Abstract
Objectives
In contrast to the extensive literature on the frequent occurrence of depressive symptoms in manic patients, there is little information about manic symptoms in bipolar depressions. Impulsivity is a prominent component of the manic syndrome, so manic features during depressive syndromes may be associated with impulsivity and its consequences, including increased risk of substance abuse and suicidal behavior. Therefore, we investigated the prevalence of manic symptoms and their relationships to impulsivity and clinical characteristics in patients with bipolar depressive episodes.
Methods
In 56 bipolar I or II depressed subjects, we investigated the presence of manic symptoms, using Mania Rating Scale (MRS) scores from the Schedule for Affective Disorders and Schizophrenia (SADS), and examined its association with other psychiatric symptoms (depression, anxiety, and psychosis), age of onset, history of alcohol and/or other substance abuse and of suicidal behavior, and measures of impulsivity.
Results
MRS ranged from 0 to 29 (25th–75th percentile, range 4–13), and correlated significantly with anxiety and psychosis, but not with depression, suggesting the superimposition of a separate psychopathological mechanism. Impulsivity and history of substance abuse, head trauma, or suicide attempt increased with increasing MRS. Receiver-operating curve analysis showed that MRS could divide patients into two groups based on history of alcohol abuse and suicide attempt, with an inflection point corresponding to an MRS score of 6.
Discussion
Even modest manic symptoms during bipolar depressive episodes were associated with greater impulsivity, and with histories of alcohol abuse and suicide attempts. Manic symptoms during depressive episodes suggest the presence of a potentially dangerous combination of depression and impulsivity.
Keywords: alcohol abuse, bipolar disorder, depression, impulsivity, mania, mixed depression, mixed state, suicide
Episodes of bipolar disorder can combine depressive and manic symptoms (1, 2). Such episodes are associated with a worse overall course of illness (3, 4) and are more difficult to treat than episodes of depression or mania alone (5). Most information about such episodes pertains to mixed manic episodes, defined in DSM-IV as manic episodes with syndromal depression. Depressive episodes accompanied by manic symptoms are less studied but may be at least as prevalent as mixed mania (6, 7). There may be a categorical subtype of depressive episodes with syndromal mania, or manic symptoms could represent a continuous dimensional property of depressed episodes. Most literature has focused on the former possibility. ‘Mixed depression’ has been defined as depressive episodes with at least three manic symptoms, although definitions based on factor analysis and on severity of manic symptom ratings appear essentially equivalent (6, 8). Patients who have depressive episodes with manic symptoms, without histories of free-standing mania or hypomania, resemble patients with bipolar disorder in terms of age of onset, family history, and other clinical characteristics (9) including prevalence of alcohol abuse (10). Patients appear to vary in lifetime susceptibility to episodes that combine depressive and manic features (11, 12). Whether as a definite subtype of depressive episodes or a threshold level of a dimensional characteristic, susceptibility to manic symptoms during depressive episodes may, therefore, be related to interepisode characteristics that are associated with an unfavorable course of illness.
We have reported that measures of impulsivity are higher in manic than in euthymic subjects with bipolar disorder (13, 14). Depressive episodes with concomitant manic symptoms could, therefore, represent a combination of depression and impulsivity. Susceptibility to manic symptoms during depressive episodes may be associated with higher trait impulsivity, potentially superimposed on the depressive syndrome. This combination is potentially dangerous in terms of risk for violence, substance abuse, or suicide (15–17).
Manic symptoms during depressive episodes could lie along a continuum, best understood as a dimensional construct, or could define a mixed subtype of depressive episodes. The purpose of the present study was: (i) to characterize the occurrence of manic symptoms in bipolar depressive episodes; (ii) to determine the relationship between manic symptoms and state or trait impulsivity; and (iii) to determine the impact of the presence of manic symptoms on suicidal behavior and course of illness.
Methods
Subjects with bipolar disorder were recruited through clinical referral and advertisements. The study procedures were described in detail and written informed consent was obtained before subjects participated in the study, which was approved by the Committee for the Protection of Human Subjects, the Institutional Review Board for the University of Texas Health Science Center at Houston. Diagnosis used the Structured Clinical Interview for DSM-IV (SCID) (18). The fifty-six subjects in this study had depression as a primary complaint and met DSM-IV criteria for bipolar disorder, type I or II, with a major depressive episode. Thirty-one of them were in a previous study of suicidal symptoms (19). Severity of depressive, manic, anxious, and psychotic symptoms was determined using the change version of the Schedule for Affective Disorders and Schizophrenia (SADS-C) (20). The SADS-C contains independent subscales measuring mania, depression, anxiety, and psychosis designed to minimize the overlap in constructs like agitation or sleep disturbance that can occur when distinct depression and mania scales are used (20). While subjects met diagnostic criteria, including duration, for a major depressive episode, this analysis did not involve duration of manic symptoms, only their presence at the time of testing. As in previous studies, we used the convention of giving a score of 0, rather than 1, if a symptom was absent, providing a more intuitive view of proportional changes or differences in severity without affecting results of statistical analysis (21, 22).
