Abstract
BK virus (BKV) is an important pathogen and cause of nephropathy in renal transplant recipients, but its significance following hematopoetic stem cell transplantation (HSCT) is less well described. We measured blood and urine BKV in 124 allogeneic HSCT patients (67 had undergone prior HSCT [surveillance cohort]; 57 were monitored from transplant day 0 [prospective cohort]). BK viruria was manifest in 64.8% of the patients; 16.9% developed viremia. In the prospective cohort, the median time from transplantation to BK viremia development (128 days) was longer than for viruria (24 days; P < 0.0001). Among clinical factors (gender, disease, transplant type, alemtuzumab use, CMV viremia, GVHD, donor HLA C7 allele), only CMV viremia was more common in patients with BKV infection (P ≤ 0.04). There was a direct relationship between blood and urine BKV levels and the occurrence, and degree, of hematuria (P ≤ 0.03). Finally, BKV infection was analyzed along with other clinical factors in relation to the development of post-HSCT renal impairment. On multivariate analysis, only BK viremia (P = 0.000002) and alternative-donor transplantation (P = 0.002) were independent predictors of development of post-HSCT renal impairment, with BK viremia associated with a median 1.62 mg/dL rise in creatinine from the pre-transplant baseline. Among eight patients in the surveillance cohort with BK viremia, two developed biopsy-proven BKV nephropathy requiring hemodialysis. Investigation of whether prophylaxis against, or treatment of, BKV in the post-HSCT setting mitigates the associated morbidities, especially kidney injury, warrants prospective evaluation.
INTRODUCTION
BK virus (BKV), a human polyomavirus1, was first recognized in 1971 after it was isolated from the urine of a Sudanese renal transplant patient who was hospitalized with acute renal failure and ureteral stenosis2. Most people are exposed to BK virus during childhood, and 75–80% of adults have antibodies3. Primary infection occurs at 4–5 years of age and manifests as a subclinical or nonspecific “flu-like” illness4,5. BKV then establishes latency primarily in the genitourinary tract4,6, and viral reactivation generally occurs in immunocompromised patients1,4.
BKV is an important pathogen and cause of nephropathy in recipients of renal transplants. Kidney transplant patients can become infected through reactivation of latent virus, through primary infection transmitted from the donor organ, or via blood transfusion3. Importantly, BKV infection can transition and escalate from viruria to viremia to nephropathy7. BKV nephropathy (BKN) begins as localized viral presence in the tubular epithelial cells of the kidney and progresses to a diffuse and destructive T cell-mediated interstitial nephritis8. The interstitial infiltrate can cause scarring of the kidney parenchyma, loss of function, and kidney failure. Since 1995, an increase in BKN from 1% in 1995 to 5% in 2001 has been observed in renal transplant patients9.
In patients receiving hematopoetic stem cell transplant (HSCT), the presence of BK viruria has long been appreciated10, and BKV has been implicated in the pathogenesis of post-transplant hemorrhagic cystitis (HC)10,11, although its exact role in that situation remains unclear12. HC with documented BK viruria is commonly treated with intravenous cidofovir, a drug with considerable nephrotoxic and myelosuppressive side-effects13. Therefore, a better understanding of the clinical importance of BK viruria in the post-HSCT setting is needed. Furthermore, while BKN has been documented in native kidneys5 and has recently been described in stem cell transplant patients14,15, the prevalence and clinical importance of BK viremia and BKN in HSCT patients have not been previously described. We hypothesized that BKV infection is an important and under-appreciated pathologic phenomenon associated with hematuria and the development of BKN in the post-HSCT setting. For a period of 16 months, we systematically monitored BKV infection in all allogeneic HSCT recipients in order to better understand the potentially important relationships between BK viruria, BK viremia, hematuria, and BKN in these patients.
MATERIALS AND METHODS
Study Participants
Beginning in September 2006, our transplant program implemented routine monitoring of BKV in the urine and blood of all adult patients who had already undergone, or who were undergoing, an allogeneic HSCT at the University of Chicago hospitals. All living transplant patients who presented for medical care, either as inpatients or outpatients, anytime after day 0 (their date of transplant), were monitored. Patients were enrolled into the study when their first BKV level (either urine or blood) was drawn. Data collection ended on December 31, 2007. One hundred twenty-four patients were monitored. Sixty-seven had undergone transplant prior to September 2006 (the surveillance cohort); 57 underwent transplant between September 2006 and December 2007 and were monitored from the time of transplant (the prospective cohort). The data were analyzed in the spring of 2008. Institutional Review Board approval was received for analysis of the data.
Monitoring Methods and Data Included for Analysis
At any presentation to the University of Chicago Transplant Program for medical care during the period of study monitoring, HSCT patients submitted blood and urine samples for BKV detection and quantification. The frequency of sampling depended on how often the patient was seen. Typically, during a single inpatient stay, only one set of BKV samples (blood and urine) was collected, unless the patient had new hematuria later in the hospital stay. Blood and urine BKV samples were also collected at every outpatient visit. Patients were typically seen at least weekly until day 100 after transplant, at least every month until six months after transplant, at least six times between six months and 24 months after transplant, and at least once per year thereafter. The clinical data that were routinely captured from each inpatient or outpatient visit, when available, included: graft-versus-host disease (GVHD) assessment, microscopic urinalysis (U/A) results to measure urinary red blood cell (RBC) count, serum creatinine, blood tacrolimus level, and blood cytomegalovirus (CMV) status. Supportive care and infection prophylaxis at the University of Chicago transplant center have been previously described16.
BKV Polymerase Chain Reaction (PCR) Analysis
Quantitative PCR was performed at the University of Chicago hospital laboratories on blood and urine samples to detect BKV DNA. Samples were placed in lysis buffer and stored at −70°C until processed. Using 200 μL aliquots of whole blood (in EDTA) or urine, DNA extraction was performed on the Roche MagNA Pure instrument (Roche Diagnostics, Indianapolis, IN, USA), using the Roche Total NA kit, eluted into a final volume of 50 μL. In each extraction run, two different known concentrations of positive control containing target DNA were processed as well as a negative control containing bacterial DNA.
Master mix was prepared using the Roche FastStart Plus kit and primers and probes were purchased from TIB MolBiol (Berlin, Germany). We used a multiplex assay for BK and JC viruses. The BKV target is the gene for large T antigen. To 5 μLof patient eluate was added 15 μLof master mix, and the samples were run on a Roche LightCycler 1.2. The protocol included 45 cycles of PCR. Five dilutions of a stock solution of cloned target DNA were used to generate a standard curve to determine the absolute quantification of DNA present in positive samples. Based on the standard curve, quantification was reported in a range from 2,500 – 25,000,000 copies/mL of patient sample; positives outside the range are reported as > 25,000,000 or < 2,500. The detection limit for the assay was validated as equivalent to 500 copies/mL of patient sample (10 copies/reaction). For positive samples, melting curve analysis was performed to verify the amplified product. An internal control was added to confirm negative samples.
