Abstract
The proportion of fat-free mass (FFM) as body cell mass (BCM) is highly related to whole body resting energy expenditure. However, the magnitude of BCM/FFM may have been underestimated in previous studies. This is because Moore’s equation [BCM (kg) =0.00833 × total body potassium (in mmol)], which was used to predict BCM, underestimates BCM by ~ %. The aims of the present study were to develop a theoretical BCM/FFM model at the cellular level and to explore the influences of sex, age, and adiposity on the BCM/FFM. Subjects were 112 adults who had the following measurements: total body water by 2H2O or 3H2O dilution; extracellular water by NaBr dilution; total body nitrogen by in vivo neutron activation analysis; and bone mineral by dual-energy X-ray absorptiometry. FFM was calculated using a multicomponent model and BCM as the difference between FFM and the sum of extracellular fluid and solids. The developed theoretical model revealed that the proportion of BCM to FFM is mainly determined by water distribution (i.e., E/I, the ratio of extracellular to intracellular water). A significant correlation (r = 0.90, P < 0.001) was present between measured and model-predicted BCM/FFM for all subjects pooled. Measured BCM/FFM [mean (SD)] was 0.584 ± 0.041 and 0.529 ± 0.041 for adult men and women (P < 0.001), respectively. A multiple linear regression model showed that there are independent significant associations of sex, age, and fat mass with BCM/FFM.
Keywords: body cell mass, extracellular water, intracellular water, nutrition status, total body potassium
At the molecular body composition level, fat-free mass (FFM) includes water, protein, bone mineral, soft tissue mineral, and glycogen. At the cellular level, these FFM compartments are organized into body cell mass (BCM), extracellular fluid (ECF), and extracellular solids (ECS) (24). According to Moore et al. (15), BCM is a “component of body composition containing the oxygen-exchanging, potassium-rich, glucose-oxidizing, and work-performing tissue.”
Both BCM and FFM are considered “metabolically active” components and are often used interchangeably to adjust for between-individual differences in resting energy expenditure (REE). Accordingly, interpretation of REE results may vary depending on the adjusting component BCM or FFM.
Forbes (6) was one of the first investigators to explore the association between BCM and FFM. Forbes’s study was based on two assumptions: FFM contains 68.1 mmol of potassium/kg and BCM contains 120 mmol of potassium/kg. The fraction of FFM as BCM was thus assumed stable at 68.1/120 or 0.57 (6). However, later studies showed that the potassium concentration of FFM and the potassium concentration of BCM suggested by Forbes were not accurate. First, the total body potassium mass (TBK)-to-FFM ratio (TBK/FFM) often differs from the assumed value of 68.1 mmol/kg. Many investigators have consistently found lower potassium concentrations in FFM, 54–59 mmol/kg for women and 59–62 mmol/kg for men (4). Second, the potassium content of BCM is not 120 mmol/kg. Both experimental and modeling estimates indicate a mean TBK/BCM of 109 mmol/kg (1, 27). These observations suggest that the BCM content of FFM may not be constant.
In addition, a growing number of experimental studies reveal that the proportion of FFM as BCM varies with some biological factors such as age and adiposity (12, 14, 19). Gallagher et al. (9) reported a sex difference in BCM/FFM, with men and women at 0.51 ± 0.05 and 0.48 ± 0.05, respectively. Wang et al. (20) reported differences between African Americans, Asians, and Caucasians in the fraction of FFM as BCM.
However, all of these previous studies have a now-recognized inherent limitation. BCM was calculated from TBK using Moore’s equation (15), BCM (kg) = 0.00833 × TBK (mmol), which underestimates BCM by ~ 11% (1, 27). Thus previous studies may be inaccurate in their assessment of the BCM-FFM relationship, notably in underestimating the magnitude of BCM/FFM.
The aim of the present study was to develop a cellular-level BCM/FFM model that provides new insights into the quantitative associations between BCM and FFM and to identify the major determinants of variation in BCM/FFM. Specifically, the present study was designed to explore the magnitude and variation of the BCM content of FFM in healthy adults.
