Abstract
A straightforward and original methodology allowing the synthesis of Janus-type dendrimer-like poly(ethylene oxide)s (PEOs) carrying orthogonal functional groups on their surface is described. The use of 3-allyloxy-1,2-propanediol (1) as a latent AB2-type heterofunctional initiator of anionic ring-opening polymerization (AROP) of ethylene oxide (EO) and of selective branching agents of PEO chain ends served to construct the two dendrons of these dendrimer-like PEOs, following a divergent pathway. Thus, the first PEO generation of the first dendron was grown by AROP from 1 followed by the reaction of the corresponding α-allyl,ω,ω′-bishydroxy- heterofunctional PEO derivative with 2-(3′-chloromethybenzyloxymethyl)-2-methyl-5,5-dimethyl-1,3-dioxane (2) used as a branching agent. This afforded the dendron A with four latent peripheral hydroxyls protected in the form of two ketal rings. The remaining α-allylic double bond of the PEO thus prepared was transformed into two hydroxyl groups using OsO4 in order to create the first PEO generation of the dendron B by AROP of EO. Allyl chloride (3) was then used as another (latent) branching agent to react with the terminal hydroxyl of the corresponding PEO chains. Deprotection under acidic conditions of the ketal groups of dendron A, followed by AROP of EO, afforded the second PEO generation on this face. This alternate and divergent procedure, combining AROP of EO and selective branching of PEO branches, could be readily iterated, one dendron after the other up to the generation six, leading to a Janus-type dendrimer-like PEO exhibiting a total mass of around 300 kg/mol and possessing 64 peripheral groups on each face. The possibility of orthogonal functionalization of the surfaces of such Janus-type dendritic PEOs was exploited. Indeed, a dendron of generation 4 was functionalized with hydroxyl functions at its periphery, whereas the other was end-capped with either tertiary amino or disulfide groups. In a variant of this strategy, azido groups and acetylene could also be orthogonally introduced at the periphery of the fourth generation Janus-type dendrimer-like PEO and subjected to polycondensation by a 1,3-dipolar cycloaddition reaction. This afforded a necklace-like covalent assembly of dendrimer-like PEOs through the formation of stable [1,2,3]-triazole linkages.
Introduction
Dendritic macromolecules encompass several subclasses of molecular compounds.1 Among them, a distinction is generally made between regular dendrimers that are macromolecules characterized by a precise size1 and structures such as dendri-grafts,2 dendronized3 and dendrimer-like polymers4 that exhibit some fluctuations of their size and molar mass. Beyond differences in the perfection of their structure and in the methods of synthesis, all dendritic macromolecules share common features such as the persistence of their overall shape due to their highly branched nature and also their multivalency materialized by a large number of peripheral functions.
After an initial period marked by the development of the methodologies of synthesis of these dendritic macromolecules,1 the time of applications in various fields—catalysis,5 light harvesting systems or energy and electron transfer,6 molecular encapsulation,7 contrast agents,8 drug delivery vehicles9—has come. One strong demand arising from the biomedical field is the availability of Janus-type dendritic structures that can assume two, or even more, different tasks such as targeting a particular site, transporting at this site a medicinally active drug and imaging it with a diagnostic label.7–10 This demand has propelled intense research into the design of dendritic species that carry at least two different types of functional groups that may be located at their periphery and/or at their core and in the inner layers. Majoros et al. have for instance statistically functionalized the periphery of their poly(amidoamine) (PAMAM) fifth generation dendrimers with folic acid as the targeting moiety, fluorescein isothioisocyanate as the fluorescence probe, and taxol as the drug.11 The design of two-faced or multifaced—also called Janus-type or hybrid or block codendrimers or asymmetrically arranged—dendrimers with different types of peripheral functions for surface manipulation or orthogonal surface functional groups for multifunctionalization is another challenging approach that was scarcely addressed.11–31 For instance, Fréchet et al. have synthesized “bow-tie” hybrids possessing polyester dendrons for the attachment of therapeutically active molecules and decorated with solubilizing poly(ethylene oxide) (PEO) chains.12 Wu et al. have derived by convergent approach dendrimers containing mannose units on one face and coumarin on the other using click chemistry.17 Similarly, Dirksen et al. have assembled two dendritic polylysines, one functionalized with a label and the other with peptides,18 whereas Lukin et al. combined divergent and convergent approaches to prepare Janus-type sulfonamides dendrimers.19 More recently, Deng et al. developed hybrid polyether-poly(ether amide) dendritic catalysts,20 while Fre´chet et al. reported on the synthesis of heterobifunctional dendritic polyesters.21 In some of these dendrimers designed for therapeutic applications,8–12,17,18,21,23,31 in vivo studies showed that they seldom reach their target because of their cytotoxicity and their uptake by the reticular endothelial system.7–10 This toxicity is generally overcome by grafting PEO, also referred to as poly(ethylene glycol) (PEG), on the outer surface of dendrons, which has proven essential to protect dendritic nanodevices from opsonization and to enhance their circulation time.12,32,33
This dire necessity has prompted us to undertake a program of research devoted to the synthesis of dendritic systems based uniquely on PEO and to their applications in the biomedical field. As each generation of these PEO-based dendritic objects originates from the chain polymerization of ethylene oxide, this entails some fluctuation of their molar mass; the term coined to name them is “dendrimer-like polymer”,4 the rest of their attributes being the same as those of regular dendrimers. In a first contribution, we described the methodology of synthesis of high generation symmetrical dendrimer-like PEOs that involved the repetition of the same sequence of reactions, i.e., polymerization of EO and a branching reaction at arm ends.34 Upon modification of their outer hydroxyl functions with glycosidic units, dendrimer-like PEOs were shown to exhibit anti-inflammatory properties.35 In a variant of this divergent approach and to better address the need of therapeutic applications, we prepared dendrimer-like PEOs that can expand and shrink as a function of pH36 and also bouquet-type dendritic PEOs that can accommodate proteins such as albumin at the core and therapeutic agents at their periphery.37 The need for dendritic PEO possessing asymmetrical faces in the biomedical field has motivated us to evolve an original synthetic strategy. The dendrimer-like PEOs described in this contribution can be viewed as the assembly of two PEO-based dendritic faces connected one to the other through their focal point and end-fitted with two different types of functional groups at their periphery. The strategy of synthesis is novel and original: in contrast to the heterodifunctional dendrimers reported so far that were generally prepared following a convergent approach, it indeed relies on a divergent approach, 11–30 though Tomalia et al. previously reported the synthesis of segmented dendrimers by a divergent method as well.38 From the same heterodifunctional initiator, each dendron was built independently one from the other which means that the successive generations were grown alternately. The chemistry used to obtain the two PEO-based dendrons only differs one from the other by the type of branching agent introduced at each generation arm tip. Reiteration of living anionic polymerization of EO and these chain end functionalization/branching reactions afforded Janus-type dendrimer-like PEOs up to the sixth generation. This alternate and divergent strategy was further exploited to functionalize them dissimilarly. As a proof of concept, one of the two dendrons based on PEO was functionalized with peripheral hydroxyl functions, whereas the other was end-capped with either tertiary amino or disulfide groups with a view of modifying surfaces such as that of mica. In a last addition, azide and acetylene functions were respectively introduced as end groups on each face of these dendrimer-like PEOs, their copper(I)-catalyzed cycloaddition affording the formation of a necklace-type morphology through stable [1,2,3] triazole linkages.
Experimental Section
Materials
Ethylene oxide (EO) (Fluka, 99.8%) was distilled over sodium into a buret. Diphenylmethylpotassium (DPMK) was prepared and titrated with acetanilide (4.5 × 10−4 mol· mL−1) as described in ref 34. Dimethyl sulfoxide (DMSO) and dimethylformamide (DMF) were distilled over CaH2 prior to use. The initiator, namely, 3-allyloxy-1,2-propanediol (1), was purified by distillation prior to use. The branching agent, 2-(3′-chloromethybenzyloxymethyl)-2-methyl-5, 5-dimethyl-1,3-dioxane (2), was prepared as described previously.36 All star-like and dendrimer-like PEO precursors were freeze-dried from a dioxane solution. All other chemicals and solvents (Aldrich) were used as received without further purification.
General Procedure of Polymerization
Anionic ring-opening polymerization of EO was carried out using Schlenk equipment following a slightly modified procedure as that already reported:34–37 for precursors possessing both primary and secondary hydroxyls deriving from branching agent 3, a few drops of EO were first introduced and the rest of the monomer was added after 2 h. Growth of PEO generations from branching agent 2 was achieved by adding EO in one shot.
