Abstract
Objective
To determine if antioxidant supplementation during pregnancy reduces the incidence of premature rupture of the membranes (PROM).
Study Design
A placebo-controlled, double-blind trial was conducted. PROM and preterm PROM (PPROM) were planned secondary outcomes of the trial. Women between 120/7 and 196/7 weeks of gestation and diagnosed to have chronic hypertension or a prior history of preeclampsia were randomized to daily treatment with both vitamin C (1000 mg) and E (400 IU) or placebo.
Results
Outcome data for PROM were available for 697 of 739 patients. The rates of PROM 37/349 [10.6%] versus 19/348 [5.5%]; adjusted risk ratio [RR] 1.89 [95.42% confidence interval CI) 1.11, 3.23]; p=0.015), and PPROM (16/349 [4.6%] versus 6/348 [1.7%]; RR 2.68 [1.07, 6.71]; p=0.025) were increased in the antioxidant group.
Conclusion
Contrary to expectations, vitamins C and E supplementation in this dose combination may be associated with an increased risk of PROM and PPROM.
Keywords: antioxidants, prematurity, premature rupture of the membranes, prevention, vitamin C, vitamin E
Introduction
Premature rupture of the membranes (PROM) prior to 37 weeks of gestation (PPROM) precedes approximately 35% of patients delivering prematurely. Among patients with a prior history of PROM, its recurrence in subsequent pregnancies is substantially increased.1 In 2001, Woods et al.2,3 proposed that generation of reactive oxygen species may be a potentially reversible pathophysiologic pathway leading to preterm premature rupture of the membranes. They hypothesized that reactive oxygen species generated by the body's response to diverse insults such as infections, cigarette smoking, bleeding, or cocaine use could activate collagenolytic enzymes and impair fetal membrane integrity. Because Vitamin E, a lipid-soluble antioxidant, inhibits membrane-damaging effects of reactive oxygen species-induced lipid peroxidation, and Vitamin C, a water-soluble antioxidant in plasma, stimulates and protects collagen synthesis while recycling vitamin E, these authors called for clinical trials to be done. In response, we prospectively evaluated the occurrence of premature rupture of membranes in a cohort of patients randomized to vitamins C and E supplementation versus placebo during a trial of their use for preeclampsia prevention. 4 The null hypothesis was that supplementation of Vitamin C and Vitamin E would not alter the frequency of PROM.
Materials and Methods
This clinical trial was conducted as a protocol within the National Institute of Child Health and Human Development (NICHD) Global Network for Women’s and Children’s Health Research. The primary clinical center (Recife) and three additional clinical sites (Campinas, Botucatu, and Porto Alegre) are staffed by the Senior Foreign Investigator (SFI) (Recife) or Senior Collaborating Investigators (Campinas, Botucatu, and Porto Alegre), a program coordinator, research physicians and a data manager or staff nurse. Each site’s major teaching hospital serves a primarily urban low-income population.
The trial enrolled women seeking prenatal care who were 120/7 to 196/7 weeks’ pregnant and diagnosed with non-proteinuric chronic hypertension or a prior history of preeclampsia in their most recent pregnancy that progressed beyond 20 weeks gestation. Exclusion criteria were planned delivery elsewhere, multifetal gestation, allergy to vitamin C or vitamin E, requirement for aspirin or anticoagulant medication, 24-hour urinary protein ≥ 300 mg, prepregnancy diabetes mellitus, known fetal anomaly incompatible with life, or prior participation in the study.
The protocol was approved by the NICHD and the institutional review boards at the University of Cincinnati, each participating site, and the data coordinating center (DCC). Each woman gave written informed consent. Planned interim analyses were monitored by the Global Network’s independent Data Monitoring Committee.
Women were assigned randomly to receive daily vitamin C 1000 mg and vitamin E 400 IU or placebo. All clinicians and clinical investigators were blinded to group assignment. The medications were manufactured as softgel capsules by J R Carlson Laboratories (Arlington Heights, IL). Each active treatment gel cap contained 500 mg of ascorbic acid, 100 IU of d-alpha tocopherol, 100 IU of d-alpha tocopherol acetate, and excipients (gelatin, soybean oil, glycerin, water lecithin, and caramel color). The placebo gel caps contained excipients only and were externally identical to the active drug. Participants were instructed to ingest two gel caps daily from enrollment until delivery or until the diagnosis of preeclampsia. Correct supplier randomization assignment was verified by the DCC. The randomization sequence was constructed by the DCC as permuted blocks of random size, stratified by clinical center, and implemented via a program residing on the clinical center’s study computer.
