Abstract
CXCR4 is a chemokine receptor which has been shown to be exploited by various tumors for increased survival, invasion, and homing to target organs. We developed a one step radiosynthesis for labeling the CXCR4-specific antagonist AMD3100 with Cu-64 to produce 64Cu-AMD3100 with a specific activity of 11.28 Ci/μmol (417 GBq/μmol) at the end of radiosynthesis. Incorporation of Cu(II) ion into AMD3100 did not change its ability to inhibit cellular migration in response to the (only) CXCR4 ligand, SDF-1/CXCL12. 64Cu-AMD3100 binding affinity to CXCR4 was found to be 62.7 μM. Biodistribution of 64Cu-AMD3100 showed accumulation in CXCR4-expressing organs and tissues, a renal clearance pathway, and an anomalous specific accumulation in the liver. We conclude that 64Cu-AMD3100 exhibits promise as a potential PET imaging agent for visualization of CXCR4-positive tumors and metastases and might be used to guide and monitor anti-CXCR4 tumor therapy.
Keywords: AMD3100, CXCR4, PET, copper-64
Introduction
Chemokines are small (8–14 kDa), mostly basic, structurally related peptides, that serve as chemotactic factors to guide migratory cells within an organism. 1 Chemokines mediate their effects through a subfamily of seven transmembrane domain, G protein-coupled receptors. The ability of chemokines to control cell trafficking is essential in various physiological processes. 2–7 More than forty chemokines 3–7 and nineteen chemokine receptors (CKRs) have been identified in humans. Chemokines play an essential role in the recruitment and activation of cells of the immune system 3. In addition, there is strong evidence that tumor-derived chemokines are responsible for recruitment of host cells that support tumor progression, 8, 9 and CKRs were also shown to be involved in various tumor processes and metastasis. 10
CXCR4 was originally discovered as the putative co-receptor for entry of T-tropic, but not M-tropic, strains of HIV-1 viruses into CD4+ T cells; 11, 12 and extensive research has elucidated the functions of this receptor. CXCR4 has been highly conserved during evolution and can be detected in a diverse range of cells, including peripheral blood lymphocytes and monocytes, mast cells, adult CD34+ bone marrow progenitor cells, endothelial cells, intestinal and alveolar epithelial cells, astrocytes, microglia, and neurons. 11, 13–17
The native ligand for CXCR4 is the chemokine stromal cell-derived factor-1 (SDF-1), also known as CXCL12. Like CXCR4, SDF-1 has been highly conserved during evolution. SDF-1 is expressed constitutively in a number of tissues including liver, lungs, lymph nodes, adrenal glands and bone marrow. Together, the SDF-1/CXCR4 ligand-receptor pair constitutes an axis that has been shown to be involved in directing cells to organ sites with high levels of SDF-1 expression, suggesting that this interaction plays a key role in the chemotaxis, retention, and homing of hematopoietic cells during homeostasis, and in the process of cancer metastases. 18, 19 In addition, the CXCR4/SDF-1 axis plays a fundamental role in the migration of progenitor cells during embryonic development of the cardiovascular, hemopoietic, and central nervous systems, 20–24 as revealed through studies of both CXCR4 and SDF-1 knockout mice. 24–26 On the other hand, CXCR4 and SDF-1 axis have also been shown, as noted above, to be exploited by HIV infection and to have roles in other disease processes such as cancer cell metastasis, leukemia progression, rheumatoid arthritis and pulmonary fibrosis. 27–31, 10
More than twenty-three different human tumors, including breast, prostate, lung, ovarian, pancreatic, esophageal, colorectal, and renal carcinoma, melanoma, neuroblastoma, and osteosarcoma, over-express CXCR4. 21, 22, 23, 32, 29, 9, 33 As one example of the possible importance of CXCR4 in cancer, Marchesi F. et al have shown that CXCR4 stimulates pancreatic tumor cells motility and invasion, and promotes their survival and proliferation. 34 Similarly, CXCR4 expression by tumor cells was shown to contribute to tumor growth, vascularization, and metastasis in prostate cancer xenografts in mice. 35 In accord with these data, inhibition of CXCR4 resulted in lower metastatic loads in the bones of mice injected with prostate cancer cells. 36 Up-regulation of CXCR4 has been also implicated in the pathogenesis of various neuro-degenerative and neuro-inflammatory diseases. 37–39
CXCR4 inhibitors, both peptide-based and small molecules, have been developed as potential therapeutics. AMD3100, (Fig. 1, compound 1), a bicyclam molecule, in which the two cyclam moieties are linked by a 1,4-phenylenebis(methylene)-bridge (Fig. 1), 40 –44 has been identified as a specific inhibitor of CXCR4. AMD3100 was originally developed as an inhibitor of HIV infection that worked by blocking the interaction of viral gp120s of some strains of HIV-1 with CXCR4 on CD4 T cells, and has been shown to block HIV infection in clinical trials. Clinical trials also demonstrated that 1 is an effective mobilizer of hematopoietic stem cells from the bone marrow (BM) in both healthy individuals and some individuals with cancer, 45, 46, 47 and is likely to enter clinical practice for this indication. 1 was also found to inhibit the SDF-1-induced chemotaxis through CXCR4 by blocking migration of monocytic cells toward higher concentration of SDF-1, 48 suggesting that it might also be used to inhibit cancer metastasis.
Figure 1.
Structure of AMD3100 (1).
Positron emission tomography (PET), a non-invasive molecular imaging modality, uses short-lived positron-emitting bioprobes to obtain four-dimensional, quantitative determination of the distribution of radioactivity within the body. 49, 50, 51 64Cu, (t1/2= 12.7 h., β+ 17.9%, electron capture 45%, β− 37.1%), can be used for PET imaging due to its positron emission and for radiotherapy due to its electron capture and μ− emissions in cancer treatment. 52–59
Previous attempts to image CXCR4 for single photon emission computed tomography (SPECT) have been reported. The CXCR4 peptide antagonist Ac-TZ14011 labeled with 111In, accumulated in the spleen (8% ID/g), had high accumulation in the kidneys and liver, and displayed low accumulation in tumors (0.51% ID/g). 60 SDF-1 has been labeled with 99Tc 61 and was evaluated as an imaging agent for myocardial infarction. A high dose of 99Tc- SDF-1 was required to assess the accumulation in the myocardium; 0.6% ID/g accumulation was observed in the infracted myocardium compared with 0.1% ID/g in normal myocardium. 61
In this manuscript we describe a novel radiochemical synthesis of 64Cu-AMD3100 (4) and its evaluation as a potential imaging agent of CXCR4 expression in a living organism. We describe binding and inhibitory properties of 64Cu-AMD3100 (4) in vitro and/or in vivo.