General Procedure for Chemical Modification of PEO Chain Ends
Etherification reactions from OH-containing PEO precursors and either allyl chloride or 2-(3′-chloromethybenzy-loxymethyl)-2-methyl-5,5-dimethyl-1,3-dioxane as well as bis-hydroxylation of allylic double bonds and acidic deprotection of ketal groups were performed following the procedures published elsewhere.34–37
Synthesis of PEO-Gk4(OH)32-Ga4(NMe2)16
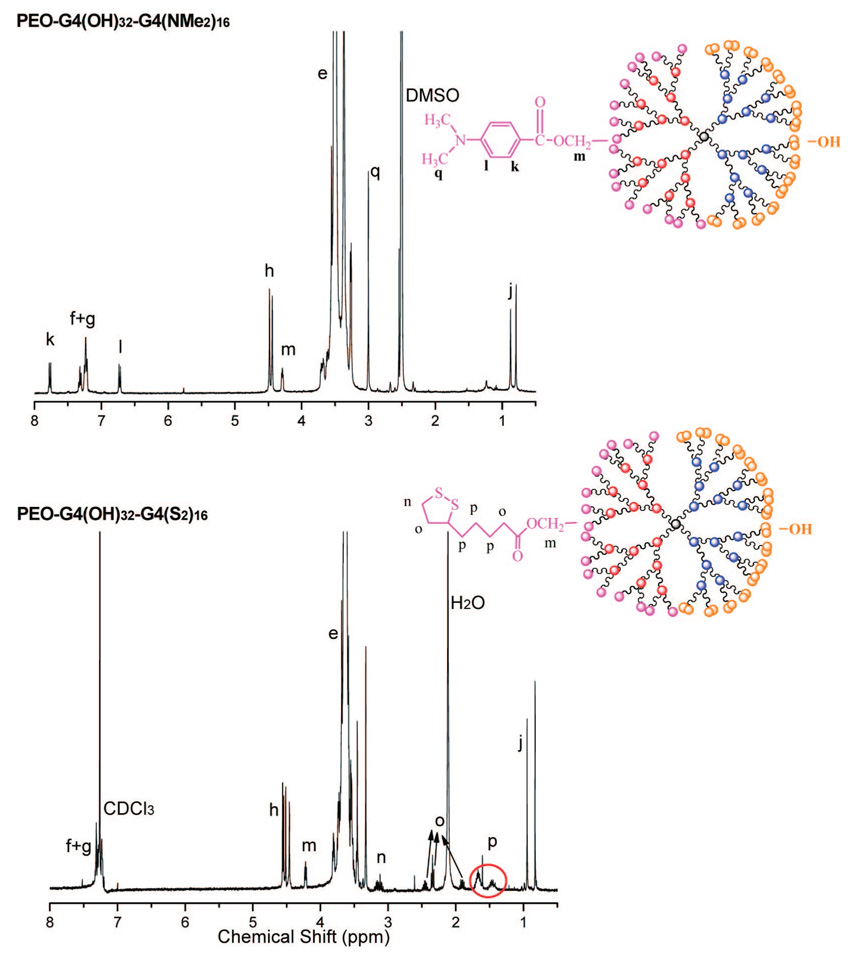
To a solution of PEO-Gk4(ket)16-Ga4(OH)16 (3.0 g, 0.6 mmol OH) in 20 mL of dried DMF, 4-dimethylaminobenzoic acid (0.5 g, 3 mmol), dicyclohexy-lcarbodimide (DCC) (0.93 g, 4.5 mmol), and 4-(dimethylamino)py-ridinium-4-toluenesulfonate (DPTS) (0.18 g, 0.6 mmol) were added under nitrogen. After stirring at room temperature (rt) overnight, the precipitate was filtered off and the filtrate was precipitated in a large excess of cold diethyl ether. After filtration, the material was redissolved in toluene and centrifuged to remove any solid impurities. The clear solution thus obtained was again precipitated in cold diethyl ether. The solid compound was then dissolved in 10 mL (0.1M) of HCl in THF/MeOH (1:1 vol.), and the solution was stirred at room temperature for 6 h. After concentration, the solution was extracted with dichloromethane twice. The organic layers were dried over anhydrous MgSO4 and concentrated, and the solution was precipitated in cold diethyl ether. The compound (2.5 g, 83%) was filtered and dried under vacuum at rt. The 1H NMR spectrum of PEO-Gk4(OH)32-Ga4(NMe2)16 is shown in Figure 6.
Figure 6.
1H NMR of PEO-Gk4(OH)32-Ga4(NMe2)16 and PEO-Gk4(OH)32-Ga4(S2)16.
The synthesis of PEO-Gk4(OH)32-Ga4(S2)16 was accomplished (in total yield 73%) following the same procedure except that 1,2-dithiolane-3-pentanoic acid was used for esterification. The 1H NMR spectrum of this compound is also shown in Figure 6.
Synthesis of 4-Chloromethyl-1-azidomethylbenzene
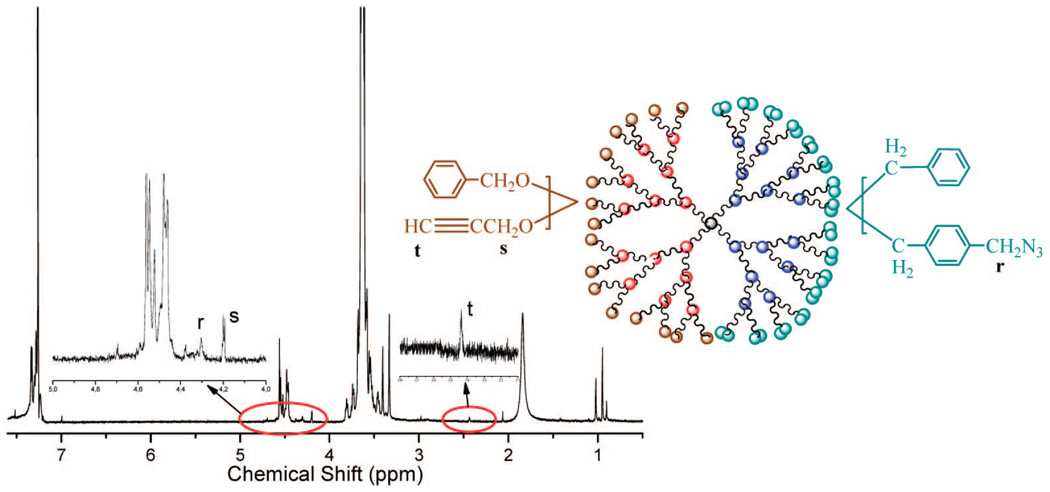
This compound was synthesized according to the procedure reported by Wu et al.39 To a 250 mL flask, 60 mL of a solution of acetone/ water (4/1 vol.), α,α′-dichloro-p-xylene (8.80 g, 50 mmol), and sodium azide (3.60 g, 55 mmol) were added. The mixture was stirred at 60 °C for 4 h. The organic phase was dried with magnesium sulfate and concentrated, and the crude product was obtained by vacuum distillation. Refractional distillation afforded a pure monoazido compound (6.8 g, 75%). 1H NMR (δppm, CDCl3): 7.40, 7.33 (two t, 4H, aromatic protons), 4.59 (d, 2H, ClCH2), 4.36 (d, 2H, N3CH2).13C NMR (δppm, CDCl3): 135.4, 129.1, 128.6, 54.4, 45.7.
Synthesis of
To a solution of tetrabutylammonium bromide (TBAB) (14 mg, 0.042 mmol) and NaOH (0.33 g, 8.4 mmol) in 0.33 g of water, PEO-Gk4(ket)16-Ga4(OH)16 (2.5 g, 0.42 mmol OH), and THF (3 mL) were added. After stirring for 30 min at 50 °C, a mixture of benzyl bromide and propargyl bromide (8.4 mmol total, 8.5:1.5 molar ratio) was added under N2. The solution was kept for 24 h at 50 °C under vigorous stirring. After removal of volatiles, the residues were extracted with dichloromethane and the solution was concentrated. The product (2.3 g, 92%) was obtained by precipitation with excess cold diethyl ether. A similar procedure was used for the deprotection of the ketal groups to afford . The latter compound (2.2 g, 0.74 mmol OH) was submitted to azidation following the same procedure as that described above, using in this case a 20-fold molar excess of a mixture of benzyl chloride and 4-chloromethyl-1-azidomethylbenzene (9:1 molar). Drying under vacuum at room temperature gave (2.0 g, 90%). 1H NMR is shown in Figure 7.
Figure 7.
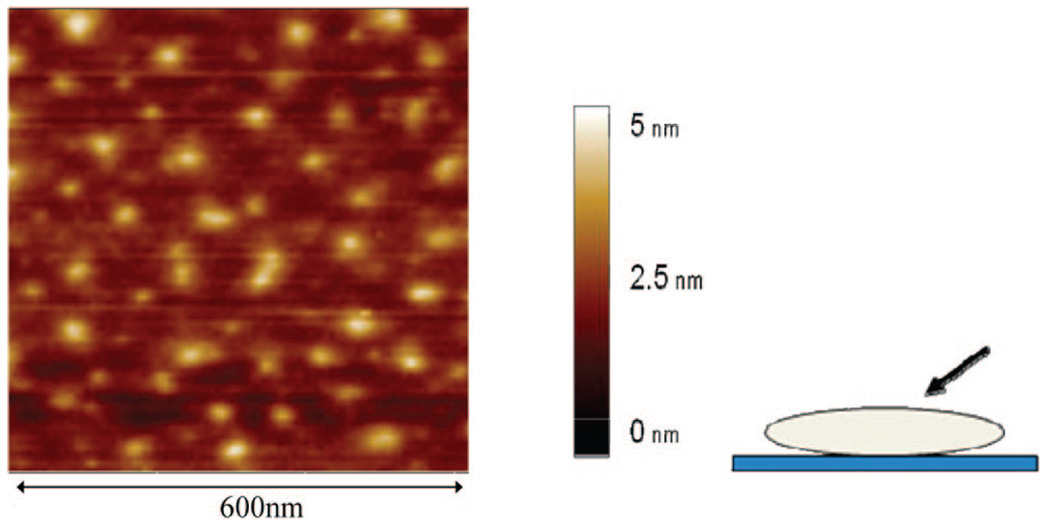
AFM image of Mica functionalized with the quaternized Janus-type dendrimer-like PEO-Gk4(OH)32-Ga4(NMe2)16.
