Abstract
The integration of implanted cartilage is a major challenge for the success of tissue engineering protocols. We hypothesize that in order for effective cartilage integration to take place, matrix-free chondrocytes must be induced to migrate between the two tissue surfaces. A chondrocyte/collagen-scaffold implant system was developed as a method of delivering dividing cells at the interface between two cartilage surfaces. Chondrocytes were isolated from bovine nasal septum and seeded onto both surfaces of a collagen membrane to create the chondrocyte/collagen-scaffold implant. A model of two cartilage discs and the chondrocyte/collagen-scaffold sandwiched in between was used to effect integration in vitro. The resulting tissue was analysed histologically and biomechanically. The cartilage–implant–cartilage sandwich appeared macroscopically as one continuous piece of tissue at the end of 40 day cultures. Histological analysis showed tissue continuum across the cartilage–scaffold interface. The integration was dependent on both cells and scaffold. Fluorescent labeling of implanted chondrocytes demonstrated that these cells invade the surrounding mature tissue and drive a remodelling of the extracellular matrix. Using cell-free scaffolds we also demonstrated that some chondrocytes migrated from the natural cartilage into the collagen scaffold. Quantification of integration levels using a histomorphometric repair index showed that the chondrocyte/collagen-scaffold implant achieved the highest repair index compared to controls, reflected functionally through increased tensile strength. In conclusion, cartilage integration can be achieved using a chondrocyte/collagen-scaffold implant that permits controlled delivery of chondrocytes to both host and graft mature cartilage tissues. This approach has the potential to be used therapeutically for implantation of engineered tissue.
Keywords: Cartilage tissue engineering, Cartilage integration, Collagen biomaterial, Cartilage repair, Osteoarthritis
1. Introduction
Cartilage tissue engineering provides a potential method for the production of three-dimensional implants [1,2]. There are two major biological challenges associated with three-dimensional cartilage regeneration. The first is creating a repair tissue that has similar structural and mechanical properties to articular cartilage [3]. To this end, effective engineering protocols have already been developed in which chondrocytes, usually from young animals, are seeded onto biodegradable scaffolds and cultured in a bioreactor [4,5]. More recently, we have demonstrated that it is possible to engineer hyaline cartilage using bone marrow mesenchymal stem cells derived from elderly patients with OA [6]. None of these engineered tissues are identical to natural hyaline cartilage; in particular they all have a significantly lower collagen content than is found in normal cartilage. However there is evidence that even very immature tissue engineered cartilage will mature into normal tissue once implanted inside the joint [7]. The second major challenge is the need to achieve successful integration across the interface between the host and repair tissue [8–10]. Integration is imperative in order to achieve enduring healing and biomechanical competence [11]. Integrative cartilage repair is thought to be hindered by the lack of matrix-producing cells in the cartilage–cartilage interface area [9,12]. This relative acellularity is due to a combination of chondrocyte loss from lesion edges, avascularity, and the absence of multipotent progenitor cells. Furthermore, blunt trauma has been shown to cause apoptosis of chondrocytes in the defect walls [8,13].
Therefore integration of the repair tissue with surrounding native cartilage must be considered as a critical step in the development of cartilage tissue engineering strategies. Initial, temporary fixation of an engineered cartilage implant may be achieved using sutures or fibrin glue. However there will be a clear discontinuity between the implant and host cartilage, creating a focus for failure [10].
In this study we have explored the role of cell migration between tissue surfaces in driving the process of integration. Chondrocytes residing within the lacunae of natural or engineered cartilage do not normally migrate in this way because they are surrounded by a dense extracellular matrix. Indeed there is evidence that the more mature the cartilage to be integrated, the less effective the integration [14]. Previous studies have proposed coating the surface of engineered cartilage with isolated chondrocytes [15–17]. While these cells may have greater propensity to migrate than endogenous chondrocytes, the number of cells seeded is difficult to control because they are likely to attach only loosely to the seeded tissue or clump together, generating only focal connections between tissue surfaces (unpublished observations).
Therefore, in order to test the hypothesis that integration of cartilage with cartilage requires cell migration between the two tissue surfaces, we have developed a chondrocyte/collagen-scaffold implant in which a thin biodegradable collagen membrane is used to deliver cells across the integrating zone. We demonstrate that this approach allows controlled delivery of proliferating cells that can actively unite one cartilage surface with the other.
2. Materials and methods
2.1. Collagen matrices
The scaffold used was Chondrogide and was a kind gift from Geistlich Biomaterials, Wolhusen, Switzerland. The membrane is a bilayered structure composed of porcine type I and type III collagens with a smooth, dense side and a rough porous side. Each scaffold was individually packed after γ-irradiation in 30 × 40 mm sheets, with a thickness of 1.5–2.0 mm. For experiments, 8 mm discs were punched from the sheet of scaffold using a dermal biopsy punch (Schuco International London Ltd).
2.2. Cartilage explants
Tissue from adult (24–30 months old) cows were obtained at a local abattoir and used within a few hours of slaughter. Natural cartilage cylinders (8 mm in diameter and 4.0 mm thick) were harvested from adult bovine nasal cartilage using a dermal biopsy punch (Schuco International London Ltd). The discs were 8 mm diameter × 4 mm thickness, obtained from the middle of nasal septum. They were rinsed and incubated with phosphate buffered saline (PBS) containing 10% (v/v) Penicillin G (10,000 units/ml)/streptomycin (10,000 μg/ml) antibiotic mixture (P/S; Sigma, Poole, UK) and 1% (v/v) amphotericin B (250 mg/ml; Sigma) for 20 min. Harvested nasal cartilage discs was maintained by culture in basic medium containing Dulbecco's modified Eagle's medium (DMEM, Sigma) with 10 mm Hepes buffer (Sigma), 1.0% (v/v) P/S, 1.0% (v/v) non-essential amino acids (NEAA; Sigma), Glutamax (Sigma), and 10% amphotericin B (Sigma) at 37 °C in a 5% CO2 environment. The remaining cartilage was used for isolation of chondrocytes.
