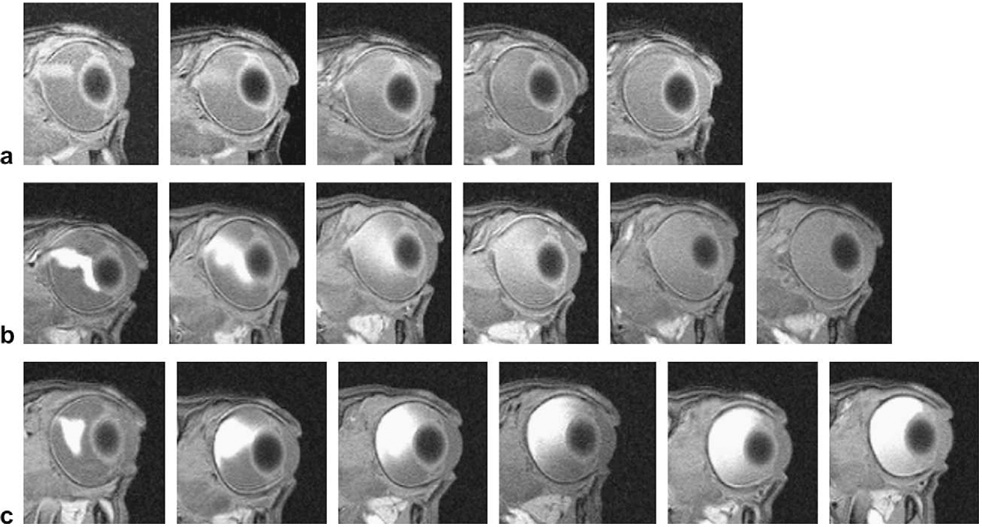
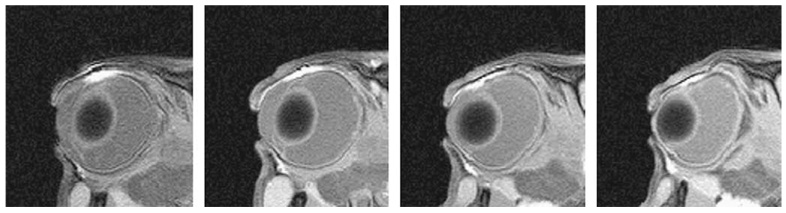
Fig. 4 shows the MR images in the 0.25,1, and 4 mg/ml Galbumin™ intravitreal injection study. The injection spot was in the vitreous usually towards the back of the eye near the retina. When the injection spot was closer to the lens, the spreading of Galbumin™ behind the lens was suggestive of a less resistive region to the flow of the solution introduced by the needle into this region during injection (Fig. 4b). Within 4 h after injection, Galbumin™ rapidly diffused from the spot of the injection and spread in the vitreous. At 24 h, the macromolecule almost completely filled the vitreous humor. No significant amount of Galbumin™ was detected in the anterior chamber over the duration of the study.
Fig. 4.
Representative MR images after intravitreal injection of (a) 0.25, (b) 1, and (c) 4 mg/ml Galbumin™ in vivo. From left to right: (a) at 20 min, 4 h, 7 h, 12 h, and 22 h, (b) at 20 min, 4 h, 13 h, 23.5 h, 39.5 h, and 58.5 h and (c) at 20 min, 4 h, 13 h, 23.5 h, 39.5 h, and 58.5 h, respectively.
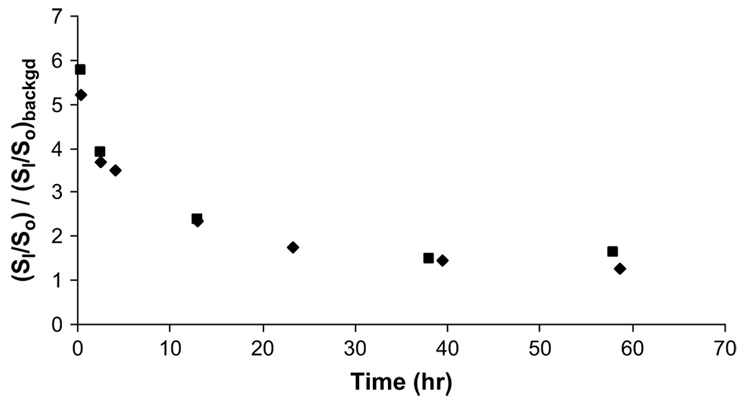
To quantitatively study the diffusion of Galbumin™ in the eye after intravitreal injection, the signal intensity in the ROI, which was the position of injection and had the highest concentration of Galbumin™ (highest signal), was monitored. Fig. 5 shows that the signal at the ROI dropped rapidly in the first 4 h followed by a slower decrease from 10 to 60 h. The diffusivity of Galbumin™ in the vitreous after intravitreal injections was estimated using equation (2) to be 8 × 10−7 cm2/s (uncertainty: ±3 × 10−7 cm2/s), essentially the same as its free aqueous diffusion coefficient (8 × 10−7 cm2/s; see Section 3.1).