SUBJECTS AND METHODS
Subjects
The subject pool consisted of male and female adults who were recruited through local sources, including flyers posted in the medical center and newspaper advertisements. Each subject completed a medical history, physical examination, and screening blood studies to establish the absence of disease. Subjects participated in recreational physical activities but were not actively engaged in any sports training programs. All participants signed an informed consent that was approved by the hospital’s Institutional Review Board.
Body Composition Measurements
Consenting subjects were studied after an overnight fast. Body weight was measured (±0.1 kg) with a digital scale (Weight Tronix, New York, NY) and height (±0.5 cm) with a wall-mounted stadiometer (Holtain, Crosswell, UK). Each subject completed five studies: 3H2O or 2H2O dilution for total body water (TBW); prompt-γ in vivo neutron activation (IVNA) for total body nitrogen (TBN); dual-energy X-ray absorptiometry (DEXA) for body fat and BMC; sodium bromide (NaBr) dilution for extracellular water (ECW), and whole body 40K counting for TBK.
TBW
Each subject completed either tritium (3H2O) or deuterium (2H2O) dilution (17). 3H2O dilution volume was measured at the Body Composition Unit of St. Luke’s-Roosevelt Hospital, and 2H2O dilution volume was measured at the Brookhaven National Laboratory, Upton, NY. The precision was 1.5% [coefficient of variation (CV)] for dilution volume estimation at the two laboratories. The 3H2O and 2H2O dilution volume (in liters) was then converted into TBW mass by multiplying with the correction factor for nonaqueous hydrogen exchange and water density at 36°C [TBW (kg) =0.96 × 0.994 × 3H2O or 2H2O dilution volume (in liters)].
TBN
TBN was quantified using the prompt-γ IVNA facilities at Brookhaven National Laboratory with a CV of 2.7% (3). The TBN (in kg) was then converted to total body protein (TBPro, in kg) as TBPro = 6.25 × TBN (2).
Bone mineral content
A DEXA scanner (Lunar DPX with software version 3.6; Madison, WI) was used in this study to estimate bone mineral content (BMC). The technique used X-rays of two distinct energy levels (40 and 70 keV) that are differently attenuated by BMC, fat, and fat-free soft tissues (16). BMC measured by DEXA represents ashed bone. Bone mineral (Mo) produces 0.9582 g of BMC due to loss of labile components, including bound water and CO2 with combustion. BMC was thus converted to bone mineral (Mo) as Mo = BMC/0.9582. The DEXA system CV is 1.3% for Mo estimation (10).
ECW
ECW was measured by NaBr dilution with a CV of 1.4%. The bromide dilution volume (in liters) was then converted to ECW (in kg) by correcting for the Gibbs-Donnan effect (0.95), and the penetration of bromide into the intracellular space of erythrocytes (0.90) [ECW (kg) =0.95 × 0.90 × NaBr dilution volume (in liters)] (17).
ECW/ICW
TBW is equal to the sum of two compartments: ECW and intracellular water (ICW). The distribution of water between the ECW and ICW was calculated as the E/I ratio
| (1) |
FFM and fat
At the molecular level, FFM can be expressed as the sum of five components: water, protein, bone mineral, soft-tissue mineral (Ms), and glycogen (24):
| (2) |
where TBW is measured by 3H2O or 2H2O dilution (17); TBPro is calculated from TBN measured by prompt-γ IVNA, TBPro = 6.25 × TBN (2); Mo is measured by DEXA (13); Ms is calculated from TBW as Ms = 0.0129 × TBW (25); and glycogen is calculated from TBN as glycogen = 0.275 × TBN (11). Body fat mass was calculated as the difference between body weight and FFM.
BCM
At the cellular level, FFM is equal to the sum of BCM, extracellular fluid (ECF), and extracellular solids (ECS) (24). BCM can thus be expressed as
| (3) |
where ECF is calculated from ECW as ECF = (1/0.98) ×ECW, where 0.98 is the mean hydration of ECF (21); and ECS is calculated from Mo as ECS = 1.732 × Mo (26). The measurements of BCM and FFM were then used to calculate the ratio BCM/FFM.