2.3. Devitalisation
In some experiments the explant discs were subjected to three cycles of freeze/thawing to kill the chondrocytes. Devitalisation was evaluated in a group of samples by measuring lactate concentrations in the culture media before and after freezing/thawing cycles in a BioProfile 400 Analyser (Nova Biomedical, Waltham, USA).
2.4. Cell isolation and expansion
Nasal chondrocytes (BNCs) were obtained from nasal septum cartilage fragments. The fragments were minced in PBS containing 10% (v/v) P/S (Sigma) and 1% (v/v) amphotericin B (250 mg/ml; Sigma). Chondrocytes were isolated by sequential digestion at 37 °C with 0.25% (w/v) trypsin (Sigma) for 30 min, followed by incubation in 1.5 mg/ml of bacterial collagenase (Sigma) in complete medium containing 10% (v/v) foetal calf serum (FCS, Sigma) at 37 °C for 14 h in a rotator. Isolated chondrocytes were centrifuged and resuspended in Complete Medium containing DMEM supplemented with basic fibroblast growth factor (FGF-2, 10 ng/ml; Peprotech, London, UK), 10% (v/v) FCS, 1.0% NEAA (Sigma), HEPES buffer (Sigma), P/S (Sigma), Glutamax (Gibco), and 2.0% (v/v) amphotericin B at 37 °C in a 5% CO2 environment. The cells were expanded in Complete Medium containing FGF-2 to increase their number and inhibit their dedifferentiation in culture [18]. Cells were expanded in monolayer for one week and for all the experiments cells from the second passage (P2) were used. The culture medium was changed twice a week.
2.5. Cell labeling
In some experiments, chondrocytes were labeled with the fluorescent dye PKH26 (Sigma) at the end of the isolation and expansion period. The labeling procedure was performed according to the manufacturer's protocol. Briefly, after trypsin release, 10 × 106 cells were washed once in basic medium and resuspended into 1 ml of dilution buffer provided by the manufacturer in the labeling kit. The cell suspension were mixed with same volume of the labeling solution containing PKH26 in a dilution buffer to the final concentration of 4 μm. Labeling was allowed for 7 min at room temperature. The labeling reaction was stopped by adding 1 ml of FCS. The pellet was transferred to a new tube and washed three times in Complete Medium before seeding the labeled cells onto scaffolds.
2.6. Cell seeding
Collagen scaffolds were seeded with BNCs at a concentration of 1.65 × 106 cells/cm2 (or 2.5 × 106 cells/scaffold) in 30 ml of complete medium and placed in ultra low attachment wells of a 24-well plate (Corning®, Acton, USA). Seeding was performed in a dropwise fashion onto the scaffold. After 4 h, 1.5 ml of Complete Medium containing FGF-2 (10 ng/ml) was added and changed daily. Seeded scaffolds were incubated for 48 h at 37 °C in an orbital shaker at 50 rpm.
2.7. Assembling and culture of constructs
Sandwich constructs of two bovine nasal septum discs with a seeded scaffold in between were assembled using skin clips and kept in differentiation medium in vitro for up to 40 days. In all groups, sandwich constructs were cultured in ultra low attachment 6-well plates (Corning®, Acton, USA) in expansion complete medium with FGF-2 (10 ng/ml) for 7 days followed by culture in a differentiation medium consisting of Complete Medium with insulin (10 mg/ml; Sigma) and long acting ascorbic acid (50 mg/ml; Sigma) for 33 days. The medium was replenished twice every week.
The constructs were prepared in several groups: (1) seeded scaffold group (chondrocyte/collagen-scaffold), (2) unseeded scaffold group (membrane only control), (3) cells without scaffold group (cells only control) and (4) without scaffold or cells group (negative control). After 40 days in culture, constructs were divided in three groups and fixed accordingly: (a) in 10% (v/v) neutral buffered formalin for histological analysis, (b) frozen and stored at −80 °C prior to sectioning for cell migration, and (c) placed in complete medium containing 10% (v/v) dimethyl sulfoxide (DMSO; Sigma) and stored at −80 °C prior to biomechanical tests.
2.8. Histological analysis
After 40 days in culture the explants were fixed in 10% neutral buffered formalin, dehydrated and paraffin embedded. Samples were then cut into 4 mm sections and stained with Toluidine blue (Sigma) for assessing morphological details and proteoglycan distribution.
2.9. Histomorphometric image analysis
All histological sections were photographed using a digital Spot camera (Diagnostic Instruments Sterling Heights, MI) and histomorphometric analysis was performed with ImagePro Discovery software (Media Cybernetics, Wokingham, UK). Two perpendicular sections, one at the edge and another at the centre of each construct, were used for histomorphometric analysis. The entire lengths of the scaffold/cartilage or cartilage/cartilage (for controls) were measured with a cursor using a computer mouse to assess the integration. The specimen parameter measured was Repair Index. The repair index was used to quantify the amount of integration the scaffold makes with the surrounding cartilage. This parameter is expressed as a percentage of the total interface lengths of the interface that is connected or bonded to cartilage [19–22]. In each of the samples, three interfaces were visible:
-
1.
Unbound Interface (Disintegration), in which there is no apposition or bonding between the scaffold and surrounding tissues.
-
2.
Bonded Interface (Apposition), scaffold and cartilage are in direct apposition but there is still a clear demarcation of the cell scaffold.
-
3.
Integrated Interface (Integration), the scaffold/cartilage interface is not only joined and continuous but there is no clear demarcation of the interface, with cell migration and matrix remodelling being clearly visible.
To calculate the repair index, we applied the following equations:
d1 and d2 = unbound interface (length, μm), dt = total interface (μm).
d3 and d4 = bonded interface (length, μm), dt = total interface (μm).
d5 and d6 = integrated interface (length, μm), dt = total interface (μm).