Study participants were discouraged from the use of antioxidant vitamins, calcium supplements, and chronic use of aspirin. The women were followed at routine prenatal visits, typically every 4 weeks until 26 to 28 weeks of gestation, every 2 to 3 weeks until 36 weeks of gestation, and then weekly until delivery or the onset of preeclampsia.
Compliance with treatment was assessed by counting residual pills at monthly return visits. A computerized bottle cap, the MEMS® V TrackCap Child Resistant (APREX, a division of AARDEX®, Ltd., Union City, CA) which internally records the date and time of each opening of the pill bottle, was placed on the first pill bottle and then transferred to sequential bottles. Information from the TrackCap was shared with the patients at monthly intervals to motivate optimal compliance through encouragement and constructive problem solving.
The primary outcome of the study was the development of preeclampsia. The planned secondary outcomes, PROM and PPROM, are the primary focus of this report. Additional secondary outcomes evaluated included abruptio placentae, preterm birth, and small-for-gestational age (SGA) and low birthweight infants. PROM was defined as spontaneous rupture of membranes prior to the onset of labor. PROM was diagnosed at the time of admission by the admitting physician through assessment of patient history and clinical findings including pooling of amniotic fluid, positive nitrazine testing and/or positive ferning of amniotic fluid. SGA was defined as a birth weight below the 10th percentile according to the growth tables of Alexander et al.5 Abruptio placentae was diagnosed according to clinical findings or placental examination.
PROM was to be treated as a surrogate outcome for preterm PROM (PPROM) (PROM prior to 37 weeks of gestation). Although PPROM would be examined directly, it was expected to occur in only 4–5% of control patients. For a 2-sided alpha of 0.05, the power to detect a 50% reduction in PPROM in the vitamin group relative to the placebo group would be low (31–37%). PROM was expected to occur in approximately 10% of control patients.
Data were analyzed using the intent-to-treat principle: all randomized subjects were included in the treatment group to which they were originally assigned. Participants with missing outcomes due to withdrawal of consent or loss to follow-up were excluded from the analysis of outcomes. Additional planned analyses of subjects who had received at least 80% of the intended doses as assessed by returned pill counts were performed. Two interim analyses that corresponded to 34 percent and 68 percent of the total planned results were performed according to the Lan-DeMets approach using an α-spending function analogous to the