Abstract
Background
Ischemic heart disease is the most common cause of mortality in diabetic patients. Although therapeutic angiogenesis is an attractive option for these patients, they appear to have reduced collateral formation in response to myocardial ischemia. The aims of this study were to establish a large animal model of diabetes and chronic myocardial ischemia, evaluate the effects of diabetes on the angiogenic response, and elucidate the molecular pathways involved.
Methods and Results
Diabetes was induced in male Yucatan miniswine using a pancreatic β-cell specific toxin, alloxan (150 mg/kg; n=8). Age-matched swine served as controls (n=8). Eight weeks after induction, chronic ischemia was induced by ameroid constrictor placement around the circumflex coronary artery. Myocardial perfusion and function were assessed at 3 and 7 weeks after ameroid placement using isotope-labeled microspheres. Endothelial cell density and myocardial expression of angiogenic mediators was evaluated. Diabetic animals exhibited significant endothelial dysfunction. Collateral dependent perfusion and LV function were significantly impaired in diabetic animals. Diabetic animals also demonstrated reduced endothelial cell density (173±14 versus 234±23 cells/hpf, P=0.03). Expression of VEGF, Ang-1, and Tie-2 was reduced, whereas antiangiogenic proteins, angiostatin (4.4±0.9-fold increase, P<0.001), and endostatin (2.9±0.4-fold increase, P=0.03) were significantly elevated in the diabetic myocardium.
Conclusions
Diabetes results in a profound impairment in the myocardial angiogenic response to chronic ischemia. Pro-and antiangiogenic mediators identified in this study offer novel targets for the modulation of the angiogenic response in diabetes.
Keywords: diabetes, angiogenesis, endothelium, ischemia, growth factors
More than 35 million people in the United States are affected by diabetes and glucose intolerance. These individuals carry up to 8 times the risk of cardiovascular events compared with nondiabetic individuals, making cardiovascular disease the largest cause of mortality in this population.1 Diabetic patients experience accelerated atherosclerosis and also exhibit a diminished angiogenic response to myocardial ischemia as shown angiographically2 and in autopsy studies.3 Although, growth factor or cell-based angiogenic therapies are an attractive therapeutic option for these patients, a better understanding of pro- and antiangiogenic influences in diabetic patients is crucial to maximize the effects of such therapies.
Various mechanisms have been postulated to explain the impaired angiogenic response in diabetes. First, the presence of vascular dysfunction, characterized by both endothelial and vascular smooth muscle impairments, is a well-documented phenomenon in this setting.4–7 Second, exposure to chronic hyperglycemia leads to the nonenzymatic glycation of proteins and in particular, glycation of the extracellular matrix has been shown to impair the formation of new blood vessels.8,9 Lastly, the presence of diabetes is associated with abnormalities in growth factor signaling which have not been well characterized, particularly in the myocardium.10,11
An important limitation to the study of myocardial angiogenesis in the setting of diabetes is the lack of a validated large animal model that captures the functional, microcirculatory, and molecular abnormalities associated with diabetes, and is suitable for the induction of chronic myocardial ischemia. Although murine and rodent hind-limb ischemia models have been used to study angiogenesis, their applicability to the setting of myocardial angiogenesis is limited. The aims of this study were to (1) develop a large animal model of diabetes and chronic myocardial ischemia, (2) examine the effects of diabetes on the coronary microcirculation, myocardial angiogenesis, and myocardial function in response to chronic ischemia, and (3) to elucidate the molecular pathways involved with the goal of finding novel targets to modulate the myocardial angiogenic response in diabetes.
Methods
General Experimental Sequence and Diabetes Induction
Sixteen Yucatan miniswine of male sex (Sinclair Research Inc, Colombia, Mo) were used for the studies. Diabetes was induced when animals were 8 months old using a single intravenous injection of alloxan (150 mg/kg). Only animals that achieved and maintained blood glucose levels greater than 200 mg/dL were included in the diabetes group. Animals with blood glucose levels exceeding 400 mg/dL were treated with daily insulin (70% amorphous, 30% crystalline) given intramuscularly to maintain blood glucose levels between 250 and 400 mg/dL. Fasting blood glucose was monitored every 2 days early after diabetes induction and weekly thereafter by obtaining a small blood sample (50 µL) from a capillary bed in the ear. Diabetes was maintained for 8 weeks before surgical instrumentation. Age matched miniswine (n=8) served as controls.