Statistical Methods
The following variables were evaluated for their association with BK viruria and BK viremia: recipient gender, donor type, diagnosis, presence or absence of GVHD, use of alemtuzumab in the conditioning regimen, presence of CMV viremia at any time during the monitoring period, and donor HLA C7 allele status. Donor HLA C7 allele status was included based upon evidence in renal transplant patients that absence of the HLA C7 allele in donor is associated with development of BK viremia in the recipient17.
To evaluate the potential effect of BK viruria or BK viremia on hematuria, hematuria was defined as absent, trivial (< 3 RBC/high power field [hpf] on urinalysis), or clinically significant (≥ 3 RBC/hpf). For each patient, correlation between hematuria and the median as well as the maximum level of BKV in the urine was interrogated. The median level of BKV was the median of all samples obtained in an individual patient. The maximum concentration was the highest level measured in each individual patient. We also evaluated the correlation between presence of BK viremia at any time post-transplant and the occurrence of hematuria.
To evaluate the potential effect of BK viruria or viremia on kidney function, we measured the creatinine increment in each patient. This was defined as the difference between the serum creatinine at the time of transplant and the highest creatinine observed during the time of monitoring. We then evaluated the association between creatinine increment and the following factors: recipient gender, diagnosis, donor type, presence of acute or chronic GVHD at any time during the monitoring period, use of alemtuzumab in the conditioning regimen, presence of CMV viremia at any time during the monitoring period, presence of hematuria at any time during the monitoring period, presence of BK viruria at any time during the monitoring period, presence of BK viremia at any time during the monitoring period, and donor HLA C7 allele status. Post-HSCT tacrolimus levels and their potential impact on creatinine changes were also considered in the analysis of each clinical variable’s effect.
Univariate statistical comparisons between patients based on the clinical factors of interest were analyzed using the student t-test. All P values are two-sided. The multivariate analysis of predictors of renal function decrement used multiple regression, with stepwise backward-elimination variable selection18. All covariates that were significant with a P value < 0.05 in the univariate analysis were retained in an initial regression model. (Because all patients with BK viremia also had BK viruria, and to determine whether BK viruria itself was an independent predictor, BK viruria in the absence of BK viremia was incorporated as its own covariate in the regression model, and BK viremia was incorporated as a separate covariate). Factors not statistically significant (P ≥ 0.05) were removed from the model one at a time, with re-estimation of all model variables after each step. Variable elimination (or re-insertion) was stopped when all remaining factors were significant at P < 0.05. Because of multiple comparisons in the multivariate analysis, we considered only P values < 0.01 as statistically significant.
RESULTS
Patient Characteristics
The clinical characteristics of the entire study cohort as well as the prospective cohort are summarized in Table 1. The entire cohort included patients undergoing HSCT as early as 1998, although the large majority was transplanted after 2002. The median age of participants at the time of their transplant was 49 years, and 61.3% of patients were males. Many diagnoses were included, but the predominant underlying diseases were acute myelogenous leukemia (AML) in 40.3% and non-Hodgkin’s lymphoma in 21.8%. The majority of patients (n = 70, 56.5%) received cells from an HLA-identical donor, defined as a matched-related (n = 66) or syngeneic donor (n = 4); the remainder received alternative-donor transplants, defined as matched-unrelated (n = 36), mismatched-related (n = 2), mismatched-unrelated (n = 6), cord (n = 4), or haploidentical/cord (n = 6) donors. Most patients (n = 90, 72.6%) received conditioning regimens that included alemtuzumab, which is routinely used at our transplant center. Some form of GVHD (acute and/or chronic) was diagnosed in 67 patients (54.0%), with 40 patients (32.0%) manifesting acute GVHD (grade II–IV). In our study, 61 patients had donors lacking the HLA C7 allele (while in another 18/124 patients the donor HLA C7 status was unknown; one additional patient received a haploidentical/cord transplant in which only one of the donor sources lacked HLA C7). CMV viremia was detected in 26/122 patients (21.3%) during the monitoring period.
Table 1.
Clinical and transplant characteristics of the 124 patients and of the prospective cohort.
| Patient Demographics | Entire Study Group | Prospective Cohort |
|---|---|---|
| Number of patients | 124 | 57 |
| Median age (range) | 49 (17–72) | 52 (18–71) |
| Male | 76 (61.3%) | 38 (66.7%) |
| Female | 48 (38.7%) | 19 (33.3%) |
| Year of Transplant | ||
| 1998–2002 | 14 (11.3%) | 0 (0.0%) |
| 2003-August 2006 | 54 (43.5%) | 4 (7.0%) |
| September 2006-December 2007 | 56 (45.2%) | 53 (93.0%) |
| Transplant Donor | ||
| HLA-identical | 70 (56.5%) | 29 (50.9%) |
| Matched-related | 66 (53.2%) | 27 (47.4%) |
| Syngeneic | 4 (3.2%) | 2 (3.5%) |
| Alternative | 54 (43.5%) | 28 (49.1%) |
| Matched-unrelated | 36 (20.0%) | 18 (31.6%) |
| Mismatched-related | 2 (1.6%) | 0 (0.0%) |
| Mismatched-unrelated | 6 (4.8%) | 4 (7.0%) |
| Cord | 10 (8.0%) | 6 (10.5%) |
| Primary Disease | ||
| CML | 8 (6.5%) | 1 (1.8%) |
| CLL | 5 (4.0%) | 2 (3.5%) |
| AML | 50 (40.3%) | 25 (43.8%) |
| ALL | 8 (6.5%) | 2 (3.5%) |
| MDS | 9 (7.3%) | 4 (7.0%) |
| Aplastic anemia | 3 (2.4 %) | 1 (1.8%) |
| Non-Hodgkin’s lymphoma | 27 (21.8%) | 16 (28.0%) |
| Hodgkin’s lymphoma | 5 (4.0%) | 3 (5.3%) |
| Multiple myeloma | 3 (2.4%) | 1 (1.8%) |
| Amyloidosis | 1 (0.8%) | 1 (1.8%) |
| Waldenstrom’s | 1 (0.8%) | 0 (0.0%) |
| Myelofibrosis | 1 (0.8%) | 0 (0.0%) |
| Pure red cell aplasia | 1 (0.8%) | 0 (0.0%) |
| PNH | 1 (0.8%) | 0 (0.0%) |
| Plasmacytoid dentritic cell tumor | 1 (0.8%) | 1 (1.8%) |
| Conditioning Regimen | ||
| Fludarabine/melphalan/alemtuz | 62 (50.0%) | 26 (45.6%) |
| Fludarabine/busulfan/alemtuz | 21 (16.9%) | 13 (22.8%) |
| TBI/etoposide | 9 (7.2%) | 2 (3.5%) |
| Clofarabine/melphalan/alemtuz | 8 (6.5%) | 6 (10.5%) |
| Fludarabine/melphalan/ATG | 5 (4.0%) | 2 (3.5%) |
| TBI/fludarabine/thiotepa/ATG | 4 (3.2%) | 3 (5.3%) |
| TBI/cyclophosphamide | 4 (3.2%) | 0 (0%) |
| TBI/fludarabine/thiotepa | 3 (2.4%) | 2 (3.5%) |
| Fludarabine/cyclophosphamide | 3 (2.4%) | 1 (1.8%) |
| BEAM | 1 (0.8%) | 0 (0%) |
| Busulfan/cyclophosphamide | 1 (0.8%) | 0 (0%) |
| Melphalan | 1 (0.8%) | 1 (1.8%) |
| TBI/etoposide/alemtuz | 1 (0.8%) | 1 (1.8%) |
| TBI/fludarabine | 1 (0.8%) | 0 (0%) |
| Graft Versus Host Disease | ||
| Acute graft verus host disease, grade II–IV | 40 (32.0%) | 14 (24.6%) |
| Any graft versus host disease | 67 (54.0%) | 23 (40.4%) |
| CMV Viremia | ||
| CMV viremia at any time post transplant | 26 (21.3%) | 16 (28.6%) |
The clinical characteristics of the 57 patients enrolled from day 0 of their transplant (the prospective cohort) were not statistically different from those of the entire study group. CML = chronic myelogenous leukemia; CLL = chronic lymphocytic leukemia; AML = acute myelogenous leukemia; ALL = acute lymphocytic leukemia; MDS = myelodysplastic syndrome; PNH = paroxysmal nocturnal hemoglobinuria; TBI = total body irradiation; alemtuz = alemtuzumab; ATG = anti-thymocyte globulin; BEAM = carmustine, etoposide, cytarabine, and melphalan.