2.10. Cell migration
Construct seeded with cells labeled with the red fluorescence PKH26 dye were frozen and later embedded in O.C.T. compound (BDH Chemical, London, UK). 7 μm sections were obtained using a cryostat (Cryosect, Seward Ltd, UK). Slides were air dried for at least 1 h at room temperature and mounted using 1–2 drops DPX mounting media (Fisher Scientific Ltd, Leicestershire, UK). Images of samples were then obtained using a fluorescence microscope. Sections from the same area were also stained with Toluidine blue as a point of reference and comparison. Labeled cells migrating or crossing the scaffold-cartilage interface were observed and photographed to determine if any cell migration occurred.
2.11. Biomechanical tests
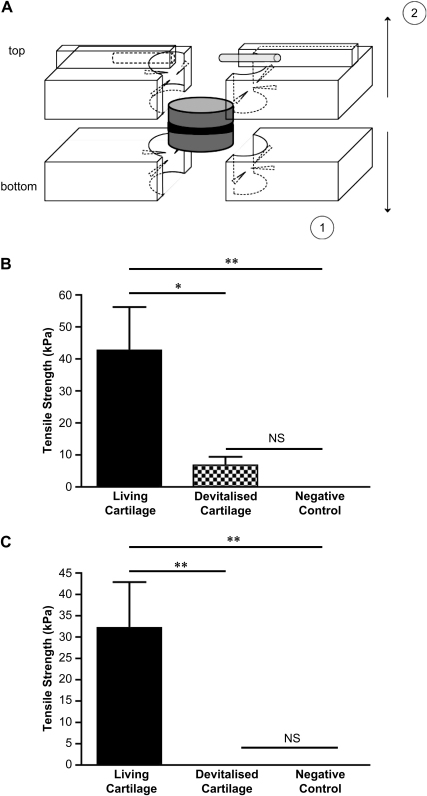
After 40 days in culture, chondrocyte/collagen-scaffold implant and control constructs were placed in Complete Medium containing 10% (v/v) DMSO and stored at −80 °C until mechanical testing. Before testing, samples were thawed and skin clips removed. Thickness and diameter were recorded for each sample using a caliper. Construct thickness ranged from 4.0 to 6.0 mm. Construct diameter was approximately 8 mm. The construct was mounted in the lower half of the holding device of an Instron 6022 mechanical testing frame (Instron, High Wycombe, UK), by clamping the “lower” half of the construct disc between two sliding Perspex sheets fitted with needles to affect gripping and bolted to the Instron (Fig. 5A). The upper half of the device with similar Perspex sheets was closed around the “upper” half of the construct and in turn held in the pneumatic clamp of the Instron. Load/displacement data was logged throughout testing to failure using a 486 PC equipped with series IX data acquisition and analysis software (Instron). This data was used to build a load/deformation curve from which the maximum load at failure (Fmax) at the interface cartilage/scaffold was obtained. Maximum tensile stress (MPa) was then calculated as Fmax [N]/original cross sectional area [mm2].
Fig. 5.
Mechanical strength of integrated cartilage. Collagen membranes were seeded with bovine nasal chondrocytes to create a chondrocyte/collagen-scaffold implant, as described under Materials and methods. The chondrocyte/collagen-scaffold implant was placed between two discs of living or devitalised bovine nasal cartilage and cultured for 40 days. Tensile strength was determined as described under Materials and methods. (A) A diagram of the new biomechanical test configuration, described under Material and methods. (B) Results using the chondrocyte/collagen-scaffold implant and (C) the results using a membrane only control (i.e., no implanted cells). In both panels the negative control (no implanted cells or membrane) is shown for comparison. Each bar is the mean ± SEM (n = 3–5). Data were analysed by ANOVA followed by a Student's t-test with a post hoc Bonferroni correction for multiple comparisons. *p < 0.05; **p < 0.01; NS = not significant.
2.12. Statistical analysis
For mechanical testing, multiple groups were compared by 2 way ANOVA with p < 0.05 taken as significant. Where significant variance was demonstrated, differences between individual groups were then determined using the two-tailed Student's t-test with a Bonferroni post hoc correction. p < 0.05 was taken as significant.
For histomorphometric analysis, multiple groups were compared by analysis of variance using the non-parametric Kruskal–Wallis test with p < 0.05 taken as significant. Where significant variance was demonstrated, differences between individual groups were then determined using the two-tailed Mann–Whitney U-test with a Dunn's post hoc correction. p < 0.05 was taken as significant.
3. Results
3.1. Macroscopic appearance of cartilage integration
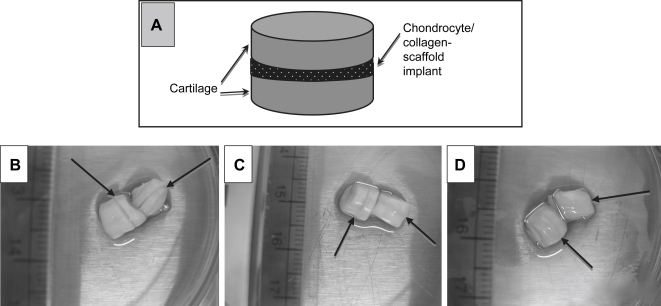
In order to investigate if chondrocytes seeded onto a sheet of biomaterial will facilitate the bonding and integration of fully mature cartilage, we developed a cartilage integration model comprising a chondrocyte/collagen-scaffold implant sandwiched between two discs of mature cartilage (Fig. 1A). The sandwiched constructs were allowed to mature in culture conditions that support matrix deposition. After 40 days in culture, the construct appeared as one piece showing the integrating chondrocyte/collagen-scaffold implant as a very faint line between the bonding cartilage pieces (Fig. 1D). The negative control (no cells or membrane) showed no evidence of integration (Fig. 1B) whilst the membrane only control (cell-free collagen scaffold) showed some apparent integration, but not as marked as that observed when the chondrocyte seeded scaffold implant was used (Fig. 1C).