Fig. 5.
(SI/So)/(SI/So)backgd at the region of interest versus time after 1 mg/ml intra-vitreal injection. The ROI is the location of the highest signal in the vitreous – the injection spot. Each symbol represents the data from an individual experiment. (SI/So)backgd is the ratio of the vitreous signal in the absence of contrast agent (SI)backgd, which is approximately 260, to So. The ratio of (SI/So)/(SI/So)backgd was determined by SI and (SI)backgd and the control signal (usually muscle) in the image slice.
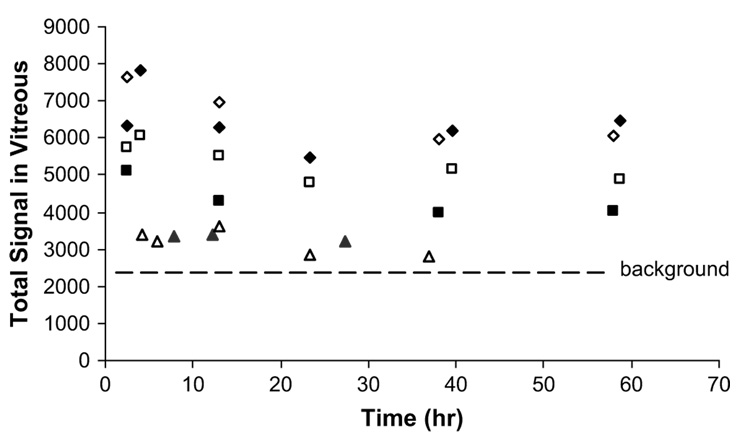
To study the clearance of Galbumin™ in the vitreous, Fig. 6 presents the total signal in the vitreous at approximately 0.4, 4, 12, 23, 40, and 60 h after injection with the assumption that the signal intensity is proportional to Galbumin™ concentration. This is a reasonable assumption at the low Galbumin™ concentrations (≤1 mg/ml) used in the present study (see Fig. 2). As a result, the 4 mg/ml data were not included in the present analysis. Within the data scatter, the clearance of Galbumin™ in the vitreous (up to 60 h) after the 0.25 and 1 mg/ml injections can be interpreted as zeroth-order clearance (r2 ≥ 0.57). A comparison of the 0.25 and 1 mg/ml data further supports that the clearance follows zeroth-order kinetics - the rates of the decrease in the total vitreous concentration of Galbumin™ after 0.25 and 1 mg/ml injections are not significantly different (average rate = −15 and −18 total MRI signal/h, respectively). In summary, the present results are consistent with slow Galbumin™ clearance in the vitreous with a half-life of approximately 2 days after 1 mg/ml injection and 0.7 day after 0.25 mg/ml injection with a substantial amount of Galbumin™ still present in the vitreous over the duration of the study.
Fig. 6.
Total average signal of the vitreous from the 10 slides covering the majority of the eye after intravitreal injection of 0.25,1, and 4 mg/ml Galbumin™. Total average signal = Σ10 image slides (average signal of all the voxels in the vitreous in an MR image). Symbols: 0.25 mg/ml, triangles; 1 mg/ml, squares; 4 mg/ml, diamonds. The open and closed symbols represent the data from the replicate under each condition. The line shows the background average signal in the vitreous from 10 slides without the injection.