All animals underwent an identical experimental protocol involving 3 separate procedures on each animal. Anesthesia was performed as reported previously,12 and animals received humane care in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and the National Research Council’s Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animals and published by the National Institutes of Health (NIH publication No. 5377-3 1996). Briefly, for all surgical procedures, anesthesia was induced with ketamine (10 mg/kg IM), thiopental (5 to 10 mg/kg IV), and thiopental 2.5% and maintained with a gas mixture of oxygen at 1.5 to 2 L/min and isoflurane at 0.75 to 3.0%. The animals were intubated and mechanically ventilated at 12 to 20 breaths/min.
The first procedure, performed via small left anterolateral thoracotomy at 8 weeks after diabetes induction, consisted of the placement of a 1.75-mm ameroid constrictor around the proximal circumflex artery and the injection of 1.5×107 gold-labeled microspheres into the left atrium during temporary circumflex coronary occlusion, to subsequently allow for identification, by shadow labeling, of the myocardial territory at risk.
The second procedure, also performed via left anterolateral thoracotomy, 3 weeks after ameroid placement, consisted of 1.5×107 Lutetium microspheres injected in the left atrium during rest conditions and 1.5×107 Europium microspheres injected during rapid atrial pacing (150 bpm) to allow for determination of baseline perfusion after ameroid closure. To document ameroid closure, left coronary angiography was performed through an 8F sheath (Cordis Corporation) surgically inserted in the femoral artery, using a catheter with the appropriate distal angulation and high atomic weight contrast (Mallinckrodt Inc).
The third procedure was carried out 4 weeks after the second procedure and 7 weeks after ameroid placement. Sternotomy was performed, 1.5×107 Samarium microspheres were injected into the left atrium during rest conditions, and 1.5×107 Lanthanum microspheres were injected during pacing (150 bpm). Euthanasia was then performed with 10 mL/kg of a saturated KCl solution administered intravenously. Cardiac samples were harvested and snap frozen for molecular studies, sectioned, weighed, and refrigerated for myocardial microsphere analyses, and put in 4°C Kreb’s solution for in vitro assessment of coronary microvascular reactivity. Ameroid constrictors were resected along with a segment of circumflex artery and examined under low power magnification to confirm occlusion.
In Vitro Assessment of Coronary Microvessel Reactivity
After cardiac harvest, epicardial coronary arterioles (80 to 150 µm in diameter and 1 to 2 mm in length) originating from branches of the left anterior descending and circumflex arteries were dissected from the surrounding tissue with a ×40 dissecting microscope and examined in isolated organ chambers, as described previously.13 The responses to sodium nitroprusside (SNP; 1 nmol/L to 100 µmol/L), an endothelium-independent cGMP-mediated vasodilator, as well as adenosine 5′-diphosphate (ADP; 1 nmol/L to 10 µmol/L), substance P (1 fmol/L to 1 nmol/L), and VEGF (1 fmol/L to 1 nmol/L), 3 endothelium-dependent receptor-mediated vasodilators that act via bioavailable NO, were studied after precontraction by 20% to 50% of the baseline diameter with the thromboxane A2 analog U46619 (0.1 to 1 µmol/L). Relaxation responses were defined as the percent relaxation of the precontracted diameter and 6 to 8 vessels were examined in each group from the left anterior descending and the circumflex territories.
Assessment of Myocardial Perfusion
Myocardial perfusion was assessed during each procedure with isotope-labeled microspheres (ILM; Biophysics Assay Laboratories) using methods previously reported.12 Isotope-labeled microspheres, 15 µm in diameter, of different isotopic mass were used at each experimental stage. Gold-labeled microspheres were injected during temporary circumflex occlusion at the time of ameroid placement to identify myocardial samples that originated from the circumflex coronary distribution (those with the lowest count of gold-labeled microspheres). Lutetium and Europium-labeled ILM were used during the second procedure to determine baseline blood flow at rest and with atrial pacing at 150 bpm. Samarium and Lanthanum-labeled ILM were injected at rest and during pacing during the third procedure. Reference blood samples were obtained from the femoral artery during the second and third procedures. After euthanasia, 10 circumferential, transmural left ventricular sections were collected for ILM assays in each animal, weighed, and dried. Each sample was exposed to neutron beams and microsphere densities measured in a γ counter. Adjusted myocardial blood flow (at rest and with pacing), reflecting changes in lateral myocardial perfusion, was determined from the 2 myocardial samples which showed the lowest count of gold microspheres by using the following equations:
Assessment of Myocardial Function
Before tissue harvest, a catheter was surgically inserted through the femoral artery, was passed into the left ventricle, and left ventricular pressure was recorded over 10-second intervals. Global systolic and diastolic function was determined using Cardiosoft (Sonosoft Inc) software by taking the first derivative of LV pressure (dP/dt). Next, maximum +dP/dt (systolic function) and minimum −dP/dt (diastolic function) measurements were obtained and averaged over 15 to 20 cardiac cycles.
