Abstract
Growing evidence supports the efficacy of cord blood transplantation (CBT), and the number of CBTs is increasing. Numerous studies confirm the presence of a graft-versus-leukemia effect following CBT, and preliminary data suggests that double unit CBT may be associated with a decreased risk of relapse. We have observed a low relapse rate following CBT among patients with acute leukemias in morphologic CR at the time of myeloablative transplant. To further assess this observation we conducted a matched cohort analysis comparing relapse rates and outcomes for patients receiving CBTs versus patients receiving matched unrelated donor (MURD) and mismatched unrelated donor (MMURD) transplants at our center. Thirty-one consecutive CBT patients (ages 0.6–42, median 22) transplanted between April 2006 and June 2008 were compared to matched subjects selected on the basis of disease type and remission number, cytogenetic risk status, minimal residual disease status (MRD), time from diagnosis to first relapse (for patients beyond CR1), use of imatinib for CML and Philadelphia chromosome positive ALL patients, age, and date of transplant. With a median follow-up among surviving CBT patients of 21.1 months (range 6.6–32.6), there has been one relapse among cord patients versus eight relapses among MURD patients (p=0.018) and seven relapses among MMURD patients (p=0.019). Transplant related mortality (TRM) between cohorts is comparable. Though we have observed a high incidence of acute graft-versus-host disease (GVHD) following CBT, the incidence of National Institutes of Health (NIH) consensus criteria chronic GVHD has been low. These data support increased investigation of the use of CBT.
Introduction
Cord blood transplantation (CBT) is rapidly evolving, and the number of CBTs is increasing. Establishment of thresholds for infused cell doses and requirements for HLA matching have significantly decreased transplant related mortality (TRM), and the introduction of double unit transplants has improved engraftment rates and appears to have improved outcomes among adults and large children.1–5 Growing inventories of higher quality cord units should continue to result in improved outcomes. Reports of lower incidences of chronic graft-versus-host disease (GVHD) and more treatment responsive GVHD as compared to other donor sources further enhance the appeal of CBT.6–8 Relative delays in immune reconstitution and prolonged time to engraftment, however, remain important challenges.
In spite of improvements in TRM following CBT and hematopoietic cell transplantion (HCT) in general, disease relapse remains a prominent cause of death following allogeneic transplant. As non-transplant based therapies improve, the relapse risk of patients for whom HCT is indicated will likely increase, and strategies to reduce relapse are crucial. Multiple studies confirm the potency of the graft-versus-leukemia (GVL) effect following CBT. Numerous series including patients with heterogeneous status of disease at the time of transplant suggest a comparable if not lower risk of relapse following CBT as compared to HCT with other donor sources.7,9–14 A growing body of evidences suggests that, especially among patients with good disease control at the time of transplant, double unit CBT may be associated with particularly low relapse rates.3,15,16 To evaluate relapse rates following CBT, we conducted a matched cohort analysis comparing relapse rates and outcomes for patients with acute leukemias in morphologic complete remission (CR) at the time of transplant receiving myeloablative CBTs, matched unrelated donor (MURD) transplants, and mismatched unrelated donor (MMURD) transplants at our center.
Patients and methods
Patients
Between April 2006, when our current cord blood protocols opened, and June 2008, 31 consecutive patients underwent myeloablative CBT for acute leukemias in morphologic CR (n=29) or chronic myeloid leukemia not in blast crisis (n=2). Results were analyzed through December 2008. To provide cohorts of MURD and MMURD subjects that were as comparable as possible, one of each type of patient was selected from our center’s database for each CBT patient without knowledge of transplant outcome. Potential matched subjects were selected first on the basis of disease status including disease type and remission number, cytogenetic risk status, minimal residual disease status (MRD), time from diagnosis to first relapse (for patients beyond CR1), and use of imatinib for CML and Philadelphia chromosome positive ALL patients. MRD was defined as the presence of detectable disease by flow cytometry, cytogenetic analysis, or fluorescent in-situ hybridization (FISH) in patients with less than 5% morphologic marrow blasts. From strata of subjects matched on the above characteristics, final cohorts were then selected based on closest possible matching of age, date of transplant, and, for patients in CR1 at the time of transplant, time from diagnosis to transplant. All patients signed consent forms and this study was approved by the center’s Institutional Review Board. General patient details are summarized below and in Table 1. Details of individual matched pairs are summarized in Table 2.
Table 1.