History of a substance use disorder was determined using the SCID (18). Subjects for whom the presence of substance abuse was unclear were excluded from analyses relevant to substance abuse. History of head trauma was determined by clinical history corroborated by medical records. Subjects for whom the significance of head trauma was unclear were excluded from relevant analyses.
Impulsivity as a stable trait was evaluated using the 30-item Barratt Impulsiveness Scale (BIS-11) (23). This scale has three second-order oblique factors: attentional impulsivity, an inability to tolerate cognitive complexity; motor impulsivity, a tendency to act impetuously; and non-planning impulsivity, a lack of sense of the future (24).
State-dependent human laboratory performance impulsivity was measured using the Immediate and Delayed Memory Task (IMT–DMT), based on the Continuous Performance Test (25). In brief, for the IMT, subjects view five-digit numbers on a computer screen for 0.5 s with a 0.5-s interval between numbers, and are instructed to respond if a number matches the preceding number. The DMT is similar except that the number ‘12345’ is shown three times for 0.5 s, at 0.5-s intervals, between the index number and the number to which the subject must respond. Greater impulsivity is associated with commission errors (CE), where a subject responds to a number in which four of five digits are correct (26). Impulsive errors are expressed as CEs corrected for the rate of correct detections (CD). This measure of impulsivity is elevated during manic episodes (14) and in other impulsive populations including subjects with disruptive behavior disorders (26, 27) and substance abuse (28, 29). The time of latency to CDs or CEs is also measured as an indicator of response speed. These methods have been described in detail (26, 30).
Statistical analyses used conventional analysis of variance, t-tests, or correlational analysis when data were normally distributed. Otherwise, appropriate non-parametric tests were used. For correlations involving variables whose distributions were not normal, Kendall tau was used because of its balance between power and control of type 1 error compared with Pearson or Spearman correlation coefficients (31). Effect sizes for two-way comparisons were calculated as the difference in means divided by the pooled standard deviation, as described by Cohen (32: p. 20). Receiver-operating characteristic (ROC) analysis (33) was carried out using SAS, version 9.1.
Results
Distribution of manic symptoms in bipolar depressed subjects
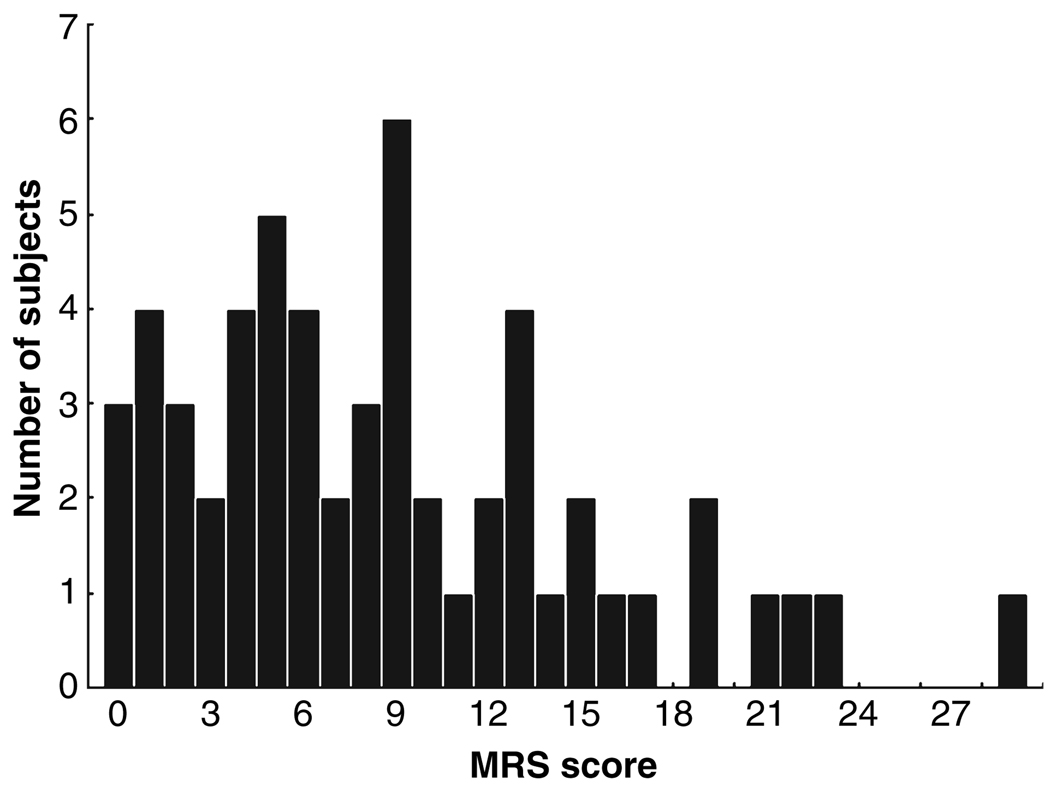
Schedule for Affective Disorders and Schizophrenia Mania Rating Scale (MRS) scores in patients with bipolar depressive episodes ranged from 0 to 29, as shown in Fig. 1. The mean ± SD was 8.75 ± 6.5, median was 8, and the 25th–75th percentile range was 4–13. Only three subjects had scores of 0. The distribution did not depart significantly from normal [Kolmogorov–Smirnov d = 0.13; χ2 (5 df) = 8.4, p = 0.13].