The clinical characteristics of the 57 patients enrolled from day 0 of their transplant (the prospective cohort) were reflective of (and not statistically different from) the entire study group with respect to age, gender, predominant underlying diseases, transplant type, use of alemtuzumab, GVHD, donor HLA C7 allele status, and CMV viremia prevalence (see Table 1).
Prevalence and Timing of Onset of BKV Infection
In the entire cohort, the median time after HSCT to first virus sampling was 58 days (range: 1 – 3196 days), and the median time to last follow-up virus sampling was 454 days (range: 3 – 3554 days). Seventy-nine patients (64.8%) had BK viruria at some time during monitoring post-HSCT. The remainder (43/122 = 35.3%) never had BKV detected in the urine during post-HSCT follow-up. Two patients did not have urinary BKV measured post-HSCT (both died shortly after transplant, precluding obtainment of samples; neither had oliguria/anuria nor renal failure). Twenty-one patients (16.9%) had BK viremia detected at some time during monitoring. The remainder (103/124 = 83.1%) never demonstrated BKV in the blood post-HSCT.
Approximately one-quarter of all patients were persistently positive for BKV in the urine, while most others fluctuated between exhibiting low-level positive and negative urine samples with a usual duration of positivity of approximately one month (Table 2). In contrast, when BK viremia occurred it was almost always at low levels and was transient or intermittent. Only two patients had persistent BK viremia for the duration of the observation period.
Table 2.
Measure of the degree to which BKV infection status changes for individual patients over time in the post-HSCT period.
| n | Median BKV Level (copies/mL) | Median Duration of Observation (days) | Median Duration of Positivity (days) | Median No. of Samples Tested | |
|---|---|---|---|---|---|
| BK Viruria | |||||
| Persistently Positive | 27 | 25,000,000 (13,500–25,000,000) | 157 (16–532) | 157 (16–532) | 11 (2–44) |
| Variable | 52 | 2,500 (0–25,000,000) | 322 (1–441) | 32 (1–411) | 11 (1–47) |
| Persistently Negative | 43 | 0 (0–0) | 188 (1–429) | 0 (0–0) | 5 (1–21) |
| BK Viremia | |||||
| Persistently Positive | 2 | 1,147,250 (228,000–2,066,500) | 286 (194–378) | 286 (194–378) | 22 (10–35) |
| Variable | 19 | 0 (0–133,000) | 220 (60–427) | 1 (1–42) | 17 (5–37) |
| Persistently Negative | 103 | 0 (0–0) | 213 (1–532) | 0 (0–0) | 9 (1–47) |
Approximately one-quarter of all patients were persistently positive for BKV in the urine, while most fluctuated between exhibiting low-level positive and negative urine samples with a usual duration of positivity of approximately one month. In contrast, when BK viremia occurred it was almost always at low levels and was transient or intermittent. Only two patients had persistent BK viremia for the duration of the observation period. NOTES: Persistently positive patients were those who had two or more samples collected, and all samples were positive. Variable patients produced both positive and negative samples during the observation period, or they produced one positive sample and then had no other samples drawn. Persistently negative patients never exhibited a positive sample. In patients who had more than one period of viral positivity, the longest period was used to calculate the cohort median. Two patients did not have urinary BKV measured post-HSCT.
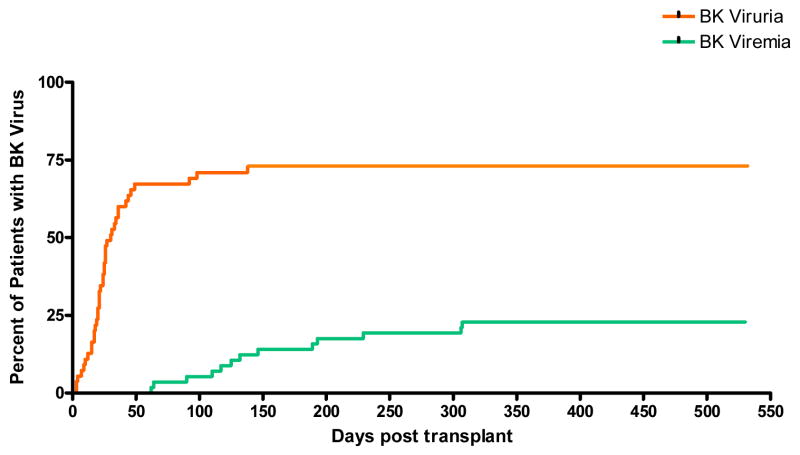
In the prospective cohort, the median time to first virus sampling was 20 days (range: 1 – 34 days), and the median time to last virus sampling was 146 days (range: 3 – 454 days). Among these patients, 40 (72.7%) developed BK viruria and 13 (22.8%) developed BK viremia. In this group the median time from transplantation to development of BK viruria was 24 days (range: 3 – 138 days), while BK viremia occurred at a significantly longer median time of 128 days (range: 62 – 307 days) (P < 0.0001). The cumulative incidence and timing of development of BK viruria and viremia for the prospective cohort are depicted in Figure 1.