Material and methods
2.1 General methods
All solvents were purchased as anhydrous from Sigma-Aldrich Co. (MO, USA). 1H-NMR spectra were recorded on an Avance 300 (Bruker, USA) in D2O or CDCl3. 1H-NMR signals are reported in parts per million (ppm) and are referenced to the residual proton (4.70 ppm for D2O and 7.26 ppm for CDCl3) of the deuterated solvent. HPLC mass spectra were obtained on a Q-Tof premier-UPLC system equipped with an electrospray interface (ESI) (Waters, USA). Eluant A was composed of 95% (0.1% formic acid, 2 mM ammonium formate) and 5 % CH3CN. Eluant B was composed of 5% (0.1% formic acid, 2 mM ammonium formate) and 95% acetonitrile. UPLC conditions utilized a BEH RPC18 2.1 × 150 mm column eluted with a 30% A, 70% B at 0.4 mL/min. Retention times for compounds 1 and 3 were 0.83 min and 3.56 min, respectively.
Gas chromatography mass spectra (GCMS) were performed on a Thermo Finnigan Trace DSQ GC-MS in CI mode with CH4 as reagent gas. IR spectra were recorded on a Perkin-Elmer Spectrum GX FTIR spectrometer in KBr pellets. Radio-TLC were analyzed on Bioscan 200 imaging scanner (Bioscan Inc., USA).
The progress of reactions was monitored by TLC (SiO2, Macherey-Nagel, USA) and visualized by UV light. Flash chromatography was carried out on SiO2 (12–40 g) (Analogix, Varian, USA). Elemental analysis was performed by Galbraith Laboratories (TN, USA).
64Cu was produced at the NIH by the irradiation of a thin layer of 64Ni (Isoflex, USA) electroplated on a solid gold internal target plate of the CS-30 cyclotron utilizing the nuclear reaction 64Ni(p,n)64Cu and separated from the target material as [64Cu]CuCl2 by anion chromatography as previously described. 62
2.2 Chemistry
2.2.1. 1-[4,8-Bis-(2,2,2-trifluoro-acetyl)-1,4,8,11-tetraaza-cyclotetradec-1-yl]-2,2,2-trifluoro-ethanone (2)
This reaction was done similarly to the known procedure. 63 Briefly, to a solution of cyclam (1.05 g, 5.2 mmol) and triethylamine (0.710 mL, 5.2 mmol) in methanol (5 mL), was added ethyl trifluoroacetate (1.86 mL, 15.6 mmol). The reaction was stirred under argon at room temperature (RT) for 5 h. The volatile solvents were removed in vacuo and the crude product was purified on a silica gel flash chromatography and eluted with 100% EtOAc. The eluent was evaporated to give 2 as white foam (2.2 g, 4.5 mmol, 86%). 1H NMR (300 MHz, CDCl3): δ 1.4–1.2 (m, 2H), 1.95–1.65 (m, 2H), 2.80–2.60 (m, 2H), 3.05–2.88 (m, 2H), 3.85–3.40 (m, 12H). GC-MS (CI-CH4) 489 (M+, 100%).
2.2.2. 1-(4,8-Bis-(2,2,2-trifluoro-acetyl)-11-{4-[4,8,11-tris-(2,2,2-trifluoro-acetyl)-1,4,8,11-tetraaza-cyclotetradec-1-ylmethyl]-benzyl}-1,4,8,11-tetraaza-cyclotetradec-1-yl)-2,2,2-trifluoro-ethanone (3)
This reaction was conducted as previously described. 40 To a solution of 2 (1.78 g, 3.64 mmol) in CH3CN (20 mL) were added α , α ′-dibromoxylene (0.481 g, 1.82 mmol) and K2CO3 (1.5 g, 10.9 mmol). The reaction was refluxed over night. After solvent evaporation, the crude product was extracted with CH2Cl2 and H2O, dried over MgSO4, evaporated and purified on silica gel flash chromatography using gradient of CH2Cl2/MeOH as eluent, to give 3 as a white solid in a yield of 66% (2.6 g, 2.41 mmol). 1H NMR (300 MHz, CDCl3): δ 1.86–1.65 (m, 4H), 2.56–2.01 (m, 8H), 2.80 (s, 4H), 3.78–3.34 (m, 28H), 7.15 (s, 4H). LC-MS: 1079 [M+H+].
2.2.3 1,1′-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-tetraaza-cyclotetradecane} –AMD3100 (1)
This reaction was conducted as described elsewhere. 64 In brief, to a solution of 3 (1.1 g, 1.02 mmol) in 5 mL of methanol were added K2CO3 (0.43 g, 3.1 mmol). The suspension was refluxed for 3 h. Then the reaction was cooled and toluene (10 mL) was added. The methanol was evaporated and MgSO4 was added to the toluene suspension. The solution was filtered and toluene was concentrated to give 1 as a free base with a yield of 66% (0.34 g, 0.67 mmol).
1H NMR (300 MHz, D2O): δ 1.80–1.45 (m, 8H), 2.72–2.35 (m, 32H), 3.50 (s, 4H), 7.30 (s, 4H). LC-MS: 503 [M+H+]. IR (KBr) ν (cm−1) 553, 742, 768, 1076, 1218, 1478, 1587, 2662, 2962, 3411.
2.2.4 1, 1′-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-tetraaza-cyclotetradecane}copper acetate (4)
This reaction was done similarly to the known procedure. 40 To a stirred solution of 1 (0.3 g, 0.6 mmol) in 5 mL methanol were added Cu(II)acetate (0.108 g, 0.6 mmol) in a solution of 0.4M NH4OAc, pH = 5.5. The solution became blue-purple almost immediately. The mixture was stirred for 1 h and then triturated with diethyl ether to give 4 (0.2 g, 0.354 mmol) as a blue-purple precipitate, which was filtered and dried in vacuo to give 59% chemical yield.
1H NMR (300 MHz, D2O): δ 1.90 (CH3CO2H, s, 9H), 1.80–1.45 (m, 8H), 2.90–2.40 (m, 32H), 3.50 (s, 3H), 7.30 (s, 4H).