Fig. 1.
Distribution of Schedule for Affective Disorders and Schizophrenia Mania Rating Scale (MRS) scores in subjects with bipolar depression.
Relationships between manic and other psychiatric symptoms
Table 1 summarizes correlations among severity of depression, mania, anxiety, and psychosis scores across the entire group of bipolar depressed subjects. Severity of psychosis and of anxiety correlated with severity of mania; severity of anxiety correlated significantly with severity of depression. Severity of depression did not correlate significantly with severity of mania. Because both depression and mania correlated similarly with anxiety scores, it is possible that a relationship between depression and mania scores could have been obscured by the common correlation with anxiety. This did not appear to be the case, as the correlation between residual scores for depression and mania after regression with anxiety scores was 0.072.
Table 1.
Correlations among psychiatric symptom scores in bipolar depressed patients
| Depression | Mania | Anxiety | Psychosis | |
|---|---|---|---|---|
| Depression | – | 0.240 | 0.450** | 0.068 |
| Mania | 0.240 | – | 0.425** | 0.249* |
| Anxiety | 0.450** | 0.425** | – | 0.151 |
| Psychosis | 0.068 | 0.249* | 0.151 | – |
The table shows subscales of the Schedule for Affective Disorders and Schizophrenia, change version. Pearson r is shown except for correlations involving psychosis scores, where Kendall tau is given; n = 56.
Significance: *p < 0.05.
p < 0.01.
Relationship to clinical features and complications of illness
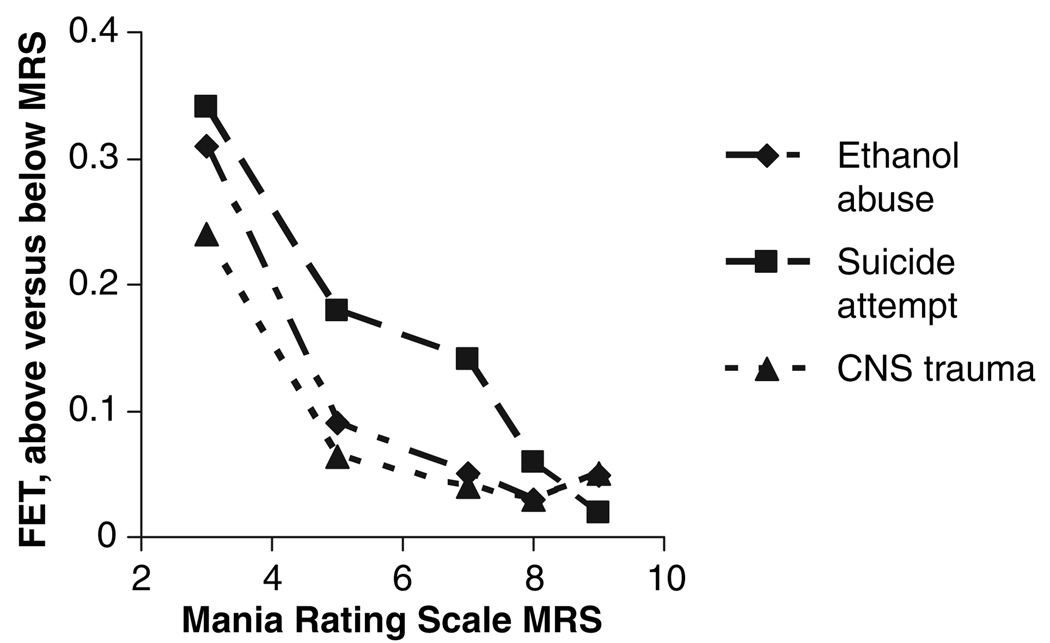
To determine whether increasing MRS scores were associated with changes in clinical characteristics of depressed subjects, we compared the Fisher’s Exact Test score for clinical differences between subjects with higher versus lower MRS scores. Fig. 2 shows that, as MRS scores increased, the Fisher’s Exact Test scores for history of an alcohol use disorder, head trauma, or of a suicide attempt decreased. This is consistent with an increased probability of alcohol abuse, head trauma, or suicide attempt history as MRS score increased.
Fig 2.
Fisher’s Exact Test (FET) for comparison of depressed subjects above versus below each MRS score. The comparison becomes significant for ethanol abuse and for head trauma at MRS > 7 (with 27 subjects scoring lower and 29 higher), and for suicide attempt at MRS > 8 (with 30 subjects lower and 26 higher).