Statistical Analysis
Group results are presented as means (SD). Differences in body composition between men and women were tested using Student’s t-test, and P < 0.05 was considered statistically significant. Simple linear regression analysis was used to describe the association between measured BCM/FFM and model-predicted BCM/FFM. A multiple linear regression model [BCM/FFM = α + β1 × sex + β2 × age + β3 × %fat + ∊] was applied to analyze the correlation of measured BCM/FFM with biological factors including sex, age, and percent fat. Effect size was expressed as η2 (eta squared). Means of individual differences between measured and model-predicted BCM/FFM ratios were tested for significance by Student’s t-tests. Data were analyzed using SAS v. 8.2 (SAS Institute, Cary, NC).
RESULTS
Development of BCM/FFM Model
At the cellular body composition level, FFM is composed of three compartments: BCM, ECF, and ECS. The primary BCM/FFM model can be written as
| (4) |
In the next stage of model development, our aim was to resolve Eq. 4 into relevant compartment ratios. ICW and ECW are the largest compartments of BCM and ECF, respectively. As noted in our previous study (21), BCM can be expressed as ICW/a and ECF as ECW/b, where a and b are the proportions of BCM and ECF as water, respectively. Similarly, extracellular solids can be expressed as a function of TBW, ECS = c × TBW = c × (ICW + ECW), where c is the ratio of ECS to TBW. In addition, both ICW and ECW are water compartments, and ECW can be expressed as a function of ICW, ECW = (E/I) × ICW, where E/I is the ratio of ECW to ICW.
Equation 4 can thus be converted and simplified to a secondary cellular level BCM/FFM model as
| (5) |
Simplified BCM/FFM Model
Equation 5 reveals that BCM/FFM is determined by four factors: fraction of BCM as ICW (a); fraction of ECF as ECW (b); ratio of ECS to TBW (c); and ratio of ECW to ICW (E/I). Each of the four determinants may vary within a range for healthy adults (21). The approximate mean value and variation range of each determinant in adults is summarized in Table 1. One can thus estimate the influence of each determinant on BCM/FFM value if a determinant takes its extreme values and the other three determinants take their mean value.
Table 1 .
Influence of model determinants on BCM/FFM value
| Determinant | Mean Magnitude of Determinant |
Variation Range of Determinant |
Influence on BCM/FFM Value |
|---|---|---|---|
| a | 0.70 | 0.69–0.71 | 0.548–0.541 |
| b | 0.98 | 0.97–0.99 | 0.542–0.546 |
| c | 0.14 | 0.12–0.16 | 0.552–0.537 |
| E/I | 0.91 | 0.54–1.40 | 0.651–0.447 |
E/I, ratio of extracellular water (ECW) to intracellular water (ICW) (ECW/ICW); a, fraction of body cell mass (BCM) as water (ICW/BCM); b, fraction of extracellular fluid as water (ECW/ECF); c, ratio of extracellular solids to total body water (ECS/TBW); FFM, fat-free mass. The influence of each determinant on BCM/FFM value can be estimated if a determinant takes its extreme values and the other 3 determinants take their mean values.