3.4. Postmortem MRI
In transscleral iontophoresis postmortem, deeper penetration reaching approximately 3 mm underneath the electrode (e.g., deeper into the ciliary body) than that in vivo was observed in the earlier time ~1 h after iontophoresis (Fig. 7). The clearance of Galbumin™ in the postmortem study was also found to be slower than that in vivo. Similar to the results in the in vivo study, no penetration of the macromolecule into the vitreous was detected postmortem. It was also noted that some eyes were slightly deformed at > 13 h after the animals were sacrificed, indicated by the unusual eye shapes seen in a few MR images at these later time points. Although the eye tissues were suggested to remain viable several hours postmortem (Kim et al., 2004), caution must be exercised in the interpretation of the postmortem results, especially at the later time points, due to potential postmortem tissue changes and the lower body temperature postmortem than in vivo.
Fig. 7.
Representative MR images at 50 min, 3 h, 13.5 h, and 22.5 h, from left to right, respectively, after 20-min 4-mA transscleral iontophoresis of Galbumin™ (concentration in the device ≈ 100 mg/ml) postmortem.
3.5. In vivo traditional pharmacokinetics
Table 1 shows the amount of Galbumin™ in the C/M and S/C/R tissue composites within approximately 0.4 cm2 around the site of electrode application at 30 min and 12 h after 20-min 4-mA transscleral iontophoresis in vivo. These results are consistent with the MRI results and show that most of the Galbumin™ delivery occurs in the conjunctiva region. Smaller amounts of Galbumin™ were found to be present in the S/C/R composite. The pharmacokinetic data show that the amount of Galbumin™ in the C/M and S/C/R composites at 30 min after transscleral iontophoresis (n ≥ 3) was in the range of 19–38 and 9–17 µg, respectively. At 12 h after iontophoresis, the remaining amount of Galbumin™ in the C/M composite was approximately 2.9–4.5 µg and that in the S/C/R composite was 0.3–5.2 µg. Assuming that the concentration of Galbumin™ in the sponge was 100 mg/ml, with 20 µl volume, the total amount of Galbumin™ in the iontophoresis device was 2 mg. The amounts delivered to the C/M and S/C/R composites at 30 min after iontophoresis therefore correspond to approximately 1–2% and 0.4–1% of the total dose in the device, respectively.
Table 1.
Amounts of Galbumin™ delivered into the eye tissues by 4 mA transscleral iontophoresis for 20 min.
| Time after iontophoresis | µg Galbumin™/mg tissue in the conjunctiva (C/M) compositea | µg Galbumin™/mg tissue in the sclera (S/C/R) compositea | Total µg Galbumin™ in the conjunctiva (C/M) composite | Total µg Galbumin™ in the sclera (S/C/R) composite |
|---|---|---|---|---|
| 30 min | 0.13 ± 0.07 | 0.04 ± 0.02 | 28 ± 9 | 13 ± 4 |
| 12 h | 0.03 ± 0.01 | 0.02 ± 0.01 | 3.7 ± 0.8 | 2.7 ± 2.5 |
Mean ± SD, n ≥ 3.
4. Discussion
4.1. Ocular delivery and distribution after iontophoresis
In the present study, transscleral iontophoretic delivery of Galbumin™ to the conjunctiva and sclera was demonstrated. The delivery of the macromolecule was primarily to the conjunctiva and nearby tissue below the site of iontophoresis application. No Galbumin™ was detected in the vitreous (i.e., below the detection limit) in both the in vivo and postmortem studies. A possible explanation is that the retinal pigmented epithelium barrier does not allow any significant penetration of the macromolecule (80 kDa globular protein) used in the present study. Clearance such as blood vasculature and lymphatic clearance may also reduce iontophoretic macromolecular delivery (Kim et al., 2004; Robinson et al., 2006; Molokhia et al., 2008). Previous studies have proposed that small molecules are mainly eliminated by the blood capillaries and macromolecules such as Galbumin™ (radius ≈ 4 nm) are cleared primarily by the lymphatic system (Supersaxo et al., 1990; McLennan et al., 2005; Kim et al., 2008). In the present study, the pharmacokinetic data at 12 h after iontophoresis show that the clearance of Galbumin™ in the C/M tissues is faster than that in the S/C/R tissues (Table 1). A comparison of the present and previous pharmacokinetic data suggests that the difference between the half-lives of the small molecules in the previous studies and Galbumin™ in the present study is related to their diffusion coefficients. For example, an approximately 10× decrease in the concentration of small molecules in the conjunctiva and sclera was observed in the first 2 h after delivery (Kondo and Araie, 1989; Voigt et al., 2002), suggesting initial elimination half-lives of around 0.6 h in these tissues. The half-life of Galbumin™ clearance in the same tissues was approximately 5 h in the present study, which is roughly 8× longer than those of the small molecules (free aqueous diffusion coefficients of these small molecules and Galbumin™ were ~6 × 10−6 cm2/s and 8 × 10−7 cm2/s, respectively). This suggests that tissue clearance was mainly diffusion-controlled. The present postmortem data also suggest that the dynamic barrier (i.e., clearance) is not the main factor for the lack of Galbumin™ penetration into the vitreous. This is consistent with the previous observation that the rate of clearance after subconjunctival injection of Gd-DTPA in vivo was substantially higher than that postmortem, but the in vivo and postmortem clearance rates of Galbumin™ did not differ significantly (Kim et al., 2008). Similarly for iontophoretic delivery, although significant enhancement of small molecule delivery into the vitreous was previously observed postmortem (compared to those in vivo) after iontophoresis (Molokhia et al., 2008), there was no significant enhancement in Galbumin™ delivery into the vitreous postmortem versus in vivo in the present study.