Polycondensation of by Copper-Catalyzed Huisgen’s 1,3-Dipolar Cycloadditions (Click Chemistry)
50 mg of were dissolved in 10 mL of a water solution of LiCl deionized (1 M). After stirring at rt for 1 week, the solution was filtered with a 0.1 µm Millipore filter. Only one single population of unimer was observed by dynamic light scattering (DLS). Solutions of copper sulfate (0.8 mg) and ascorbic acid (1.3 mg) (prefiltered with 0.1 µm Millipore filter) were added into the above unimer solution of , and the mixture was stirred for 2 weeks at rt before characterization by DLS and AFM.
Characterization
1H NMR spectra were recorded on a Bruker AC 400 spectrometer.
The molar masses were determined by size exclusion chromatography (SEC) that was performed using a PSS column (8 mm × 300 mm, 5 µm), equipped with a refractive index detector (Varian RI-4) in tetrahydrofuran (THF) as eluent (1 mL/min) at 25 °C. SEC columns were calibrated with linear polystyrene samples. High generation PEO samples were analyzed with SEC equipment fitted with three TSK-gel columns (7.8 cm × 30 cm, 5 µm, G 2000, 3000, and 4000 HR with pore sizes of 250, 1500, and 10 000 Å, respectively) and a refractive index (RI) detector (Jasco, RI-1530) using DMF as eluent (0.7 mL/min) calibrated with linear polystyrene or linear poly(ethylene oxide) standards with no significant difference observed between the two types of standards.
MALDI-TOF mass spectrometry was performed using a Micromass TofSpec E spectrometer equipped with a nitrogen laser (337 nm), a delay extraction, and a reflector. The MALDI mass spectra represent averages over 100 laser shots. This instrument operated at an accelerating potential of 20 kV. The polymer solutions (10 g · L−1) were prepared in THF. The matrix solution (1,8-dithranol-9(10H)-anthracenone, dithranol) was dissolved in THF. The polymer solution (2 µL) was mixed with 20 µL of the matrix solution, and 2 µL of a sodium iodide solution (10 g · L−1 in methanol) were added to favor ionization by cation attachment. The final solution (1 µL) was deposited onto the sample target and dried in air at room temperature.
Dynamic light scattering (DLS) experiments were performed using an ALV Laser Goniometer, which consists of a 22 mW HeNe linear polarized laser with a 632.8 nm wavelength and an ALV-5000/EPP Multiple Tau Digital Correlator with a 125 ns initial sampling time. The samples were kept at constant temperature (25 °C) during all experiments. The accessible scattering angular range varied from 40° up to 150°. The solutions were introduced into 10 mm diameter glass cells. The minimum sample volume required for the experiment was 1 mL. The data acquisition was done with the ALV-Correlator Control Software, and the counting time varied for each sample from 300 s up to 600 s. Millipore water was thoroughly filtered through 0.1 µm filters and directly used for the preparation of the solutions. All the solutions showed a monomodal distribution with a translational diffusive mode. The hydrodynamic radius (RH) of these dendrimer-like samples was then calculated from the diffusion coefficient using the Stokes-Einstein relation D = kT/6πηRH, where η is the viscosity of the medium (water).
Atomic Force Microscopy (AFM)
A dilute solution (0.01 wt%) was spin cast on 1 × 1 cm2 freshly cleaved mica. Samples were analyzed after complete evaporation of the solvent at room temperature. All AFM images were recorded in air with a Dimension Microscope (Digital Instruments, Santa Barbara, CA), operated in tapping mode. The probes were commercially available silicon tips with a spring constant of 40 N/m, a resonance frequency lying in the 270–320 kHz range, and a radius of curvature in the 10–15 nm range.
Results and Discussion
Synthesis of Janus-Type Dendrimer-like PEOs
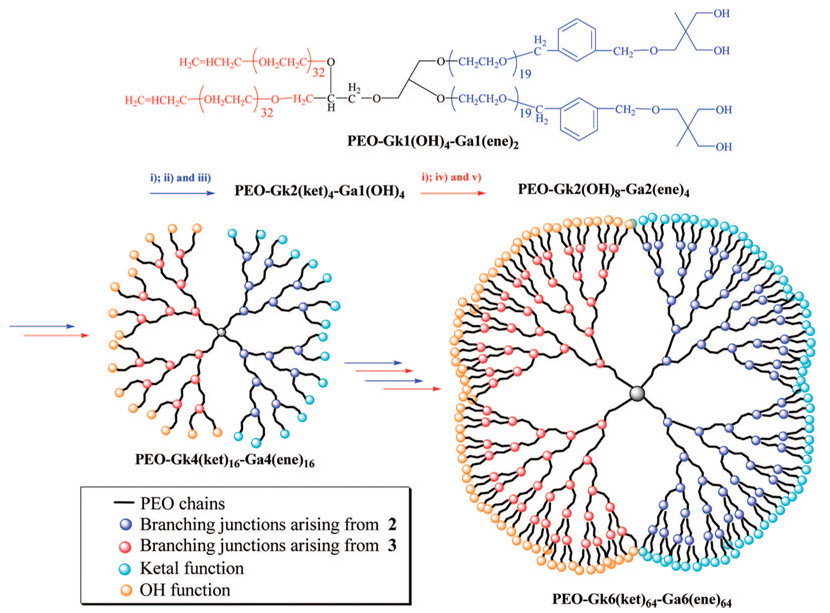
The design of Janus-type dendrimer-like PEOs fitted with two types of peripheral functions hinges on a novel synthetic method, involving the divergent and alternate growth of two different PEO-based dendrons. Grown from a unique heterodifunctional initiator, both dendrons were synthesized by repeating the three steps of anionic ring opening polymerization (AROP) of EO, chain end functionalization through the use of orthogonal branching agents, and activation/multiplication of initiating sites for the next generation to grow (Scheme 1).
Scheme 1.