Immunohistochemistry
Myocardial sections from the circumflex territory of control and diabetic animals were stained with anti–PECAM-1 (CD-31) antibody diluted to 1:600 (BD Biosciences) as previously described.12 The sections were counterstained with methyl green, and examined for capillary endothelial cell density in a triplicate, blinded fashion from 600×440 µm (0.264 mm2) cross-sectional fields randomly selected from the center of circumflex territories.
Western Blotting
Whole-cell lysates were isolated from the homogenized myocardial samples with a RIPA buffer (Boston Bioproducts) and centrifuged at 12 000g for 10 minutes at 4°C to separate soluble from insoluble fractions. Protein concentration was measured spectrophotometrically at a 595-nm wavelength with a DC protein assay kit (Bio-Rad). Forty to 80 micrograms of total protein were fractionated by 4% to 20% gradient, SDS polyacrylamide gel electrophoresis (Invitrogen) and transferred to PVDF membranes (Millipore). Each membrane was incubated with specific antibodies as follows: anti-VEGF antibody (dilution 1:250; Calbiochem), anti-eNOS antibody (1:2500; BD Biosciences), anti–phospho-Akt (Ser473) antibody (1:1000), anti-Akt antibody (1:1000), anti–phospho-eNOS (Ser1177) antibody (1:1000; Cell Signaling), anti–Tie-2 antibody (1:200; Santa Cruz), anti-endostatin antibody (1:1000; Upstate), anti-angiostatin antibody. Then the membranes were incubated for 1 hour in diluted appropriate secondary antibody (Jackson Immunolab). Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham). Bands were quantified by densitometry of radioautograph films.
Data Analysis
Data are reported as means±SEM. Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using 2-way repeated measures analysis of variance examining the relationship between vessel relaxation, log concentration of the vasoactive agent of interest, and the experimental group (SAS Version 9.1). Immunoblots are expressed as a ratio of protein to loading band density and were analyzed after digitization and quantification of x-ray films with ImageJ 1.33 (National Institutes of Health, USA). Blots and ILM data were analyzed with 2-tailed analyses of variance. Bonferroni corrections were applied to multiple tests, and probability values of less than 0.05 were considered statistically significant.
Statement of Responsibility
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Experimental Model
Diabetes was successfully induced in 8 of 10 miniswine (80%). Four animals required small doses of intramuscular insulin (10 to 20 u/d) to keep blood glucose levels below 400 mg/dL at certain points during the experimental period. Blood glucose levels were 319±31 mg/dL in diabetic animals compared with 69±2 mg/dL in control animals (P<0.001). All animals survived the entire experimental protocol. Gross examination of harvested tissues did not show any evidence of myocardial hypertrophy, fibrosis, or scarring. Histological examination, using hematoxin and eosin staining, did not reveal any major abnormalities.
Coronary Microvessel Reactivity
Results of microvessel relaxation studies are summarized in Figure 1. U46619 was used at a dose of 1×10−6 M to preconstrict coronary microvessels by an average of 40±4% of the baseline diameter. Compared with the control group, diabetic animals demonstrated mild impairment in microvessel relaxation to endothelium-dependent vasorelaxants, ADP, and substance P (both P<0.001) in the ischemic territory, suggesting reduced NO bioavailability. Furthermore, microvessel relaxation in response to VEGF was markedly impaired in diabetic animals (P<0.001). Endothelium-independent relaxation, in response to sodium nitroprusside, was similar between groups (P=0.13).
Figure 1.
Coronary microvascular reactivity. Percent relaxation to increasing concentrations of vasodilating agents after preconstriction with U46619. Responses to endothelium-dependent vasodilators (A) ADP, (B) Substance P, and (C) VEGF, as well as endothelium-independent vasodilator (D) SNP in the ischemic territory of control and diabetic animals. ADP indicates adenosine 5′ diphosphate; SNP, sodium nitroprusside; VEGF, vascular endothelial growth factor. *P<0.001.
Myocardial Perfusion
Crude circumflex territory myocardial blood flow was similar between control and diabetic animals at 3 weeks after ameroid placement, both at rest (0.43 ± 0.07 versus 0.33 ± 0.02 mL/min/g respectively, P=0.14) and with pacing (0.34 ± 0.04 versus 0.39 ± 0.03 mL/min/g, P=0.39; Figure 2A). At 7 weeks, whereas the control group had an increase in circumflex territory perfusion at rest (+0.23 ± 0.07 mL/min/g), diabetic animals demonstrated a reduction in perfusion (−0.18 ± 0.02 mL/min/g; P<0.001). These differences were also evident during rapid atrial pacing with the baseline-adjusted circumflex flow significantly higher in the control group (+0.21 ± 0.05 versus −0.22±0.04 mL/min/g; P<0.001; Figure 2B).