O’Brien-Fleming procedure6 at an overall level of α=0.05 (two-tailed); the significance level for the final analysis was α=0.0458. Comparisons between study drug and placebo groups were stratified by site and risk group using Cochran-Mantel-Haenszel (CMH) chi-square statistics7 for binary outcomes and analysis of variance for continuous outcomes, with CMH row mean tests with modified ridit scores (the nonparametric van Elteren test)7 where distributions were skewed. Analyses that adjusted for additional covariates used logistic regression models.8 Data were analyzed using SAS/STAT software, version 9.1.3 (SAS Institute, Inc., Cary, NC, 2003). Exact CMH procedures in StatXact, version 7 (Cytel, Inc., Cambridge, MA, 2005) were used when binary outcomes were sparse, yielding odds ratios instead of risk ratios. Lan-DeMets calculations were performed using East, version 4 (Cytel, Inc., Cambridge, MA, 2005).
Results
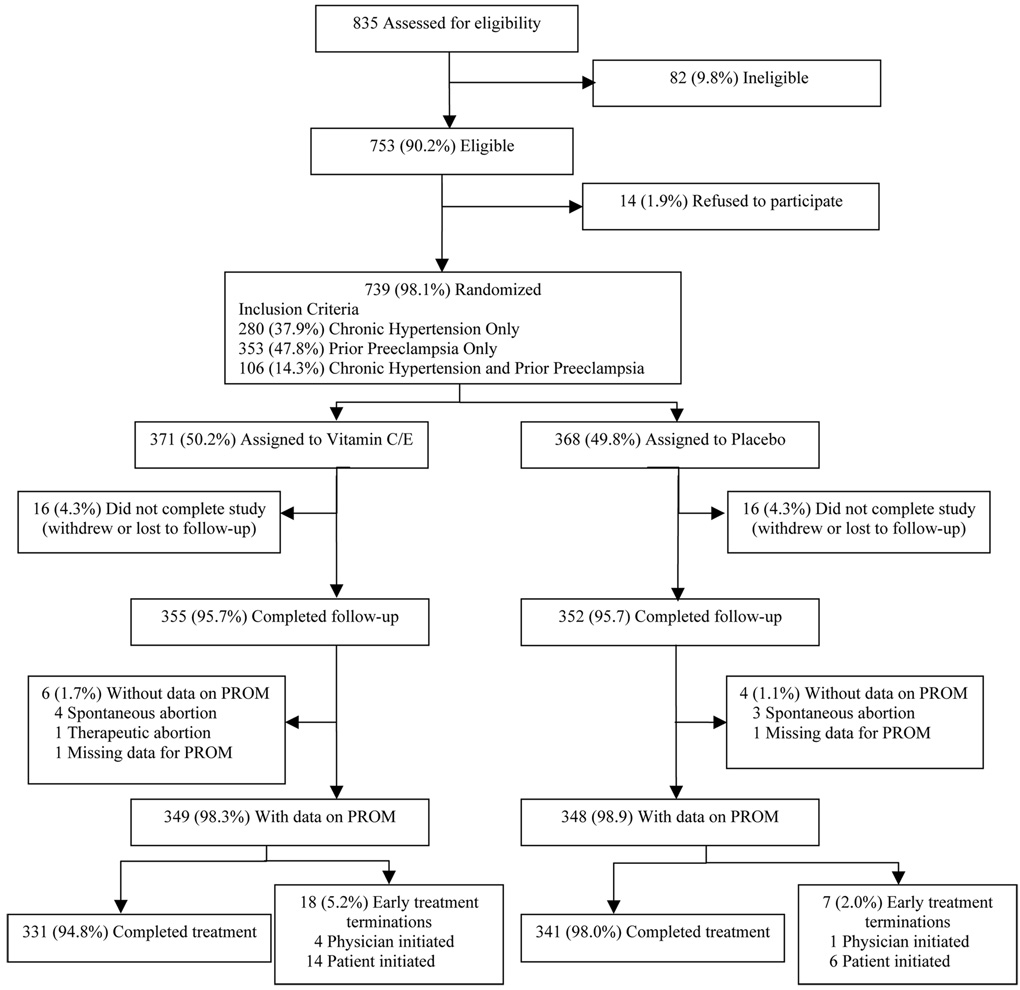
Screening for enrollment began on July 2, 2003 at Recife; May 19, 2004 at Botucatu; July 5, 2004 at Porto Alegre, and February 2, 2005 at Campinas; and concluded on May 15, 2006. Follow-up was completed on November 23, 2006. From the general population of obstetric clinic patients and of 835 women satisfying inclusion criteria, 739 of 753 (98%) eligible women were enrolled in the study (Recife, 265; Campinas, 202; Botucatu, 152; Porto Alegre, 120). Figure 1 describes the enrollment flow. The specific reasons for ineligibility, refusal to participate, and withdrawal from the study are as reported in our previous publication.4 In each treatment group, 4.3% of subjects were lost to follow-up or withdrew consent.
Figure 1.
Enrollment Flow Chart
The demographic and clinical characteristics of the enrolled subjects, including the criteria for inclusion in the study, were similar between the two treatment groups both overall (Table 1) and within the chronic hypertension and prior preeclampsia only subgroups.4 Gestational age at enrollment was determined by best obstetric estimate; ultrasound dating was performed on 61.3% of the women and rejected the last menstrual period dating in about half of those. Violations of inclusion or exclusion criteria occurred in 25 subjects. Twenty-three women were enrolled outside the 12 to 19 week window for gestational age (7–11 weeks, n=13; 20–23 weeks, n=10). The majority were discovered by ultrasound examinations performed after enrollment. There were two twin gestations, discovered after enrollment; one was lost to spontaneous abortion, and one delivered liveborns, resulting in one more infant analyzed than mothers in the group receiving study drug. All 25 women remained on their assigned study treatment and continued in follow-up.
Table 1.