Although Galbumin™ was not observed in the vitreous in the present study, the delivery of macromolecules from the conjunctiva and sclera to the posterior retina cannot be ruled out. Literature has shown that drugs delivered by intrascleral injection or implant could reach the retina and choroid via the suprachoroidal space (Gilger et al., 2006; Kim et al., 2007). This suggests that the delivery of a drug into and across the sclera could eventually result in the delivery of the drug to the choroid and retina. The results in the present study suggest the possibility of using transscleral iontophoresis to deliver macromolecules into these posterior tissues. Future studies are needed to test this hypothesis and to optimize the electrode position, the current intensity, and the duration of iontophoresis for transscleral iontophoretic delivery of macromolecules.
4.2. Ocular distribution after intravitreal injection
The diffusion of Galbumin™ in the rabbit vitreous was fast after intravitreal injection. Within 24 h after the injection, the macromolecule was observed to fill almost the whole vitreous independent of the variability associated with the injection location. Despite the relatively high viscosity of the vitreous humor (Xu et al., 2000), no significant hindrance of Galbumin™ diffusion was observed in the vitreous; the diffusion coefficient of Galbumin™ in the vitreous based on equation (2) was essentially the same as that in free aqueous diffusion. This is consistent with previous studies of small permeants (Berkowitz et al., 1994; Xu et al., 2000; Li et al., 2004a) and radioactive proteins (Maurice, 1959). Together, the previous and present findings suggest that the microscopic viscosity of vitreous humor is similar to that of saline/water and that the hyaluronic acid in rabbit eyes, providing the viscosity of the vitreous humor, may not create a polymer network dense enough to significantly hinder Galbumin™ diffusion. The present results show that the half-life of the macro-molecule (free aqueous diffusion coefficient ≈ 8 × 10−7 cm2/s) is concentration dependent and approximately 2 days after the 1 mg/ml injection.
It has been suggested that drugs are eliminated by two main routes after intravitreal injection: anterior and posterior (Worakul and Robinson, 1997; Maurice, 2001; Urtti, 2006). In general, hydrophilic drugs have been suggested to utilize the anterior route and lipophilic drugs the posterior route (Worakul and Robinson, 1997). In the anterior route, the drug diffuses across the vitreous to the posterior chamber and, thereafter, cleared from the posterior and anterior chambers. Elimination can then be through uveal blood flow or Schlemm’s canal in the anterior chamber although a previous study did not find Gd-albumin permeation across the blood-aqueous barrier (Wu et al., 1992). In the posterior route, the drug is eliminated across the blood-retina barrier into the blood circulation. Therefore, posterior elimination requires the permeation of the drug across the retinal pigmented epithelium in the blood-retina barrier, and molecules with high molecular weight and high water-solubility tend to have long half-lives in the vitreous (Urtti, 2006). The present data show no significant increase in Galbumin™ concentration in the anterior chamber in the MR images after 0.25 and 1 mg/ml injections. In the 4 mg/ml intravitreal injection study, a slight increase in anterior chamber signal was observed (Fig. 4) but the increase was not statistically significant. The present results suggest that (a) the concentration of the macromolecule in the anterior chamber was below detection due to the fast aqueous humor turnover and slow diffusion from the vitreous to the anterior chamber, (b) clearance occurred at the uvea/ciliary body as the main elimination pathway before the accumulation of the macromolecule in the anterior chamber, or (c) posterior clearance remained the main ocular elimination route for the macromolecule.