IR (KBr) ν (cm−1) 518, 597, 721, 799, 835, 1131, 1204, 1429, 1474, 1677, 3438. Anal. Calcd for C28H51N8·Cu·(OAc)3·2H2O: C, 52.59%; H, 8.31%; N, 14.43%; Cu, 8.18%. Found: C, 52.70%; H, 7.94%; N, 14.38%; Cu, 6.21%.
2.3 Radiochemistry
2.3.1 [64Cu]-1′-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-tetraaza-cyclotetradecane}copper acetate (64Cu-4)
64Cu-chloride was converted to 64Cu-acetate by adding 0.5 mL of 0.4 M NH4OAc pH = 5.5 solution to 20 μL 64Cu-chloride. 64Cu-acetate solution (0.4 mL; 3.5–8 mCi, 130–296 MBq) was added into a solution of various amounts of 1 dissolved in 0.4 M NH4OAc pH = 5.5 as indicated. The reaction was stirred for 1 h at RT. Thereafter, the radiochemical purity was determined using C-18 TLC plates (KC18F, 60 A, 200 μm, Whatman USA), developed in 2% ethylenediaminetetraacetic acid (EDTA) in water.
2.4 Biology
2.4.1 Animals
C57BL/6 mice were purchased from Taconic (Germantown, NY) and housed under specific pathogen free conditions. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals on approved studies from the National Institutes of Health Institutional Animal Care and Use Committee.
2.4.2 Cells
Jurkat cells were purchased from the ATCC and grown in RPMI supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 2 mM L-glutamine and Penicillin-Streptomycin (Gibco, CA).
A single cell suspension of mouse splenocytes was prepared by disrupting a spleen and lysing red cells using ACK solution (Quality Biological, MD).
2.4.3 Transwell migration assays
600 μL of migration medium (RPMI supplemented with 1% fetal bovine serum) containing SDF-1 (PeproTech, NJ), at the concentration indicated, were placed into the lower chamber of a Costar 24-well transwell (Corning, NY). 105 Jurkat cells in 100 μL migration medium were placed into the upper chamber (pore size 5 μm) and cells were collected from lower chamber after 3 h of migration at 37°C, and counted by flow cytometry using counting beads. Control migrations were performed without the chemokine in the lower chamber.
2.4.4 Binding assay
Cells (104 per well) were incubated with increasing concentrations of 4 and 250 nCi (9.25 KBq) of 64Cu-4 for 1 h, after which cells were harvested onto a filter (Perkin-Elmer, USA) using Cell Harvester 96 (Tomtec, USA). Thereafter, scintillation fluid (Packard Bioscience, CT, USA) was added to the filter. Disintegrations were measured using a β− counter (1450 Microbeta, Perkin-Elmer, USA).
2.4.5 Biodistribution
25 μCi (0.925 MBq, total mass ≤ 2 ng) of 64Cu-4 in a volume of 100 μL were injected through the tail vein of C57BL/6 mice. One, two and 6 h post injection, blood was drawn from the heart under anesthesia and the mice were sacrificed. Spleen, liver, muscle, kidney, mesenteric lymph nodes (LN), intestine and femurs were removed. BM was flushed from within the bones, and the remaining organs were weighed. All organs were assayed for radioactivity using a gamma counter (1480 Wizard 3, Perkin-Elmer, USA). In blocking experiments, the tracer was injected together with 50 μg of 4. Blocking experiments with SDF-1 were done by injecting the mice with 10 μg of the chemokine 20 min prior to- and together with the tracer (total of 20 μg per mouse). The results are presented as percent injected dose per gram tissue (%ID/g). Each group contained 5–6 mice.
2.4.6 PET studies
Mice (18 – 22 g) were anesthetized using isoflurane/O2 (1.5–5% v/v) and injected with 25–26 μCi (0.925–0.962 MBq, total mass ≤ 2 ng) of 64Cu-4 or free 64Cu-acetate via the tail vein in a total volume of 100 μL PBS. For blocking experiments, the tracer was injected together with 50 μg of 4. PET scans were performed using the Advanced Technology Laboratory Animal Scanner (ATLAS) PET scanner. 65 Whole-body (40, 80 and 120 min; five bed positions, each 8 min) scans were started approximately two min after radiotracer injection and recorded with a 100–700 keV energy window. Each group consisted of at least five mice. The images were reconstructed by a two-dimensional ordered subsets expectation maximum (2D-OSEM) algorithm, and no correction was applied for attenuation or scatter. Image analysis was done using ImageJ version 1.4g (NIH, USA) and OsiriX version 2.7.5 (Switzerland) software. The results were calculated as percentage injected dose per gram (%ID/g). At the end of each experiment a 64Cu source of known activity was imaged to obtain KBq of 64Cu per counts per seconds for the imaging system (calibration factor). Then, every ROI (counts per second per cubic centimeter) was multiplied by this factor and divided by injected activity.
2.4.7 Flow cytometry
Jurkat cells (106 in 100 μL of PBS supplemented with 4% FBS) were blocked with serum for five min on ice and then stained with PE-conjugated anti-human CXCR4 or isotype control (R&D Systems, MN). Cells were acquired on an LSRII (BectonDickinson, CA) and analyzed using FlowJO (Tree Star, OR).
Results and Discussion
1 is a symmetrical bicyclam that is a highly specific CXCR4 antagonist and that can bind to metal ions such as Cu(II) 66. It was demonstrated that a complex between 1 and metal ions such as Ni(II), Zn(II) and Cu(II) improved the interaction of the cyclam complex with a carboxylate group of an aspartate residue within the binding pocket of CXCR4. 66 There are several synthetic routes to obtain 1 which involves optimization of reaction conditions such as pH, 67 temperature, and concentration 63. Other methods involve blocking of amino groups using phosphorus 68, 69 or metal-tricarbonyl, 70 but involve the use of anhydrous material and undesirable solvents. In addition, a direct synthetic route for 1 without the need of protecting groups, 71 requires more synthetic steps and no improvement of the yield.