Branching Reactions Used during the Alternate and Divergent Synthesis of Janus-Type Dendrimer-like PEOs
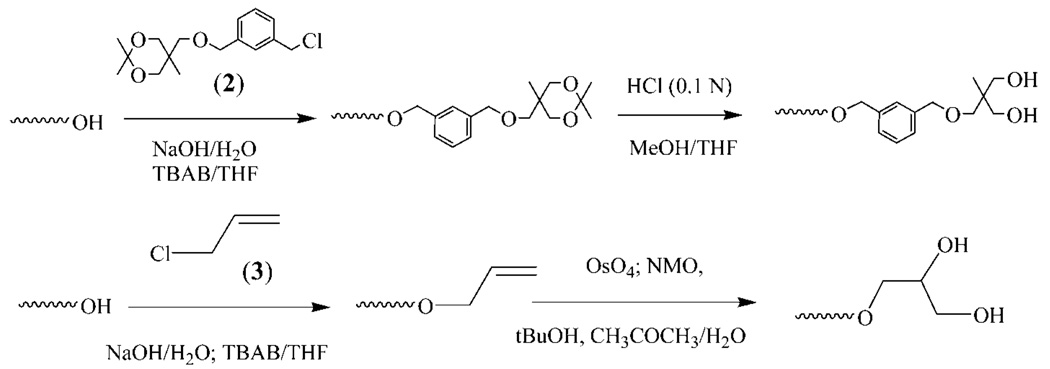
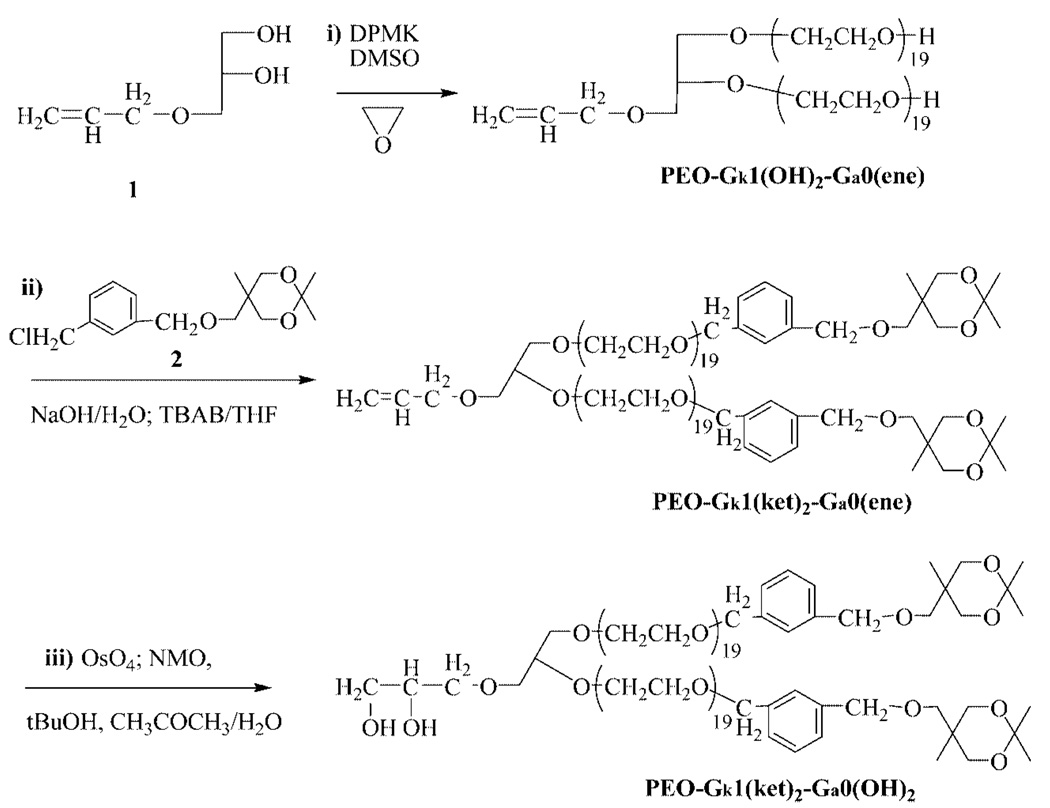
The last two steps are nominally the same for the two dendrons whereas the branching reactions of the PEO arm ends are specific to each dendron. It is thus essential that the branching/activation reactions of one particular dendron do not interfere with those of the other dendron. Only under these conditions can the sequence of activation/polymerization/ functionalization peculiar to each dendron succeed one after another and eventually permit to end functionalize dissimilarly the last generations. As shown in Scheme 2, the initiator meant to become the core of such Janus-type dendrimer-like PEOs is 3-allyloxy-1,2-propanediol (1). The two hydroxyls of this initiator were partially deprotonated (approximately 30% of hydroxyl) by a solution of diphenylmethyl potassium (DPMK), the AROP of EO being carried out in dimethylsulfoxide (DMSO) under conditions similar to those previously reported.34–37 By preventing the aggregation of propagating alkoxides and promoting a rapid exchange of protons with dormant hydroxy-lated species, these conditions permitted the AROP of EO to proceed in a controlled way, yielding PEO-Gk1(OH)2-Ga0(ene) linear samples of targeted molar masses and low polydispersities (PDIs). The designation of these Janus-type dendrimer-like PEOs will be as follows: Gkn refers to the nth generation of the dendron whose branching agent is a ketal-containing compound; Gan refers to the nth generation of the other dendron whose branching agent is allyl chloride, and OH or ene represents the functions (hydroxyl or allyl) present at each PEO chain end.
Scheme 2.
Alternate Synthetic Strategy to Dendron A of Janus-Type Dendrimer-like PEOs Using 2 as a Branching Agent
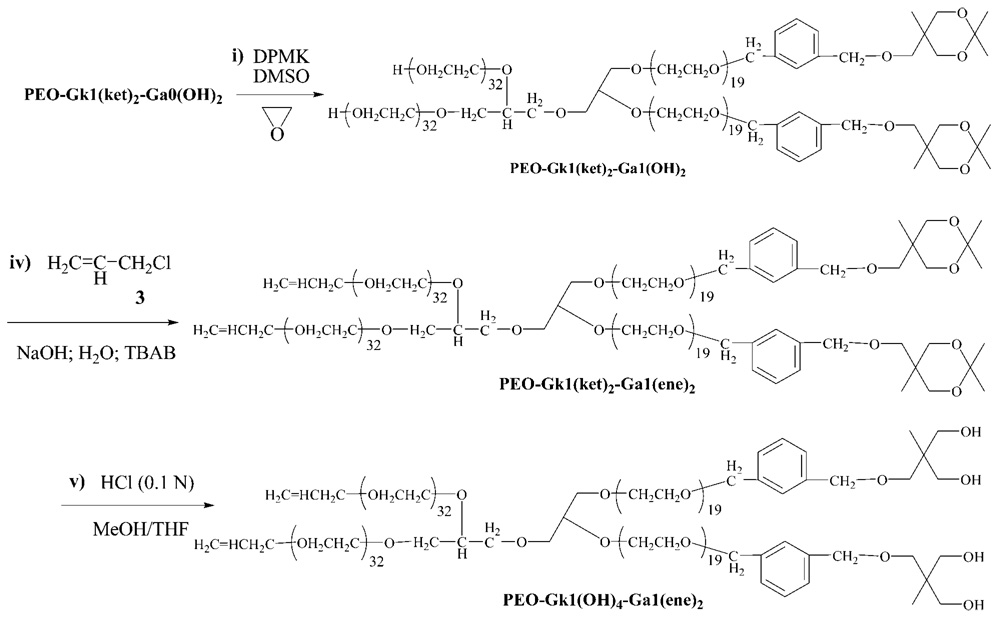
The two terminal hydroxyl groups carried by the chain ends were then submitted to reaction with 2-(3′-chloromethybenzy-loxymethyl)-2-methyl-5,5-dimethyl-1,3-dioxane (2) as branching agent using a similar etherification procedure as that already reported.34–37 This branching agent specific to the ketal-containing dendron was designed to multiply by a factor of 2 the number of hydroxyls generation after generation. A linear PEO, noted PEO-Gk1(ket)2-Ga0(ene), carrying one double bond at its focal point and two geminal hydroxyls protected in the form of a ketal ring (ket) at each arm end, was thus obtained, completing the synthesis of the first generation of the ketal-featuring dendron. The functionalization/branching/activation steps of the other dendron were performed through the introduction of allyl double bonds at PEO chain ends followed by the derivatization of the allylic groups into two hydroxyls by an osmylation reaction (Scheme 3). The allylic double bond standing at the focal point of PEO-Gk1(ket)2-Ga0(ene) was then subjected to this osmylation reaction, affording PEO-Gk1(ket)2-Ga0(OH)2 which served as a precursor to grow the first generation of the allyl-derived dendron from its two hydroxyls. Before this, the effectiveness of the branching reactions of both the allyl-derived dendron and the ketal-featuring dendron was monitored by 1H NMR spectroscopy and by MALDI-TOF mass spectroscopy. Figure 1 shows the 1H NMR spectra of PEO-Gk1(OH)2-Ga0(ene), PEO-G1k(ket)2-Ga0(ene), and PEO-Gk1(ket)2-Ga0(OH)2 where all the peaks can be undoubtedly assigned. After etherification of PEO-Gk1(OH)2-Ga0(ene), the terminal OH protons entirely disappear and a new series of peaks due to aromatic protons, methylene protons, two geminal methyl groups, and other methyl groups, all belonging to the ketal-containing branching agent, appear at 7.25, 4.53, 1.39, and 0.88 ppm, respectively. Likewise, after osmylation and hydrolysis of the osmate ester formed, the peaks assigned to the double bond at 5.90-5.81, 5.27-5.12 ppm completely vanish in favor of two new peaks appearing at 4.62 and 4.48 ppm, respectively (although the latter peak overlaps with the signal attributable to the methylene protons of the aromatic ring). Importantly, the ketal groups in PEO-G1k(ket)2-G a0(ene) remain unaffected during osmylation. In all cases, the integral ratios are in excellent agreement with theoretical values, indicating a controlled AROP of EO, a quantitative functionalization of living chain ends and a flawless derivatization of the central allyl double bond.
Scheme 3.
Alternate Synthetic Strategy to Dendron B of Janus-Type Dendrimer-like PEOs Using 3 as a Branching Agent
Figure 1.
1H NMR of PEO-G1(OH)2-G0(ene) (A); PEO-G1(ket)2-G0(ene) (B); and PEO-G1(ket)2-G0(OH)2 (C).