Patient characteristics
| Cord | MMURD | MURD | |
|---|---|---|---|
| Number | 31 | 31 | 31 |
| Age | 22 (0.6–42) | 25 (1–48) | 25 (0.9–41) |
|
Disease |
AML n=17 ALL n=13 Biphenotypic n=1 CML n=2 |
AML n=18 ALL n=11 Biphenotypic n=2 CML n= 2 |
AML n=18 ALL n=13 Biphenotypic n=0 CML n=2 |
|
Donor Source |
Cord blood n=31 |
Bone marrow n=15 Peripheral blood n=16 |
Bone marrow n=6 Peripheral blood n=25 |
|
Matching |
4/6:4/6 n=13 4/6:5/6 n=5 5:6:5/6 n=7 6/6:6/6 n=1 5/6 n=4 |
9/10 n=28 8/10 n=3 |
10/10 n=31 |
|
Conditioning |
CY/TBI/FLU n=31 |
CY/TBI based n=21 BU/CY based n=10 |
CY/TBI based n=24 BU/CY based n=5 TREO/FLU n=2 |
Table 2.
Individual cases and matched pairs
| AML CR1/CML | ALLCR1 | AML/ALL beyond CR1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Age | Dz | Dz status | Transplant date | Age | Dz | Dz status | Transplant date | Age | Dz | Dz status | Days dx to relapse | Transplant date |
| Cord | 22 | AML | CR1 | 5/24/2007 | 0.6 | BALL | CR1 | 11/1/2006 | 30 | AML | CR2 | 456 | 10/18/2007 |
| MMURD | 25 | AML | CR1 | 9/9/2004 | 1 | BALL | CR1 | 11/1/2005 | 37 | AML | CR2 | 674 | 6/7/2005 |
| MURD | 26 | AML | CR1 | 9/7/2007 | 1 | BALL | CR1 | 8/24/2006 | 29 | AML | CR2 | 717 | 11/23/2004 |
| Cord | 11 | AML | CR1 | 11/17/2007 | 14 | BALL | CR1 | 10/5/2007 | 38 | AML | CR2 | 229 | 1/5/2007 |
| MMURD | 22 | AML | CR1 | 7/15/2004 | 8 | BALL | CR1 | 2/23/2008 | 48 | AML | CR2 | 244 | 12/21/2004 |
| MURD | 18 | AML | CR1 | 8/30/2006 | 13 | TALL | CR1 | 6/17/2006 | 40 | AML | CR2 | 313 | 5/19/2004 |
| Cord | 31 | AML | CR1 | 6/26/2006 | 22 | TALL | CR1 | 1/17/2008 | 29 | AML | CR2 | 305 | 6/23/2008 |
| MMURD | 44 | AML | CR1 | 7/13/2006 | 10 | TALL | CR1 | 10/8/2003 | 24 | AML | CR2 | 201 | 11/23/2002 |
| MURD | 29 | AML | CR1 | 6/16/2003 | 20 | BALL | CR1 | 10/12/2007 | 30 | AML | CR2 | 489 | 5/4/2005 |
| Cord | 20 | AML | CR1 | 10/20/2006 | 0.9 | BALL | CR1 | 5/16/2008 | 14 | AML | CR2 | 598 | 12/22/2006 |
| MMURD | 26 | AML | CR1 | 2/6/2004 | 2 | BALL | CR1 - MRD | 9/28/2006 | 25 | AML | CR2 | 456 | 5/14/2003 |
| MURD | 17 | AML | CR1 | 7/22/2003 | 0.9 | BALL | CR1 | 2/28/2003 | 17 | AML | CR2 | 670 | 9/12/2005 |
| Cord | 30 | AML | CR1 | 11/22/2006 | 38 | BALL | CR1 - MRD | 8/11/2006 | 42 | AML/MDS | CR2 | 531 | 10/9/2006 |
| MMURD | 30 | AML | CR1 | 8/31/2006 | 39 | Biphenotypic | CR1 | 7/26/2006 | 44 | AML | CR2 | 365 | 2/15/2003 |
| MURD | 30 | AML | CR1 | 1/6/2005 | 38 | BALL | CR1 - MRD | 6/27/2007 | 31 | AML | CR2 | 366 | 12/10/2004 |
| Cord | 3 | AML | CR1 | 4/21/2006 | 21 | BALL | CR1 | 4/17/2006 | 39 | AML | CR2-MRD | 391 | 10/3/2007 |
| MMURD | 8 | AML | CR1 | 10/18/2002 | 22 | Biphenotypic | CR1 | 6/11/2005 | 37 | AML | CR2-MRD | 306 | 7/9/2004 |