Fig. 1.
Macroscopic appearance of the zone of cartilage integration. Collagen membranes were seeded with bovine nasal chondrocytes to create a chondrocyte/collagen-scaffold implant, as described under Materials and methods. The scaffold was placed between two discs of bovine nasal cartilage, as shown in (A). In all cases the pieces of cartilage were held together using clinical grade skin clips and cultured for 40 days. (B)–(C) Typical examples of the macroscopic appearance of these cartilage integration constructs. Each photograph shows 2 separate constructs. (B) The negative control (no membrane or cells), (C) the membrane only control (collagen scaffold with no cells) and (D) the chondrocyte/collagen-scaffold implant (collagen membrane with cells). Arrows indicate the site of integration between the two pieces of cartilage in each construct.
3.2. Matrix formation at the zone of integration
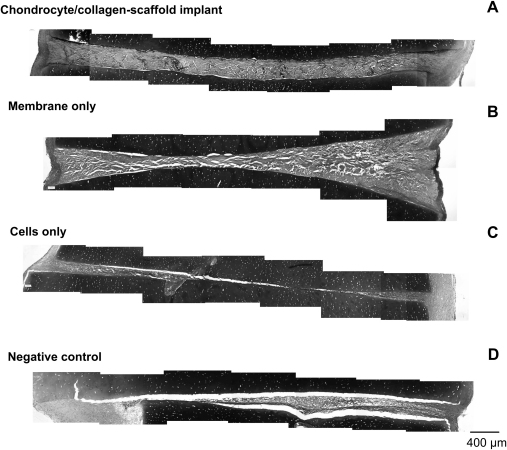
Constructs created using chondrocyte/collagen-scaffold implants showed dense tissue that bridged the space between the two pieces of cartilage (Fig. 2A), creating a close interface between the scaffolds and the cartilage. A similar picture was observed when the cell-free scaffold was used, although the bridging tissue stained less strongly with Toluidine blue, indicating that a less mature extracellular matrix had been formed (Fig. 2B). In control constructs seeded with chondrocytes but without a scaffold (Fig. 2C) or negative controls (no cell or scaffold implant; Fig. 2D), there was tissue formation at the edges of the construct but most of the interface between the cartilage pieces was empty or filled with a quite thin cell layer and minimal extracellular matrix.
Fig. 2.
Histological appearance of the zone of cartilage integration. Collagen membranes were seeded with bovine nasal chondrocytes to create a chondrocyte/collagen-scaffold implant, as described under Materials and methods. The scaffold was placed between two discs of bovine nasal cartilage, as shown in Fig. 1A. All constructs and controls were cultured for 40 days and then processed for histology. Each panel shows a typical paraffin embedded section stained with Toluidine blue at 10× magnification. The images are compilations of serial lateral sections spanning the full width of the integration zone. (A) The chondrocyte/collagen-scaffold implant. (B) The membrane only control (collagen scaffold with no cells), (C) the cells only control (chondrocytes seeded onto the surface of each piece of cartilage) and (D) the negative control (no cells and no membrane).
3.3. The role of endogenous chondrocytes
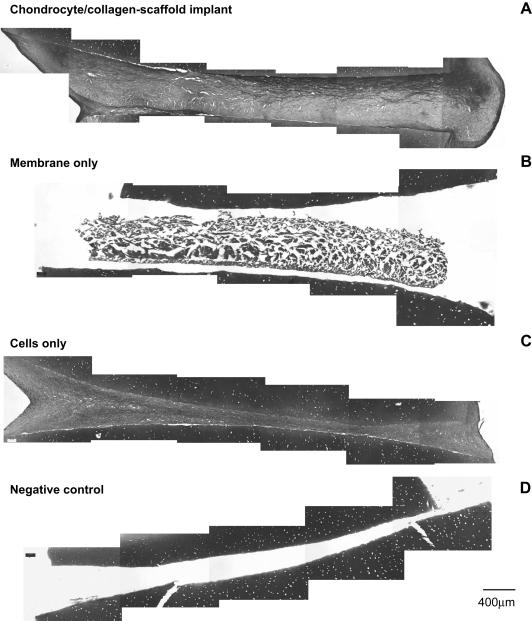
The observation that cell-free scaffold induced tissue formation between cartilage pieces (Fig. 2B) suggested that chondrocytes within the natural cartilage might interact with the scaffold material and contribute to the integration process. To test this possibility, constructs were created using devitalised cartilage (Fig. 3). The chondrocyte/collagen-scaffold formed tissue that integrated with the surrounding devitalised tissue in a similar way to that observed when using living cartilage (compare Fig. 3A with Fig. 2A, respectively) suggesting that implant-induced integration is largely independent of the endogenous cells. However, when the scaffold was used without chondrocyte seeding (membrane only), there was no formation of tissue in the integrating zone between the devitalised cartilage pieces (Fig. 3B). This contrasts with the moderate level of tissue formation in this zone with living cartilage (compare Fig. 3B with Fig. 2B). Control constructs created using living cells seeded onto devitalised cartilage without a scaffold were no different to those created using living cartilage (compare Fig. 3C with Fig. 2C, respectively). Negative control constructs created from devitalised cartilage without implanted cells or scaffold formed no integrating tissue at all (Fig. 3D).
Fig. 3.
Histological appearance of the zone of integration using devitalised cartilage. Collagen membranes were seeded with bovine nasal chondrocytes to create a chondrocyte/collagen-scaffold implant, as described under Materials and methods. The scaffold was placed between two discs of bovine nasal cartilage, as shown in Fig. 1A, that had been devitalised by repeated freeze-thawing. All constructs and controls were cultured for 40 days and then processed for histology. Each panel shows a typical paraffin embedded section stained with Toluidine blue at 10× magnification. The images are compilations of serial lateral sections spanning the full width of the integration zone. (A) The chondrocyte/collagen-scaffold implant. (B) The membrane only control (collagen scaffold with no cells), (C) the cells only control (chondrocytes seeded onto the surface of each piece of devitalised cartilage) and (D) the negative control (no cells and no membrane).