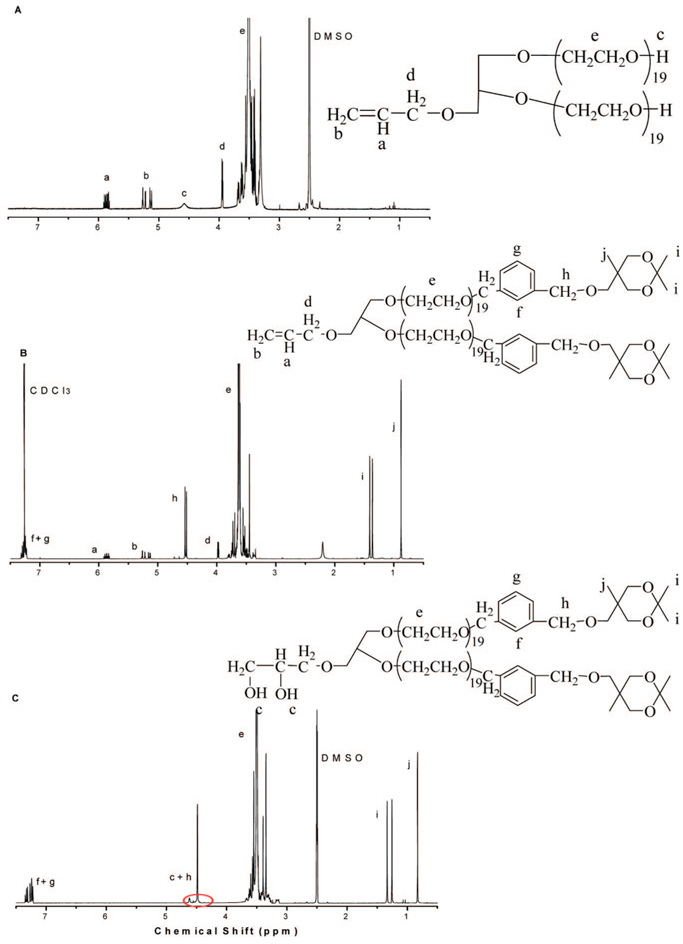
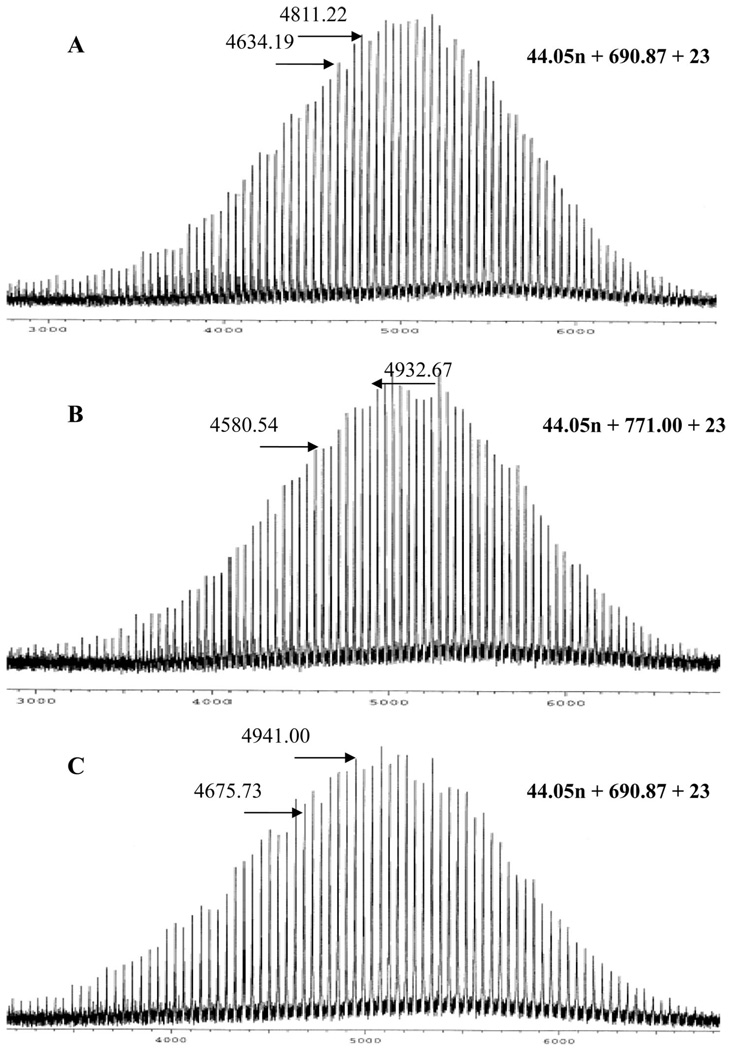
The MALDI TOF characterization of these samples fully corroborates the results of the 1H NMR analysis. Only one population is observed in all cases with a peak-to-peak mass increment of 44.05 g•mol−1 corresponding to the molar mass of one EO unit (Figure 2). By taking into account the molar mass of chain ends and that of the initiator, the distribution of chains reflected in the MALDI-TOF spectrum could be perfectly accounted for. The peaks, indeed, appeared at m/z = 44.05n + Mtermi + 23, where n is the degree of polymerization, 23 is the molar mass of the sodium ion generated during the ionization process, and Mtermi is the molar mass of the end groups which is equal to 132.16 for PEO-Gk1(OH)2-Ga0(ene), 656.86 for PEO-Gk1(ket)2-Ga0(ene), and 690.87 for PEO-Gk1(ket)2-Ga0(OH)2, respectively.
Figure 2.
MALDI TOF mass spectrometry of 1H NMR of PEO-G1(OH)2-G0(ene) (A); PEO-G1(ket)2-G0(ene) (B); and PEO-G1(ket)2-G0(OH)2 (C).
The latter sample was then used to grow the two PEO arms of the first generation of the allyl-derived dendron. The same conditions as those previously reported were applied to polymerize EO, the sample eventually obtained, PEO-Gk1(ket)2-Ga1(OH)2, corresponding to the first generation of Janus-type dendrimer-like PEO comprised of its two first generation dendrons. Next, the two terminal hydroxyl groups of PEO-Gk1(ket)2-Ga1(OH)2 were functionalized with allyl chloride (Scheme 3), affording PEO-Gk1(ket)2-Ga1(ene)2 whose allyl termini and the corresponding dendron are expected to remain inert during the sequence of reactions applied to the ketal-endowed dendron. The ketal groups of PEO-Gk1(ket)2-Ga1(ene)2 were then deprotected under acidic conditions which generated PEO-Gk1(OH)4-Ga1(ene)2 and four terminal hydroxyls used to grow the next generation of ketal-featuring dendrons.
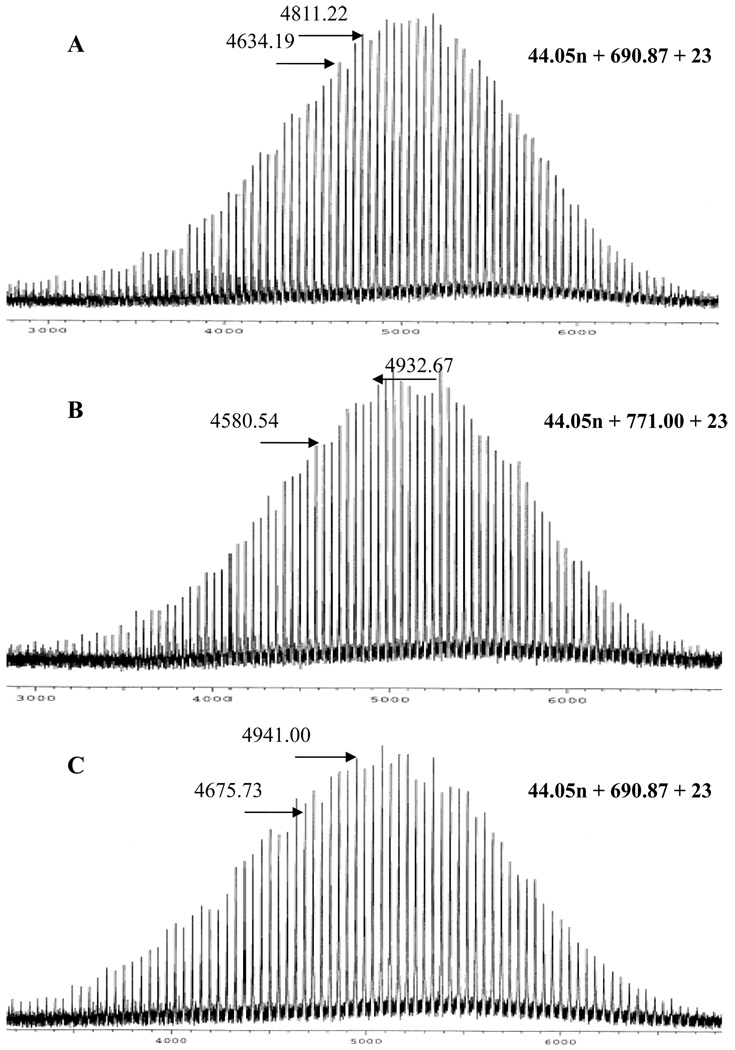
Prior to the AROP of EO from PEO-Gk1(OH)4-Ga1(ene)2, the latter sample and its precursors PEO-Gk1(ket)2-Ga1(ene)2 and PEO-Gk1(ket)2-Ga1(OH)2 were thoroughly characterized by 1H NMR and MALDI TOF. In Figure 3A, the integral ratio of peak i to peak j, attributable to the two geminal methyl protons of ketal ring and to the methyl protons of the same ketal, respectively, is the same as the one determined in Figure 1B and Figure 1C, indicating that the ketal groups were stable and left untouched during the AROP of EO. In Figure 3B, the peaks characteristic of the allylic double bond of PEO-Gk1(ket)2-Ga1(ene)2 clearly appear at 5–6 ppm, and in Figure 3C, no signal due to ketal ring is observed after acidic treatment undergone by PEO-Gk1(ket)2-Ga1(ene)2. The peaks due to the methyl protons of this protecting group is indeed replaced by a triplet appearing at 4.33 ppm, attributable to the four hydroxyl protons of PEO-Gk1(OH)4-Ga1(ene)2. Equally important, is the unchanged intensity of peaks assigned to the allylic double bond with respect to the others, meaning that the latter functions were not affected by the acidic conditions used for deprotection. In all cases, the integral ratios of corresponding peaks are in excellent agreement with theoretical values, indicating a controlled AROP of EO as well as a quantitative functionalization and deprotection step. The MALDI TOF mass spectra of the three first generation samples PEO-Gk1(ket)2-Ga1(OH)2, PEO-Gk1(ket)2-Ga1(ene)2, and PEO-Gk1(OH)4-Ga1(ene)2 unambiguously demonstrate that AROP of EO and the chemistry used to allylate hydroxyls and deprotect ketals were flawless (Figure 4). Indeed, one single population is observed with a peak-to-peak mass increment of 44.05 g·mol−1. The distribution of chains seen in Figure 4 corresponds perfectly to the one calculated by taking into account the molar mass of a sodium ion generated during the ionization process and the molar mass of the respective end groups. Both 1H NMR and MALDI TOF mass spectrometry as well as the SEC analyses confirm that the functionalization/branching, activation steps specific to each dendron occurred quantitatively and selectively without side reactions. These steps could thus be applied alternately to grow the two dendrons generation after generation.