| MURD | 6 | AML | CR1 | 10/18/2005 | 25 | BALL | CR1 | 10/1/2005 | 39 | AML | CR2-MRD | 628 | 12/21/2002 |
| Cord | 42 | AML | CR1 | 7/27/2006 | 23 | ALLPh+ | CR1 - MRD | 10/30/2006 | 5 | BALL | CR3-MRD | 1038 | 12/18/2006 |
| MMURD | 45 | AML | CR1 | 12/6/2005 | 25 | ALLPh+ | CR1 - MRD | 3/9/2006 | 25 | BALL | CR3-MRD | 1492 | 4/13/2007 |
| MURD | 40 | AML | CR1 | 7/16/2007 | 37 | ALLPh+ | CR1 - MRD | 7/29/2003 | 8 | BALL | CR2-MRD | 1067 | 5/16/2007 |
| Cord | 26 | Biphenotypic | CR1 | 6/5/2008 | 28 | ALLPh+ | CR1 - MRD | 2/4/2008 | 2 | BALL | CR2-MRD | 214 | 6/18/2007 |
| MMURD | 26 | AML | CR1 | 2/7/2003 | 38 | ALLPh+ | CR1 - MRD | 9/7/2006 | 22 | BALL | CR2-MRD | 177 | 2/20/2004 |
| MURD | 21 | AML | CR1 | 5/2/2002 | 39 | ALLPh+ | CR1 - MRD | 6/3/2005 | 11 | BALL | CR2-MRD | 352 | 11/22/2007 |
| Cord | 10 | AML | CR1 - MRD | 3/5/2007 | 42 | ALLPh+ | CR1 | 6/16/2008 | 42 | TALL | CR2/aplastic | 426 | 6/11/2007 |
| MMURD | 26 | AML | CR1 - MRD | 2/1/2008 | 43 | ALLPh+ | CR1 | 8/18/2006 | 25 | TALL | CR2/aplastic | 396 | 1/14/2004 |
| MURD | 5 | AML | CR1 - MRD | 12/22/2003 | 41 | ALLPh+ | CR1 | 1/7/2005 | 40 | BALL | CR2 | 334 | 11/20/2001 |
| Cord | 13 | CML | CP2 s/p BC | 4/12/2007 | 11 | ALLPh+ | CR1 | 12/12/2006 | 15 | BALL | CR3-MRD | 1389 | 5/8/2008 |
| MMURD | 29 | CML | CP2 s/p BC | 12/14/2004 | 6 | ALLPh+ | CR1 | 3/22/2005 | 11 | BALL | CR3-MRD | 1127 | 8/7/2003 |
| MURD | 24 | CML | CP2 s/p BC | 5/2/2006 | 28 | ALLPh+ | CR1 | 10/20/2006 | 11 | BALL | CR3 | 2009 | 3/4/2003 |
| Cord | 24 | CML | CP1 | 7/20/2007 | |||||||||
| MMURD | 17 | CML | CP1 | 8/8/2003 | |||||||||
| MURD | 22 | CML | CP1 | 12/19/2003 | |||||||||
Among CBT patients, 27 patients received two units (six of whom had one of the two units CD34+ selected and ex vivo expanded) and four received single units. Matching was performed at low resolution for HLA A and B and high resolution (allele level) for HLA DRB1. Matching details are summarized in Table 1. For patients receiving CBT with unmanipulated units, single unit CBT was permitted for 6/6 units with total nucleated cell count (TNC) ≥ 3.0 × 107/ kg, 5/6 units with NC ≥ 4.0 × 107/ kg, and 4/6 units with TNC ≥ 6.0 × 107 /kg. If these thresholds were not met, double unit CBT was performed and each unit was required to have a TNC ≥ 1.5 × 107 /kg. For patients receiving an ex vivo expanded unit, the unmanipulated unit was required to have a TNC ≥ 2.5 × 107 /kg. Conditioning regimen for 30 patients was 120 mg/kg cyclophosphamide (CY), 75 mg/m2 fludarabine (FLU), and 13.2 Gy total body irradiation (TBI). One CBT patient received decreased CY dosing of 90 mg/kg because of a history of liver abnormalities and esophageal varices. For all CBT patients, GVHD prophylaxis was cyclosporine (CSP) and mycophenolate mofetil (MMF).