Of the four determinants, as Table 1 shows, water distribution (i.e., E/I) has the largest influence on BCM/FFM value. Comparatively, the influences of a, b, and c on BCM/FFM are much smaller than the influence of E/I. Therefore, the mean magnitudes (i.e., a = 0.70, b = 0.98, and c = 0.14) are assumed for modeling purposes to be stable in healthy adults. Equation 5 can thus be converted to a simplified model:
| (6) |
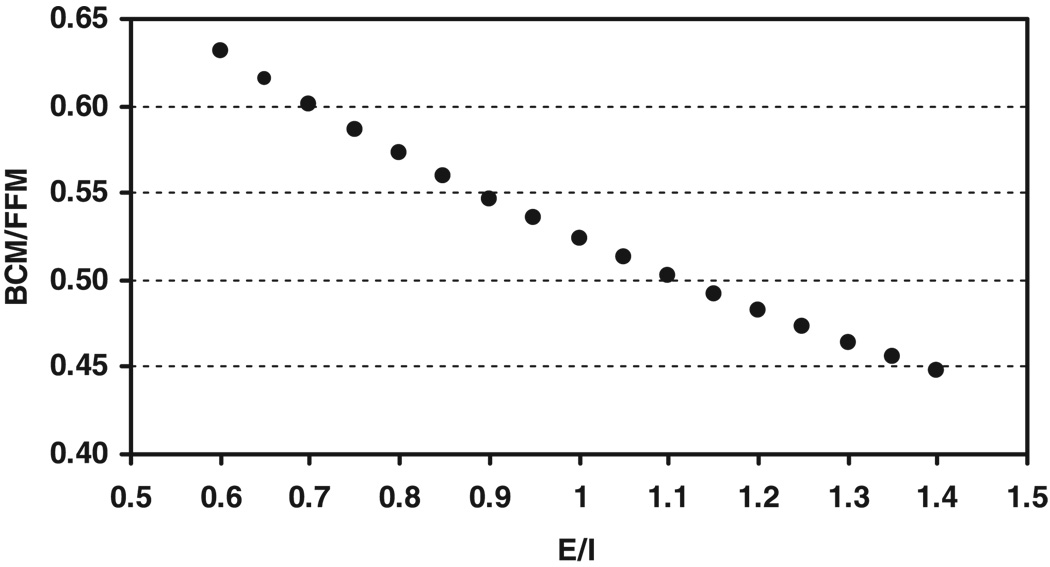
Equation 6 shows that BCM/FFM vs. E/I is a concave curve, as shown in Fig. 1. Although this simplified model is a decreasing nonlinear function, the nonlinear component is small over the usual range of E/I values.
Fig. 1.
Proportion of fat-free mass (FFM) as body cell mass (BCM) by the simplified model (i.e., Eq. 6) on the ordinate and the ratio E/I as extracellular water (ECW) to intracellular water (ICW) on the abscissa.
In the following sections, we use measured BCM/FFM values to evaluate the developed BCM/FFM model in a sample of men and women.
Sample Characteristics
The study group consisted of 112 adult subjects, 14 men and 98 women, ranging in age from 22 to 74 yr, body weight from 42.1 to 105.7 kg, and BMI from 16.8 to 34.7 kg/m2 (Table 2). The women were overweight as a group, with a BMI of 28.3 ± 4.9 kg/m2, whereas the men’s BMI was within the normal range, 21.5 ± 2.3 kg/m2. Three race/ethnicity groups were represented in the sample: African American (n = 55), Caucasian (n = 46), and Hispanic (n = 11).
Table 2.
Baseline characteristics and body composition in 112 adult subjects
| Total (n = 112) | Men (n = 14) | Women (n = 98) | P | |
|---|---|---|---|---|
| Age, yr | 43.0 (10.6) | 40.5 (11.2) | 43.3 (10.5) | 0.39 |
| Body wt, kg | 73.8 (15.0) | 65.1 (7.6) | 75.1 (15.4) | <0.001 |
| Height, cm | 164.1 (7.7) | 174.2 (4.8) | 162.6 (6.9) | <0.001 |
| BMI, kg/m2 | 27.1 (5.2) | 21.5 (2.3) | 28.3 (4.9) | <0.001 |
| %Fat | 34.0 (11.7) | 12.5 (6.5) | 37.0 (8.6) | <0.001 |
| TBW, kg | 33.2 (5.7) | 40.6 (5.2) | 32.2 (4.9) | <0.001 |
| TBN, kg | 1.42 (0.26) | 1.76 (0.23) | 1.37 (0.22) | <0.001 |
| BMC, kg | 2.56 (0.42) | 2.82 (0.31) | 2.52 (0.42) | <0.004 |
| FFM, kg | 45.2 (6.8) | 55.1 (6.8) | 43.8 (5.5) | <0.001 |
| ECW, kg | 16.0 (2.9) | 17.5 (3.2) | 15.8 (2.8) | 0.084 |
| TBK, mmol | 2,690 (487) | 3,396 (488) | 2,589 (396) | <0.001 |
| BCM, kg | 24.2 (4.5) | 32.2 (4.7) | 23.1 (3.2) | <0.001 |
| E/I | 0.958 (0.204) | 0.765 (0.159) | 0.986 (0.195) | <0.001 |
| BCM/FFM (measured) | 0.535 (0.045) | 0.584 (0.041) | 0.529 (0.041) | <0.001 |
| BCM/FFM (predicted) | 0.537 (0.047) | 0.585 (0.043) | 0.530 (0.044) | <0.001 |
| Δ | −0.002 (0.021) | −0.001 (0.009) | −0.002 (0.022) |
Values are means (SD). BCM, body cell mass; BCM/FFM, proportion of FFM as BCM; BMC, bone mineral content; BMI, body mass index; TBK, total body potassium; TBN, total body nitrogen. P, Student’s t-test between men and women; Δ, difference between measured and predicted BCM/FFM.