Figure 3.
1H NMR of PEO-G1(ket)2-G1(OH)2 (A); PEO-G1(ket)2-G1(ene)2 (B); and PEO-G1(OH)4-G1(ene)2 (C).
Figure 4.
MALDI TOF mass spectrometry of PEO-G1(ket)2-G1(OH)2 (A); PEO-G1(ket)2-G1(ene)2 (B); and PEO-G1(OH)4-G1(ene)2 (C).
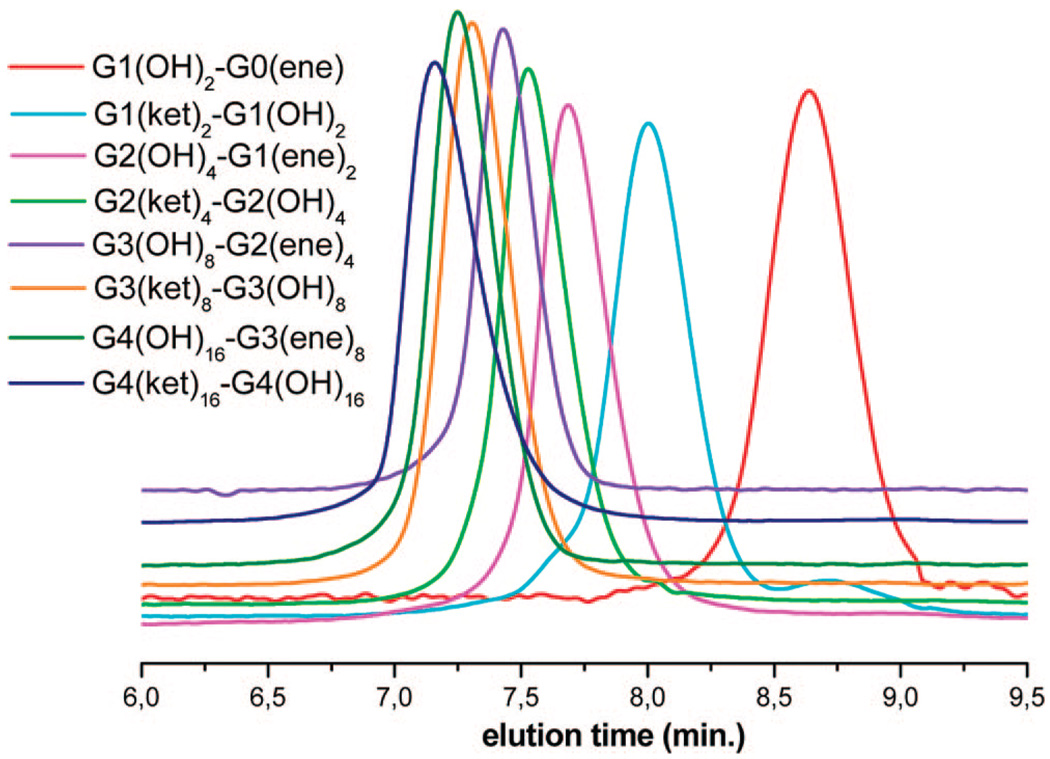
The sequence of reactions described above was thus repeated alternately up to the sixth generation with a total molar mass of 330 000 g · mol−1, the PEO segments having an average molar mass of 1000 g · mol−1, i.e., an average degree of polymerization of 24 (Scheme 4). The chemistry associated with the growth of each dendron is versatile and flexible enough to be adapted at the gram scale to the preparation of a library of Janus-type dendrimer-like PEOs comprised, for instance, of faces with an unequal number of generations and of different size. In this work, only dendrimer-like PEOs composed of two dendrons that include a same number of generations that were dissimilarly functionalized at their periphery are described. In Table 1 are gathered all data pertaining to 1H NMR and SEC characterization of Janus-type dendrimer-like PEOs from generation 1 to generation 6 including all intermediates. In samples Gkn(ket)2n-Gan(OH)2n, the peak j due to the branching agent 2-(3′-chloromethybenzyloxymethyl)-2-methyl-5,5-dimethyl-1,3-di-oxane appearing at 0.88 ppm in the NMR spectrum was used as a reference to calculate the molar mass; in samples Gkn(ket)2n-Ga(n–1)(ene)2n−1 we used the signal due to allyl double bonds as a reference. In all cases, the experimental values of molar masses fall close to the theoretical ones. As expected with the increase of the branched character of these dendrimer-like samples, discrepancies can be noted between the theoretical values of molar masses or those drawn from 1H NMR characterization with values obtained by SEC analysis from linear PEO standards. This merely reflects the highly branched nature of the dendrimer-like PEOs whose hydrodynamic volumes are obviously smaller than those of linear polymers of the same molar mass used for the calibration of SEC. As shown in Figure 5, the SEC traces exhibit a unimodal and narrow molar mass distribution with PDIs remaining below 1.2. Due to the strong aggregation of the samples of high generations in THF, the latter solvent was used for SEC characterization only for the four first generations. DMF was indeed preferred as the eluent for all other samples: the extent of aggregations reduces to a very small amount and even vanishes when the GPC analysis was carried out at 80 °C.
Scheme 4.
Representation of Janus-Type Dendrimer-like PEOs
Table 1.
Molecular Characteristics of Janus-Type Dendrimer like PEOs
| code of PEO derivative | Mn theo × 103a (g · mol−1) | Mn NMR × 103b (g · mol−1) | Mn SEC × 103c (g · mol−1) | PDIc | DPe (CH2CH2O) | OHf | tot.g |
|---|---|---|---|---|---|---|---|
| Gk1(OH)2-Ga0(ene) | 2.20 | 1.85 | 2.84 | 1.07 | 19 | 2 | 3 |
| Gk1(ket)2-Ga1(OH)2 | 5.97 | 5.20 | 7.58 | 1.12 | 32 | 2 | 4 |
| Gk2(OH)4-Ga1(ene)2 | 10.8 | 8.62 | 10.7 | 1.12 | 18 | 4 | 6 |
| Gk2(ket)4-Ga2(OH)4 | 16.2 | 12.0 | 14.2 | 1.14 | 16 | 4 | 8 |
| Gk3(OH)8-Ga2(ene)4 | 24.5 | 22.5 | 20.2 | 1.11 | 29 | 8 | 12 |
| Gk3(ket)8-Ga3(OH)8 | 36.7 | 29.3 | 25.6 | 1.11 | 16 | 8 | 16 |
| Gk4(OH)16-Ga3(ene)8 | 51.1 | 49.4 | 27.9 | 1.11 | 27 | 16 | 24 |
| Gk4(ket)16-Ga4(OH)16 | 71.3 | 64.0 | 29.8 | 1.17 | 18 | 16 | 32 |
| Gk5(OH)32-Ga4(ene)16 | 112 | 101 | 102d | 1.19 | 26 | 32 | 48 |
| Gk5(ket)32-Ga5(OH)32 | 163 | 146 | 119d | 1.19 | 28 | 32 | 64 |
| Gk6(OH)64-Ga5(ene)32 | 233 | 220 | 143d | 1.32 | 25 | 64 | 96 |
| Gk6(ket)64-Ga6(OH)64 | 330 | 299 | 191d | 1.29 | 24 | 64 | 128 |
Theoretical molar mass based on the molar ratio of the monomer to the (macro)initiator.
Molar mass determined by 1H NMR using DMSO-d6 as solvent (see text).
Molar mass and polydispersity index determined by SEC using linear polystyrene standards for calibration.
The elution solvent used for SEC was DMF.
The average degree of polymerization of the grown PEO segments drawn from 1H NMR data.
Theoretical number of peripheral OH groups.