Figure 2.
Myocardial perfusion. Isotope-labeled microspheres were used to determine crude (A) and baseline-adjusted (B) circumflex territory myocardial blood perfusion in control and diabetic swine after circumflex ameroid occlusion. Crude circumflex territory perfusion was similar between groups at rest (P=0.14) and with pacing (P=0.39), whereas baseline adjusted circumflex territory flow was significantly reduced in diabetic animals both at rest and with pacing. *P<0.001.
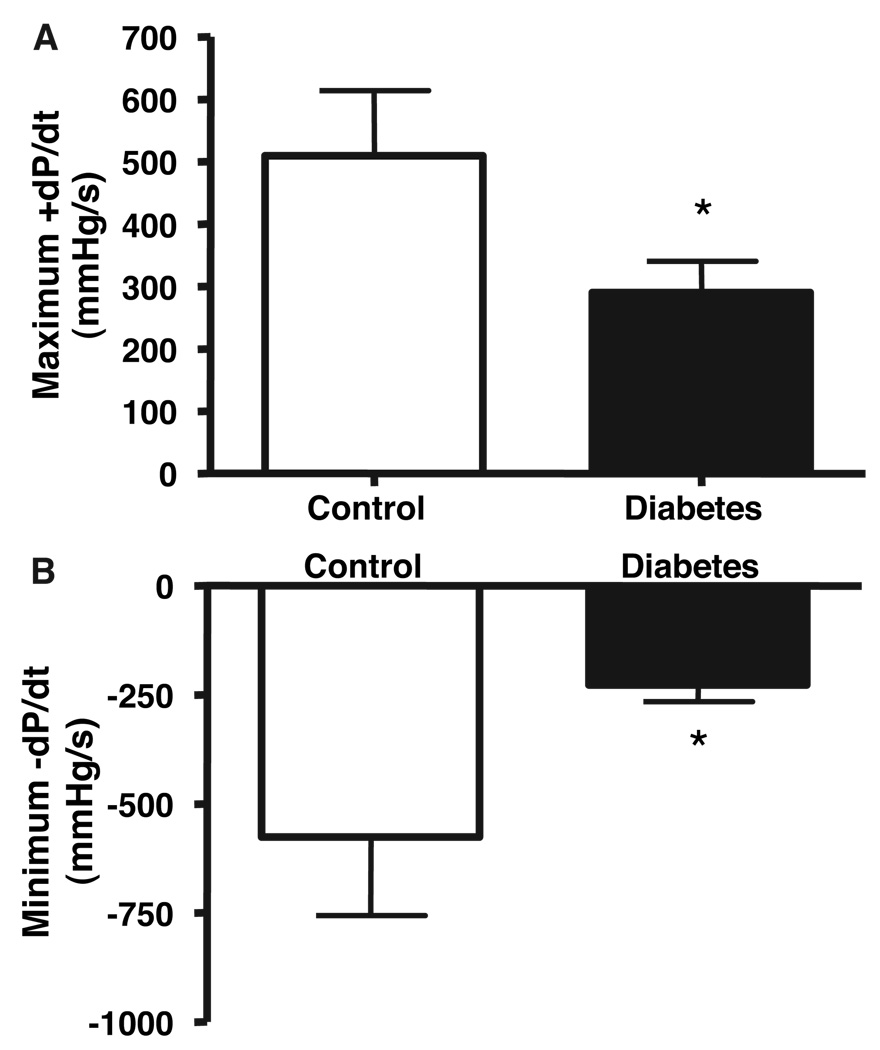
Myocardial Function
Global left ventricular function was measured at the time of the final procedure, 7 weeks after ameroid placement, and systolic and diastolic function were quantified using maximum positive dP/dt (first derivative of LV pressure) and minimum negative dP/dt, respectively. Diabetic animals demonstrated reduced LV contractility as well as impaired LV relaxation (both P<0.05; Figure 3).
Figure 3.
Left ventricular function. Maximum +dP/dt and minimum −dP/dt, representing systolic and diastolic function, respectively, were derived from left ventricular pressure measurement obtained before harvest. Diabetic animals demonstrated reduced LV contractility as well as impaired LV relaxation. *P<0.05.