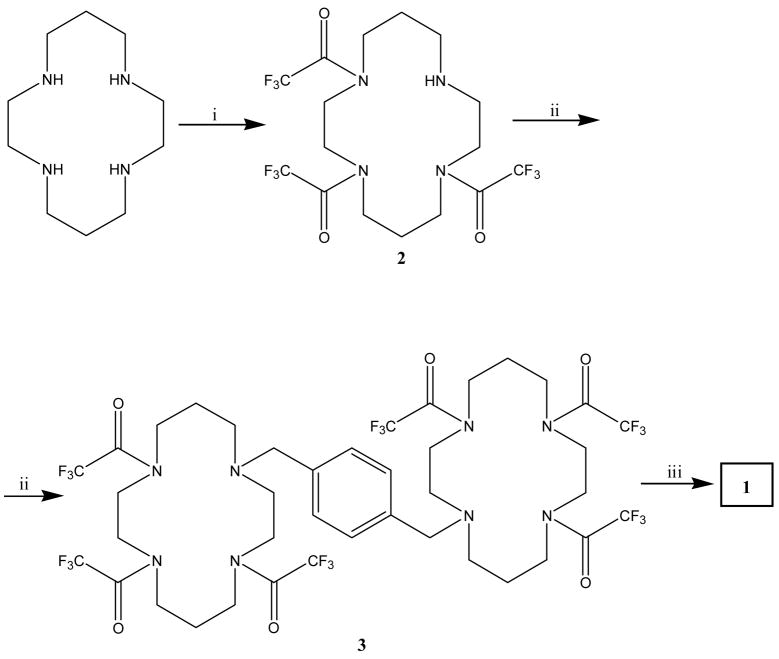
The synthesis of 1, as a free base, included three steps (Fig. 2); (i) protecting on the cyclam (ii) bis-alkylation - dimerization and (iii) deprotection. 63, 40 The protection of the amine on the cyclam ring was done similarly to the procedure of Yang et. al., 63, 72 using four equivalents of ethyl trifluororacetate (EtOTFA) as a selective acetylation agent. However, we obtained a mixture of 60% fully protected cyclam and only 40% of the desired product, 2, which contains one free amine as established by GCMS and NMR. Hence, we modified the synthesis and used only three equivalent of EtOTFA under the same reaction conditions as described in the experimental section, and we succeeded in obtaining 2 with a conversion > 99% and 86% yield. The dimerization of two cyclam rings and the de-protection of the TFA groups to furnish 1 as a free base were done as previously published. 40, 64
Figure 2.
Synthesis of 1. Reagents and conditions: (i) ethyl trifluoroacetate, triethylamine, methanol 5 h, RT; (ii) α , α –dibromoxylene, K2CO3, CH3CN, reflux, over-night; (iii) K2CO3, methanol, reflux 3 h.
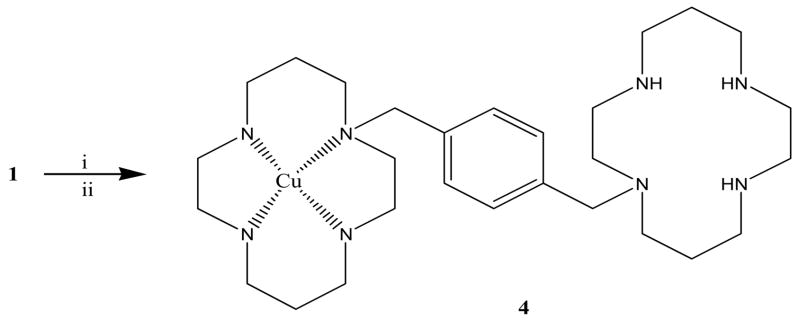
1 was used for the preparation of cold standard, 4, containing Cu(II) ion (Fig. 3), and as a precursor for the radiosynthesis with 64Cu (Fig. 3). The incorporation of a single Cu(II) ion into 1 was assured by using 1 equivalent of Cu(OAc)2. The incorporation reaction was conducted in methanol; however, in order to obtain better solubility, Cu(OAc)2 was first dissolved in a small volume of NH4OAc buffer (0.1–0.2 mL). The incorporation was verified using IR and elemental analysis.
Figure 3.
Synthesis of 4. Reagents and conditions: (i) Non-radioactive synthesis: Cu(II)acetate, methanol, NH4- acetate, 1 h, RT; (ii) Radioactive synthesis: 64Cu-Acetate, 1h, RT.
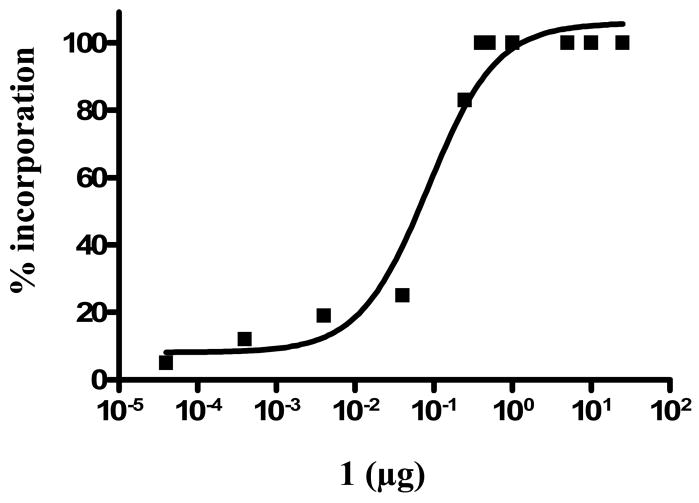
The incorporation reaction was optimized for the smallest amount of substrate because we wished to avoid the need to remove excess unlabeled ligand (1). Increasing amounts of 1 were added to aqueous [64Cu]-Cu(OAc)2 solution at pH 5.5 and incubated for 1 h at RT. The percent of incorporation was monitored by radio TLC. At substrate masses less than 0.4 μg of 1, two peaks were observed by radio TLC (Rf ~ 0.1, 64Cu-4; Rf ~ 0.6 free 64Cu). At mass greater than or equal to 0.4 μg, only the desired product, 64Cu-4 was observed as a single peak. The absence of free 64Cu-acetate at Rf ~ 0.6 indicated that the formation of complex 64Cu-4 was completed. The lowest amount of 1 needed for 100 % incorporation was determined as 0.4 μg (Fig 4) providing a highest specific activity (SA) of 11,280 Ci/mmol (4.17·105 GBq/mmol) at the end of radiosynthesis (n=10). The ability to obtain 100% complex formation has been shown previously by McCarthy et al. for the incorporation of 64Cu into a TETA chelator. 52
Figure 4.