Theoretical number of total peripheral groups.
Figure 5.
SEC traces (THF) of Janus-type dendrimer-like PEOs (see also Table 1).
The protocol described here thus allowed us to obtain two types of dendrimer-like PEOs for subsequent peripheral derivatization with dissimilar functions. Indeed, either PEO-Gkn(ket)2n-Gan(OH)2n or PEO-Gkn(OH)2n-Ga(n-1)(ene)2n−1 can serve as precursors for such a derivatization resulting in the introduction of two different functions at the periphery of each dendron. For instance, PEO-Gkn(ket)2n-Gan(OH)2n can be modified with functional groups reacting with its 2n hydroxyls, and its 2n ketal ring can well be deprotected so as to generate PEO-Gkn(ket)2n-Gan(X)2n dendrimer-like samples fitted with 2n X functions on one face and 2n+1 hydroxyls on the other. In the case of PEO-Gkn(OH)2n-Ga(n–1)(ene)2n−1, one can derivatize the hydroxyls of the ketal-featuring dendron and transform the 2n−1 allyl double bonds into twice as many hydroxyls by osmylation/ hydrolysis so as to obtain PEO-Gkn(X)2n-Ga(n–1)(OH)2n. We opted for the first alternative and describe in the next section two examples of Janus-type dendrimer-like PEOs fitted with hydroxyls on one face and a tertiary amine or disulfide on the other.
Derivatization of the Periphery of Janus-Type Dendrimer-like PEOs
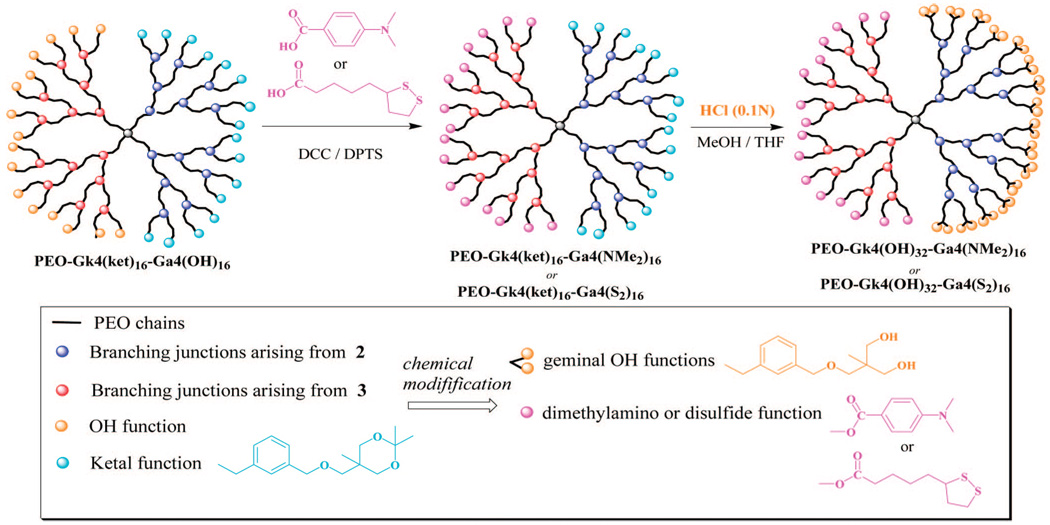
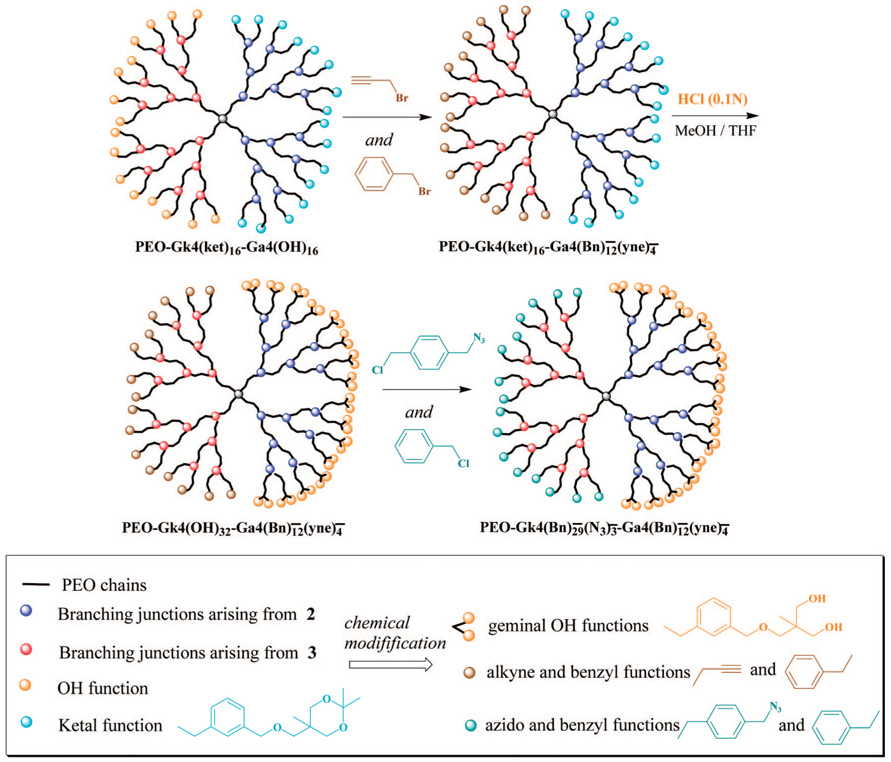
Knowing that disulfide-containing molecules bind strongly to gold surfaces and form thiolate covalent bonds and that cationic polymers derived from amino-containing polymers adsorb on opposite charges substrates such as mica, we prepared a dendritic PEO fitted with peripheral disulfides or tertiary amines on one face and hydroxyls on the other for the purpose of surface modification. In addition, the hydroxyls carried by the noninteracting dendron could well be used to conjugate biomolecules for the purpose of biorecognition and biosensing; the latter aspect will be described in a forthcoming paper. In a two-step sequence of reactions (Scheme 5), the 16 hydroxyls of the allyl-featuring dendron in PEO-Gk4(ket)16-Ga4(OH)16 were first esterified with either 4-dimethylaminobenzoic acid or 1,2-dithiolane-3-pentanoic acid, using DPTS and DCC as catalysts. The 16 ketal rings of the ketal-terminated dendron were subsequently deprotected under acidic conditions. Characterization by 1H NMR of the obtained structures PEO-Gk4(OH)32-Ga4(NMe2)16 and PEO-Gk4(OH)32-Ga4(S2)16 revealed the disappearance of the double peak due to the two geminal methyl protons at 1.39 ppm, indicating a quantitative deprotection of the ketal rings (Figure 6). Moreover, the six methylene protons belonging to the disulfide moiety of PEO-Gk4(OH)32-Ga4(S2)16 could be clearly seen in the NMR spectrum through the n, o, p peaks, the signal of the single methine proton overlapping with that of PEO chains. On the other hand, introduction of the 4-dimethylaminobenzoic moiety in PEO-Gk4(OH)32-Ga4(NMe2)16 was revealed by peaks due to the aromatic protons and the six methyl protons at 7.77 (k), 6.72 (l), and 3.00 (q) ppm. Noteworthy is the direct evidence of esterification brought by the peaks appearing at 4.22, 4.30 ppm in both spectra, which are attributable to the terminal methylene groups of PEO chains that carry the ester functions. Figure 7 shows a representative AFM image obtained from the coating of a mica surface with our Janus-type dendrimer-like PEOs using a tapping mode. Spherical particles corresponding to individual dendritic PEOs can be clearly seen with an average diameter of 25 nm.40
Scheme 5a.
a Chemical modification of the periphery of Janus-type dendrimer-like PEOs: introduction of tertiary amino groups or disulfides on one face and of geminal hydroxyls on the other.
Polycondensation of Heterodifunctionalized Janus-Type Dendrimer-like PEOs by “Click Chemistry”
Regular dendrimers are known to exhibit a noncollapsed globular shape, but dendrons and dendronized polymers1–3,29,41 can be engineered to self-assemble into a variety of supramolecular objects of unique morphologies. Starting from dendritic building blocks that were provided with structural information, Percec’s group has generated unique synthetic assemblies resembling spherical micelles, cylinders, helical porous columns, etc.29,41 In this investigation, we took advantage of the Janus-type character of the two dendrons to link them one to another and elaborate a very unique necklace morphology, corresponding to a string of dendrimer-like PEOs. Our first move was to modify the outer part of the two dendrons with antagonist functions. As each dendron of a given generation contains not less than 2n outer functions, the introduction of as many antagonist functions on each dendron followed by their subsequent condensation would have resulted in cross-linked dendrimer-like PEOs. To avoid the formation of such a macroscopic network and obtain the expected necklace morphology, only a small percentage of the 2n peripheral groups (between 10 and 25%) were transformed into antagonist functions, the rest being modified into inert functions. In that case, it can be expected that each face provides the way and room for only one reaction with an antagonist function carried by another dendron, the steric hindrance generated after one such reaction with another dendron preventing any branching through a second reaction. The two antagonist functions chosen to end-cap the periphery of dendrons are azide and alkyne, two functional groups known to undergo a “click reaction” by 1,3-dipolar cycloaddition42,43 in the presence of Cu(I) as catalyst (Scheme 5).