Manic symptoms and impulsivity in depressive episodes
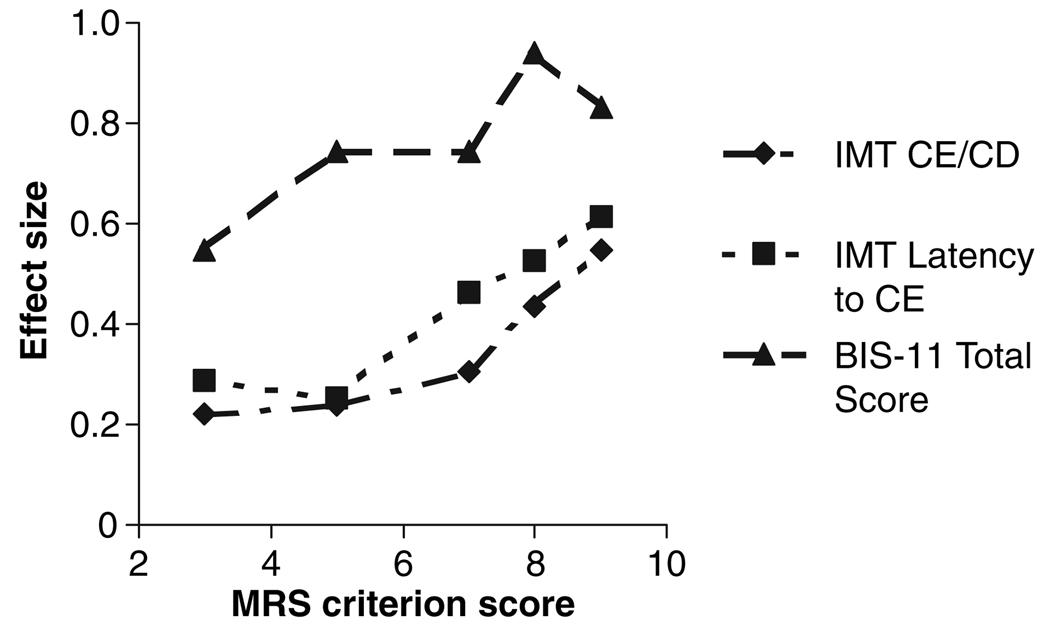
Fig. 3 shows effect sizes comparing measures of impulsivity in subjects scoring above and below a series of MRS scores. There were progressive increases in effect size as the MRS score increased. For MRS score of 8 or higher, effect sizes for the IMT measures reached 0.5, described by Cohen as moderate or ‘visible to the naked eye’ (32: p. 26). The BIS score was more sensitive than eitherIMT–DMT measure, with a ‘large’ effect size [>0.8; overlap between groups of less than 50% (32)] at MRS of 8.
Fig 3.
Effect sizes for comparisons of impulsivity measures in bipolar depression as a function of Mania Rating Scale (MRS) score. The graph shows effect size for comparing those above versus below each MRS score. The Immediate Memory Task (IMT) measures required MRS scores of about 8 to reach moderate effect sizes, while the Barratt Impulsiveness Scale (BIS) total score had a comparable effect size at a MRS of only 3 or 4 (at MRS > 3, 12 subjects scored lower and 44 higher). CE = commission errors; CD = correct detections.
Relationships between impulsivity and psychiatric symptoms
Barratt Impulsiveness Scale scores correlated significantly with MRS scores, as shown in Table 2. Correlations with psychosis and anxiety scores were not significant after correction for multiple analyses, and there were no significant correlations with depression scores. Multiple linear regression confirmed that only the MRS score contributed to variance in BIS scores (partial r = 0.388, p < 0.005) without significant contributions from anxiety (partial r = 0.083) or depression (partial r = 0.010) scores. Measures of IMT performance had no significant univariate correlations with symptom ratings, although for CEs/CDs, there were trends at 0.05 < p < 0.1 for psychosis, MRS, and depression.
Table 2.
Correlations between symptom ratings and impulsivity measures
| MRS | Depression | Anxiety | Psychosis | |
|---|---|---|---|---|
| IMT CE/CD | 0.234 | 0.249 | 0.203 | 0.172 |
| BIS Total | 0.388* | 0.135 | 0.243 | 0.105 |
| BIS Attention | 0.398* | 0.124 | 0.319 | 0.220 |
| BIS Motor | 0.411* | 0.095 | 0.207 | 0.032 |
| BIS non-planning | 0.193 | 0.115 | 0.146 | 0.073 |
Pearson r is shown except for correlations involving psychosis scores, where Kendall tau is given; n = 56.
Significance after correction for multiple comparisons: *p < 0.01.
MRS = Mania Rating Scale; IMT = Immediate Memory Task; CE = commission errors; CD = correct detections; BIS = Barratt Impulsiveness Scale.
Manic symptoms and clinical features: ROC analysis
The data summarized above suggest that, as MRS scores increased, subjects scoring above versus below each score diverged with respect to alcohol use disorder or suicide attempt history and measures of impulsivity. These differences become statistically significant for MRS scores of about 8 or more. This suggests that severity of manic symptoms may distinguish two groups of bipolar depressed patients who differ in their clinical characteristics.