Figure 1.
Cumulative incidence and timing of development of BK viruria and viremia for the prospectively-monitored cohort (n = 57).
Clinical Factors Impacting BKV Infection
Since previous studies have suggested that increased age is associated with an increased prevalence of BK viruria, at least in immunocompetent populations19,20, we analyzed the median age of our patients who had BK viruria and compared it to the median age of our patients without urinary BKV. The median age of the two groups was not statistically different (BK viruria group = 48 years, no urinary BKV group = 52 years, P = 0.07).
In a univariate analysis of all of the clinical factors examined, only CMV viremia was significantly associated with an increased risk of also having BK viruria (P = 0.001) and BK viremia (P = 0.04) (Table 3). Notably, BK viruria and viremia were not more prevalent in patients receiving alemtuzumab.
Table 3.
Association between various clinical factors and prevalence of BK viruria and viremia.
| Clinical Factor | BK Viruria | BK Viremia | ||
|---|---|---|---|---|
| n (%) | P | n (%) | P | |
| Females | 28/46 (61%) | 0.48 | 12/48 (25%) | 0.06 |
| Males | 51/76 (67%) | 9/76 (12%) | ||
| HLA identical donor | 41/71 (58%) | 0.09 | 8/71 (11%) | 0.06 |
| Alternative donor | 38/51 (75%) | 13/53 (25%) | ||
| CML | 6/8 (75%) | 0.77 | 2/8 (25%) | 0.53 |
| CLL | 2/5 (40%) | 0.12 | 0/5 (0%) | 0.30 |
| AML | 30/49 (61%) | 0.50 | 9/50 (18%) | 0.79 |
| ALL | 7/8 (88%) | 0.27 | 2/8 (25%) | 0.53 |
| MDS | 6/9 (67%) | 0.90 | 1/9 (11%) | 0.63 |
| AA | 2/3 (67%) | NT | 0/3 (0%) | NT |
| NHL | 20/26 (77%) | 0.14 | 5/27 (19%) | 0.80 |
| HD | 4/5 (80%) | 0.47 | 1/5 (20%) | 0.85 |
| MM | 1/3 (33%) | NT | 0/3 (0%) | NT |
| Alemtuzumab | 61/90 (68%) | 0.24 | 16/90 (18%) | 0.68 |
| No alemtuzumab | 18/34 (53%) | 5/34 (15%) | ||
| GVHD | 34/67 (51%) | 0.72 | 8/67 (12%) | 0.47 |
| No GVHD | 45/57 (79%) | 13/57 (23%) | ||
| Donor HLA C7 positive* | 32/44 (73%) | 0.41 | 9/44 (20%) | 0.75 |
| Donor HLA C7 negative* | 42/61 (69%) | 11/61 (18%) | ||
| CMV viremia† | 24/26 (92%) | 0.001 | 8/26 (31%) | 0.04 |
| No CMV viremia† | 5/96 (5%) | 13/96 (14%) | ||
Only occurrence of CMV viremia at any time post-transplant was significantly associated with an increased risk of also having BK viruria (P = 0.001) and BK viremia (P = 0.04). Within disease sub-categories, P values reflect the comparison between patients with the listed disease and all other patients in the study. For two patients in the study, urine samples for BK were not obtained, thus the n denominator for some clinical factors is different between the BK viruria and BK viremia columns.
For donor HLA C7 allele status, information was not available for 18 patients, and one patient received a haploidentical/cord transplant in which only one of the donor sources was positive for the HLAC7 allele--this patient was not included in the above table.
For presence of CMV viremia, two patients did not have blood CMV samples drawn. CML = chronic myelogenous leukemia; CLL = chronic lymphocytic leukemia; AML = acute myelogenous leukemia; ALL = acute lymphocytic leukemia; MDS = myelodysplastic syndrome; AA = aplastic anemia; NHL = non-Hodgkin’s lymphoma; HD = Hodgkin’s disease; MM = multiple myeloma. NT = not tested.
Relationship Between BKV Infection and Hematuria
One hundred twenty of the 124 study patients had at least one U/A performed. Only 19 patients (15.8%) never demonstrated microscopic hematuria at any time (“no hematuria group”). Most patients with microscopic hematuria had trivial hematuria (n = 84/101). Seventeen patients had clinical hematuria.
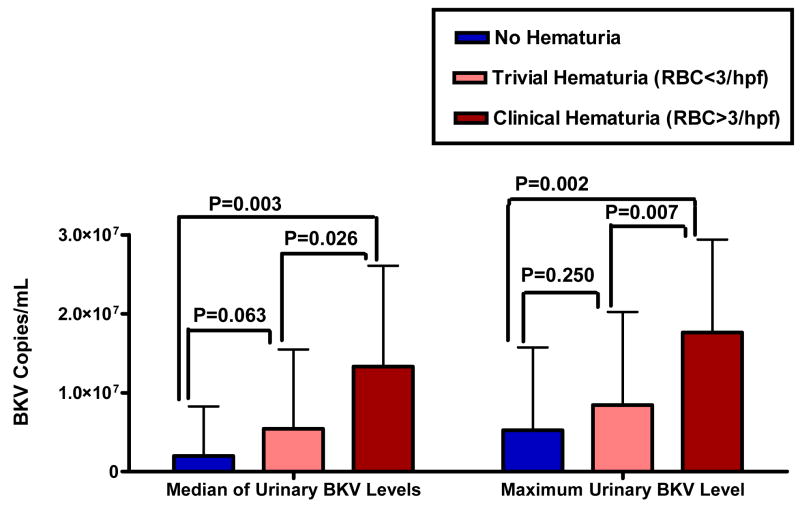
The dichotomous presence or absence of BK viruria in the post-HSCT period was not a predictor of development of hematuria, since there was no difference in the prevalence of BK viruria in those who demonstrated hematuria compared to those who did not. There was however a significant association between the median amount of BK viruria and the degree of hematuria. The average of all patients’ median BK viruria levels was 1.99(106) copies/mL in the “no hematuria” group, lower than the average of the median BK viruria levels in the “trivial hematuria” group (5.44(106) copies/mL, P = 0.06), which in turn was significantly lower than the average of the median BK viruria levels in the “clinical hematuria” group (13.32(106) copies/mL, P = 0.03) (Figure 2). This suggests that there is a direct relationship between the median amount of urinary BKV in the post-HSCT period and both the presence, and the degree, of microscopic hematuria.
Figure 2. Relationship between both median (LEFT PANEL) and maximum (RIGHT PANEL) levels of urinary BKV and degree of microscopic hematuria post-HSCT.