Measured and Predicted BCM/FFM
FFM and BCM were measured on the basis of Eq. 2 and Eq 3, respectively, and water distribution was calculated on the basis of Eq. 1 (Table 2).
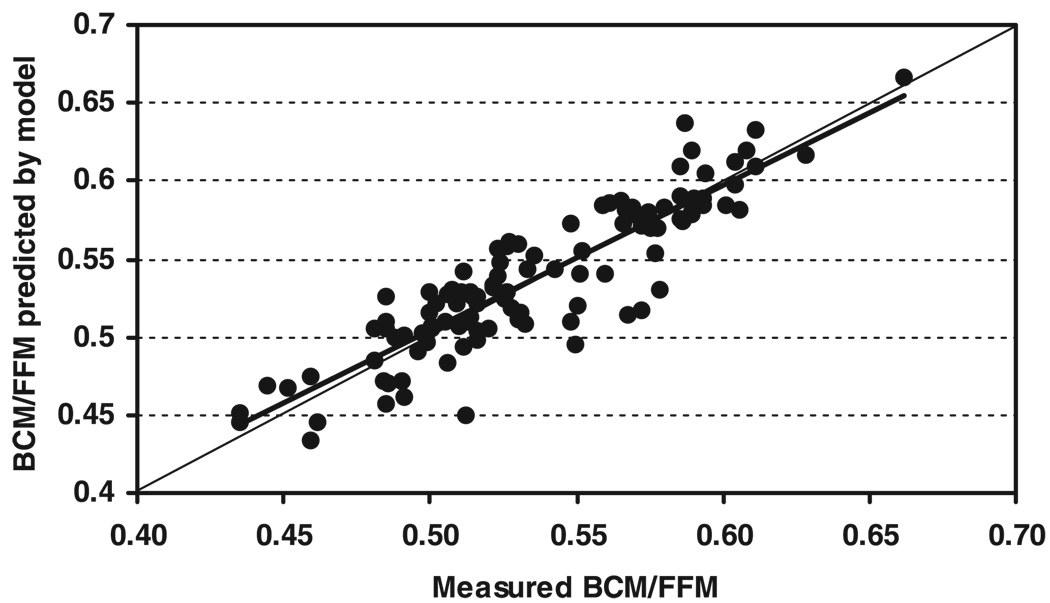
The individual E/I values were applied in Eq. 6 to predict individual BCM/FFM in the men and women. The model-predicted values of BCM/FFM were highly correlated with the corresponding measured BCM/FFM values for all subjects pooled (r = 0.89, P < 0.001; Fig. 2). The measured and predicted mean values also did not differ: 0.585 ± 0.043 vs. 0.584 ± 0.041 (paired t-test, P = 0.76) for the men and 0.530 ± 0.044 vs. 0.529 ± 0.041 (paired t-test, P = 0.44) for the women. The variance of the differences between measured and predicted BCM/FFM in the pooled sample is only 21% that of BCM/FFM, indicating that the simplified model (i.e., Eq. 6) effectively accounts for ~ 80% of the variability in BCM/FFM.
Fig. 2.
Proportion of FFM as BCM predicted by the simplified model (i.e., Eq. 6) on the ordinate and the measured BCM/FFM on the abscissa. Model-predicted BCM/FFM = 0.933 × measured BCM/FFM + 0.037, r = 0.90, P < 0.001; n = 112 adults. Line of identity is shown in the figure.