Percent incorporation of 64Cu into 1
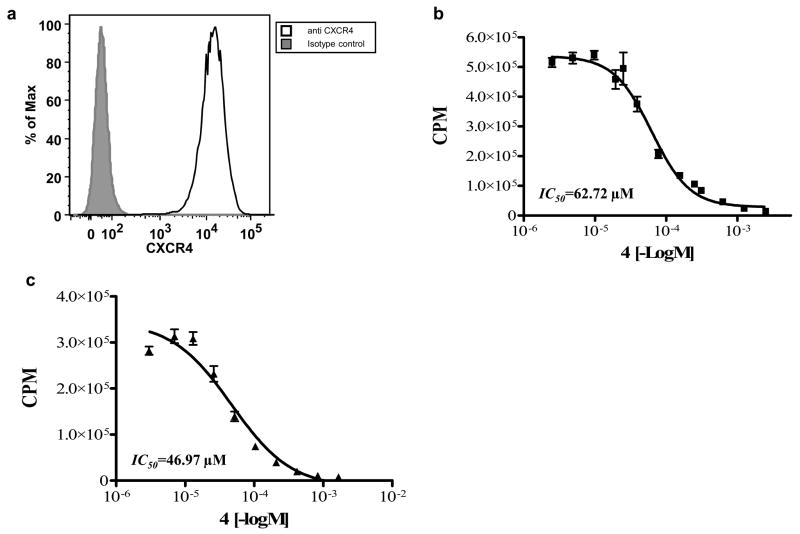
Gerlach et al. demonstrated that incorporation of metal ions into 1 improved the binding affinity towards CXCR4, as measured by inhibiting binding of SDF-1 and anti-CXCR4 antibody. 66 In order to verify that incorporation of Cu(II) ion enhances the binding of 1 to CXCR4, we evaluated binding in Jurkat T cells. We established that the Jurkat T-cells express high levels of CXCR4 using flow cytometry (Fig. 5a). The cells were then incubated with a constant amount of 64Cu-4 and increasing concentrations of non-radioactive 4 for 1 h at RT (Fig 5b). The IC50 of 64Cu-4 binding to Jurkat cells was 62.7 μM. In order to evaluate the binding of 64Cu-4 to mouse cells we repeated the experiment with mouse splenocytes, and obtained a similar IC50 of 46.9 μM (Fig. 5c). This is the first published result of homologous displacements for 1. Heterologous displacement of radiolabeled antibody with 4 showed lower IC50 of 0.1 μM by Gerlach et al., 66 and an IC50 of 15.2 μM was reported by Gupta et al., which was done by competition of 1 with 125I-SDF-1 using HL-60 cells. 73
Figure 5.
(a) CXCR4 expression by Jurkat cells evaluated by flow cytometry. Gray histogram represents isotype control staining, black line represents anti CXCR4 staining (b) binding assay of 4 using Jurkat cell line (c) binding assay of 4 using mouse splenocytes. Results shown are average of three experiments.
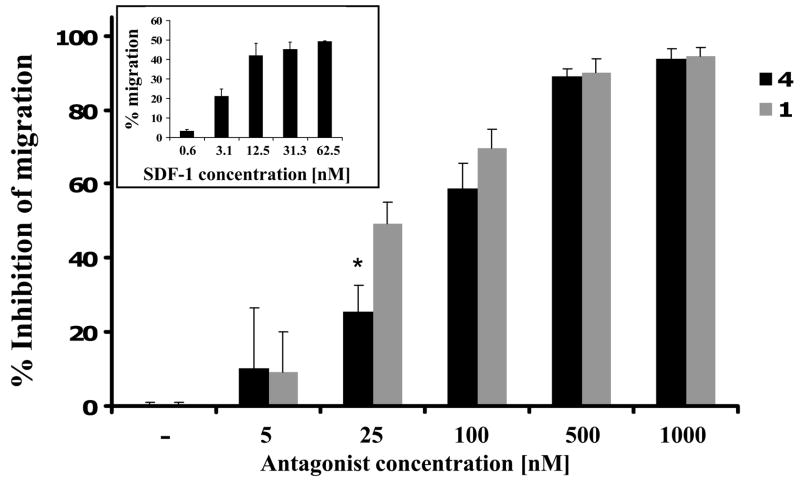
In order to verify that the CXCR4 inhibitory characteristics of 4 are not affected by the incorporation of Cu(II) ion, we evaluated the ability of 64Cu-4 to inhibit Jurkat cells migration towards SDF-1. Migration was tested through 5 μm pore membrane in a standard assay format. We used SDF-1 in the lower wells at 100 ng/mL, the lowest concentration that induces maximal migration, and tested various concentrations of 4 and 1 in the upper wells. Both 1 and 4 were found to inhibit Jurkat migration to SDF-1 in a similar manner with IC50’s of 27.4 nM and 75.4 nM, respectively (Fig 6). This result indicates that 4 retain the ability to inhibit SDF-1-induced chemotaxis through CXCR4. A comparison between the binding affinity of 4 towards CXCR4, versus its migration inhibition, revealed a 1000 fold discrepancy between the binding as measured by homologous displacement and functional activity. A similar discrepancy has been observed between activity of AMD3100 and heterologous displacement of SDF-1. 66 Another interesting observation was reported for a different chemokine receptor, CCR1. Jensen et al. showed that a low affinity small molecule agonist can act as an allosteric enhancer for CCL3 and at the same time as a competitive blocker of the binding of CCL5 through binding deep in the main ligand binding pocket. 74 Although we cannot explain these discrepancies, we postulate that AMD3100 may show high-affinity interactions to the SDF-1-bound form of CXCR4 (positive co-operativity), producing a non-signaling conformation.
Figure 6.
Inhibition of SDF-1 induced migration of Jurkat cells by 1 and 4. The lowest concentration of SDF-1 to induce maximal migration was 31.3 nM and was used in the inhibition experiments (small box). Results shown are average of 3 experiments ± SE. * P<0.05 calculated by Students two-tailed t-test comparing inhibition by 1 and 4.