Baseline characteristics of enrolled patients
| Treatment | |||
|---|---|---|---|
| Characteristic | Total | ||
| Vit C&E | Placebo | ||
| Total number randomized – N | 371 | 368 | 739 |
| Study site – n (%) | |||
| Botucatu | 78 (21.0) | 74 (20.1) | 152 (20.5) |
| Campinas | 100 (26.9) | 102 (27.7) | 202 (27.3) |
| Porto Alegre | 60 (16.1) | 60 (16.3) | 120 (16.2) |
| Recife | 133 (35.8) | 132 (35.9) | 265 (35.9) |
| Preeclampsia risk group – n (%) | |||
| Prior preeclampsia only | 178 (48.0) | 175 (47.6) | 353 (47.8) |
| Chronic hypertension | 193 (52.0) | 193 (52.4) | 386 (52.2) |
| Prior preeclampsia | 49 (13.2) | 57 (15.5) | 106 (14.3) |
| No prior preeclampsia | 144 (38.8) | 136 (37.0) | 280 (37.9) |
| Age (years) – mean ± s.d. | 28.9 ± 6.3 | 29.7 ± 6.2 | 29.3 ± 6.3 |
| Race (self identified) – n (%) | |||
| Brown | 166 (44.7) | 167 (45.4) | 333 (45.1) |
| Black | 40 (10.8) | 45 (12.2) | 85 (11.5) |
| White | 165 (44.5) | 156 (42.4) | 321 (43.4) |
| Yellow | 0 ( 0.0) | 0 ( 0.0) | 0 ( 0.0) |
| Marital status – n (%) | |||
| Married | 157 (42.3) | 168 (45.7) | 325 (44.0) |
| Co-habiting | 182 (49.1) | 175 (47.6) | 357 (48.3) |
| Other | 32 ( 8.6) | 25 ( 6.8) | 57 ( 7.7) |
| Primigravida – n (%) | 37 (10.0) | 34 ( 9.2) | 71 ( 9.6) |
| Gestational age at enrollment (weeks) – mean ± s.d. | 15.6 ± 2.7 | 15.7 ± 2.5 | 15.6 ± 2.6 |
| Source of estimated date of delivery (EDD) – n (%) | |||
| Date of last menstrual period (LMP) | 137 (36.9) | 149 (40.5) | 286 (38.7) |
| LMP confirmed by ultrasound | 115 (31.0) | 98 (26.6) | 213 (28.8) |
| Ultrasound rejects LMP | 119 (32.1) | 121 (32.9) | 240 (32.5) |
| Prepregnancy body mass index* (kg/m2) – mean ± s.d. | 28.5 ± 7.1 | 28.8 ± 7.0 | 28.7 ± 7.1 |
| Smoking during gestation – n (%) | 52 (14.0) | 50 (13.6) | 102 (13.8) |
| Smoking at enrollment – n (%) | 27 ( 7.3) | 31 ( 8.4) | 58 ( 7.8) |
| < 1 pack per day | 23 (85.2) | 27 (87.1) | 50 (86.2) |
| ≥1 pack per day | 4 (14.8) | 4 (12.9) | 8 (13.8) |
Weight in kg divided by the square of height in m.
Data on secondary outcomes, including PROM and PPROM, were not available for 7 women with spontaneous abortion (4 in the vitamin group, 3 in the placebo group) and 1 woman (vitamin group) with a therapeutic abortion. In addition, for 2 women (1 in each treatment group) information on the time of rupture of membranes was unavailable, leaving 697 women (349 treatment group, 348 placebo group) for this analysis. Of these, 18 (5.2%) and 7 (2.0%) had early treatment termination but remained in follow-up. The percentage of patients judged by returned pill counts as having received at least 80% of the intended doses was substantial (599 of 697 patients [85.9%]) and was similar between treatment groups (84.8%, 87.1%).
Table 2 notes the labor and delivery experience of the study participants. The distributions of gestational age at delivery, route of delivery, and type of membrane rupture did not differ between treatment groups. The type of labor differed significantly between treatment groups (p=0.040), with a somewhat greater percentage of augmented spontaneous labor (7.4% versus 3.7%) and a smaller percentage of induced labor (14.6% versus 18.1%) among women in the vitamin group. Two hundred ninety-eight of 697 patients (42.8%) were delivered without prior labor. Site differences were pronounced. The distribution of gestational age at delivery differed among sites (p=0.0029), with medians of 37.7, 38.0, 38.9, and 38.7 weeks at Botucatu, Campinas, Porto Alegre, and Recife; the incidence of preterm birth varied similarly (30.4%, 27.8%, 24.3%, and 21.2%), but the differences were not statistically significant (p=0.27). Delivery without preceding labor was differentially distributed among sites: Botucatu, 71/148 (48.0%); Campinas, 50/193 (25.9%); Porto Alegre, 42/102 (41.2%); and Recife 135/254 (53.2%); p<0.0001. Cesarean delivery was performed in 467 patients (67.0%): 91 (61.5%) at Botucatu; 121 (62.7%) at Campinas; 49 (48.0%) at Porto Alegre; and 206 (81.1%) at Recife; p<0.0001. Spontaneous rupture of the membranes was noted among 184 (26.4%) patients: 42 (28.4%), 57 (29.5%), 32 (31.4%), and 53 (20.9%), respectively; p<0.0001. The remaining patients underwent amniotomy or membrane rupture during Cesarean delivery.
Table 2.