Association Between BCM/FFM and Biological Variables After Adjusting for E/I
An interesting question is whether variation in E/I accounts for reported associations of BCM/FFM with age, sex, fat mass and similar variables (9, 12, 14, 19, 20). We first applied a general linear regression model to analyze the associations of measured BCM/FFM with biological factors including sex, race (i.e., African American, Caucasian, Hispanic), age, fat mass, and height. The model accounted for 33% of the total variability, but only fat mass had an independent significant association with BCM/FFM (P < 0.001). We then added E/I to the model to determine whether any independent associations persisted after adjusting for E/I. This model accounted for 89% of the variance. The E/I variable showed the strongest association by far with BCM/FFM (P < 0.001, η2 = 0.55), but fat mass remained significant (P < 0.001, η2 = 0.09), and height also reached significance (P < 0.002, η2 = 0.01). The significant coefficients for fat mass and height indicate that there are independent associations of these variables with BCM/FFM after variation in E/I is taken into account.
DISCUSSION
A theoretical BCM/FFM model was developed in the present study. The model with empirically derived coefficients indicates that water distribution is strongly associated with BCM/FFM. This finding helps to explain the subsequent observations of sex, age, and adiposity effects on BCM/FFM. Another finding of the present study was that the BCM/FFM values are much higher than those reported by previous studies: 0.584 ± 0.041 vs. 0.51 ± 0.05 for men and 0.529 ± 0.041 vs. 0.48 ± 0.05 for women (9).
E/I and BCM/FFM
Previous studies reported variation in BCM related to FFM. Mazariegos et al. (14) and Kyle et al. (12) observed a lower BCM/FFM in older adults vs. young adults. Gallagher et al. (9) and Wang et al. (20) found that the fraction of FFM as BCM appears smaller in women than in men. With increasing adiposity there was a corresponding reduction in BCM relative to FFM (19). Although these studies indicate sex, age, and adiposity influences on BCM/FFM, mechanistic explanations were not provided.
Our simplified model (i.e., Eq. 6) reveals that water distribution is the major determinant of BCM relative to FFM. When E/I increases from 0.70 to 1.00, for example, BCM/FFM correspondingly decreases from 0.60 to 0.52 (Fig. 1). Women as a group have a higher E/I value than men; elderly women may have a higher E/I value than young women; and obese women have a higher E/I than women with normal body weight. The whole body, as well as each organ and tissue, is composed of two compartments, cells (including ICW and residuals) and non-cells (including ECW and solids). The E/I ratio can thus be considered as a substitution for the ratio of non-cells to cells. As sex, age, and adiposity influence E/I on a group basis, according to our simplified model, BCM/FFM appears smaller in women than in men, and the magnitudes of BCM/FFM decrease with increasing adiposity and age in elderly subjects. Generally speaking, any biological and pathological factors, which affect water distribution, may influence the BCM/FFM.
BCM/FFM and REE
Body composition has a quantitative association with whole body resting energy expenditure (REE), a major component of total energy expenditure (23). Whole body REE (in kcal/day) can be expressed as the sum of energy expended by individual organs and tissues with the following general model:
| (7) |
where Mi is individual organ-tissue mass (in kg), i is a specific organ-tissue component (i = 1, 2, … n), and ki is the specific resting metabolic rate of individual organs and tissues (in kcal·kg−1·day−1) (4). On the basis of Eq. 7, our group measured metabolically active organs (liver, brain, heart, and kidneys) and two other tissues (skeletal muscle and adipose tissue) by MRI in a cohort of young healthy adults. The predicted REE was nearly identical to REE measured by indirect calorimetry (8).