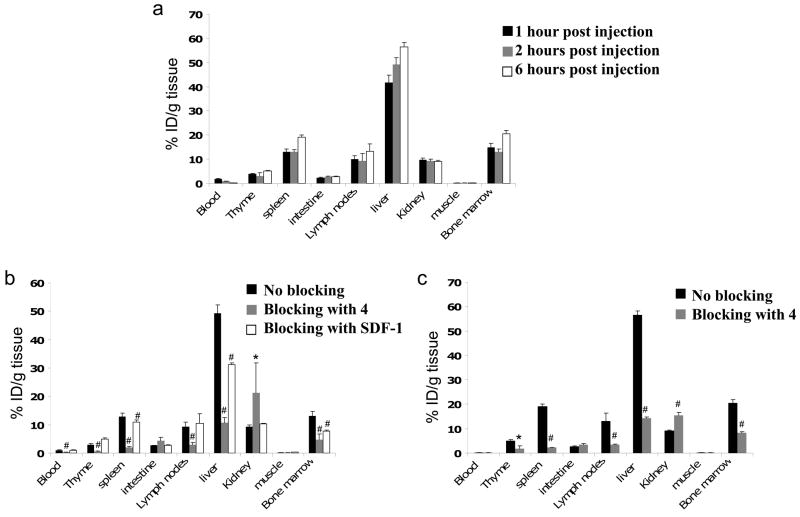
The biodistribution of 64Cu-4 was analyzed in immune competent C57BL/6 mice using both PET scans in live animals and organ dissection and gamma counting. Since multiple immune cells express CXCR4, we would expect the tracer to accumulate in immune-related organs such as the spleen, lymph nodes and bone marrow. Mice were divided into six groups: three groups were injected (i.v.) only with 64Cu-4 and sacrificed after 1, 2 and 6 h. A forth and fifth groups were designed to test the specific binding of 4 in vivo and was co-injected with excess of cold tracer and sacrificed 2 or 6 h post injection. The sixth group was injected with SDF-1 to evaluate specific binding of 64Cu-4 to CXCR4, and sacrificed after 2 h. Spleen, intestine, LN, femoral BM, blood, liver, muscle and kidney were removed for gamma-counting (Fig 7).
Figure 7.
(a) Biodistribution of 64Cu-4 in normal C57Bl/6 mice. (b) Blocking of 64Cu-4 accumulation 2 h after co-injection with 50 μg of 4 or 20 μg of SDF-1. (c) Blocking of 64Cu-4 accumulation 6 h after co-injection with 50 μg of 4. Each group contains at least 5 mice. Results shown are average ± SE. * P<0.05, # P<0.01 calculated by Students two-tailed t-test comparing blocking with 4 or SDF-1 groups to 2 h or 6 h control group.
Uptake of 64Cu-4, measured by percent ID/g of tissue, was detected after 1 h in the liver (41%) and kidneys (10%). High accumulation of 64Cu-4 was also observed in immune related organs; spleen (13%), BM (14%) and LN (10%). 64Cu-4 uptake was low in blood and muscle (1.7% and 0.3%, respectively). 2 h-post injection there were significant changes in 64Cu-4 accumulation only in the blood which was reduced to <1%, and a slight increase in the liver to 49% (Fig 7a). A slight increase of 64Cu-4 was observed in the spleen, LN, liver and BM 6 h post injection (Fig 7a). Co-injection of excess of non-labeled 4 resulted in reduced accumulation of 64Cu-4 in all immune related organs by 60–80 % in comparison to the accumulation after 2 or 6 h (Figs 7b, 7c). Surprisingly, the accumulation of 64Cu-4 in the liver was also reduced by 79% and 74% after 2 and 6 h respectively, which suggests specific binding of 4 in the liver. There was a corresponding increase in accumulation of 64Cu-4 in the kidneys, presumably due to a reduction in specific binding and enhanced renal clearance. 75 Injection of 10 μg SDF-1 20 min prior to and together with the 64Cu-4 reduced the accumulation of 64Cu-4 in the liver, BM and slightly in the spleen (Fig. 7b). The lower reduction of the tracer accumulation after excess of SDF-1 injection in comparison to excess of 4 could be due to differences in distribution and/or pharmaco-kinetics of the SDF-1 protein vs. the small molecule AMD3100. In addition, the possible binding of SDF-1 to glycosaminoglycans and heparin sulphate in-vivo might reduce the bioavailability of SDF-1 to bind CXCR4. 76 CXCR4 expression in the liver is reportedly limited to sinusoidal endothelial cells77, oval cells, 78, 79 and to immune cells, 80 which are unlikely to account for the high hepatic accumulation of 64Cu-4 – although inhibition of liver binding by SDF-1 indicates a CXCR4-dpendent component. It is possible that hepatocytes or other liver cells take up 64Cu-4 through CXCR4-independent pathways, which are blocked by excess cold 4, but not by SDF-1. In accordance, Misra et al. found that 99Tc labeled SDF-1 did not accumulate in the liver. 61 Hence, the specificity of 1 for CXCR4 should be re-examined in binding assays using liver membrane preparations.
The differences between our biodistribution results and other reported biodistribution of labeled CXCR4 ligands SDF-1 61 and Ac-TZ14011, 60 might be explained by the different pharmaco-kinetics of small protein and small peptide respectively, and the pharmaco-kinetics of a small molecule such as AMD3100.
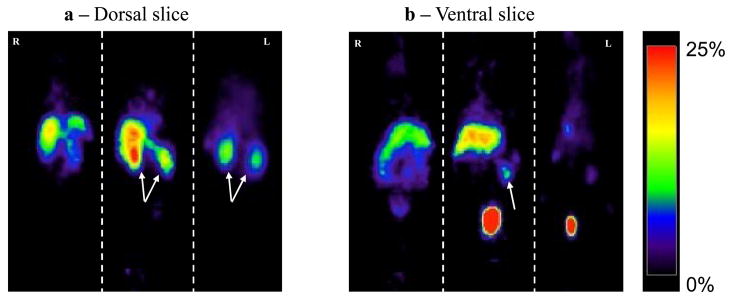
In addition to biodistribution experiments, mice were also scanned with ATLAS, to visualize the uptake in the various organs. The rapid renal clearance of 64Cu-4 resulted in high accumulation of 64Cu-4 in the bladder within 2 h (Fig. 8b). As was revealed by organ analyses, there was high accumulation of 64Cu-4 in the liver, kidney and spleen. Blocking with excess of non-radioactive 4 reduced the uptake in the spleen and liver to undetectable levels, and increased the accumulation in the kidneys, in accordance with the biodistribution results (Figs. 8a and 8b). In addition, to verify that the activity was due to accumulation of 64Cu-4 rather than free 64Cu, mice were scanned after injection of free 64Cu. The uptake of the free isotope was in the liver and intestine (Figs. 8a and 8b). There was no uptake in the spleen, and low accumulation in the bladder. These results indicate that free 64Cu does not accumulate in immune organs.
Figure 8.