A direct relationship was seen between higher median and maximum levels of urinary BKV with greater amounts of microscopic hematuria. RBC = red blood cells.
The group with no hematuria had an average maximum urinary BKV level of 5.27(106) copies/mL, which was not significantly different than that of those with trivial hematuria (8.44(106) copies/mL, P = 0.25). Yet, both groups’ levels were significantly lower than the maximum BK viruria levels of those with clinical hematuria (17.65(106) copies/mL, P < 0.007) (Figure 2). These data suggest that maximum BKV urinary load, even as measured by one U/A, is directly associated with both the occurrence, and the degree, of hematuria.
We also examined whether BK viremia was associated with hematuria. In our study, interestingly, all 19 patients without hematuria were BK blood negative, while 21 of 101 patients with hematuria were BK blood positive (P = 0.03).
Impact of BKV Infection on Renal Function
Nearly all transplant patients were observed to incur a decrement in renal function simply by undergoing HSCT. The median baseline (day 0) serum creatinine value for the entire cohort was 0.80 mg/dL. This was significantly lower than the median of the peak post-HSCT creatinine values for the cohort, 1.30 mg/dL (P < 10−12). Of the 124 patients, only 12 did not have higher maximal post-HSCT creatinine values compared to their day 0 creatinine baselines.
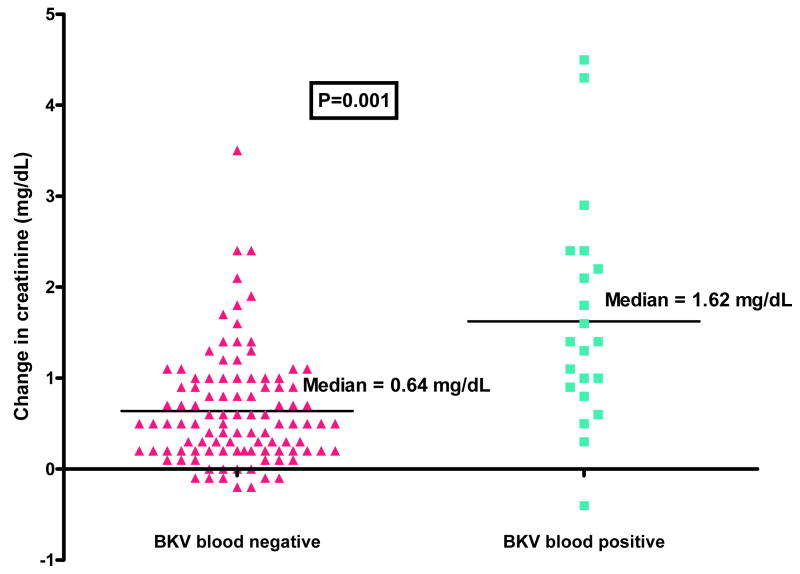
On univariate analysis, six clinical factors were associated with a concomitant decrement in renal function: female gender; having undergone an alternative-donor transplant; having an underlying disease which was not myelodysplastic syndrome; having CMV viremia; having BK viruria; and having BK viremia (Table 4). Median post-HSCT tacrolimus levels for patients with these characteristics were not significantly higher and did not explain any of these associations. On multivariate analysis, only two factors were independently associated with a significant change in creatinine: undergoing an alternative-donor transplant (P = 0.002) and having BK viremia (P = 0.000002). The factor with the greatest absolute effect on creatinine elevation was BK viremia: the creatinine rise was 1.62 mg/dL in patients with BK viremia, but only 0.64 mg/dL in those without BKV in the blood (P = 0.001) (Figure 3).
Table 4.
Average creatinine rise post-HSCT as a function of various clinical factors.
| Risk Factor | n | Creatinine Rise (mg/dL) | Creatinine Rise of Comparison Group (mg/dL) | Difference | P value |
|---|---|---|---|---|---|
| Female gender | 48 | 1.02 | 0.67 | +0.35 | 0.04 |
| Alternative-donor | 54 | 1.10 | 0.58 | +0.52 | 0.0009 |
| CML | 8 | 0.71 | 0.81 | −0.10 | 0.67 |
| CLL | 5 | 0.88 | 0.80 | +0.08 | 0.60 |
| AML | 50 | 0.96 | 0.70 | +0.26 | 0.12 |
| ALL | 8 | 1.01 | 0.79 | +0.22 | 0.41 |
| MDS | 9 | 0.47 | 0.83 | −0.36 | 0.02 |
| NHL | 27 | 0.60 | 0.86 | −0.26 | 0.07 |
| HD | 5 | 1.42 | 0.78 | +0.64 | 0.46 |
| Hematuria | 101 | 0.87 | 0.62 | +0.25 | 0.26 |
| Alemtuzumab | 90 | 0.81 | 0.78 | +0.03 | 0.93 |
| GVHD | 68 | 0.90 | 0.69 | +0.21 | 0.17 |
| Donor HLA C7 positive | 45 | 0.93 | 0.78 | +0.15 | 0.41 |
| CMV viremia | 26 | 1.21 | 0.70 | +0.51 | 0.03 |
| BK viruria | 79 | 0.97 | 0.53 | +0.44 | 0.001 |
| BK viremia | 21 | 1.62 | 0.64 | +0.98 | 0.001 |
For each clinical factor listed, the comparison group is comprised by all patients not demonstrating that clinical characteristic (e.g., for “females”, the comparison group is all males; for “HLA alternative” the comparison group is all patients who received an HLA-identical transplant; for “BK viremia” the comparison group is all patients without BK viremia; for the disease sub-types, the comparison group is all other patients in the study who did not have that disease). Patients with aplastic anemia (n = 3), myeloma (n = 3), and one of the six other singularly-represented diseases (n = 6) were not tested due to the small sizes of these sub-categories. On univariate analysis, six of the above clinical factors were significantly associated with a concomitant decrement in renal function: female gender; having undergone an alternative-donor transplant; having an underlying disease which was not MDS; having CMV viremia; having BK viruria; and having BK viremia. Patients with MDS had a significantly smaller rise in creatinine (0.47 mg/dL) than all other patients (0.83 mg/dL, P = 0.02). On multivariate analysis, only two factors were independently associated with a significant change in creatinine: undergoing an alternative-donor transplant (P = 0.002) and having BK viremia (P = 0.000002).
Figure 3. Impact of BK viremia on change in creatinine post-HSCT.
Change in creatinine represents the difference between the maximum post-HSCT creatinine level and the pre-transplant baseline creatinine. BK viremia was the factor having the greatest absolute effect on creatinine elevation in this study: the creatinine rise was 1.62 mg/dL in patients with BK viremia, but only 0.64 mg/dL in those without BKV in the blood (P = 0.001).