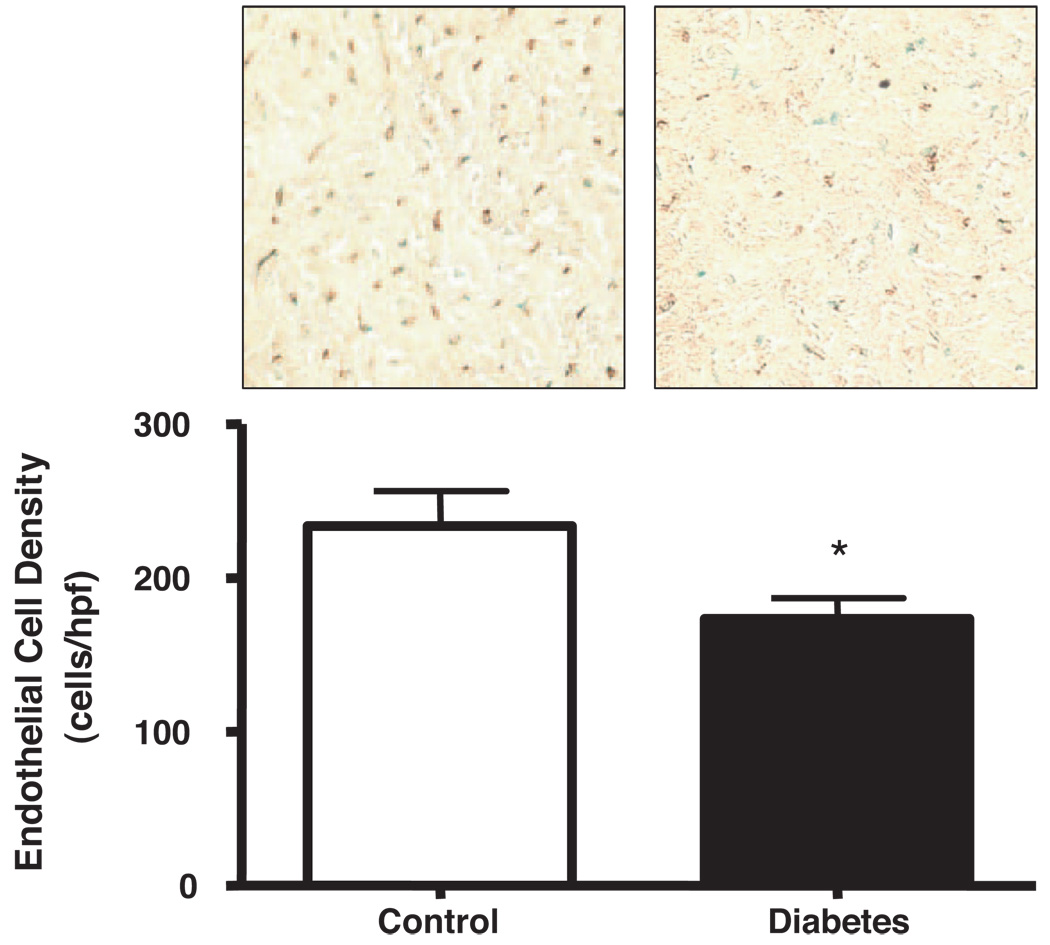
Capillary Endothelial Cell Density
Figure 4 shows the density of CD31+ capillary endothelial cells (cells/high power field) in the ischemic territory of pigs from both groups 7 weeks after ameroid placement. Diabetic animals had significantly reduced endothelial cell density compared with controls (173 ± 14 versus 234 ± 23; P=0.03).
Figure 4.
Endothelial cell density. CD31+ endothelial cell density per high power field (0.264 mm2) was determined in the ischemic territory of control and diabetic animals. Diabetic animals had reduced endothelial cell density compared with controls (234 ± 23 versus 173 ± 14; *P=0.03).
Western Blotting
To elucidate molecular mechanisms for the impaired angiogenic response in diabetes, we examined the expression of pro- and antiangiogenic mediators in the myocardium of diabetic and control animals.
Proangiogenic Mediators: VEGF and Angiopoietin Pathways
Vascular endothelial growth factor and angiopoiten-1 are important proangiogenic growth factors that mediate their effects through binding to their respective receptors, flk-1 and Tie-2, leading to the downstream activation of Akt and endothelial nitric oxide synthase (eNOS; Figure 5A). The results of the Western blots and the densitometric analyses of proangiogenic mediators involved in the VEGF and Ang-1 pathway are summarized in Figure 5B and 5C. The expression of VEGF was markedly reduced in the diabetic myocardium compared with controls (−59 ± 8%; P<0.001), whereas the difference in Ang-1 expression was not significant (P=0.14). Using Western blotting techniques, we were unable to measure the levels of flk-1. Tie-2 receptor expression, however, was significantly reduced in the myocardium of diabetic animals (−33 ± 7%, P<0.001). Akt expression and phosphorylation as well as eNOS expression was similar between groups, whereas eNOS phosporylation was significantly reduced in diabetic animals (−55±15%, P=0.008). These findings suggest that VEGF and Ang-1 signaling is affected at multiple levels in the diabetic myocardium.