Representative PET images (%ID/g) of C57Bl/6 mice 2 h after injection with 26 μCi of free 64Cu (left), 64Cu-4 (middle) or co-injection of 64Cu-4 and 50 μg of 4 (right). (a) Dorsal slice. Arrows indicate the kidneys. (b) Ventral slice. Arrow indicates the spleen.
Biodistribution and PET scans revealed that 64Cu-4 accumulates mainly in organs known to contain high densities of CXCR4-expressing cells, as well as liver and kidney. The accumulation of 64Cu-4 in CXCR4 expressing tissues, along with evidence of specific competition in vivo by SDF-1, support further evaluation of 64Cu-4 as a potential marker for the imaging of CXCR4 in tissues such as tumors.
Conclusions
We prepared 64Cu-4, a potent CXCR4 antagonist, in high radiochemical yield and with high radiochemical purity. 64Cu-4 was as potent as 1 in inhibiting the activity of CXCR4. Thus, we expected no alteration in in vivo characteristics of 64Cu-4 vs. 1, including binding to CXCR4 and acting as a CXCR4 antagonist. Biodistribution studies revealed uptake by organs and tissues involved in the immune system of mice as well as in the liver. Uptake in the liver, which although specific, may have a CXCR4-independent component and may detract from the usefulness of this radiotracer for imaging cancers in the liver. We conclude that 64Cu-4 might be useful for discriminating CXCR4 positive and negative tumors, and for directing the use and monitoring the effectiveness of CXCR4-targeted therapies.
Acknowledgments
This research was supported by Intramural Research Programs of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and the National Institute of Allergy and Infectious Diseases (NIAID). We would like to thank Dr. Amnon Peled from Hadassah-Hebrew University Medical Center, Jerusalem, Israel, for fruitful discussions and Dr. Ying Ma for HPLC-MS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zlotnik A. J Pathol. 2008;215:211. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A, Yoshie O. Immunity. 2000;12:121. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 3.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Cancer Res. 2002;62:5930. [PubMed] [Google Scholar]
- 4.Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Inflamm Bowel Dis. 2008;14:1000. doi: 10.1002/ibd.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rollins BJ. Blood. 1997;90:909. [PubMed] [Google Scholar]
- 6.Murphy PM. Methods Mol Biol. 2000;138:89. doi: 10.1385/1-59259-058-6:89. [DOI] [PubMed] [Google Scholar]
- 7.Rossi D, Zlotnik A. Annu Rev Immunol. 2000;18:217. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Vicari AP, Caux C. Cytokine Growth Factor Rev. 2002;13:143. doi: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 10.Schols D. Antiviral Res. 2006;71:216. doi: 10.1016/j.antiviral.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Horuk R. Cytokine Growth Factor Rev. 2001;12:313. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 12.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. Am J Respir Crit Care Med. 2003;167:1676. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 13.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. Proc Natl Acad Sci U S A. 1997;94:1925. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. J Biol Chem. 1998;273:4282. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 15.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. Curr Biol. 1997;7:112. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 16.Patrussi L, Baldari CT. Immunol Lett. 2008;115:75. doi: 10.1016/j.imlet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen WJ, Jayawickreme C, Watson C, Wolfe L, Holmes W, Ferris R, Armour S, Dallas W, Chen G, Boone L, Luther M, Kenakin T. Mol Pharmacol. 1998;53:177. doi: 10.1124/mol.53.2.177. [DOI] [PubMed] [Google Scholar]
- 18.Li JK, Yu L, Shen Y, Zhou LS, Wang YC, Zhang JH. World J Gastroenterol. 2008;14:2308. doi: 10.3748/wjg.14.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. Cancer Res. 2007;67:7518. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 20.Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ. Blood. 2002;100:2597. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 21.Redjal N, Chan JA, Segal RA, Kung AL. Clin Cancer Res. 2006;12:6765. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- 22.Balkwill F. Semin Cancer Biol. 2004;14:171. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, Bai Z, Srinoulprasert Y, Yang BG, Hayasaka H, Miyasaka M. Cancer Sci. 2005;96:317. doi: 10.1111/j.1349-7006.2005.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Proc Natl Acad Sci U S A. 1998;95:9448. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasawa T, Tachibana K, Kishimoto T. Semin Immunol. 1998;10:179. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 26.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Nature. 1998;393:595. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 27.Burger JA, Burkle A. Br J Haematol. 2007;137:288. doi: 10.1111/j.1365-2141.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 28.Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Cancer Res. 2003;63:1969. [PubMed] [Google Scholar]
- 29.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Nature. 2001;410:50. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 30.Murphy PM. N Engl J Med. 2001;345:833. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 31.Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. FEBS Lett. 2003;550:79. doi: 10.1016/s0014-5793(03)00824-x. [DOI] [PubMed] [Google Scholar]
- 32.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Cancer Res. 2002;62:1832. [PubMed] [Google Scholar]
- 33.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. Proc Natl Acad Sci U S A. 2003;100:13513. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Cancer Res. 2004;64:8420. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 35.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. Faseb J. 2004;18:1240. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 36.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. J Bone Miner Res. 2005;20:318. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 37.Xia MQ, Hyman BT. J Neurovirol. 1999;5:32. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- 38.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. Nat Neurosci. 2001;4:702. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 39.Rauer M, Pagenstecher A, Schulte-Monting J, Sauder C. J Neurovirol. 2002;8:168. doi: 10.1080/13550280290049741. [DOI] [PubMed] [Google Scholar]