Labor and delivery characteristics of patients evaluable for PROM.*
| Treatment | |||
|---|---|---|---|
| Characteristic | Total | ||
| Vit C&E | Placebo | ||
| Number of mothers – N | 349 | 348 | 697 |
| Gestational age at delivery – median | 38.1 | 38.4 | 38.3 |
| (25th, 75th percentile) | (36.9, 39.4) | (37.1, 39.4) | (36.9, 39.4) |
| Type of labor – n (%)† | |||
| No labor | 150 (43.0) | 148 (42.5) | 298 (42.8) |
| Spontaneous | 148 (42.4) | 137 (39.4) | 285 (40.9) |
| Not augmented | 122 (35.0) | 124 (35.6) | 246 (35.3) |
| Augmented with oxytocin | 26 ( 7.4) | 13 ( 3.7) | 39 ( 5.6) |
| Induced | 51 (14.6) | 63 (18.1) | 114 (16.4) |
| Route of delivery – n (%) | |||
| Vaginal | 118 (33.8) | 112 (32.2) | 230 (33.0) |
| Cesarean | 231 (66.2) | 236 (67.8) | 467 (67.0) |
| Type of membrane rupture - n (%) | |||
| Spontaneous | 99 (28.4) | 85 (24.4) | 184 (26.4) |
| Amniotomy | 54 (15.5) | 61 (17.5) | 115 (16.5) |
| During cesarean section | 196 (56.2) | 202 (58.0) | 398 (57.1) |
The total number with data on PROM is 697 = 739 enrolled – 24 lost to follow-up – 8 consent withdrawn – 8 patients without delivery data (7 spontaneous and 1 therapeutic abortion) – 2 patients with missing values for PROM.
p=0.040 for the difference in distribution between treatment groups, Cochran-Mantel-Haenszel method, adjusting for study site and enrollment preeclampsia risk group (prior preeclampsia only, chronic hypertension only, chronic hypertension and prior preeclampsia).
Table 3 reports the rate of PROM and PPROM by treatment for the intent-to-treat cohort and the compliant subgroup. In the intent-to-treat cohort, both PROM (37/349 [10.6%] versus 19/348 [5.5%]; adjusted risk ratio [RR] 1.89 [95.42% confidence interval [CI] 1.11, 3.23]; p=0.015) and PPROM (16/349 [4.6%] versus 6/348 [1.7%]; RR 2.68 [1.07, 6.71]; p=0.025) were significantly more likely in the vitamin group. The incidence of PROM varied by study site (Botucatu, 10.1%; Campinas, 12.4%; Porto Alegre, 11.8%; Recife, 2.0%; p=0.0002), but the treatment differences for PROM were consistent in direction across the sites. Among compliant patients, the PROM difference remained significant (p=0.029) while the PPROM difference, though in the same direction and similar in magnitude, did not (p=0.16). The treatment difference for PROM remained significant after covariate adjustment by logistic regression for maternal age, smoking, and body mass index (quadratic), both overall (p=0.017) and for the compliant subset (p=0.045). The frequency of preterm birth was significantly increased when PROM occurred (22/56 [39.3%], versus 154/641 [24.0%]; p=0.015).
Table 3.
Effect of antioxidants on the incidence of PROM and preterm PROM.*
| Treatment | ||||
|---|---|---|---|---|
| Group | Adjusted Risk Ratio (95.42% Confidence Interval)† | P-value† | ||
| Vit C&E n/N (%) |
Placebo n/N (%) |
|||
| Intent-to-treat | ||||
| PROM | 37/349 (10.6) | 19/348 ( 5.5) | 1.89 (1.11, 3.23) | 0.015 |
| Preterm PROM | 16/349 ( 4.6) | 6/348 ( 1.7) | 2.68 (1.07, 6.71) | 0.025 |
| Compliant patients‡ | ||||
| PROM | 30/296 (10.1) | 16/303 ( 5.3) | 1.89 (1.05, 3.40) | 0.029 |
| Preterm PROM | 10/296 ( 3.4) | 5/303 ( 1.7) | 2.08 (0.72, 6.04) | 0.16 |
The total number with data on PROM is 697 = 739 enrolled – 24 lost to follow-up – 8 consent withdrawn – 8 patients without delivery data (7 spontaneous and 1 therapeutic abortion) – 2 patients with missing values for PROM.
Cochran-Mantel-Haenszel method, adjusting for study site and enrollment preeclampsia risk group (prior preeclampsia only, chronic hypertension only, chronic hypertension and prior preeclampsia).
Patients whose returned pill counts indicated that they received at least 80% of the intended doses from enrollment to delivery or to development of preeclampsia.
Table 4 details perinatal outcomes. No significant between group differences were noted. Abruptio placentae was observed in 4 (1.1%) and 8 (2.3%) of patients in the vitamin and placebo groups (adjusted odds ratio, 0.52 [0.11, 2.03]; exact p=0.38).
Table 4.