3.4. Cell migration from scaffold to cartilage
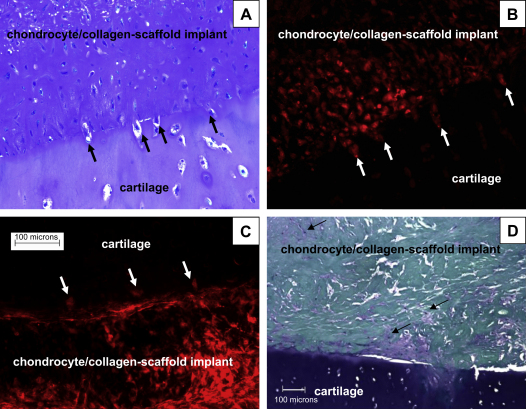
We hypothesized that in order for cartilage integration to occur, chondrocytes from the scaffold must invade the surrounding tissue to form a tissue continuum. To test this hypothesis, seeded chondrocytes were pre-labeled with a fluorescent dye (PKH26) and the cells were used to create constructs as described under Materials and methods. Migrating cells were clearly observed at the cartilage/scaffold interface using fluorescence microscopy of tissue cryosections (Fig. 4B and C). The scaffold-derived chondrocytes migrated into the surrounding fresh cartilage tissue (Fig. 4B) and this process appeared to involve remodelling of the extracellular matrix, shown by the lack of peri-cellular Toluidine blue staining around the migrating cells and the increased intensity of staining along the interface (Fig. 4A). In order to exclude the possibility that PKH26 was leaching out of the implanted cells and being taken up by endogenous cells in the cartilage, we performed the same experiment using devitalised tissue. Once again the scaffold-derived chondrocytes had the capacity to leave the collagen membrane and penetrate the cartilage tissue (Fig. 4C), confirming the chondrocyte/collagen-scaffold implant origin of the labeled cells.
Fig. 4.
Cell migration in cartilage integration constructs. Cell migration from the chondrocyte/collagen-scaffold implant to the surrounding nasal cartilage was determined by pre-labeling the bovine nasal chondrocytes with fluorescent dye PKH26 before seeding them onto collagen membranes, as described under Materials and methods. In (A) and (B) the pre-labeled chondrocyte/collagen-scaffold implant was inserted between two pieces of living bovine nasal cartilage and one of the two interfaces is shown. (A) Stained with Toluidine blue and viewed under white light whilst (B) is viewed under fluorescent light (both 200× magnification). Arrows indicate cells in the process of migrating across the border between the cartilage and the membrane. (C) Cell migration from the collagen membrane into devitalised cartilage (200× magnification). In (D), migration of endogenous chondrocytes from surrounding cartilage into the collagen membrane was determined in membrane only constructs (i.e., no implanted cells), stained with Toluidine blue (100× magnification). Areas of cell infiltration and matrix formation are indicated with arrows.
3.5. Cell migration from cartilage to scaffold
The observation that membrane only implants led to some matrix formation in the originally empty collagen scaffold (Fig. 2B; 100× magnification) led us to investigate this phenomenon further using higher power magnification (200×). There was clear evidence of endogenous chondrocytes from the surrounding tissue invading the cell-free scaffold and elaborating an extracellular matrix (Fig. 4D). This migration of the endogenous cells appeared to involve degradation of the extracellular matrix in some areas of the natural cartilage, adjacent to the collagen membrane, as demonstrated by loss of Toluidine blue staining. These data suggest that reverse migration of endogenous cartilage chondrocytes into the scaffold may play a role in the process of integration.
3.6. Tensile strength of the integration zone
Biomechanical tests were performed to determine the tensile strength of the interface between chondrocyte/collagen-scaffold implant and pairs of cartilage discs cultured in vitro. We devised a new biomechanical test configuration that avoided the classical use of glue (Fig. 5A). Load increased linearly with displacement until the maximum (ultimate) load was achieved. At this load, specimen failed and the construct separated. In all cases, fracture appeared at the interface region between the scaffold and one of the cartilage discs. The interface tissue was considered to have failed at the maximum load, and the tensile strength was calculated as the measured ultimate load divided by the original surface area, giving the stress at failure in kPa [9].
The average tensile strength of chondrocyte-scaffold and control groups (fresh and devitalised) is shown in Fig. 5. Living cartilage constructs containing the chondrocyte/collagen-scaffold implants showed greatest tensile strength, at an average of 42 kPa, which was significantly greater than the negative control, which had no measurable tensile strength (Fig. 5B). Devitalised cartilage constructs containing the chondrocyte/collagen-scaffold implants had only limited tensile strength (Fig. 5B), suggesting that interaction between the collagen scaffold and endogenous cells is necessary for maximal integration.
Surprisingly, the cell-free scaffold (membrane only) used with living tissue also had a measurable and significant tensile strength (Fig. 5C), with a mean of 32 kPa, suggesting that invasion by endogenous cells and limited extracellular matrix formation is enough to induce some degree of functional integration. In support of this conclusion, devitalised tissue implanted with the membrane only had no measurable tensile strength (Fig. 5C).
Controls with cells only (living or devitalised cartilage and cells with no membrane) all had no measurable tensile strength (data not shown).