Figure 5.
Myocardial expression of proangiogenic mediators. Expression of various proteins involved in VEGF and angiopoiten-1 signaling (A) was assessed using Western blotting. Diabetic animals had reduced myocardial expression of VEGF and Tie-2, but Ang-1 expression was not significantly different between groups (P=0.14). Akt expression and phosphorylation and eNOS expression was similar between diabetic and control animals, but eNOS phosphorylation was markedly reduced in the diabetic myocardium. VEGF indicates vascular endothelial growth factor; eNOS, endothelial nitric oxide synthase. *P<0.01.
Antiangiogenic Mediators: Angiostatin and Endostatin
Figure 6 displays the results of Western blots of antiangiogenic mediators, endostatin and angiostatin. The expression of both endostatin (2.9 ± 0.4-fold, P=0.03) and angiostatin (4.4 ± 0.9-fold, P<0.001) was profoundly increased in the myocardium of diabetic animals. Expression of these antiangiogenic proteins was similar between the ischemic circumflex and the nonischemic LAD territories.
Figure 6.
Myocardial expression of antiangiogenic mediators. Expression of (A) Angiostatin and (B) Endostatin was significantly increased in the myocardium of diabetic animals. *P<0.001; **P=0.03.
Discussion
The presence of diabetes is associated with accelerated coronary artery disease as well as alterations in the angiogenic response to ischemia. The study of the myocardial angiogenic response in diabetes is limited, in large part, because of the unavailability of a clinically relevant large animal model of chronic myocardial ischemia that captures the functional, microvascular, and molecular abnormalities associated with diabetes. In this study, we report the successful development of a porcine model of alloxan-induced diabetes and chronic myocardial ischemia, with a high rate of successful induction (80%) and maintenance of diabetes as well as excellent animal retention throughout the experimental protocol. We found that a 15-week exposure to diabetes resulted in coronary endothelial dysfunction, as shown by impaired coronary microvessel relaxation to ADP and substance P as well as a profound impairment in VEGF signaling in the coronary microvasculature. Diabetic animals also demonstrated a significantly impaired angiogenic response to chronic myocardial ischemia. This was observed functionally as reduced perfusion of the collateral-dependent circumflex territory and morphologically as reduced endothelial cell density in the ischemic myocardium of diabetic animals. The reduction in the angiogenic response was associated with systolic and diastolic left ventricular dysfunction. Lastly, these functional alterations were associated with increased expression of antiangiogenic proteins, angiostatin and endostatin, as well as alterations in VEGF and Ang-1 signaling. In summary, this study provides an in-depth characterization of the myocardial angiogenic response in diabetes at the functional, cellular, and molecular levels, and identifies molecular targets for the modulation of the myocardial angiogenic response in diabetes.
Endothelial dysfunction in the presence of diabetes has been demonstrated in various murine,14,15 rodent,7,16,17 and swine models18 as well as in coronary and noncoronary vasculature of humans with type I19–21and type II diabetes. 5,6,22–25 The majority of this information has been derived from noninvasive studies of the human forearm circulation or using rodent models of diabetes. As such, little is known about the effects of diabetes on the coronary microcirculation. In this study, diabetic animals demonstrated impaired coronary microvessel relaxation to ADP and substance P whereas relaxation to endothelium-independent vasorelaxant, SNP, was similar between groups, suggesting endothelial dysfunction and reduced NO bioavailability. Furthermore, the reduction in microvessel relaxation was greatly exaggerated in response to VEGF, suggesting impairment in VEGF signaling in addition to reduced NO bioavailability.