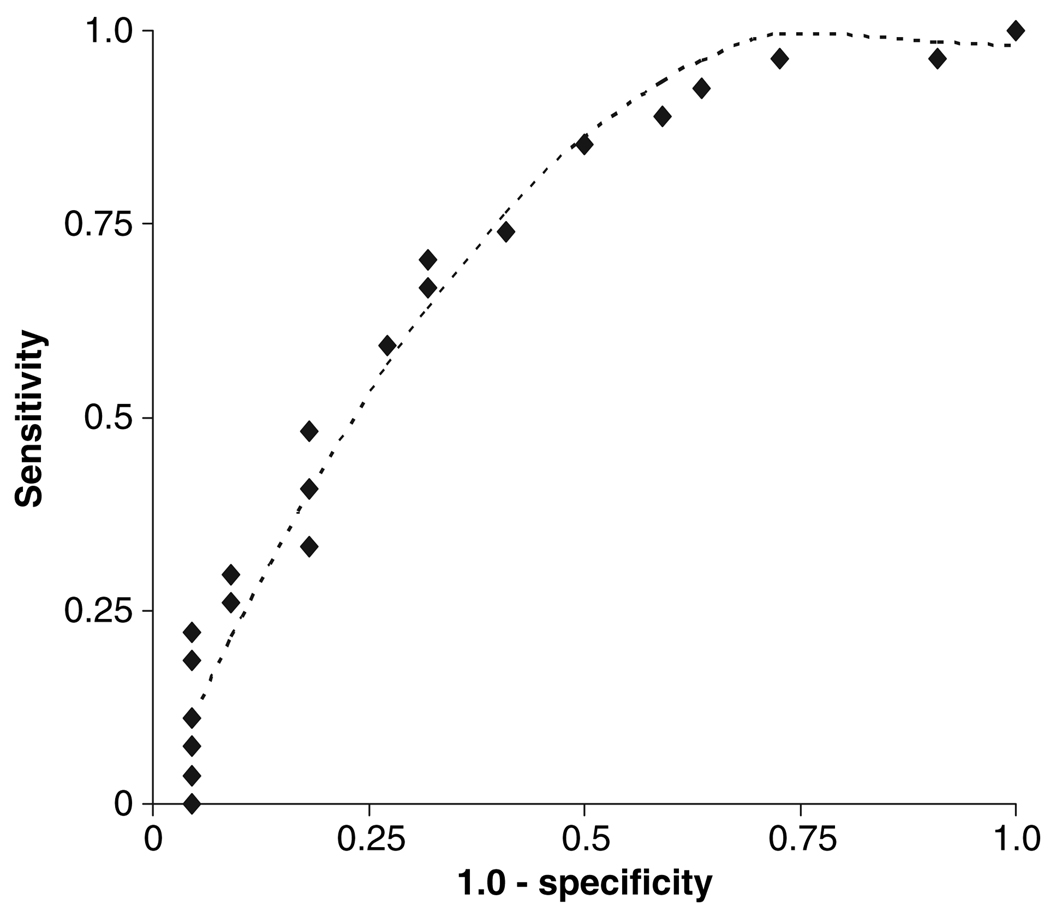
To determine whether severity of manic symptoms could potentially differentiate depressed patients with bipolar disorder, we conducted a ROC analysis using MRS score as a predictor for having history of both alcohol abuse and attempted suicide. The results are shown in Fig. 4. MRS scores were a significant predictor (AUC = 0.726, maximum-likelihood χ2 = 4.54, p = 0.03).
Fig 4.
Receiver operating characteristic curve for Mania Rating Scale (MRS) as a predictor of having history of alcohol abuse and suicide attempt. As described in the text, area under the curve = 0.72, and the inflection point occurred at the point corresponding to MRS = 6, where sensitivity = 0.85 and specificity = 0.55. The dashed line shows the curve for least squares fitting to a quadratic equation.
At the inflection point of the ROC curve, MRS = 6, sensitivity = 0.85, and specificity = 0.55. Inspection of the data in Fig 2 and Fig 3 confirms that a more stringent criterion for mania scores might provide a more clinically useful way to distinguish subtypes of bipolar depression. The ROC analysis shows that MRS scores could potentially be used to classify depressed subjects (34).
Discussion
Manic symptoms, although generally mild, were common in bipolar depressive episodes. The distribution of mania scores was unimodal. This differs from bimodal distributions of depression scores reported by us and others in manic episodes (35–37). As MRS scores increased, there were progressive changes in clinical characteristics and in impulsivity.
Episode characteristics and course of illness
Susceptibility to episodes of illness that contain both depression and mania appears to be associated with a worse course of illness (3, 4). For example, this report (Fig. 2) confirms an earlier report that episodes with prominent depressive and manic symptoms appear related to previous neurological insults (38).