3.7. Quality of integration
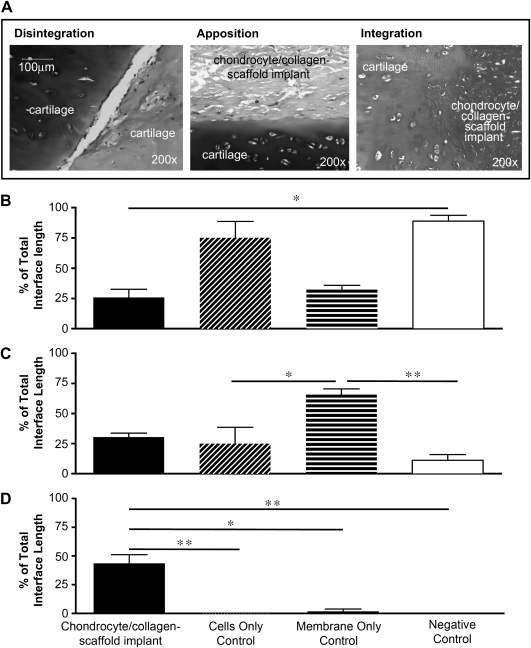
In order to quantify the degree and quality of cartilage integration in the sandwich constructs, we adapted a histomorphometric repair index [19–22] that we have defined as a percentage of the total interface length of the scaffold that is connected or bonded to cartilage. The total potential integration interface was analysed and the proportion falling into each of three quality parameters (Fig. 6A) was expressed as a percentage of the total: (1) Disintegration: an open interface in which both surfaces (cartilage/cartilage or cartilage/scaffold) remain open, without contact; (2) Apposition: both surfaces are in contact but there is a clear demarcation between them; (3) Integration: a fully integrated interface in which there is no clear demarcation, characterised by cell migration and matrix remodelling.
Fig. 6.
Histomorphometric analysis of cartilage integration. Collagen membranes were seeded with bovine nasal chondrocytes to create a chondrocyte/collagen-scaffold implant, as described under Materials and methods. The scaffold was placed between two discs of bovine nasal cartilage, as shown in Fig. 1A. Controls included cells with no membrane, seeded onto the surface of each piece of cartilage (cells only control), collagen scaffold with no cells (membrane only control) and no membrane or cells (negative control). In all cases the constructs were cultured for 40 days and then processed for histology. Morphometric analysis was used to measure the quality of integration across the complete integrating interface (i.e., the full length of both cartilage surfaces). The classification of morphological patterns is shown in (A). The % of integrating surface falling into each morphological category was calculated as described under Materials and methods. (B) The % disintegration, (C) the % apposition and (D) the % integration. Each bar is the mean ± SEM (n = 3–5). Data were analysed by Kruskal–Wallis non-parametric ANOVA followed by a Mann–Whitney U-test with a post hoc Dunn correction for multiple comparisons. *p < 0.05; **p < 0.01. All comparisons not indicated were not significant.
Using this histomorphometric approach, we observed that there was a high level of disintegration in negative controls (no scaffold or cells) and constructs seeded with cells but without a scaffold, accounting for approximately 100% and 75%, respectively of the total interface (Fig. 6B). In constructs with membrane only (cell-free scaffold), or chondrocyte/collagen-scaffold implants (cells seeded onto scaffold), there was little disintegration, accounting for just 25% of the interface, which was significantly lower than the negative control.
Apposition was observed in all constructs containing cell-loaded or unloaded scaffold as well as constructs with cells only (Fig. 6C). There was very little apposition (less than 16%) in negative controls. The percentage of apposition was found to be significantly higher in cell-free scaffold compared to negative controls or cells only controls.
There was no detectable integration (loss of demarcating border) in negative control constructs or those seeded with cells but no scaffold, whilst the membrane only controls only had up to 3% integration. In contrast, those constructs created using a chondrocyte/collagen-scaffold implant showed an extensive integration, reaching up to 50% of the total interface (Fig. 6D). These results demonstrate the requirement for the chondrocyte/collagen-scaffold implant scaffold in order to produce a clear integration with loss of the demarcating border between cartilage and implant.
4. Discussion
We set out to test the hypothesis that integration of cartilage with cartilage requires migration of chondrocytes between the two surfaces. We have demonstrated that this is the case and that the migrating chondrocytes may be derived either from cells that have been grown in vitro and then seeded onto a collagen membrane, or from the cartilage tissue itself. Our observation that a cell-free collagen membrane may stimulate cartilage integration with some degrees of mechanical stability was unexpected. The observation, however, may be misleading because the quality of integration was not as good as that achieved with the c chondrocyte/collagen-scaffold implant. With the cell-free scaffold there was no loss of a demarcating border, only apposition of cartilage tissue with the membrane and partial filling of the membrane with new extracellular matrix. It seems reasonable to presume that any mechanical stability provided by this partial integration will be transient, because the collagen membrane is biodegradable and so cannot provide a permanent focus for integration. In contrast, the loss of demarcating border observed with the chondrocyte/collagen-scaffold implant indicates an integration that is likely to be stable over time because of the continuous nature of the extracellular matrix across the cartilage/implant interface, and is likely to increase as new matrix is deposited. It remains possible that alternative biomaterials with a longer half-life could be developed as cell-free implants for inducing integration.
Until now, there has been no consistent method for assisting the integration of mature cartilage implants with host tissue. Building on our methods for cartilage tissue engineering using different cells and biomaterials [6,23], we have explored the factors that are most important in driving an effective integration between tissues. We used tensile testing to measure any increase in mechanical stability and histomorphometry to estimate the quality of integration, as an indicator of longevity of the integration, with loss of the demarcating border as the decisive factor. Furthermore we explored the role of cell migration through the use of PKH26, a vital dye that permitted the microscopic tracking of the scaffold chondrocytes across the interface. We also explored the role of chondrocytes within the natural cartilage through comparison of living and devitalised tissue. In this way we have built up a comprehensive picture of the key factors that regulate integration.