Perinatal Outcomes
| Treatment | ||||
|---|---|---|---|---|
| Outcome | Adjusted Risk Ratio (95.42% Confidence Interval)* | P-value* | ||
| Vit C&E | Placebo | |||
| Number of infants – N† | 350 | 348 | ||
| Fetal and neonatal deaths – n (%) | 13 ( 3.7) | 15 ( 4.3) | 0.94 (0.45, 1.96) | 0.87 |
| Stillbirth | 7 ( 2.0) | 9 ( 2.6) | 0.80 (0.30, 2.17) | 0.66 |
| Neonatal death | 6 ( 1.7) | 6 ( 1.7) | 1.16 (0.37, 3.60) | 0.79 |
| Preterm delivery – n (%) | ||||
| < 37 weeks’ gestation | 96 (27.4) | 81 (23.3) | 1.17 (0.90, 1.52) | 0.23 |
| < 34 weeks’ gestation | 29 ( 8.3) | 25 ( 7.2) | 1.13 (0.67, 1.90) | 0.64 |
| Birth weight (g) – mean ± s.d. | 3,018.7 ± 780.1 | 3,047.2 ± 755.6 | −25.1 (−139.8, 89.5)‡ | 0.66‡ |
| Low birth weight (<2500 g) – n (%) | 61 (17.4) | 61 (17.5) | 1.00 (0.72, 1.38) | 0.98 |
| Very low birth weight (<1500 g) – n (%) | 20 ( 5.7) | 18 ( 5.2) | 1.12 (0.60, 2.11) | 0.72 |
| Small for gestational age – n (%) | 49 (14.0) | 48 (13.8) | 1.03 (0.71, 1.49) | 0.87 |
| Apgar scores – n (%) | ||||
| < 4 at 1 minute | 15 ( 4.4) | 21 ( 6.2) | 0.72 (0.38, 1.40) | 0.33 |
| < 7 at 5 minutes | 8 ( 2.3) | 12 ( 3.5) | 0.72 (0.30, 1.78) | 0.47 |
Cochran-Mantel-Haenszel method, adjusting for study site and preeclampsia risk group (prior preeclampsia only, chronic hypertension only, chronic hypertension and prior preeclampsia).
Excludes infants of mothers who withdrew consent or were lost to follow-up (n=32), who had a spontaneous or therapeutic abortion (n=8), or who had missing data for PROM (n=2); denominators vary due to missing responses.
Adjusted mean difference, 95.42% confidence interval, and p-value from analysis of variance adjusting for study site and preeclampsia risk group (prior preeclampsia only, chronic hypertension only, chronic hypertension and prior preeclampsia).
Comment
The findings of this investigation were unexpected. Given the number of enrolled patients, it was anticipated that PROM would be a necessary outcome surrogate for PPROM. However, a statistically significant negative effect of antioxidant supplementation increasing the risk of both PROM and PPROM was found. The direction of the effect on PROM was consistent across each of our sites. These findings were observed despite the heterogeneity of the populations enrolled, the need for early delivery for preeclampsia or chronic hypertension that may have attenuated a more pronounced effect on PROM frequency, and differences in obstetric practices among sites. Among compliant patients similar overall treatment results for PROM were noted and the lack of significance for PPROM in the compliant subgroup may represent a Type II error, given the similarity of the direction and magnitude of the treatment effect to the intent-to-treat analysis. However, we did not identify a deleterious influence of supplementation on the frequency of low birthweight, small for gestational age, stillbirth, or Apgar score. This is likely due to the infrequency of PPROM in the population studied.
These findings stand in contrast to the accumulation of reports suggesting the protective importance of antioxidant vitamins in the pathophysiology of PROM. In 1994, Barrett et al.9 collected amniotic fluid and venous blood specimens from 80 pregnant women with or without PROM and analyzed them for ascorbic acid, alpha-tocopherol, retinol and beta-carotene concentrations. No differences in retinol and alpha-tocopherol were found between the PROM and control groups. PROM and control subjects had similar serum ascorbic acid concentrations. However, PROM subjects had significantly lower amniotic fluid ascorbic acid and serum beta-carotene levels suggesting a possible pathophysiologic role.
Plessinger et al.10 noted extensive damage to in vitro amniotic epithelium and collagen I but not collagen IV resulting from hypochlorous acid exposure that was dose related. Pretreatment with vitamins C and E prevented this damage in all cases. The authors concluded that the protection against hypochlorous acid-induced damage provided by antioxidant therapy (vitamins C and E) is of therapeutic significance. This prompted these authors2,3,10,11 to suggest the theory that premature rupture of the membranes is in part due to reactive oxygen species and antioxidant deficit that resulted in membrane damage.