4.3. Ocular drug delivery in human
Although the rabbit is the most commonly used animal model for ocular research due to its similarities to human such as permeability characteristics and anterior chamber volume, there are major differences between the rabbit and human eye tissues (Mitra, 2003). For example, the thickness of the sclera in the New Zealand rabbits is between 0.2 and 0.3 mm at equator whereas that in humans is 0.4–0.5 mm. The volume of the vitreous humor in rabbits is approximately 1.5 ml, smaller than the volume of 4 ml in humans. The components of vitreous humor such as hyaluronic acid can also be different in animals and humans, and the vitreous humor properties such as viscosity can change with age and disease states. As a result, there are limitations in extrapolating the results in the present study to human. For example, in the intravitreal injection study, if vitreous clearance is diffusion-controlled or via the anterior route, the rate of clearance can be slower in humans due to the larger volume of human vitreous compared to the rabbit. On the contrary, if the clearance is barrier-controlled via the posterior route, the rate of clearance can be faster in humans due to the larger retinal surface area when the permeability of the retina-blood barrier is the same. Despite this, some data obtained in rabbits can be useful to understand the mechanisms of ocular drug delivery such as the location and distribution of Galbumin™ in the eye after iontophoresis application and the pathway of Galbumin™ clearance after intravitreal injection. At the anatomical and histological levels, human and rabbit vitreous matrices are sufficiently similar to make the rabbit a promising animal model for the study of vitreous matrix differentiation and degeneration (Los, 2008). In summary, caution should be exercised to utilize the present results in ocular delivery development.
5. Conclusion
The distribution of a globular macromolecule in the eye was studied with MRI after transscleral iontophoresis and intravitreal injection. Traditional pharmacokinetic study was also conducted. The results in the present study with Galbumin™ provide insights into the distribution and clearance of macromolecules of sizes and shapes similar to those of ranibizumab in ocular drug delivery. In the iontophoresis study, the potential of using iontophoresis as a noninvasive delivery method for macromolecules to the posterior segment of the eye was investigated. Transscleral iontophoresis primarily delivered the macromolecule to the conjunctiva and nearby tissues under the site of the iontophoresis application. No Galbumin™ was detected in the anterior chamber or vitreous after iontophoresis. At 12 h after iontophoresis, a significant amount of Galbumin™ was cleared from the tissues. In the intravitreal injection study, the distribution of Galbumin™ in the vitreous humor was fast with a diffusion coefficient estimated to be close to that of free aqueous diffusion.
Acknowledgements
This research was supported by NIH Grant EY 015181. The authors thank Xin Liu for his help in the MRI experiments and Aciont Inc. (Salt Lake City, UT) for providing the iontophoresis electrode device. The authors also thank Dr. Steven Kern, Dr. James Herron, and Dr. Paul Bernstein for their helpful discussions and Dr. Paul Cobine for his help with the ICP-OES.