The collagen membrane was decisive in the process of initiating integration. When cells alone were used there was some filling of the space with proliferating cells but little or no extracellular matrix to bind the pieces of cartilage together. Therefore there was no integration and limited apposition. The collagen membrane on its own led to a moderate degree of extracellular matrix formation and this was a result of infiltration of the membrane by cells migrating from the cartilage. The mechanism by which this infiltration was stimulated by the collagen is unknown, but it apparently involved some degradation of the proteoglycan in the extracellular matrix at the junction of the cartilage and scaffold (Fig. 4D). This moderate degree of matrix formation nevertheless led to an increase in tensile strength that was almost as great as that achieved using the chondrocyte/collagen-scaffold implant. However detailed histomorphometric analysis demonstrated very little true integration as the demarcating border was still clear throughout the integration zone. Therefore, although the collagen membrane is an essential component for driving the integration process, it is clearly not sufficient for a long-term, stable outcome. However combining the collagen membrane with chondrocytes into a chondrocyte/collagen-scaffold implant did lead to complete loss of the demarcating border in up to 50% of the integration zone after just 40 days of in vitro culture. Fluorescent labeling demonstrated clearly that the implanted chondrocytes migrated from the collagen membrane into the cartilage and histological analysis showed that an extensive extracellular matrix formed between the two pieces of cartilage, creating a continuum of tissue.
Although we have gone some way to defining the factors that are most important in cartilage integration, there remain a number of questions that must be answered if this chondrocyte/collagen-scaffold implant approach is to be developed as a practical method for implanting engineered tissue. In this study we have used a single type of collagen membrane and a single cell type, bovine nasal chondrocytes, future studies will need to extend our work to explore the use of articular chondrocytes and stem cells as alternatives. Secondly, we have demonstrated an increase in tensile strength after 40 days in vitro, however successful tissue survival in vivo will require a much greater degree of integration with a far larger increase in tensile strength. Therefore longer term studies and in vivo implantation studies are required in order to determine if this approach is viable clinically.
A range of methods has been developed for the repair of articular cartilage lesions [10,24]. These include osteochondral transplantation [25], microfracture [26] and autologous chondrocyte implantation (ACI) [27,28] with or without the assistance of a scaffold matrix to deliver the cells [29]. A feature of all of these techniques is that their use is limited to the repair of focal lesions and patients with osteoarthritis (OA) are mostly excluded from treatment. OA cartilage lesions are generally large and unconfined [30] and so do not provide an appropriate environment for chondrocytes or stem cells to be retained long enough to elaborate an extracellular matrix. Therefore successful repair of OA cartilage lesions is only likely to be achieved when mature, three-dimensional cartilage implants can be generated that have enough extracellular matrix for fixation and permanent integration within the joint.
A number of studies have investigated methods for enhancing integration of cartilage with cartilage. Bos et al. [31] investigated the effects of enzymic matrix digestion on cartilage integration. Their hypothesis was that accumulation of dead cells at the wound edge inhibits integration and removal of this layer of tissue leads to a higher number of living cells at the wound edge and therefore better apparent integration. However their study did not include any mechanical testing and it was unclear if the induced integration was in any way functional. Redman et al. investigated the effects of trauma on chondrocyte apoptosis [8]. They demonstrated that blunt trauma led to a significant increase in apoptosis at the wound edge compared to injury with a sharp instrument. They concluded that integration could be enhanced by avoiding blunt traumas during surgery or by inhibiting apoptosis. Peretti has undertaken a series of studies investigating the use of chondrocytes to promote cartilage integration [17,32]. This method involves seeding chondrocytes onto a piece of devitalised cartilage matrix and sandwiching this piece between two pieces of natural cartilaginous tissue. These constructs were then allowed to mature in vivo in a sub-cutaneous pouch of nude mice, leading to enhanced integration that was dependent on the seeded cells. Taken together the studies described above highlight the importance of a fall in viable chondrocyte number in preventing integration of tissues and the need for a cell based strategy to enhance integration. Our work described here confirms the conclusions of these earlier studies and extends this body of work by demonstrating a role of both implanted cells and endogenous chondrocytes and by highlighting the key role of the collagen membrane in bringing the two cartilage surfaces together and initiating the integration response. It is important to note that, unlike the devitalised cartilage scaffolds used by Peretti, the Chondrogide scaffolds used in this study are thin sheets, ensuring a small distance between the two cartilage surfaces. This is likely to be important for production of a continuum of tissue. It should be further noted that the extensive integration induced by our chondrocyte/collagen-scaffold implant was achieved after 40 days of culture in vitro in standard culture conditions. It can be expected that implantation in vivo would enhance this integrative response [17,32].
In summary, we have provided new evidence to support the hypothesis that cell migration between cartilage surfaces is critical for integration of those tissues. The chondrocytes may be derived from the natural cartilage, from implanted scaffolds, or both. The scaffold design is also a critical factor as it can play a role in aligning the surfaces of cartilage and stimulating migration of the endogenous cells. Studies of our chondrocyte/collagen-scaffold implant using in vivo models of cartilage repair are required to determine if the integrative response that is initiated is mechanically stable under normal load in the joint. Further studies are also required to determine if the chondrocyte/collagen-scaffold implant approach could be used to integrate other types of tissue.
5. Conclusions
Our results suggest that cartilage integration can be achieved using a chondrocyte/collagen-scaffold implant that permits simple, active and controlled delivery of chondrocytes to both host and graft mature cartilage tissues. The collagen membrane is required to promote apposition of the cartilage surfaces and initiates inward migration by endogenous chondrocytes whilst the seeded cells are required to promote extracellular matrix remodelling in the zone of integration. This combined cell and membrane approach has the potential to be used therapeutically for implantation of cartilage engineered tissue as well as repair of meniscal cartilage tears.
Acknowledgments
Funding for this work was provided by a grant from the UK Arthritis Research Campaign (ARC).
Appendix.
Figures with essential color discrimination. Fig. 4 of this article may be difficult to interpret in black and white. The full color images can be found in the on-line version, at doi:10.1016/j.biomaterials.2009.02.052.