- 40.Bridger GJ, Skerlj RT, Thornton D, Padmanabhan S, Martellucci SA, Henson GW, Abrams MJ, Yamamoto N, De Vreese K, Pauwels R, et al. J Med Chem. 1995;38:366. doi: 10.1021/jm00002a019. [DOI] [PubMed] [Google Scholar]
- 41.Bridger GJ, Skerlj RT, Padmanabhan S, Martellucci SA, Henson GW, Struyf S, Witvrouw M, Schols D, De Clercq E. J Med Chem. 1999;42:3971. doi: 10.1021/jm990211i. [DOI] [PubMed] [Google Scholar]
- 42.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R, et al. Antimicrob Agents Chemother. 1994;38:668. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joao HC, De Vreese K, Pauwels R, De Clercq E, Henson GW, Bridger GJ. J Med Chem. 1995;38:3865. doi: 10.1021/jm00019a017. [DOI] [PubMed] [Google Scholar]
- 44.Rosenkilde MM, Gerlach LO, Hatse S, Skerlj RT, Schols D, Bridger GJ, Schwartz TW. J Biol Chem. 2007;282:27354. doi: 10.1074/jbc.M704739200. [DOI] [PubMed] [Google Scholar]
- 45.Fricker SP, Anastassov V, Cox J, Darkes MC, Grujic O, Idzan SR, Labrecque J, Lau G, Mosi RM, Nelson KL, Qin L, Santucci Z, Wong RS. Biochem Pharmacol. 2006;72:588. doi: 10.1016/j.bcp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Hu JS, Freeman CM, Stolberg VR, Chiu BC, Bridger GJ, Fricker SP, Lukacs NW, Chensue SW. Am J Pathol. 2006;169:424. doi: 10.2353/ajpath.2006.051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan A, Greenman J, Archibald SJ. Curr Med Chem. 2007;14:2257. doi: 10.2174/092986707781696618. [DOI] [PubMed] [Google Scholar]
- 48.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. J Exp Med. 1997;186:1383. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishani E, Abourbeh G. Curr Top Med Chem. 2007;7:1755. doi: 10.2174/156802607782507457. [DOI] [PubMed] [Google Scholar]
- 50.Elsinga PH. Methods. 2002;27:208. doi: 10.1016/s1046-2023(02)00076-2. [DOI] [PubMed] [Google Scholar]
- 51.Shiue CY, Welch MJ. Radiol Clin North Am. 2004;42:1033. doi: 10.1016/j.rcl.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 52.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Nucl Med Biol. 1997;24:35. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 53.Jones-Wilson TM, Deal KA, Anderson CJ, McCarthy DW, Kovacs Z, Motekaitis RJ, Sherry AD, Martell AE, Welch MJ. Nucl Med Biol. 1998;25:523. doi: 10.1016/s0969-8051(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 54.Lewis JS, Connett JM, Garbow JR, Buettner TL, Fujibayashi Y, Fleshman JW, Welch MJ. Cancer Res. 2002;62:445. [PubMed] [Google Scholar]
- 55.Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ. J Med Chem. 2002;45:469. doi: 10.1021/jm0103817. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Wuest M, Kovacs Z, Sherry AD, Motekaitis R, Wang Z, Martell AE, Welch MJ, Anderson CJ. J Biol Inorg Chem. 2003;8:217. doi: 10.1007/s00775-002-0408-5. [DOI] [PubMed] [Google Scholar]
- 57.Anderson CJ, Wadas TJ, Wong EH, Weisman GR. Q J Nucl Med Mol Imaging. 2008;52:185. [PMC free article] [PubMed] [Google Scholar]
- 58.Sun X, Kim J, Martell AE, Welch MJ, Anderson CJ. Nucl Med Biol. 2004;31:1051. doi: 10.1016/j.nucmedbio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Wu AM, Yazaki PJ, Tsai S, Nguyen K, Anderson AL, McCarthy DW, Welch MJ, Shively JE, Williams LE, Raubitschek AA, Wong JY, Toyokuni T, Phelps ME, Gambhir SS. Proc Natl Acad Sci U S A. 2000;97:8495. doi: 10.1073/pnas.150228297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanaoka H, Mukai T, Tamamura H, Mori T, Ishino S, Ogawa K, Iida Y, Doi R, Fujii N, Saji H. Nucl Med Biol. 2006;33:489. doi: 10.1016/j.nucmedbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Misra P, Lebeche D, Ly H, Schwarzkopf M, Diaz G, Hajjar RJ, Schecter AD, Frangioni JV. J Nucl Med. 2008;49:963. doi: 10.2967/jnumed.107.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szajek LP, Meyer W, Plascjak P, Eckelman WC. Radiochem Acta. 2005;93:1. [Google Scholar]
- 63.Wen Y, Giandomenico CM, Sartori M, Moore DA. Tetrahedron letters. 2003;44:2481. [Google Scholar]
- 64.6489472, U. P. 2002.
- 65.Seidel J, Vaquero J, Green M. IEEE Trans Nucl Sci. 2003;50:1347. [Google Scholar]
- 66.Gerlach LO, Jakobsen JS, Jensen KP, Rosenkilde MR, Skerlj RT, Ryde U, Bridger GJ, Schwartz TW. Biochemistry. 2003;42:710. doi: 10.1021/bi0264770. [DOI] [PubMed] [Google Scholar]
- 67.Zoltan Kovacs ADS. Synthesis. 1997:759. [Google Scholar]
- 68.Gardinier I, Oget ARN, Bernard H, Yaouanc JJ, Handel H. Tetrahedron letters. 1996;37:7711. [Google Scholar]
- 69.Marshall DGaGR. Synthetic communications. 1998;28:2903. [Google Scholar]
- 70.Patinec V, Clement JJYJC, Handel H, des Abbayes H. Tetrahedron letters. 1995;36:79. [Google Scholar]
- 71.Achmatowicz M, Hegedus LS. J Org Chem. 2003;68:6435. doi: 10.1021/jo030146r. [DOI] [PubMed] [Google Scholar]
- 72.Xu D, Mattner PG, Prasad K, Repic O, Blacklock TJ. Tetrahedron letters. 1996;37:5301. [Google Scholar]
- 73.Gupta SK, Pillarisetti K, Thomas RA, Aiyar N. Immunol Lett. 2001;78:29. doi: 10.1016/s0165-2478(01)00228-0. [DOI] [PubMed] [Google Scholar]
- 74.Jensen PC, Thiele S, Ulven T, Schwartz TW, Rosenkilde MM. J Biol Chem. 2008;283:23121. doi: 10.1074/jbc.M803458200. [DOI] [PubMed] [Google Scholar]
- 75.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Antimicrob Agents Chemother. 2000;44:1667. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laguri C, Arenzana-Seisdedos F, Lortat-Jacob H. Carbohydr Res. 2008;343:2018. doi: 10.1016/j.carres.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 77.Li W, Gomez E, Zhang Z. J Exp Clin Cancer Res. 2007;26:527. [PubMed] [Google Scholar]
- 78.Mavier P, Martin N, Couchie D, Preaux AM, Laperche Y, Zafrani ES. Am J Pathol. 2004;165:1969. doi: 10.1016/S0002-9440(10)63248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. Cloning Stem Cells. 002;4:339. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 80.Wald O, Weiss ID, Galun E, Peled A. Cytokine. 2007;39:50. doi: 10.1016/j.cyto.2007.05.013. [DOI] [PubMed] [Google Scholar]