Development of BKV Nephropathy
Among the eight patients in the surveillance cohort with BK viremia, two developed biopsy-proven BKV-associated interstitial nephritis with severe renal failure. No other cases of unexplained renal failure requiring kidney biopsy occurred. Interestingly, the two patients with BKV nephropathy were the only two to have persistently-positive blood BKV levels throughout the period of monitoring (Table 2). A description of these two cases of BKV nephropathy is provided here.
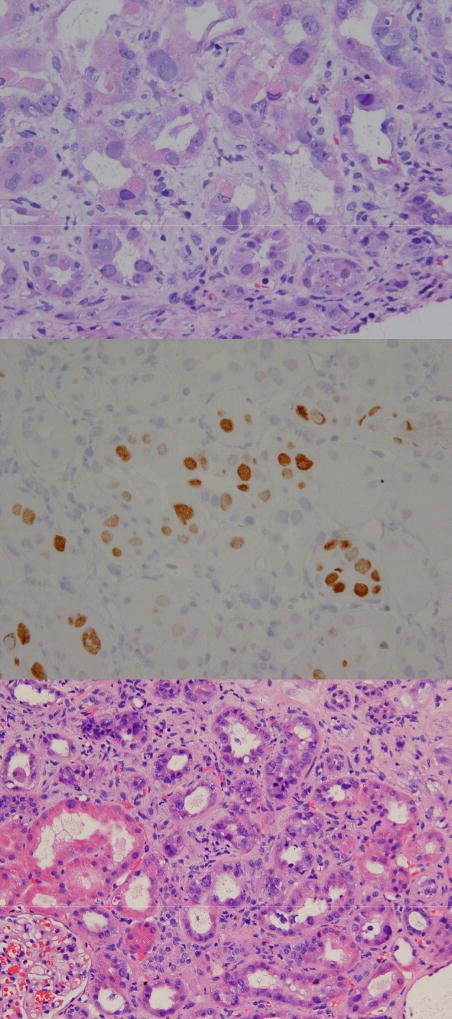
The first patient was a 36 year old female with relapsed Hodgkin’s disease who had previously undergone an autologous HSCT one year prior. Her conditioning regimen prior to matched, unrelated donor transplantation consisted of fludarabine, melphalan, and alemtuzumab. She had no prior history of kidney disease. Transplant was complicated with steroid-dependent GVHD. Her renal function began to slowly decline after transplant. BK viremia was detected, and without another cause of her renal insufficiency identified, a kidney biopsy was performed nine months after transplant. The biopsy revealed changes reflective of polyomavirus nephropathy: a diffuse, prominent, interstitial inflammatory reaction with tubulitis (Figure 4A). These cells tested strongly positive using an SV40 immunohistochemical stain (SV40 and BKV share a significant region of homology)21 (Figure 4B). Urinary BKV levels were consistently at the upper limit of the assay’s detection throughout this period, while blood BKV levels were modestly and consistently elevated. The patient was treated with leflunomide, an immunomodulatory agent, but there was no appreciable decrement in her blood or urine BKV levels, and her glomerular filtration rate continued to worsen. She required initiation of hemodialysis 18 months after her transplant.
Figure 4. Photomicrographs of the pathologic findings in two patients with biopsy-proven BK virus-induced nephropathy.
A (TOP PANEL) = high-powered view of enlarged tubular epithelial cells showing viral cytopathic effect and intranuclear inclusions in one of the two affected patients in our study. Glomeruli and vascular structures are normal; B (MIDDLE PANEL) = SV40 immunohistochemical stain further demonstrating the intranuclear viral inclusions in the same patient (SV40 and BKV share a significant region of homology); C = (BOTTOM PANEL) = similar pathologic findings in the kidney of the second patient in our study with BKV nephropathy.
The second patient was a 41 year old female with AML who underwent matched-unrelated donor HSCT in first remission after fludarabine/melphalan/alemtuzumab conditioning. Two and a half years after her transplant, she began to develop acute renal insufficiency, which corresponded temporally to a rapid spike in her blood BKV measurements to very high levels. Urinary BKV levels were also consistently at the upper limit of the assay’s detection. An alternative cause of her acute renal insufficiency was not found. Leflunomide was given, without a dramatic decrease in blood or urine BKV levels. Kidney biopsy revealed polyomavirus nephropathy (Figure 4C). Renal deterioration continued and the patient ultimately required hemodialysis.
The temporal relationship between the timing and magnitude of BK viremia and creatinine rise in these two patients is shown in Supplemental Figure S1.
DISCUSSION
The result of this analysis of a large, systematically monitored cohort of patients demonstrates that BK viruria is a common finding among HSCT recipients, while BK viremia is less common, but not rare. BK viruria is detectable early after HSCT, and in the cohort of patients who were tracked from day 0, the timing of detection of BK viruria (24 days) was similar to the median timing of first virus sampling (20 days), suggesting that BK viruria may actually be detectable even earlier, and, in a portion of patients, is likely present at baseline (pre-transplant). While we did not measure this, BKV shedding can be detected in the urine of a portion of immunocompetent individuals22,23 and in a recent preliminary analysis was detected in 46% of transplant patients pre-transplant24. The prevalence of post-HSCT BK viruria in our study (64.8%) is similar to multiple other studies which have demonstrated rates between 50–100%25–29.
In contrast, BK viremia has been less well-studied, and its timing of detection in our post-HSCT population was significantly later than that of BK viruria, suggesting that BK viremia represents a separate, pathologic disease state in this patient population. Several hypotheses have been purported3 in kidney transplant recipients which may explain the late occurrence of BK viremia in our patients: BKV may be acquired via blood transfusions (a risk which would increase over time in patients due to the chronological, cumulative need for transfusions post-HSCT); it could represent acquisition of BKV from the donor, with a period of latency or required replication post-HSCT until detection is possible; or it may represent a form of native “progression” of BKV infection in the transplant recipient, possibly in a step-wise manner (genitourinary BKV latency → detectable BK viruria → frank BK viremia)7,9,14,30,31. Our study did not measure BKV in the blood of donors, nor does our hospital routinely screen for BKV in transfused blood products, so we could not definitively differentiate between these possibilities in our study. However, the fact that all patients who had BK viremia also had BK viruria, and the fact that BK viruria is detectable much earlier, lends credence to the “progression” hypothesis.
Analysis of several transplant-related clinical factors potentially associated with the development of BKV infection was undertaken in this study, but none of the common predictors of transplant-related outcomes was associated with an increased (or decreased) occurrence of BK viruria or viremia. Having GVHD and undergoing an HLA-mismatched transplant had specifically been previously implicated as possible risk factors for BKV reactivation25,32, but these associations were not found in our study. Only CMV viremia was found to be associated with higher rates of BK viruria and viremia. One prior case report also suggested a potential correlation between CMV and BKV reactivation33. While causation cannot be determined by our data, we do not believe that CMV viremia predisposes a patient to the development of BKV infection. Rather, it is more likely that the as-yet-poorly defined factors which control CMV reactivation/replication in patients in the post-HSCT period are the same factors which govern this process for BKV.