For unrelated donors, allele level matching was performed at HLA A, B, C, DRB1, and DQ. Among MURD patients, transplants occurred between November 2001 and November 2007 (median May 2005). Conditioning regimen were: 120 mg/kg CY and 12 Gy TBI (n=12); 120 mg/kg CY and 13.2 Gy TBI (n=9); 120 mg/kg CY and oral busulfan targeted (targeted BU) to 800–900 ng/ml (n=4); targeted CY and 12 Gy TBI (n=2); 42 gm/m2 treosulfan (TREO) and 150 mg/kg FLU (n=2); targeted BU, 120 mg/m2 FLU, and 5.5 mg/kg anti-thymocyte globulin (ATG) (n=1); 95 mg/kg CY and 12 Gy TBI (decreased CY for a patient with a history of liver abnormalities) (n=1). GVHD prophylaxis was CSP and methotrexate (MTX) (n=11), tacrolimus (TAC) and MTX (n=17), and TAC, sirolimus, MTX (n=3). Among MMURD patients, transplants occurred between October 2002 and February 2008 (median December 2004). Conditioning regimen were: 120 mg/kg CY and 12 Gy TBI (n=12); 120 mg/kg CY and 13.2 Gy TBI (n=7); 120 mg/kg CY and targeted BU (n=9); targeted CY and 12 Gy TBI (n=1); 120 mg/kg CY, targeted BU, and ATG 4.5 mg/kg (n=1); 60 mg/kg etoposide and 12 Gy TBI (etoposide substituted for CY for a patient with borderline ejection fraction) (n=1). GVHD prophylaxis was CSP and MTX (n=17), TAC and MTX (n=14).
Statistical analysis
Patient characteristics and follow-up times are summarized using standard measures. Cumulative incidence estimates of acute and chronic GVHD, relapse, and TRM were utilized, with death or relapse (for GVHD), TRM (for relapse), and relapse (for TRM) included as competing risk events.17 Relapse was defined as the presence of greater than five percent morphologic marrow blasts or biopsy proven extramedullary disease. Acute GVHD was graded according to established criteria.18 Chronic GVHD was graded according to the 2005 National Institutes of Health (NIH) consensus criteria.19 Kaplan-Meier estimates were used to evaluate overall survival (OS) and relapse free survival (RFS). Censoring for all time-to event outcomes occurred at the date of last contact. Estimates are reported at 2 years post-HCT since the maximum follow-up time for the cord blood group was 2.7 years and no deaths or relapses occurred after 2 years in any group. Associated 95% confidence intervals are reported for each incidence estimate. Cause-specific hazards are compared between groups using a log rank test, with 2-sided p-values considered significant at the 0.05 level.
Results
Matching
All patients were matched successfully for all disease criteria, except in the following three cases (Table 2): a CBT patient with ALL was in CR1 with MRD (0.05% blasts by flow cytometry) while the MMURD matched pair had no MRD, a CBT patient with ALL was in CR3 with MRD (0.01% blasts by flow cytometry and cytogenetic abnormalities) while the MURD matched pair had no MRD, and a MMURD ALL patient in CR1 had MRD (flow cytometry negative, cytogenetic positive) while the CBT patient had no MRD. For patients with acute leukemias in CR1 at the time of transplant, median time from diagnosis to transplant was 177 days for CBT patients (range 90–416), 151 days for MURD patients (range 106–300), and 186 days for MMURD patients (range 113–364). The six patients with either CML or Philadelphia chromosome positive ALL were all treated with imatinib prior to transplant. Four of six patients in the CBT, MURD, and MMURD cohorts were treated with imatinib post transplant. Median age difference was 3 years between CBT and MURD pairs and 5 years between CBT and MMURD pairs. Median follow-up for surviving CBT patients is 21.1 months (range 6.6–32.6) versus 35.1 months (range 12.4–75.8) for MURD patients and 29.1 months (range 7–65.1) for MMURD patients.