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Langer R. 1994 Whitaker lecture: polymers for drug delivery and tissue engineering. Ann Biomed Eng. 1995;23:101–111. doi: 10.1007/BF02368317. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro F., Koide S., Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg. 1993;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Freed L.E., Hollander A.P., Martin I., Barry J.R., Langer R., Vunjak-Novakovic G. Chondrogenesis in a cell–polymer bioreactor system. Exp Cell Res. 1998;240:58–65. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 5.Riesle J., Hollander A.P., Langer R., Freed L.E., Vunjak-Novakovic G. Collagen in tissue engineered cartilage: types, structure and crosslinks. J Cell Biochem. 1998;71:313–327. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Kafienah W., Mistry S., Dickinson S., Sims T., Learmonth I., Hollander A. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 2007;56:177–187. doi: 10.1002/art.22285. [DOI] [PubMed] [Google Scholar]
- 7.Hollander A.P., Dickinson S.C., Sims T.J., Brun P., Cortivo R., Kon E. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 2006;12(7):1787–1798. doi: 10.1089/ten.2006.12.1787. [DOI] [PubMed] [Google Scholar]
- 8.Redman S.N., Dowthwaite G.P., Thomson B.M., Archer C.W. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthr Cartil/OARS. 2004;12(2):106–116. doi: 10.1016/j.joca.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Ahsan T., Sah R.L. Biomechanics of integrative cartilage repair. Osteoarthr Cartil/OARS. 1999;7(1):29–40. doi: 10.1053/joca.1998.0160. [DOI] [PubMed] [Google Scholar]
- 10.Hunziker E.B. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthr Cartil/OARS. 1999;7(1):15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 11.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil/OARS. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 12.Reindel E.S., Ayroso A.M., Chen A.C., Chun D.M., Schinagl R.M., Sah R.L. Integrative repair of articular cartilage in vitro: adhesive strength of the interface region. J Orthop Res. 1995;13(5):751–760. doi: 10.1002/jor.1100130515. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll S.W. Preclinical cartilage repair: current status and future perspectives. Clin Orthop Relat Res. 2001;(391 Suppl.):S397–S401. [PubMed] [Google Scholar]
- 14.Obradovic B., Martin I., Padera R.F., Treppo S., Freed L.E., Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19(6):1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 15.Schinagl R.M., Kurtis M.S., Ellis K.D., Chien S., Sah R.L. Effect of seeding duration on the strength of chondrocyte adhesion to articular cartilage. J Orthop Res. 1999;17(1):121–129. doi: 10.1002/jor.1100170118. [DOI] [PubMed] [Google Scholar]
- 16.Peretti G.M., Bonassar L.J., Caruso E.M., Randolph M.A., Trahan C.A., Zaleske D.J. Biomechanical analysis of a chondrocyte-based repair model of articular cartilage. Tissue Eng. 1999;5(4):317–326. doi: 10.1089/ten.1999.5.317. [DOI] [PubMed] [Google Scholar]
- 17.Peretti G.M., Zaporojan V., Spangenberg K.M., Randolph M.A., Fellers J., Bonassar L.J. Cell-based bonding of articular cartilage: an extended study. J Biomed Mater Res. 2003;64A(3):517–524. doi: 10.1002/jbm.a.10367. [DOI] [PubMed] [Google Scholar]
- 18.Pei M., Seidel J., Vunjak-Novakovic G., Freed L.E. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002;294(1):149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 19.Peretti G.M., Gill T.J., Xu J.W., Randolph M.A., Morse K.R., Zaleske D.J. Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med. 2004;32(1):146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 20.van de Breevaart Bravenboer J., In der Maur C.D., Bos P.K., Feenstra L., Verhaar J.A., Weinans H. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6(5):R469–R476. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu C., Kubo T., Hirasawa Y., Coutts R.D., Amiel D. Histomorphometric and biochemical effect of various hyaluronans on early osteoarthritis. J Rheumatol. 1998;25(9):1813–1819. [PubMed] [Google Scholar]
- 22.Hacker S.A., Healey R.M., Yoshioka M., Coutts R.D. A methodology for the quantitative assessment of articular cartilage histomorphometry. Osteoarthr Cartil/OARS. 1997;5(5):343–355. doi: 10.1016/s1063-4584(97)80038-6. [DOI] [PubMed] [Google Scholar]
- 23.Kafienah W., Jakob M., Demarteau O., Frazer A., Barker M.D., Martin I. Three dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 24.Smith G.D., Knutsen G., Richardson J.B. A clinical review of cartilage repair techniques. J Bone Joint Surg. 2005;87(4):445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 25.Hangody L., Feczko P., Bartha L., Bodo G., Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop. 2001;(391 Suppl.):S328–S336. doi: 10.1097/00003086-200110001-00030. [DOI] [PubMed] [Google Scholar]
- 26.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop. 2001;(391 Suppl.):S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 27.Peterson L., Minas T., Brittberg M., Nilsson A., Sjogren-Jansson E., Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Minas T., Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18(1):13–44. doi: 10.1016/s0278-5919(05)70128-9. v–vi. [DOI] [PubMed] [Google Scholar]
- 29.Trattnig S., Ba-Ssalamah A., Pinker K., Plank C., Vecsei V., Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005;23(7):779–787. doi: 10.1016/j.mri.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Bos P.K., DeGroot J., Budde M., Verhaar J.A., van Osch G.J. Specific enzymatic treatment of bovine and human articular cartilage: implications for integrative cartilage repair. Arthritis Rheum. 2002;46(4):976–985. doi: 10.1002/art.10208. [DOI] [PubMed] [Google Scholar]
- 32.Peretti G.M., Randolph M.A., Caruso E.M., Rossetti F., Zaleske D.J. Bonding of cartilage matrices with cultured chondrocytes: an experimental model. J Orthop Res. 1998;16(1):89–95. doi: 10.1002/jor.1100160115. [DOI] [PubMed] [Google Scholar]