When applied to older subjects, however, the REE predicted by equation 7 was higher than the measured REE by ~ 11% (P < 0.001) (7). Our previous study found that older subjects have a smaller fraction of FFM as BCM (14). As multiple regression analysis revealed, the BCM/FFM value was lower with greater age (β = − 0.24, P = 0.005) (17). We added a new variable (the fraction of FFM as BCM) to equation 7 (22). A modified theoretical REE model was thus developed as
| (8) |
where 0.58 is BCM/FFM value in Reference Man, and the corresponding BCM/FFM is 0.56 in Reference Women (17). Equation 8 was then applied to predict REE in adult men and women aged 23–88 yr (22). We found that REE predicted by Eq. 8 was correlated with measured REE (r = 0.92, P < 0.001), and the mean difference between measured REE and predicted REE for the whole group was not significant. This study shows that the REE prediction model performed better when BCM/FFM is included.
If age is included as a variable in estimating REE along with the predictions of Eq. 8, the role of the BCM/FFM model remains highly significant (P < 0.001), indicating that its contribution to the estimation of REE is independent of its association with age.
In conclusion, the ratio of BCM to FFM is not a constant. According to our modeling analysis, the variation in BCM/FFM is primarily dependent on water distribution. The larger the E/I, the smaller the BCM/FFM and vice versa. The magnitude of BCM/FFM is one of the major determinants of whole body REE.
Acknowledgments
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-42618 and P30-DK-26687.
REFERENCES
- 1.Cohn SH, Vaswani AN, Yasumura S, Yuen K, Ellis KJ. Assessment of cellular mass and lean body mass by noninvasive nuclear techniques. J Lab Clin Med. 1985;105:305–311. [PubMed] [Google Scholar]
- 2.Cuningham JJ. N × 6.25: recognizing a bivariate expression for protein balance in hospitalized patients. Nutrition. 1994;10:124–127. [PubMed] [Google Scholar]
- 3.Dilmanian FA, Weber DA, Yasumura S, Kamen Y, Kindofsky L, Heymsfield SB, Pierson RN, Jr, Wang J, Kehayias JJ, Ellis KJ. The performance of the BNL delayed-and prompt-gamma neutron activation systems at Brookhaven National Laboratory. In: Yasumura S, Harrison JE, McNeill KG, Woodhead AD, Dilmanian FA, editors. Advances in In Vivo Body Composition Studies. NY: Plenum: 1990. pp. 309–315. [DOI] [PubMed] [Google Scholar]
- 4.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, editor. Energy Metabolism: Tissue Determinants and Cellular Corollaries. New York: Raven; 1992. pp. 61–77. [Google Scholar]
- 5.Ellis JE. Whole-body counting and neutron activation analysis. In: Heymsfield SB, Lohman TG, Wang ZM, Going SB, editors. Human Body Composition. 2nd ed. Champaign, IL: Human Kinetics; 2005. pp. 51–62. [Google Scholar]
- 6.Forbes GB. Human Body Composition. New York: Springer-Verlag; 1987. [Google Scholar]
- 7.Gallagher D, Allen A, Wang ZM, Heymsfield SB, Krasnow N. Smaller organ tissue mass in the elderly fails to explain lower resting metabolic rate. In: In Vivo Body Composition Studies, edited by Yasumura S, Wang J, and Pierson RN Jr. Ann NY Acad Sci. 2000;904:449–455. doi: 10.1111/j.1749-6632.2000.tb06499.x. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D, Belmonte D, Deurenberg P, Wang ZM, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement by magnetic resonance imaging allows accurate in vivo modeling of resting energy expenditure and metabolically active tissue mass. Am J Physiol Endocrinol Metab. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher D, Visser M, Wang ZM, Harris T, Pierson RN, Jr, Heymsfield SB. Metabolically active component of fat-free body mass: influences of age, adiposity, and gender. Metabolism. 1996;45:992–997. doi: 10.1016/s0026-0495(96)90269-3. [DOI] [PubMed] [Google Scholar]
- 10.Heymsfield SB, Waki M, Kehayias JJ, Lichtman S, Dilmanian FA, Kamen Y, Wang J, Pierson RN., Jr Chemical and elemental analysis of humans in vivo using improved body composition models. Am J Physiol Endocrinol Metab. 1991;261:E190–E198. doi: 10.1152/ajpendo.1991.261.2.E190. [DOI] [PubMed] [Google Scholar]