Siega-Riz et al.12 studied a prospective cohort of 2064 pregnant women with singleton gestations. Women who had total vitamin C intakes of <10th percentile preconceptionally had twice the risk of preterm delivery because of premature rupture of the membranes (relative risk: 2.2; 95% CI: 1.1, 4.5). This risk was attenuated slightly for second-trimester intake (relative risk: 1.7; 95% CI: 0.8, 3.5). The elevated risk of preterm premature rupture of the membranes was greatest for women with a low vitamin C intake during both time periods. They suggested that vitamin supplementation may be a viable interventional strategy.
Recently, Casanueva et al.13 reported 109 patients randomly assigned at 20 weeks’ gestation to receive 100 mg vitamin C or placebo. Mean plasma vitamin C concentrations decreased significantly throughout the pregnancy in both groups (p=0.001), and there were no significant differences between groups. Despite this and the low dose of supplementation, the incidence of PROM was 14 per 57 pregnancies (24.5%) in the placebo group and 4 per 52 pregnancies (7.69%) in the supplemented group (relative risk: 0.26; 95% CI: 0.078, 0.837). The authors concluded that vitamin C after 20 wk of gestation effectively lessens the incidence of PROM.
Of the previously reported large preeclampsia prevention trials of vitamins C and E in the same doses as used in this trial14–17, only Rumbold et al16 reported PPROM. Among 1877 randomized patients, PPROM was observed among 30/935 (3.2%) in the supplemented group versus 23/942 (2.4%; relative risk: 1.31; 95% CI: 0.77, 2.25). Although these results trended similarly to this report, the differences were not significant. Whether reliable data regarding PROM could have been retrieved from the other studies is unknown.
This study has its limitations. The assessment of PROM/PPROM was a secondary outcome within a study of patients at risk of preeclampsia. Whether the findings would be reproduced in a more general population within or outside Brasil is unknown. We did not collect information regarding prior history of PROM among our previously pregnant patients. While it is unlikely, disproportionate representation of such patients could have populated the vitamin supplemented group. Glucocorticoid use was not assessed in the database. However, the prescription of glucocorticoids in this patient population is infrequent and its use among enrolled patients should be randomly distributed with respect to treatment.
The incidence of PROM at the site in Recife was lower than at the other sites. This is at least partly explained by the higher rate of cesarean delivery there. However, any potential under-reporting would not be differential given the blinding of the treatments, and therefore should not bias the association between treatment and PROM. Regardless, the observed treatment effect on PROM was consistent across sites, including Recife. In part due to the at risk nature of the study cohort, the cesarean delivery rate in this study (67%) is higher than that reported for public hospitals (32.9%) in Brazil but lower than that reported from private hospitals (80.4%).18 Elective cesarean delivery is not commonly performed prior to 39 weeks of gestation, and generally is not performed prior to either labor or PROM among preterm patients to some extent limiting its impact upon rates of PROM. Cesarean delivery for preterm severe and superimposed preeclampsia may have decreased the risk of PPROM to some degree.
It seems unlikely that further studies of supplementation with vitamins C and E at the doses used in this study either for the prevention of preeclampsia or, perhaps, for PROM prophylaxis are warranted. However, it would be over-reaching to suggest that these studies directly or indirectly challenge the validity of the suspected role of reactive oxygen species in the pathogenesis of PROM. Any one or combination of reasons could explain the results including selected drug, dose, population studied, timing, and pharmacologic preparation, to name a few possibilities. In this regard, the previously mentioned study by Casanueva et al.,13 wherein a daily 100 mg supplementation of vitamin C used alone, had positive results. Whether these observations are dose dependent or whether investigations using different dosing and combinations of antioxidants might be successful is unknown.
In light of the lack of effectiveness of this dose combination of vitamins to reduce the incidence of preeclampsia, as noted in consecutively reported randomized trials,4,15–17 and the concerns raised in this investigation regarding the increased risk of PROM with this combination, the empiric clinical use of this combination among patients at risk of preeclampsia or PROM should be abandoned. Future investigations of the role of antioxidant vitamin supplementation in the prevention of PROM should make note of the findings of this study and proceed cautiously.
Acknowledgements
We acknowledge the important contributions of Jutta Thornberry Janet Moore, Steve Litavecz, and Ty Hartwell, RTI International; Susie Meikle, our initial NICHD Project officer; and members of our site research teams: Cincinnati: Les Myatt; Recife: Elias Ferreira de Melo, Antonio Carlos Barbosa Lima, Angelo Manoel Barreto, José Remígio Neto, Eduardo Costa Ramos; Botucatu: José Carlos Peraçoli, Joelcio Francisco Abbade, Anice Vieira de Camargo Martins, Grasiela Bossolan, Kleber Campos, Tania Prevedel ; Porto Alegre: Melissa Prade Hemesath, Cristiano Dihl Zaffari; Campinas: Fernanda G Surita, Eliana Amaral, Mary A. Parpinelli, Fabiana Krupa.