References
- Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001;108:765–772. doi: 10.1016/s0161-6420(00)00658-8. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Wilson CA, Tofts PS, Peshock RM. Effect of vitreous fluidity on the measurement of blood-retinal barrier permeability using contrast-enhanced MRI. Magn. Reson. Med. 1994;31:61–66. doi: 10.1002/mrm.1910310110. [DOI] [PubMed] [Google Scholar]
- Cantor CR, Schimmel PR. Biophysical Chemistry: Part II: Techniques for the Study of Biological Structure and Function. first ed. W.H. Freeman; 1980. [Google Scholar]
- Eljarrat-Binstock E, Domb AJ, Orucov F, Frucht-Pery J, Pe’er J. Methotrexate delivery to the eye using transscleral hydrogel iontophoresis. Curr. Eye Res. 2007;32:639–646. doi: 10.1080/02713680701528674. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Orucov F, Frucht-Pery J, Pe’er J, Domb AJ. Methyl-prednisolone delivery to the back of the eye using hydrogel iontophoresis. J. Ocul. Pharmacol. Ther. 2008;24:344–350. doi: 10.1089/jop.2007.0097. [DOI] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Raiskup F, Stepensky D, Domb AJ, Frucht-Pery J. Delivery of gentamicin to the rabbit eye by drug-loaded hydrogel iontophoresis. Invest. Ophthalmol. Vis. Sci. 2004;45:2543–2548. doi: 10.1167/iovs.03-1294. [DOI] [PubMed] [Google Scholar]
- Gilger BC, Salmon JH, Wilkie DA, Cruysberg LP, Kim J, Hayat M, Kim H, Kim S, Yuan P, Lee SS, Harrington SM, Murray PR, Edelhauser HF, Csaky KG, Robinson MR. A novel bioerodible deep scleral lamellar cyclosporine implant for uveitis. Invest. Ophthalmol. Vis. Sci. 2006;47:2596–2605. doi: 10.1167/iovs.05-1540. [DOI] [PubMed] [Google Scholar]
- Haupert CL, Jaffe GJ. New and emerging treatments for patients with uveitis. Int. Ophthalmol. Clin. 2000;40:205–220. doi: 10.1097/00004397-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund KB, Cooney M, Fine HF. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–284. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Kamei M, Misono K, Lewis H. A study of the ability of tissue plasminogen activator to diffuse into the subretinal space after intravitreal injection in rabbits. Am. J. Ophthalmol. 1999;128:739–746. doi: 10.1016/s0002-9394(99)00239-1. [DOI] [PubMed] [Google Scholar]
- Kim H, Robinson MR, Lizak MJ, Tansey G, Lutz RJ, Yuan P, Wang NS, Csaky KG. Controlled drug release from an ocular implant: an evaluation using dynamic three-dimensional magnetic resonance imaging. Invest. Ophthalmol. Vis. Sci. 2004;45:2722–2731. doi: 10.1167/iovs.04-0091. [DOI] [PubMed] [Google Scholar]
- Kim SH, Csaky KG, Wang NS, Lutz RJ. Drug elimination kinetics following subconjunctival injection using dynamic contrast-enhanced magnetic resonance imaging. Pharm. Res. 2008;25:512–520. doi: 10.1007/s11095-007-9408-z. [DOI] [PubMed] [Google Scholar]
- Kim SH, Galban CJ, Lutz RJ, Dedrick RL, Csaky KG, Lizak MJ, Wang NS, Tansey G, Robinson MR. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Invest. Ophthalmol. Vis. Sci. 2007;48:808–814. doi: 10.1167/iovs.06-0670. [DOI] [PubMed] [Google Scholar]
- Kondo M, Araie M. Iontophoresis of 5-fluorouracil into the conjunctiva and sclera. Invest. Ophthalmol. Vis. Sci. 1989;30:583–585. [PubMed] [Google Scholar]
- Kralinger MT, Voigt M, Kieselbach GF, Hamasaki D, Hayden BC, Parel JM. Ocular delivery of acetylsalicylic acid by repetitive coulomb-controlled iontophoresis. Ophthalmic Res. 2003;35:102–110. doi: 10.1159/000069129. [DOI] [PubMed] [Google Scholar]
- Lam TT, Edward DP, Zhu XA, Tso MO. Transscleral iontophoresis of dexamethasone. Arch. Ophthalmol. 1989;107:1368–1371. doi: 10.1001/archopht.1989.01070020438050. [DOI] [PubMed] [Google Scholar]
- Lang JC. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995;16:39–43. [Google Scholar]
- Li SK, Jeong EK, Hastings MS. Magnetic resonance imaging study of current and ion delivery into the eye during transscleral and transcorneal iontophoresis. Invest. Ophthalmol. Vis. Sci. 2004a;45:1224–1231. doi: 10.1167/iovs.03-0821. [DOI] [PubMed] [Google Scholar]
- Li SK, Molokhia SA, Jeong EK. Assessment of subconjunctival delivery with model ionic permeants and magnetic resonance imaging. Pharm. Res. 2004b;21:2175–2184. doi: 10.1007/s11095-004-7669-3. [DOI] [PubMed] [Google Scholar]