Our data add supporting evidence to prior studies which have implicated urinary BKV in the development of post-HSCT hematuria11. Our findings that both a higher continuous level of urinary BKV and a higher maximum urinary BKV level are associated with greater numbers of RBC in the urine are consistent with prior similar associations28,29,34,35. While we did not assess the clinical severity of episodes of hematuria (i.e., whether there were associated urinary symptoms), our data do support the hypothesis that higher levels of urinary BKV may be correlated with greater urinary bleeding. Our finding of an association between BK viremia and hematuria was consistent with findings from two prior studies36,37 but in contrast to another report which found no correlation28. Given that hematuria (and HC) are problematic and potentially serious HSCT complications38, our data would support prospective testing of the concept that pharmacologic reduction in urinary BKV load, if achievable, might prevent more severe cases of urinary bleeding as can be seen with severe HC.
The most intriguing conclusion from this study was the demonstration of BK viremia as an independent risk factor for deterioration of renal function in the post-HSCT setting. Previously, only case reports had described an association between BKV and renal failure post-HSCT14,15,39,40. While HSCT itself resulted in an aggregate rise in creatinine above pre-transplant baselines in our study, undergoing an alternative-donor transplant and having BK viremia emerged as being independently associated with a statistically significant increase beyond the increase seen with transplantation alone. The absolute increase in creatinine observed in patients with BK viremia (median 1.62 mg/dL) was large and had the strongest statistical association. Even in the absence of frank oliguric renal failure, this degree of rise in serum creatinine represents a significant decrement in glomerular filtration rate which can complicate the ongoing treatment of patients especially as it impacts clearance of many transplant-related medications, precludes contrast-related radiologic imaging, and complicates handling of fluid volumes accompanying intravenous medications and blood products.
In contrast to BK viruria which often persisted during the period of observation, BK viremia was transient in many cases and so were elevations in creatinine. Still, two of eight patients in the surveillance cohort (which represents the patients with prolonged follow-up) developed severe interstitial nephritis that was biopsy-proven as BKV mediated. Given that these two individuals were the only two to have persistently-positive blood BKV levels in our study, this may suggest that prolonged duration of BK viremia is a risk factor for developing BKV nephropathy. It is also likely that at least some of the other patients in our study with impairment of renal function had undiagnosed mild BKV nephropathy14,15. Thrombotic thrombocytopenic purpura (TTP)-associated renal failure was not a confounder of the BK viremia-renal impairment association, as only two patients developed TTP in our study and neither had BK viremia. In a portion of the cases with BK viremia (10 of 21, including the two patients with biopsy-proven BKV nephropathy and renal failure), the occurrence of renal impairment temporally followed the patient turning positive for blood BKV or a spike in blood BKV levels, suggesting a direct causative relationship. In the other BK viremic patients, no clear temporal relationship between the timing of viremia and creatinine rise was observed, and the inciting event causing acute renal impairment was likely another cause. In these cases where BK viremia itself was not directly causative of renal impairment, it is possible that the predisposing factors for renal impairment—perhaps in the local microenvironment of the kidney itself—are the same which predispose to BKV reactivation and BK viremia, thereby explaining the strong association that was found.
One limitation of this study is that we were not able to account for all differences which may have existed in the administration of all potentially-nephrotoxic concomitant medications post-HSCT, though we did control for tacrolimus, perhaps the most commonly used nephrotoxic agent in our patients. While almost all patients at our center are treated with the same prophylactic medical regimens post-HSCT, patient-specific differences in the use of other chronic medications were not accounted for in our analysis. The use of aminoglycosides is specifically avoided in our transplant population in usual practice, and aminoglycosides were not administered to either of the two patients who ultimately developed renal failure requiring hemodialysis. In considering the impact of conditioning regimens on our outcomes, use of total body irradiation and/or cyclophosphamide did not impact creatinine change. We also evaluated whether the use of alemtuzumab, a potent immunosuppressant41,42 which is favored at our center, had an impact on the incidence or severity of BK viruria or viremia. Importantly, BKV infection was not increased in patients receiving alemtuzumab. Separately, we cannot rule out the possibility that some patients had more frequent virus sampling due to unmeasured variables, such as increased post-transplant complications. Finally, this study was conducted at one urban transplant center, and transplant-related supportive care practices which were not captured in our analysis are not necessarily similarly implemented at other centers.
In summary, BKV infection is common in post-HSCT patients, is associated with hematuria, and BK viremia, possibly by causing kidney damage which can lead to BKV nephropathy in some cases, is an independent risk factor for worsening renal function in the post-HSCT period. Investigation of whether prophylaxis against, or treatment of, BKV in the post-HSCT setting mitigates the associated morbidities, especially kidney injury, is the subject of ongoing investigation.
Supplementary Material
A = (TOP PANEL) corresponds to the first patient described in the text; B = (BOTTOM PANEL) corresponds to the second patient described in the text.
Acknowledgments
The authors would like to thank the nurses and patients of the University of Chicago bone marrow transplant program for their assistance and participation in this study. This work was supported by NCI grant 5K24CA116471-2 (K.V.B.). The authors declare no competing financial interests.
Footnotes
Presented in abstract form at the 49th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 2007.