Relapse
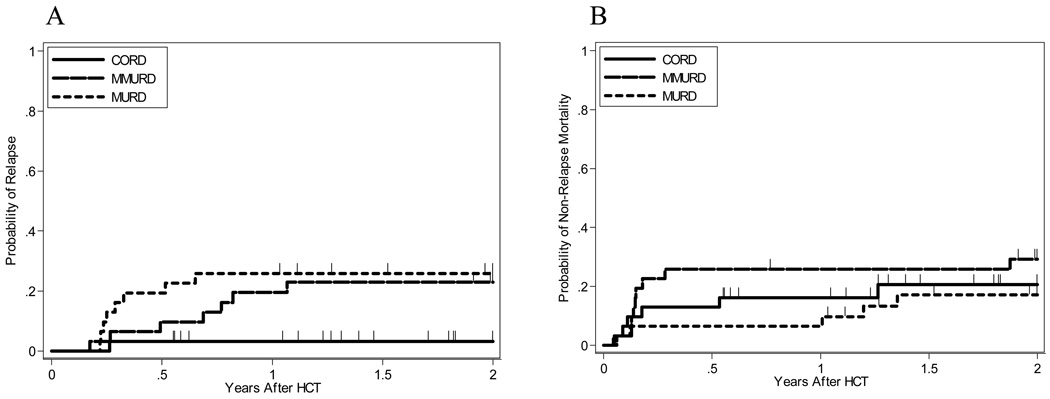
There has been one relapse among cord patients versus eight relapses among MURD patients and seven relapses among MMURD patients. Two year cumulative incidence of relapse is 3.2%, 95% CI [0.2–14.1%] for CBT patients versus 25.8%, 95% CI [12.1–41.8%] for MURD patients and 23%, 95% CI [10.1–38.9%] for MMURD patients (Table 3, Figure 1). Cause specific hazard for relapse is significantly decreased when comparing cord patients to MURD patients (p=0.018) and MMURD patients (p=0.019) by logrank test.
Table 3.
Relapse, TRM and Survival
| TnQTable2 | Two Year Survival (95% CI) | |
|---|---|---|
| Transplant Type | Overall Survival | Relapse-Free Survival |
| Cord Blood: | 74.5% (53.0%, 87.2%) | 76.2% (56.0%, 88.0%) |
| MMURD: | 50.0% (31.0%, 66.2%) | 47.8% (29.5%, 64.0%) |
| MURD: | 59.7% (39.8%, 74.9%) | 57.1% (37.7%, 72.5%) |
Figure 1. Cumulative incidence of relapse and transplant related mortality by cohort.
Relapse (A) and transplant related mortality (B).
Transplant related mortality
There have been six deaths due to TRM among CBT patients versus five among MURD patients and nine among MMURD patients. Causes of TRM among CBT patients were infection n=5 and GVHD n=1, among MURD patients were infection n=3, GVHD n=1, and regimen related toxicity (RRT) n=1, and among MMURD patients were infection n=3, GVHD n=3, RRT n=2, GVHD/infection n=1. Two year cumulative incidence of TRM is 20.6%, 95% CI [8.2–36.9%] for CBT patients versus 17%, 95% CI [6.2–32.5%] for MURD patients and 29.2%, 95% CI [14.6–45.5%] for MMURD patients (Table 3, Figure 1). Cause specific hazard for TRM is non-significant when comparing CBT patients to MURD patients (p=0.78) and MMURD patients (p=0.41) by logrank test.
Graft versus host disease
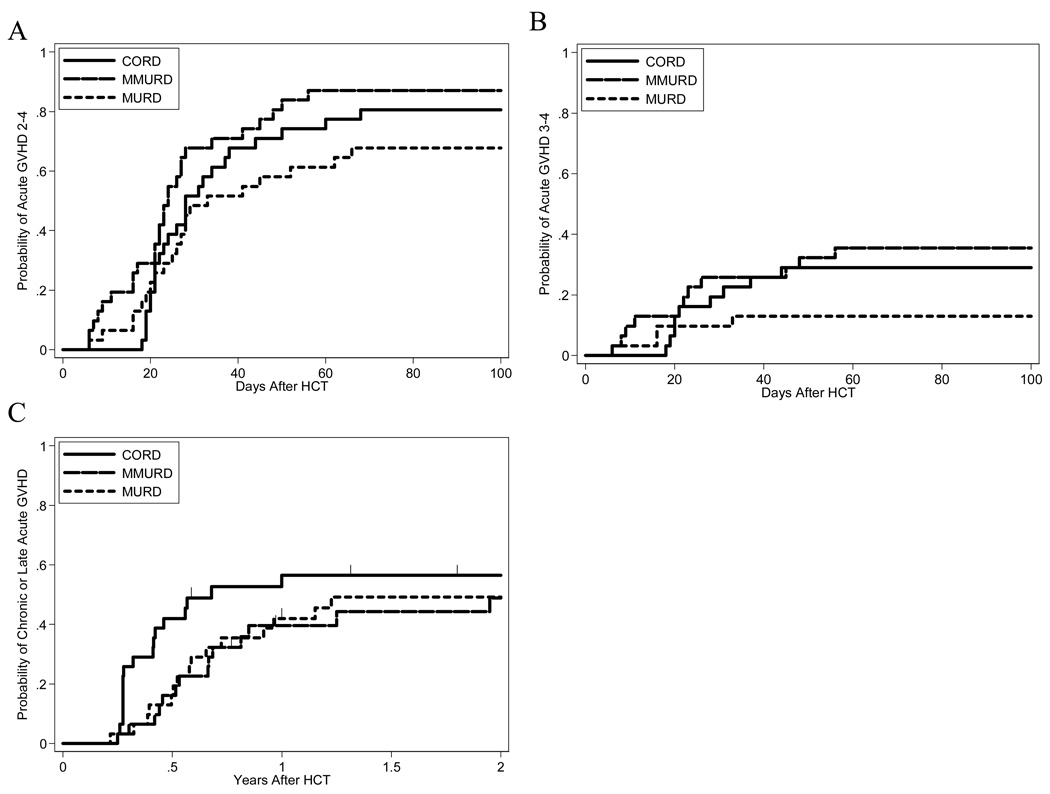