The combination of depression and impulsivity is associated with behavioral risk (16). We and others have reported increased impulsivity to be associated with substance abuse (28, 39) and with suicidal behavior (19, 40, 41). In the current study, increasing MRS score was associated with more frequent and more rapid impulsive responses on the IMT (Fig. 3), reflecting the tendency to make rapid, unplanned responses, similar to subjects with histories of suicide attempts in our previous report (19). Elevated BIS scores reflect increased trait impulsivity, not limited to the current episode (13, 23), suggesting that depressed patients with manic symptoms have a long-term susceptibility to impulsivity (Fig. 3). In the current study, increasing MRS score was associated with greater likelihood that subjects had histories of alcohol abuse, head trauma, and suicide attempts (Fig. 2). These characteristics are consistent with consequences of elevated trait impulsivity.
Because this was a cross-sectional study, the direction of causality between impulsivity and other characteristics cannot be defined. For example, risk for either substance abuse (42) or head trauma (43) appears to be associated with increased impulsivity. Conversely, either head trauma (44) or alcohol administration (45) can increase impulsivity. Subjects with histories of substance use disorders have increased impulsivity even in the absence of current substance use (28), suggesting either that impulsivity was a predisposing factor, or that the effect of substance use on impulsivity can persist for years.
Relative specificity of depressive and manic symptom ratings
Symptoms attributed to depression or mania can overlap. The SADS-C combines evaluation of depression, mania, psychosis, and anxiety (20, 37). The SADS-C is designed to minimize overlap because it is intended to measure these characteristics simultaneously (20). If anything, the SADS-C is biased against the reporting of manic symptoms during depressive episodes. There are specific instructions to avoid double-scoring of items like agitation, overt anger, irritability, and sleep disturbance (20). The lack of correlation between depression and mania scores (Table 1) suggests that mania and depression rating items were differentiated.
Agitated depression can overlap with depressive episodes that have manic symptoms (46). Some, but not all, bipolar depressive episodes meeting Research Diagnostic Criteria for agitated depression also have core features of mania, such as racing thoughts and increased goal-directed activity (47, 48). In a large outpatient population, about 70% of agitated bipolar depressive episodes were reported as mixed, i.e., having at least four manic symptoms (which could include motor agitation) (48). Therefore, some, but not all, agitated depressive episodes have manic features.
Mania during depression: dimensional or categorical?
There are two limiting conceptualizations for combinations of depressive and manic symptoms. The first is a dimensional construct in which episodes are viewed as consisting of symptoms combined in different proportions. As shown in Fig 2 and Fig 3, clinical characteristics of patients in depressive episodes appear to change in a continuous fashion as a function of mania scores. The second is a categorical model, assuming that a critical level of mania severity defines a specific type of episode, as proposed by Benazzi (6, 8). This approach has the potential to classify patients in a manner that potentially can predict course of illness and treatment response, and is consistent with Fig 2 and Fig 3. A compromise is to regard manic symptoms as dimensional, but having a threshold level at which clinical consequences are meaningful.
Our results show that, while it is possible to classify bipolar depressed subjects according to their MRS scores, a definitive criterion remains to be established. The ROC analysis was only modestly significant, and the choice of alcoholism and suicide attempt history, while clinically relevant characteristics, is an indirect measure of membership in any mixed depressive class. Choice of a different dependent variable, such as BIS score or head trauma, would have produced a ROC curve with a different inflection point. More definitive efforts at classification will require a larger group of subjects.
Mania Rating Scale scores had a unimodal distribution in bipolar depressed subjects, but Fig 2–Fig 4 show that a high MRS score was associated with greater impulsivity and a more complicated course of illness than a lower score. The range of scores over which these clinical changes take place is modest compared with the MRS score of 15 or higher required to enter an acute mania treatment study (21).
The focus of this study is on the effects of the range of manic symptoms during syndromal depressive episodes in subjects with bipolar disorder. There was no duration requirement other than that of 1 day implicit in the SADS-C, and duration of manic symptoms was generally shorter than required to meet DSM-IV criteria for a manic, or even hypomanic, episode. A longitudinal analysis of behavior would be needed to establish whether duration of symptoms or affective lability was the more relevant characteristic. Some of the subjects in this study had MRS scores high enough to potentially meet DSM-IV criteria for a mixed state, but few subjects met these criteria. It would be arbitrary not to include such subjects in this analysis.
Potential limitations of this study should be considered when interpreting the results. The number of patients was relatively small, limiting our ability to investigate the potential classification of episodes in detail. No personality measures were obtained that could have been compared with the impulsivity measures. As noted above, this was a cross-sectional study and did not consider duration of manic symptoms.
In summary, the present study investigated manic symptoms in depressive episodes among subjects already known to have bipolar disorder. Our aim was to examine relationships between the degree of manic symptoms during depressive episodes and clinical characteristics. The results showed that the presence of manic symptoms during depressive episodes was related to greater current and lifetime behavioral risk. Manic symptoms appear to be a dimensional component of bipolar depressive episodes, but may have a threshold of severity associated with increased impulsivity and associated behavioral risks. This may reflect a combination of depression with trait impulsivity. While manic symptoms were associated with more severe previous complications, their predictive value, and the validity of a subtype of depression defined on the basis of manic symptoms, must be confirmed prospectively.