In addition to microvascular dysfunction, the presence of diabetes has been associated with a number of abnormalilites that can impair the angiogenic response to myocardial ischemia. First, exposure to chronic hyperglycemia leads to the nonenzymatic glycation of protein leading to the formation of advanced glycation end products (AGEs). In vitro studies have demonstrated a diminished vascular tube formation in response to growth factors in a glycated collagen matrix.8 In a hindlimb ischemia model, Tamarat et al demonstrated reduced endogenous angiogenesis in diabetic mice which was reversed by the administration of aminoguanidine, an inhibitor of AGE formation.9
Second, diabetes is associated with alterations in proangiogenic growth factor signaling. A number of studies have demonstrated alterations in the expression of various growth factors in plasma and noncardiac tissue in the setting of diabetes.11,26 Sasso et al studied the expression of VEGF and its downstream mediators in myocardial biopsies of patients with or without type II diabetes and found that although VEGF expression was increased, that VEGF receptor activation and downstream signaling was reduced.10 In concordance with this study, we also found that diabetes was associated with impaired growth factor signaling, which was manifest as reduced Tie-2 expression and decreased eNOS phosphorylation. Contrary to the above study, however, VEGF expression was reduced in the myocardium of diabetic swine and Ang-1 expression was similar between groups. The differences in VEGF expression between the 2 studies may be attributable to differences in the nature of diabetic disease as well as different treatments used in the patients examined.
Lastly, there is little available information on the role of antiangiogenic proteins in the context of diabetes. Angiostatin and endostatin, cleavage products of plasminogen and collagen XVIII, respectively, are formed through the proteolytic actions of various matrix metalloproteinases, and are potent antiangiogenic proteins. Weirauch et al demonstrated reduced collateral-dependent perfusion in chronically instrumented dogs under hyperglycemic conditions and demonstrated the critical role of angiostatin in inhibiting collateral vessel formation in response to repetitive coronary occlusion, both in vivo and in vitro.27 We have reproduced some of their findings in a clinically relevant model of diabetes and chronic myocardial ischemia by demonstrating a profound (>4-fold) increase in angiostatin expression in diabetic animals. In addition, diabetes was also associated with a 2-fold increase in endostatin expression. Inhibition of these antiangiogenic proteins may represent a novel target for the modulation and enhancement of the angiogenic response in diabetes.
Limitations
Our model of alloxan-induced diabetes largely mimics type I diabetes, ie, hypoinsulinemic hyperglycemia. Although various aspects of the disease are common between type I and type II diabetes including chronic exposure to hyperglycemia, endothelial dysfunction, increased oxidative stress and inflammation, accelerated atherosclerosis, and diminished angiogenesis, other features may be different and may limit the generalizability of this model to type II diabetes and insulin resistance. Furthermore, this model exposes the animals to a relatively short duration (15 weeks) of severe hyperglycemia compared with the very long durations (many years) of mild to moderate hyperglycemia that diabetic patients experience. Lastly, although this model of chronic ischemia provides physiologically relevant measures of perfusion and function, it is limited by the fact that molecular markers were only assessed at a single time point, 7 weeks after ameroid placement, and therefore, acute changes in pro- and antiangiogenic mediators may not be adequately captured in this model.
Conclusions
Diabetes results in a profound impairment in endogenous myocardial angiogenesis in response to chronic ischemia. The associated alterations in growth factor signaling and increased expression of antiangiogenic mediators identified in this study may represent novel targets for the modulation of the diabetic angiogenic response. Finally, the establishment of this model will allow for the preclinical evaluation of growth factor or cell-based angiogenic therapies as well as effects of angiogenic modulating agents eg, insulin, l-arginine, statins, etc that may have therapeutic potential in patients with diabetes.
Acknowledgments
Sources of Funding
This study was funded by the grant R01 HL69024 from the National Institutes of Health (Dr Sellke). Dr Boodhwani is supported by a grant from the National Institutes of Health (HL04095-06) and the Irving Bard Memorial Fellowship.
Disclosures
F.W.S. is on the speakers bureau for Bayer Inc, a consultant for Dyax Pharmaceuticals, and has received grant support from Ikaria Pharmaceuticals.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Presented at the American Heart Association Scientific Sessions, Chicago, Ill, November 12–15, 2006.