Cumulative incidence of grade II-IV acute GVHD through day 100 among CBT patients is 80.6%, 95% CI [61.9%– 90.8%] versus 67.7%, 95% [48.4%, 81.2%] for MURD patients and 87.1%, 95% CI [69.2%, 95.0%] for MMURD patients. Cumulative incidence of grade III-IV acute GVHD among CBT patients is 29.0%, 95% CI [14.5%–45.3%] versus 12.9%, 95% CI [4.1%–27.0%] for MURD patients and 35.5%, 95% CI [19.4% – 51.9%] for MMURD patients. Two year incidence of the composite endpoint of ≥ moderate chronic GVHD or grade II-IV late, persistent or relapsing acute GVHD, or overlap syndrome is 56.5%, 95% CI [37.0%, 72.1%] for CBT patients versus 49.2%, 95% CI [30.6%–65.3%] for MURD patients and 48.5%, 95% CI [29.8%–65.4%] for MMURD patients (Figure 2). Among CBT patients, only three patients with late GVHD developed ≥ moderate chronic GVHD; 14 patients had late, persistent, or relapsing acute GVHD. No cumulative incidences of GVHD are statistically significantly decreased or increased when comparing CBT patients to MURD or MMURD patients.
Figure 2. Cumulative incidence GVHD by cohort.
Grade II-IV GVHD through day 100 (A), grade III-IV GVHD through day 100 (B), and composite endpoint of ≥ moderate chronic GVHD or grade II-IV late, persistent, or relapsing acute GVHD (C).
Overall and relapse free survival
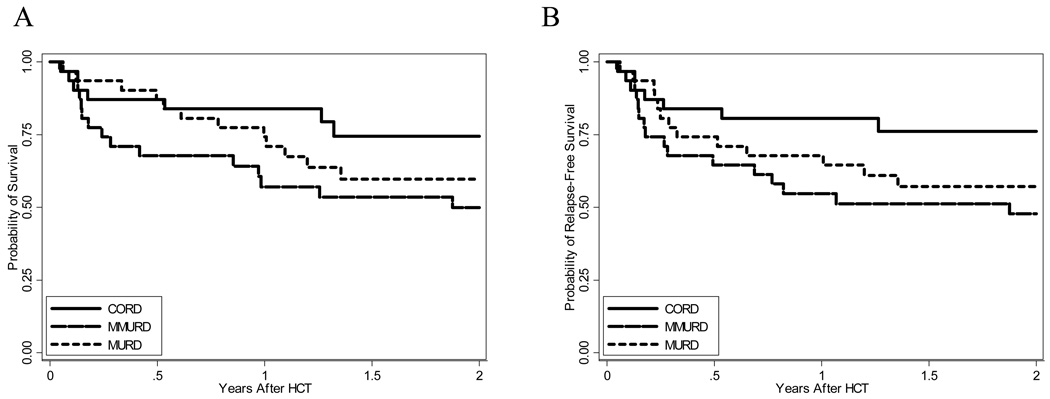
Two year OS and RFS for CBT patients are 74.5%, 95% CI [53–87.2%] and 76.2%, 95% CI [56–88%] versus 59.7%, 95% CI [39.8–74.9%] and 57.1, 95% CI [37.7–72.5%] for MURD patients and 50%, 95% CI [31–66.2%] and 47.8%, 95% CI [29.5–65%] for MMURD patients (Table 3, Figure 3). Cause specific hazard for OS and RFS are borderline significantly decreased when comparing CBT patients to MMURD patients (p=0.062 and 0.041 respectively) and non-significant when comparing CBT patients to MURD patients (p=0.27 and 0.17 respectively).