Acknowledgements
We thank Joseph R. Calabrese and John E. Overall for valuable suggestions and Glen Colton, PsyD, Mary Pham, and Sabah Abutaseh for their assistance. This study was supported in part by the Pat R. Rutherford, Jr Chair in Psychiatry (ACS) and by NIH grants RO1 MH 69944 (ACS), RO1 AA12046 (DMD), RO1 DA08425 (FGM), and KO2 DA00403 (FGM). This report is dedicated to the memory of our late, esteemed colleague Ernest S. Barratt, PhD.
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Himmelhoch JM, Mulla D, Neil JF, Detre TP, Kupfer DJ. Incidence and severity of mixed affective states in a bipolar population. Arch Gen Psychiatry. 1976;33:1062. doi: 10.1001/archpsyc.1976.01770090052004. [DOI] [PubMed] [Google Scholar]
- 2.McElroy SL, Keck PE, Jr, Pope HG, Jr, Hudson JI, Faedda GL, Swann AC. Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry. 1992;149:1633–1644. doi: 10.1176/ajp.149.12.1633. [DOI] [PubMed] [Google Scholar]
- 3.Keller MB. The course of manic-depressive illness. J Clin Psychiatry. 1988;49:4–7. [PubMed] [Google Scholar]
- 4.Turvey CL, Coryell WH, Solomon DA, et al. Long-term prognosis of bipolar I disorder. Acta Psychiatr Scand. 1999;99:110–119. doi: 10.1111/j.1600-0447.1999.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 5.Dilsaver SC, Swann AC, Shoaib AM, Bowers TC, Halle MT. Mixed mania associated with non-response to anti-manic agents. Am J Psychiatry. 1993;150:1548–1551. doi: 10.1176/ajp.150.10.1548. [DOI] [PubMed] [Google Scholar]
- 6.Benazzi F. Bipolar II depressive mixed state: finding a useful definition. Compr Psychiatry. 2003;44:21–27. doi: 10.1053/comp.2003.50000. [DOI] [PubMed] [Google Scholar]
- 7.Akiskal HS, Benazzi F. Validating Kraepelin’s two types of depressive mixed states: ‘depression with flight of ideas’ and ‘excited depression’. World. J Biol Psychiatry. 2004;5:107–113. doi: 10.1080/15622970410029919. [DOI] [PubMed] [Google Scholar]
- 8.Benazzi F. Depressive mixed state: dimensional versus categorical definitions. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:129–134. doi: 10.1016/s0278-5846(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 9.Akiskal HS, Benazzi F. Family history validation of the bipolar nature of depressive mixed states. J Affect Disord. 2003;73:113–122. doi: 10.1016/s0165-0327(02)00330-0. [DOI] [PubMed] [Google Scholar]
- 10.Ducrey S, Gex-Fabry M, Dayer A, et al. A retrospective comparison of inpatients with mixed and pure depression. Psychopathology. 2003;36:292–298. doi: 10.1159/000075187. [DOI] [PubMed] [Google Scholar]
- 11.Cassidy F, Ahearn E, Carroll BJ. A prospective study of inter-episode consistency of manic and mixed subtypes of bipolar disorder. J Affect Disord. 2001;67:181–185. doi: 10.1016/s0165-0327(01)00446-3. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Bottlender R, Sievers M, Schroter A, Kleindienst N, Moller HJ. Evaluating the inter-episode stability of depressive mixed states. J Affect Disord. 2004;81:103–113. doi: 10.1016/S0165-0327(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 13.Swann AC, Anderson JC, Dougherty DM, Moeller FG. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Res. 2001;101:195–197. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- 14.Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J Affect Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 15.Corruble E, Damy C, Guelfi JD. Impulsivity: a relevant dimension in depression regarding suicide attempts? J Affect Disord. 1999;53:211–215. doi: 10.1016/s0165-0327(98)00130-x. [DOI] [PubMed] [Google Scholar]
- 16.Simon TR, Swann AC, Powell KE, Potter LB, Kresnow M, O’Carroll PW. Characteristics of impulsive suicide attempts and attempters. Suicide Life Threat Behav. 2001;32 Suppl. 1:30–41. doi: 10.1521/suli.32.1.5.49.24212. [DOI] [PubMed] [Google Scholar]
- 17.Pezawas L, Stamenkovic M, Jagsch R, et al. A longitudinal view of triggers and thresholds of suicidal behavior in depression. J Clin Psychiatry. 2002;63:866–873. doi: 10.4088/jcp.v63n1003. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition. New York: Biometrics Research Institute, New York State Psychiatric Institute; 1996. [Google Scholar]
- 19.Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in bipolar disorder. Am J Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia: Change Version. New York: Biometrics Research, New York State Psychiatric Institute; 1978. [Google Scholar]
- 21.Bowden CL, Brugger AM, Swann AC, et al. Efficacy of divalproex versus lithium and placebo in the treatment of mania. JAMA. 1994;271:918–924. [PubMed] [Google Scholar]
- 22.Swann AC, Bowden CL, Morris D, et al. Depression during mania: treatment response to lithium or divalproex. Arch Gen Psychiatry. 1997;54:37–42. doi: 10.1001/archpsyc.1997.01830130041008. [DOI] [PubMed] [Google Scholar]