- 11.Kehayias JJ, Heymsfield SB, LoMonte AF, Wang J, Pierson RN., Jr In vivo determination of body fat by measuring total body carbon. Am J Clin Nutr. 1991;53:1339–1344. doi: 10.1093/ajcn/53.6.1339. [DOI] [PubMed] [Google Scholar]
- 12.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 13.Lohman TG, Chen Z. Dual-energy x-ray absorptiometry. In: Human Body Composition, edited by Heymsfield SB, Lohman TG, Wang ZM, and Going SB. Human Kinetics. 2005:63–78. [Google Scholar]
- 14.Mazariegos M, Wang ZM, Gallagher D, Baumgartner RN, Wang J, Pierson RN, Jr, Heymsfield SB. Aging in females: changes in the five levels of body composition and their relevance to the two-compartment chemical model. J Gerontol. 1994;49:M201–M208. doi: 10.1093/geronj/49.5.m201. [DOI] [PubMed] [Google Scholar]
- 15.Moore FD, Olsen KH, McMurray JD, Parker HV, Ball MR, Boyden CM. The Body Cell Mass and Its Supporting Enviroment: Body Composition in Health and Disease. Philadelphia, PA: Saunders; 1963. [Google Scholar]
- 16.Pietrobelli A, Formica C, Wang ZM, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol Endocrinol Metab. 1996;271:E941–E951. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- 17.Schoeller DA. Hydrometry. In: Human Body Composition, edited by Heymsfield SB, Lohman TG, Wang ZM, and Going SB. Human Kinetics. 2005:35–50. [Google Scholar]
- 18.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the Task Group on Reference Man. Oxford, UK: Pergamon; 1975. [Google Scholar]
- 19.Waki M, Kral JC, Mazariegos M, Wang J, Pierson RN, Jr, Heymsfield SB. Relative expansion of extracellular fluid in obese versus nonobese women. Am J Physiol Endocrinol Metab. 1991;261:E199–E203. doi: 10.1152/ajpendo.1991.261.2.E199. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Thornton JC, Heymsfield SB, Pierson RN., Jr The relationship between body mass index and body cell mass in African-American, Asian, and Caucasian adults. Acta Diabetol. 2003;40:S305–S308. doi: 10.1007/s00592-003-0094-y. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZM, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: new physiology modeling approach. Am J Physiol Endocrinol Metab. 1999;276:E995–E1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZM, Heshka S, Heymsfield SB, Shen W, Gallagher D. A cellular level approach to predicting resting energy expenditure across the adult years. Am J Clin Nutr. 2005;81:799–806. doi: 10.1093/ajcn/81.4.799. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZM, Heshka S, Zhang K, Boozer C, Heymsfield SB. Resting energy expenditure: systematic organization and critique of prediction methods. Obes Res. 2001;9:331–336. doi: 10.1038/oby.2001.42. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZM, Pierson RN, Jr, Heymsfield SB. The five-level model: a new approach to organizing body composition research. Am J Clin Nutr. 1992;56:19–28. doi: 10.1093/ajcn/56.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZM, Pi-Sunyer FX, Kotler DP, Wielopolski L, Withers RT, Pierson RN, Jr, Heymsfield SB. Multicomponent methods: evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. Am J Clin Nutr. 2002;76:968–974. doi: 10.1093/ajcn/76.5.968. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZM, Shen W, Kotler DP, Heshka S, Wielopolski L, Aloia JF, Nelson ME, Pierson RN, Jr, Heymsfield SB. Total body protein: a new cellular level mass and distribution prediction model. Am J Clin Nutr. 2003;78:979–984. doi: 10.1093/ajcn/78.5.979. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZM, St-Onge MP, Lecumberii B, Pi-Sunyer FX, Heshka S, Wang J, Kotler DP, Gallagher D, Wielopolski L, Pierson RN, Jr, Heymsfield SB. Body cell mass: model development and validation at the cellular level of body composition. Am J Physiol Endocrinol Metab. 2004;286:E123–E128. doi: 10.1152/ajpendo.00227.2003. [DOI] [PubMed] [Google Scholar]