Contribution: Study conception and design: P.H.O. and K.V.B. Patient enrollment and follow-up care: P.H.O., M.A.J., A.S.A., C.R., K.P., E.R., W.S., K.V.B. Data analysis and interpretation: P.H.O., K.S., S.D.P., K.V.B. Manuscript writing: P.H.O., K.S., A.S.A., C.R., K.P., W.S., K.V.B. Final approval of manuscript: P.H.O., K.S., M.A.J., A.S.A., S.D.P., C.R., K.P., E.R., W.S., K.V.B.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirsch HH, Steiger J. Polyomavirus BK. The Lancet Infectious Diseases. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 2.Coleman DV, Mackenzie EF, Gardner SD, Poulding JM, Amer B, Russell WJ. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. Journal of Clinical Pathology. 1978;31:338–347. doi: 10.1136/jcp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman JA. BK virus nephropathy--polyomavirus adding insult to injury. [comment] New England Journal of Medicine. 2002;347:527–530. doi: 10.1056/NEJMe020076. [DOI] [PubMed] [Google Scholar]
- 4.Reploeg MD, Storch GA, Clifford DB. Bk virus: a clinical review. Clinical Infectious Diseases. 2001;33:191–202. doi: 10.1086/321813. [DOI] [PubMed] [Google Scholar]
- 5.Nickeleit V, Mihatsch MJ. Polyomavirus nephropathy in native kidneys and renal allografts: an update on an escalating threat. Transplant International. 2006;19:960–973. doi: 10.1111/j.1432-2277.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 6.Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. Journal of Infectious Diseases. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. [see comment] New England Journal of Medicine. 2002;347:488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 8.Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. American Journal of Transplantation. 2004;4:2082–2092. doi: 10.1046/j.1600-6143.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 10.Apperley JF, Rice SJ, Bishop JA, et al. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987;43:108–112. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. New England Journal of Medicine. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama H, Kurosu T, Sakashita C, et al. Adenovirus is a key pathogen in hemorrhagic cystitis associated with bone marrow transplantation. Clinical Infectious Diseases. 2001;32:1325–1330. doi: 10.1086/319992. [DOI] [PubMed] [Google Scholar]
- 13.Vandercam B, Moreau M, Goffin E, Marot JC, Cosyns JP, Jadoul M. Cidofovir-induced end-stage renal failure. Clinical Infectious Diseases. 1999;29:948–949. doi: 10.1086/520475. [DOI] [PubMed] [Google Scholar]
- 14.Limaye AP, Smith KD, Cook L, et al. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. American Journal of Transplantation. 2005;5:614–620. doi: 10.1046/j.1600-6143.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 15.Stracke S, Helmchen U, von Muller L, Bunjes D, Keller F. Polyoma virus-associated interstitial nephritis in a patient with acute myeloic leukaemia and peripheral blood stem cell transplantation. Nephrology Dialysis Transplantation. 2003;18:2431–2433. doi: 10.1093/ndt/gfg361. [DOI] [PubMed] [Google Scholar]
- 16.Kline J, Pollyea DA, Stock W, et al. Pre-transplant ganciclovir and post transplant high-dose valacyclovir reduce CMV infections after alemtuzumab-based conditioning. Bone Marrow Transplantation. 2006;37:307–310. doi: 10.1038/sj.bmt.1705249. [DOI] [PubMed] [Google Scholar]
- 17.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. [see comment] American Journal of Transplantation. 2005;5:2213–2221. doi: 10.1111/j.1600-6143.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 18.Altman DG. Practical Statistics for Medical Research. 1991:365–395. [Google Scholar]
- 19.Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. Journal of Infectious Diseases. 1990;161:1128–1133. doi: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- 20.Zhong S, Zheng HY, Suzuki M, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. Journal of Clinical Microbiology. 2007;45:193–198. doi: 10.1128/JCM.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanchiere JA, Nicome RK, Greer JM, Demmler GJ, Butel JS. Frequent detection of polyomaviruses in stool samples from hospitalized children. Journal of Infectious Diseases. 2005;192:658–664. doi: 10.1086/432076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randhawa P, Uhrmacher J, Pasculle W, et al. A comparative study of BK and JC virus infections in organ transplant recipients. Journal of Medical Virology. 2005;77:238–243. doi: 10.1002/jmv.20442. [DOI] [PubMed] [Google Scholar]
- 23.Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Khoo SH. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. Journal of Clinical Virology. 2004;29:224–229. doi: 10.1016/S1386-6532(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 24.de Padua Silva L, Patah P, Saliba RM, et al. Polyoma (BK) Viruria Prior to Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) from Donors Other Than Matched Siblings: A Prospective Evaluation of Hemorrhagic Cystitis (HC) Incidence [abstract]; Blood (Proceedings and Abstracts of the 50th Annual Meeting of the American Society of Hematology); 2008. (#50) [Google Scholar]
- 25.Giraud G, Priftakis P, Bogdanovic G, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplantation. 2008;41:737–742. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 26.Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. Journal of Clinical Oncology. 1995;13:1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 27.Azzi A, Fanci R, Bosi A, et al. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplantation. 1994;14:235–240. [PubMed] [Google Scholar]
- 28.Leung AY, Suen CK, Lie AK, Liang RH, Yuen KY, Kwong YL. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98:1971–1978. doi: 10.1182/blood.v98.6.1971. [DOI] [PubMed] [Google Scholar]
- 29.Azzi A, Cesaro S, Laszlo D, et al. Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. Journal of Clinical Virology. 1999;14:79–86. doi: 10.1016/s1386-6532(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 30.Coleman DV, Gardner SD, Field AM. Human polyomavirus infection in renal allograft recipients. British Medical Journal. 1973;3:371–375. doi: 10.1136/bmj.3.5876.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drachenberg CB, Papadimitriou JC. Polyomavirus-associated nephropathy: update in diagnosis. Transplant Infectious Disease. 2006;8:68–75. doi: 10.1111/j.1399-3062.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 32.Bogdanovic G, Priftakis P, Giraud G, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. Journal of Clinical Microbiology. 2004;42:5394–5396. doi: 10.1128/JCM.42.11.5394-5396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielorai B, Shulman LM, Rechavi G, Toren A. CMV reactivation induced BK virus-associated late onset hemorrhagic cystitis after peripheral blood stem cell transplantation. Bone Marrow Transplantation. 2001;28:613–614. doi: 10.1038/sj.bmt.1703187. [DOI] [PubMed] [Google Scholar]
- 34.Wong AS, Chan KH, Cheng VC, Yuen KY, Kwong YL, Leung AY. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clinical Infectious Diseases. 2007;44:830–837. doi: 10.1086/511863. [DOI] [PubMed] [Google Scholar]
- 35.Giraud G, Bogdanovic G, Priftakis P, et al. The incidence of hemorrhagic cystitis and BK-viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica. 2006;91:401–404. [PubMed] [Google Scholar]
- 36.Cesaro S, Facchin C, Tridello G, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplantation. 2008;41:363–370. doi: 10.1038/sj.bmt.1705909. [DOI] [PubMed] [Google Scholar]
- 37.Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood. 2005;106:1130–1132. doi: 10.1182/blood-2004-12-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sencer SF, Haake RJ, Weisdorf DJ. Hemorrhagic cystitis after bone marrow transplantation. Risk factors and complications. Transplantation. 1993;56:875–879. doi: 10.1097/00007890-199310000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Bruno B, Zager RA, Boeckh MJ, et al. Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation. 2004;77:1049–1057. doi: 10.1097/01.tp.0000122421.71556.71. [DOI] [PubMed] [Google Scholar]
- 40.Iwamoto S, Azuma E, Hori H, et al. BK virus-associated fatal renal failure following late-onset hemorrhagic cystitis in an unrelated bone marrow transplantation. Pediatric Hematology & Oncology. 2002;19:255–261. doi: 10.1080/08880010252899424. [DOI] [PubMed] [Google Scholar]
- 41.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clinical Infectious Diseases. 2006;43:16–24. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 42.Peleg AY, Husain S, Kwak EJ, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clinical Infectious Diseases. 2007;44:204–212. doi: 10.1086/510388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A = (TOP PANEL) corresponds to the first patient described in the text; B = (BOTTOM PANEL) corresponds to the second patient described in the text.