- Los LI. The rabbit as an animal model for post-natal vitreous matrix differentiation and degeneration. Eye. 2008;22:1223–1232. doi: 10.1038/eye.2008.39. [DOI] [PubMed] [Google Scholar]
- Marmor MF, Negi A, Maurice DM. Kinetics of macromolecules injected into the subretinal space. Exp. Eye Res. 1985;40:687–696. doi: 10.1016/0014-4835(85)90138-1. [DOI] [PubMed] [Google Scholar]
- Maurice DM. Protein dynamics in the eye studied with labelled proteins. Am. J. Ophthalmol. 1959;47:361–368. doi: 10.1016/s0002-9394(14)78042-0. [DOI] [PubMed] [Google Scholar]
- Maurice DM. Review: practical issues in intravitreal drug delivery. J. Ocul. Pharmacol. Ther. 2001;17:393–401. doi: 10.1089/108076801753162807. [DOI] [PubMed] [Google Scholar]
- McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov. Today: Technol. 2005;2:89–96. doi: 10.1016/j.ddtec.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Li SK, Tuitupou AL, Kochambilli RP, Papangkorn K, Mix DCJ, Higuchi WI, Higuchi JW. Passive and oxymetazoline-enhanced delivery with a lens device: pharmacokinetics and efficacy studies with rabbits. J. Ocul. Pharmacol. Ther. 2008;24:385–391. doi: 10.1089/jop.2007.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra AK. Ophthalmic Drug Delivery Systems. second ed. New York: Marcel Dekker; 2003. [Google Scholar]
- Molokhia SA, Jeong EK, Higuchi WI, Li SK. Examination of penetration routes and distribution of ionic permeants during and after transscleral iontophoresis with magnetic resonance imaging. Int. J. Pharm. 2007;335:46–53. doi: 10.1016/j.ijpharm.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokhia SA, Jeong EK, Higuchi WI, Li SK. Examination of barriers and barrier alteration in transscleral iontophoresis. J. Pharm. Sci. 2008;97:831–844. doi: 10.1002/jps.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson TM, Ferguson E, Febbraro S, Bakhtyari A, King M, Mundasad M. Tolerance of ocular iontophoresis in healthy volunteers. J. Ocul. Pharmacol. Ther. 2003;19:145–151. doi: 10.1089/108076803321637672. [DOI] [PubMed] [Google Scholar]
- Peyman GA, Bok D. Peroxidase diffusion in the normal and laser-coagulated primate retina. Investig. Ophthalmol. 1972;11:35–45. [PubMed] [Google Scholar]
- Robinson MR, Lee SS, Kim H, Kim S, Lutz RJ, Galban C, Bungay PM, Yuan P, Wang NS, Kim J, Csaky KG. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 2006;82:479–487. doi: 10.1016/j.exer.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Smelser GK, Ishikawa T, Pei YF. Structure of the Eye. Stuttgart: Schattauer-Verlag; 1965. [Google Scholar]
- Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 1990;7:167–169. doi: 10.1023/a:1015880819328. [DOI] [PubMed] [Google Scholar]
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Voigt M, Kralinger M, Kieselbach G, Chapon P, Anagnoste S, Hayden B, Parel JM. Ocular aspirin distribution: a comparison of intravenous, topical, and coulomb-controlled iontophoresis administration. Invest. Ophthalmol. Vis. Sci. 2002;43:3299–3306. [PubMed] [Google Scholar]
- Vollmer DL, Szlek MA, Kolb K, Lloyd LB, Parkinson TM. In vivo transscleral iontophoresis of amikacin to rabbit eyes. J. Ocul. Pharmacol. Ther. 2002;18:549–558. doi: 10.1089/108076802321021090. [DOI] [PubMed] [Google Scholar]
- Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. Eur. J. Pharm. Biopharm. 1997;44:71–83. [Google Scholar]
- Wu JC, Jesmanowicz A, Hyde JS. Anterior segment high resolution MRI: aqueous humor dynamics observed using contrast agents. Exp. Eye Res. 1992;54:145–148. doi: 10.1016/0014-4835(92)90079-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Heys JJ, Barocas VH, Randolph TW. Permeability and diffusion in vitreous humor: implications for drug delivery. Pharm. Res. 2000;17:664–669. doi: 10.1023/a:1007517912927. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Dursun D, Dubovy S, Miller D, Alfonso E, Forster RK, Behar-Cohen F, Parel JM. Iontophoresis for the treatment of paecilomyces keratitis. Cornea. 2002;21:131–132. doi: 10.1097/00003226-200201000-00029. [DOI] [PubMed] [Google Scholar]