- 23.Barratt ES, Patton JH. Impulsivity: cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M, editor. Biological Basis of Sensation-seeking, Impulsivity, and Anxiety. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. pp. 77–116. [Google Scholar]
- 24.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comput. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- 26.Dougherty DM, Bjork JM, Harper RA, et al. Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psychiatry. 2003;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- 27.Dougherty DM, Bjork JM, Marsh DM, Moeller FG. A comparison between adults with conduct disorder and normal controls on a continuous performance test: differences in impulsive response characteristics. Psychological Record. 2000;50:203–219. [Google Scholar]
- 28.Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG. Impulsivity: a link between bipolar disorder and substance abuse. Bipolar Disord. 2004;6:204–212. doi: 10.1111/j.1399-5618.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 29.Moeller FG, Barratt ES, Fischer CJ, et al. P300 event-related potential amplitude and impulsivity in cocaine-dependent subjects. Neuropsychobiology. 2004;50:167–173. doi: 10.1159/000079110. [DOI] [PubMed] [Google Scholar]
- 30.Mathias CW, Marsh DM, Dougherty DM. Reliability estimates for the immediate and delayed memory tasks. Percept Mot Skills. 2002;95:559–569. doi: 10.2466/pms.2002.95.2.559. [DOI] [PubMed] [Google Scholar]
- 31.Arndt S, Turvey C, Andreasen NC. Correlating and predicting psychiatric symptom ratings: Spearman’s r versus Kendall’s tau correlation. J Psychiatr Res. 1999;33:97–104. doi: 10.1016/s0022-3956(98)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- 33.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 34.Stotts AL, Schmitz JM, Grabowski J. Concurrent treatment for alcohol and tobacco dependence: are patients ready to quit both? Drug Alcohol Depend. 2003;69:1–7. doi: 10.1016/s0376-8716(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 35.Cassidy F, Forest F, Murry E, Carroll BJ. A factor analysis of the signs and symptoms of mania. Arch Gen Psychiatry. 1998;55:27–32. doi: 10.1001/archpsyc.55.1.27. [DOI] [PubMed] [Google Scholar]
- 36.Dilsaver SC, Chen YR, Shoaib AM, Swann AC. Phenomenology of mania: evidence for distinct depressed, dysphoric, and euphoric presentations. Am J Psychiatry. 1999;156:426–430. doi: 10.1176/ajp.156.3.426. [DOI] [PubMed] [Google Scholar]
- 37.Swann AC, Janicak PL, Calabrese JR, et al. Structure of mania: depressive, irritable, and psychotic clusters with different retrospectively-assessed course patterns of illness in randomized clinical trial participants. J Affect Disord. 2001;67:123–132. doi: 10.1016/s0165-0327(01)00447-5. [DOI] [PubMed] [Google Scholar]
- 38.Himmelhoch JM, Garfinkel ME. Sources of lithium resistance in mixed mania. Psychopharmacol Bull. 1986;22:613–620. [PubMed] [Google Scholar]
- 39.Brady KT, Myrick H, McElroy S. The relationship between substance use disorders, impulse control disorders, and pathological aggression. Am J Addict. 1998;7:221–230. [PubMed] [Google Scholar]
- 40.Dougherty DM, Mathias CW, Marsh DM, Papageorgiou TD, Swann AC, Moeller FG. Laboratory-measured behavioral impulsivity relates to suicide attempt history. Suicide Life Threat Behav. 2004;34:374–385. doi: 10.1521/suli.34.4.374.53738. [DOI] [PubMed] [Google Scholar]
- 41.Gut-Fayand A, Dervaux A, Olie JP, Loo H, Poirier MF, Krebs MO. Substance abuse and suicidality in schizophrenia: a common risk factor linked to impulsivity. Psychiatry Res. 2001;102:65–72. doi: 10.1016/s0165-1781(01)00250-5. [DOI] [PubMed] [Google Scholar]
- 42.Moeller FG, Dougherty D, Barratt E, et al. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002;68:105. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 43.McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am. 1992;15:395–413. [PubMed] [Google Scholar]
- 44.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 45.Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC. Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in immediate and delayed memory task performance. Alcohol Clin Exp Res. 2000;24:1702–1711. [PubMed] [Google Scholar]
- 46.Benazzi F, Koukopoulos A, Akiskal HS. Toward a validation of a new definition of agitated depression as a bipolar mixed state (mixed depression) Eur Psychiatry. 2004;19:85–90. doi: 10.1016/j.eurpsy.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Swann AC, Secunda SK, Katz MM, et al. Specificity of mixed affective states: clinical comparison of mixed mania and agitated depression. J Affect Disord. 1993;28:81–89. doi: 10.1016/0165-0327(93)90036-j. [DOI] [PubMed] [Google Scholar]
- 48.Benazzi F. Agitated depression: a valid depression subtype? Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1279–1285. doi: 10.1016/j.pnpbp.2004.06.018. [DOI] [PubMed] [Google Scholar]