Figure 3. Overall and relapse free survival.
Kaplan-Meier estimates of overall survival (A) and relapse free survival (B).
Discussion
We have observed a low relapse rate following myeloablative CBT for patients with high risk acute leukemias or CML in morphologic CR at the time of transplant. A number of factors might contribute to this low relapse rate. The majority of patients in our study (n=22) underwent double unit CBT with two unmanipulated units and six additional patients underwent double unit CBT with one unmanipulated and one CD34+ selected and ex-vivo expanded unit. Clinical data suggest that for patients with good disease control at the time of transplant, double unit CBT may be associated with decreased relapse rates.3,15,16 The biologic mechanism underlying this observation is not characterized, but it may be a result of the immunologic interaction between the two units and the host and is an area of active investigation. Additionally, the majority of our CBT patients underwent transplant with multiply mismatched units; only one patient received 6/6 matched units. In the unrelated donor setting, higher degrees of mismatch are associated with lower relapse risk but increased TRM, and early data also support this observation in the CBT setting.10 It is uncertain whether a higher proportion of patients with better matched cord blood units would result in higher rates of relapse. Finally, our CBT conditioning regimen for adult patients contains 13.2 Gy TBI. Many TBI based myeloablative non-cord blood HCT conditioning regimens for adults, including those at our center, contain 12 Gy TBI. Fifteen patients in the MURD cohort and 14 patients in the MMURD cohort received only 12 Gy TBI. Higher TBI doses are associated with decreased relapse, though they have also been associated with increases in TRM.20,21
To compare this relapse rate to the relapse rate observed in comparable patients undergoing myeloablative MURD and MMURD transplants, we conducted a matched cohort analysis. Because details of the disease and disease status at the time of transplant are the most important predictors of relapse risk, we focused on matching patients very closely for these variables. Our center’s large database of MURD and MMURD transplants allowed us to match comparably aged patients who had undergone transplants in a comparable period and received similar supportive care. In the MURD cohort there were eight relapses and in the MMURD cohort there were seven relapses, in contrast to one relapse in the cord group. The lack of difference in relapse rates between the MURD and MMURD cohorts is likely attributable to the small sample size, but it is possible that unidentified risk factors for relapse in the MMURD cohort may contribute to this finding.
Though the small size of our series precludes definitive conclusions, the homogeneity of the cohorts is important. In larger series of CBT outcomes that have included analysis of relapse rates, many patients are not in morphologic remission at the time of transplant. Moreover, for larger series, disease risk is typically stratified into early (CR1), intermediate (≥CR2), and advanced (morphologic relapse).9,12,14,22 The advent of MRD monitoring and increasing refinement of cytogenetic and molecular risk markers for prognosis of acute leukemias allows increasingly sophisticated stratification of relapse risk assessment, and data supports the observation that poor risk features, even for patients in morphologic CR at the time of transplant, increase the chance of post transplant relapse.23–30 Though many reports comparing outcomes between single unit CBT and MURD or MMURD HCT find comparable relapse rates even when comparing patients in CR at the time of transplant, it is possible that bias exists. CBT is often reserved for patients with the highest risk disease and is frequently undertaken when transplant is felt to be urgent. These patients, though they may be in CR, may have disease features putting them at greater risk for relapse than patients undergoing URD transplant. Alternatively, however, investigators fearing less GVL effects with cord blood may reserve the technique for those at lower risk.
Importantly, non-relapse related outcomes were comparable between cohorts. Regarding GVHD, our observed high incidence of grade II-IV acute GVHD among CBT patients is consistent with recent reports suggesting that double unit CBT is associated with a higher incidence of acute GVHD than single unit CBT.4,5 In contrast to other reports regarding chronic GVHD, however, we have observed a relatively higher incidence of the composite endpoint of ≥ moderate chronic GVHD or grade II-IV late, persistent or relapsing acute GVHD, or overlap syndrome in our CBT patients. The majority of CBT patients experiencing this composite endpoint had either late, persistent, or relapsing acute GVHD; only three patients demonstrated features of ≥ moderate chronic GVHD. Few studies have graded chronic GVHD according to the 2005 NIH consensus criteria, and the extent to which late manifestations of GVHD among CBT patients may be different from late manifestations of GVHD in other donor settings requires further investigation. In addition, the prognostic significance of late, persistent or relapsing acute GVHD versus classic chronic GVHD is uncertain. Larger numbers and longer follow up will be needed to examine these issues. When comparing the incidence of late GVHD between the CBT, MURD, and MMURD cohorts in this study, it is important to note that the low incidence of the competing risk of relapse among CBT patients may have resulted in an increased cumulative incidence of late GVHD relative to the MURD and MMURD cohorts.