Additionally, we wish to thank Soubhi Kahhale and his research team at the University of Sao Paulo, Brasil, for their important initial contributions to this research.
This effort was supported by Grant Number 1 U01 HD40565 co-sponsored by the National Institute of Child Health and Human Development and the Bill and Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: Poster presentation at the Annual Meeting of the Society for Maternal Fetal Medicine. Poster No. 0685. Dallas Texas. February 2, 2008
ClinicalTrials.gov Identifier: NCT00097110
Condensation: Supplementation of vitamins C and E beginning in the second trimester of pregnancy was noted to increase the risk of premature rupture of the membranes.
References
- 1.Premature rupture of the membranes. ACOG Practice Bulletin. Number 80. 2007 April [Google Scholar]
- 2.Woods JR, Jr, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol. 2001 Jul;185(1):5–10. doi: 10.1067/mob.2001.115868. [DOI] [PubMed] [Google Scholar]
- 3.Woods JR., Jr Reactive oxygen species and preterm premature rupture of membranes-a review. Placenta. 2001 Apr;22 Suppl A:S38–S44. doi: 10.1053/plac.2001.0638. [DOI] [PubMed] [Google Scholar]
- 4.Spinnato JA, Freire S, Pinto e Silva JL, Cunha Rudge M, Martins-Costa S, et al. Antioxidant Therapy to Prevent Preeclampsia: A Randomized Controlled Trial. Obstet Gynecol. 2007 Dec;110(6):1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996 Feb;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 6.DeMets DL, Lan KKG. Interim analysis: the alpha spending function approach. Stat Med. 1994;13(13–14):1341–1352. doi: 10.1002/sim.4780131308. discussion 1353–1356. [DOI] [PubMed] [Google Scholar]
- 7.Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel Methods: applications and recent developments. Ann Rev Public Health. 1988;9:123–160. doi: 10.1146/annurev.pu.09.050188.001011. [DOI] [PubMed] [Google Scholar]
- 8.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 9.Barrett BM, Sowell A, Gunter E, Wang M. Potential role of ascorbic acid and beta-carotene in the prevention of preterm rupture of fetal membranes. Int J Vitam Nutr Res. 1994;64(3):192–197. [PubMed] [Google Scholar]
- 10.Plessinger MA, Woods JR, Jr, Miller RK. Pretreatment of human amnion-chorion with vitamins C and E prevents hypochlorous acid-induced damage. Am J Obstet Gynecol. 2000 Oct;183(4):979–985. doi: 10.1067/mob.2000.106676. [DOI] [PubMed] [Google Scholar]
- 11.Wall PD, Pressman EK, Woods JR., Jr Preterm premature rupture of the membranes and antioxidants: the free radical connection. J Perinat Med. 2002;30(6):447–457. doi: 10.1515/JPM.2002.071. [DOI] [PubMed] [Google Scholar]
- 12.Siega-Riz AM, Promislow JH, Savitz DA, Thorp JM, Jr, McDonald T. Vitamin C intake and the risk of preterm delivery. Am J Obstet Gynecol. 2003 Aug;189(2):519–525. doi: 10.1067/s0002-9378(03)00363-6. [DOI] [PubMed] [Google Scholar]
- 13.Casanueva E, Ripoll C, Tolentino M, Morales RM, Pfeffer F, Vilchis P, Vadillo-Ortega F. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: a randomized trial. Am J Clin Nutr. 2005 Apr;81(4):859–863. doi: 10.1093/ajcn/81.4.859. [DOI] [PubMed] [Google Scholar]
- 14.Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999 Sep 4;354(9181):810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 15.Beazley D, Ahokas R, Livingston J, et al. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2005 Feb;192(2):520–521. doi: 10.1016/j.ajog.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Rumbold AR, Crowther CA, Haslam RR, et al. ACTS Study Group. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006 Apr 27;354(17):1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 17.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006 Apr 8;367(9517):1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 18.Kilsztajn S, Carmo MS, Machado LC, Jr, Lopes ES, Lima LZ. Caesarean sections and maternal mortality in Sao Paulo. Eur J Obstet Gynecol Reprod Biol. 2007 May;132(1):64–69. doi: 10.1016/j.ejogrb.2006.06.005. Epub 2006 Jul 28. [DOI] [PubMed] [Google Scholar]