References
- 1.Grundy SM, Garber A, Goldberg R, Havas S, Holman R, Lamendola C, Howard WJ, Savage P, Sowers J, Vega GL. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group IV: lifestyle and medical management of risk factors. Circulation. 2002;105:e153–e158. doi: 10.1161/01.cir.0000014022.85836.96. [DOI] [PubMed] [Google Scholar]
- 2.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 3.Yarom R, Zirkin H, Stammler G, Rose AG. Human coronary microvessels in diabetes and ischaemia. Morphometric study of autopsy material. J Pathol. 1992;166:265–270. doi: 10.1002/path.1711660308. [DOI] [PubMed] [Google Scholar]
- 4.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48:1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 5.Momose M, Abletshauser C, Neverve J, Nekolla SG, Schnell O, Standl E, Schwaiger M, Bengel FM. Dysregulation of coronary microvascular reactivity in asymptomatic patients with type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2002;29:1675–1679. doi: 10.1007/s00259-002-0977-0. [DOI] [PubMed] [Google Scholar]
- 6.Papaioannou GI, Seip RL, Grey NJ, Katten D, Taylor A, Inzucchi SE, Young LH, Chyun DA, Davey JA, Wackers FJ, Iskandrian AE, Ratner RE, Robinson EC, Carolan S, Engel S, Heller GV. Brachial artery reactivity in asymptomatic patients with type 2 diabetes mellitus and microalbuminuria (from the Detection of Ischemia in Asymptomatic Diabeticsbrachial artery reactivity study) Am J Cardiol. 2004;94:294–299. doi: 10.1016/j.amjcard.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Rosen P, Ballhausen T, Stockklauser K. Impairment of endothelium dependent relaxation in the diabetic rat heart: mechanisms and implications. Diabetes Res Clin Pract. 1996;31 Suppl:S143–S155. doi: 10.1016/0168-8227(96)01242-9. [DOI] [PubMed] [Google Scholar]
- 8.Kuzuya M, Satake S, Ai S, Asai T, Kanda S, Ramos MA, Miura H, Ueda M, Iguchi A. Inhibition of angiogenesis on glycated collagen lattices. Diabetologia. 1998;41:491–499. doi: 10.1007/s001250050937. [DOI] [PubMed] [Google Scholar]
- 9.Tamarat R, Silvestre JS, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier MP, Wautier JL, Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci U S A. 2003;100:8555–8560. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi H, Takagi H, Koyama S, Oh H, Watanabe D, Antonetti DA, Matsubara T, Nagai K, Arai H, Kita T, Honda Y. Alterations in expression of angiopoietins and the Tie-2 receptor in the retina of streptozotocin induced diabetic rats. Mol Vis. 2004;10:608–617. [PubMed] [Google Scholar]
- 12.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 13.Tofukuji M, Metais C, Li J, Hariawala MD, Franklin A, Vassileva C, Simons M, Sellke FW. Effects of ischemic preconditioning on myocardial perfusion, function, and microvascular regulation. Circulation. 1998;98:II197–II204. discussion II204-II205. [PubMed] [Google Scholar]
- 14.Szabo C. PARP as a drug target for the therapy of diabetic cardiovascular dysfunction. Drug News Perspect. 2002;15:197–205. doi: 10.1358/dnp.2002.15.4.840052. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 16.Tada H, Muramatsu I, Nakai T, Kigoshi S, Miyabo S. Effects of chronic diabetes on the responsiveness to endothelin-1 and other agents of rat atria and thoracic aorta. Gen Pharmacol. 1994;25:1221–1228. doi: 10.1016/0306-3623(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 17.Rosen P, Ballhausen T, Bloch W, Addicks K. Endothelial relaxation is disturbed by oxidative stress in the diabetic rat heart: influence of tocopherol as antioxidant. Diabetologia. 1995;38:1157–1168. doi: 10.1007/BF00422364. [DOI] [PubMed] [Google Scholar]
- 18.Bagwell CA, Brophy C. Enhanced arterial contractile responses in diabetic hypercholesterolemic pig carotid arteries. Int J Surg Investig. 2000;1:477–481. [PubMed] [Google Scholar]
- 19.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulindependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 20.Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulindependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen MJ, Clarkson P, Donald AE, Thomson H, Thorne SA, Powe AJ, Furuno T, Bull T, Deanfield JE. Effect of enalapril on endothelial function in young insulin-dependent diabetic patients: a randomized, double-blind study. J Am Coll Cardiol. 1998;31:1330–1335. doi: 10.1016/s0735-1097(98)00099-0. [DOI] [PubMed] [Google Scholar]
- 22.Sekiya M, Suzuki J, Watanabe K, Funada J, Otani T, Akutsu H. Beneficial effect of troglitazone, an insulin-sensitizing antidiabetic agent, on coronary circulation in patients with non-insulin-dependent diabetes mellitus. Jpn Circ J. 2001;65:487–490. doi: 10.1253/jcj.65.487. [DOI] [PubMed] [Google Scholar]
- 23.van Etten RW, de Koning EJ, Verhaar MC, Gaillard CA, Rabelink TJ. Impaired NO-dependent vasodilation in patients with Type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45:1004–1010. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- 24.Watts GF, O’Brien SF, Silvester W, Millar JA. Impaired endotheliumdependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidaemia. Clin Sci (Lond) 1996;91:567–573. doi: 10.1042/cs0910567. [DOI] [PubMed] [Google Scholar]
- 25.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulindependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 26.Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis. 2005;180:113–118. doi: 10.1016/j.atherosclerosis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel PS, Warltier DC, Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]