With respect to TRM, all patients were comparably aged and underwent myeloablative conditioning, indicating good performance status and limited comorbidities and decreasing the likelihood of selection bias affecting TRM. While delayed engraftment and immune reconstitution remain important issues following CBT and CBT patients frequently require intensive supportive care in the early post-transplant period, we did not observe excess TRM in our small cohort of CBT patients.
Our data suggest that patients undergoing myeloablative CBT for high risk acute leukemias in morphologic CR have low relapse rates with acceptable TRM. The use of double unit transplant may contribute to these results. These data suggest that consideration should be given to proceeding rapidly to transplant with CBT for patients with high risk malignancies while disease status is under good control. While our series is small, and these findings may not hold true for patients in relapse at the time of transplant or for older or infirm patients unable to tolerate myeloablative conditioning, they do support increased investigation of CBT. Larger numbers and longer follow-up will be necessary to confirm these observations.
Acknowledgements
The authors Denise Ziegler and Judy Schramm for their assistance in care of the patients.
This study was supported by NIH Grant T32 CA 009515-24
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein C, Weisdorf D, DeFor T, et al. Favorable Leukemia-Free Survival (LFS) for Adults and Children Undergoing Myeloablative Umbilical Cord Blood (UCB) Transplantation with Cyclophosphamide (CY), Fludarabine (FLU) and Total Body Irradiation (TBI): A Single Center Analysis of 194 Patients. Blood. 2008;112:A1957. [Google Scholar]
- 4.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora M, Nagaraj S, Wagner JE, et al. Chronic graft-versus-host disease (cGVHD) following unrelated donor hematopoietic stem cell transplantation (HSCT): higher response rate in recipients of unrelated donor (URD) umbilical cord blood (UCB) Biol Blood Marrow Transplant. 2007;13:1145–1152. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 8.Barker JN, Davies SM, DeFor T, et al. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 9.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 11.Hwang WY, Samuel M, Tan D, et al. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444–453. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Ooi J, Tomonari A, Konuma T, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Defor TE, Brunstein C, et al. Allogeneic hematopoietic stem cell transplantation in adult acute lymphocytic leukemia: impact of donor source on survival. Biol Blood Marrow Transplant. 2008;14:1394–1400. doi: 10.1016/j.bbmt.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 15.Verneris MR, Brunstein C, DeFor TE, et al. Risk of Relapse after Umbilical Cord Blood Transplantation (UCBT) in Patients with Acute Leukemia: Marked Reduction in Recipients of Two Units. Blood. 2005;106:A305. [Google Scholar]
- 16.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of Risk Factors for Outcomes After Unrelated Cord Blood Transplantation in Adults With Lymphoid Malignancies: A Study by the Eurocord-Netcord and Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Onc. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. NewYork: John Wiley; pp. 168–171. [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host-disease: 1. Diagnosis and Staging Working Group Report. Bio Blood and Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 21.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77:1660–1665. [PubMed] [Google Scholar]
- 22.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagel JM, Gooley T, Estey E, et al. Impact of Pre-Transplant Minimal Residual Disease Assessed by Flow Cytometry on Outcome Following Myeloablative Hematopoietic Cell Transplantation for Patients with AML-CR1. Blood. 2008;112:A3253. [Google Scholar]
- 24.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallman MS, Dewald GW, Gandham S, et al. Impact of cytogenetics on outcome of matched unrelated donor hematopoietic stem cell transplantation for acute myeloid leukemia in first or second complete remission. Blood. 2007;110:409–417. doi: 10.1182/blood-2006-10-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 27.Paganin M, Zecca M, Fabbri G, et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed "high-risk" acute lymphoblastic leukemia. Leukemia. 2008;22:2193–2200. doi: 10.1038/leu.2008.227. [DOI] [PubMed] [Google Scholar]
- 28.Schetelig J, Bornhäuser M, Schmid C, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 29.Cornelissen JJ, van der Holt B, Verhoef GE, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. 2008 Nov 14; doi: 10.1182/blood-2008-07-168625. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2008 Dec 4; doi: 10.1182/blood-2008-07-163212. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]