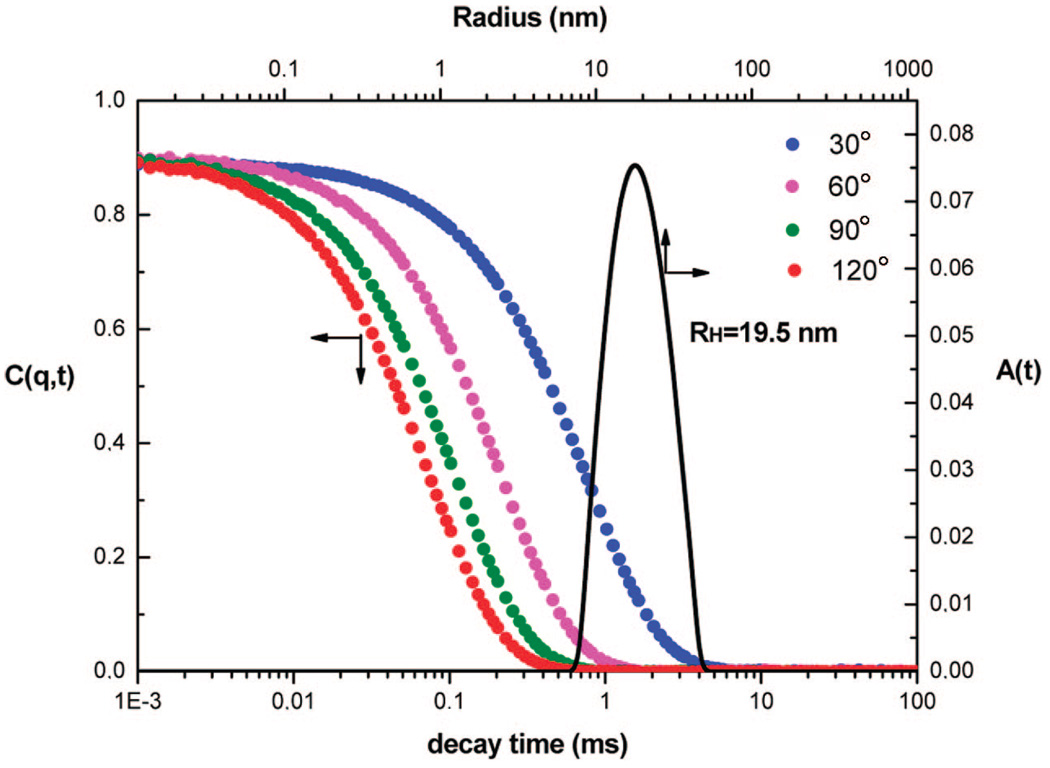
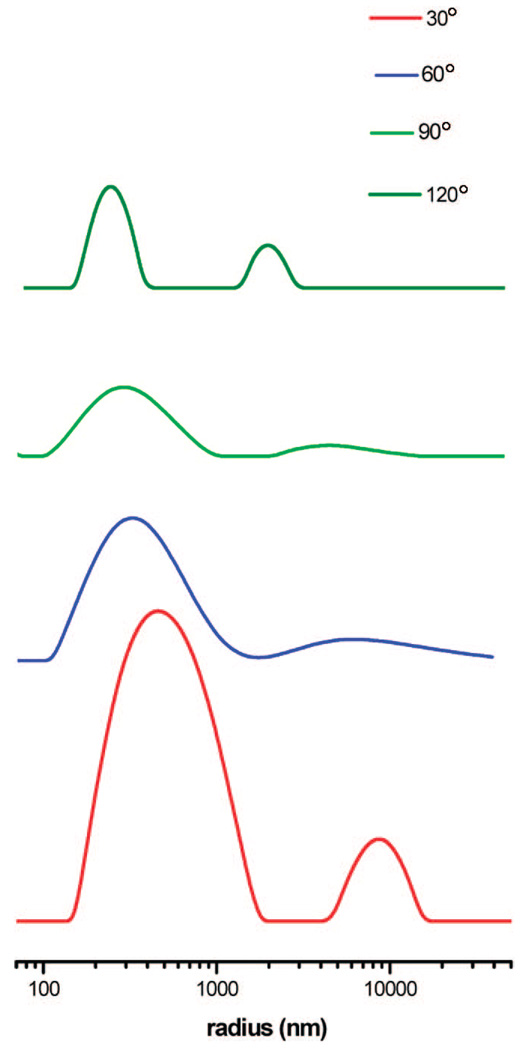
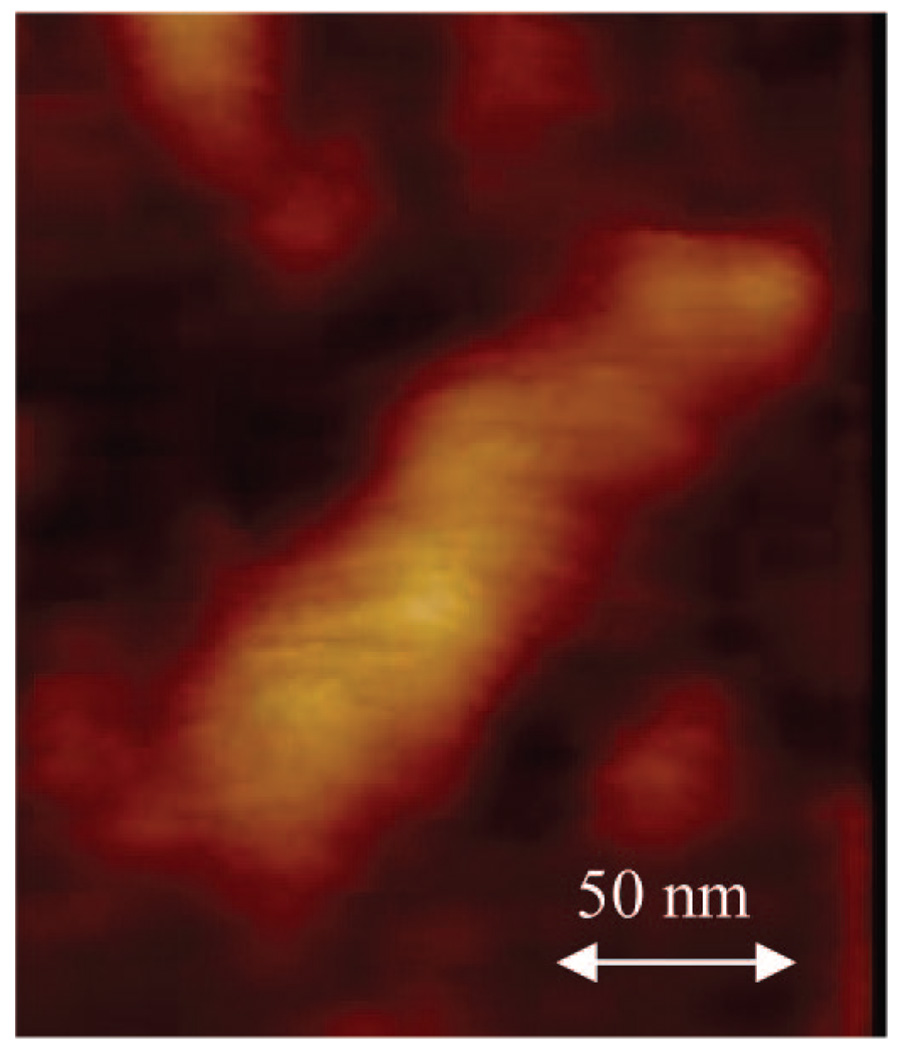
From the precursor PEO-Gk4(ket)16-Ga4(OH)16 selected, the targeted dendrimer could be obtained in three steps. First, only 25% of the 16 hydroxyls of the precursor were transformed into 4-azido methyl benzoyl moieties, the remaining hydroxyls being neutralized into benzoyloxy moieties by reaction with benzyl chloride. The obtained product was subjected to acidic treatment of its 16 ketal rings and generated 32 hydroxyls; only 10% of the latter functions were modified into propargyl groups using propargyl bromide, the remaining 90% being converting into benzoyloxyl moieties. The presence of azide and acetylene groups was confirmed by 1H NMR, as shown in Figure 8. The signal of methylene protons adjacent to azido group is seen at 4.30 ppm while the signal of propargyl protons appears at 4.20 and 2.44 ppm, respectively. Based on the integral intensities of these peaks, the average numbers of azido and propargylic groups introduced on each face correspond closely to the expected values. The characteristic absorption of the azide groups and propargyl triple bonds were also well detected by IR at around 2095 and 2160 cm−1. Before carrying out the azide-alkyne Cu(I)-mediated cycloaddition of this asymmetric dendrimer-like PEO, the latter was treated with LiCl in water to avoid any possible aggregation. As shown by dynamic light scattering, only one population is observed (Figure 9) and a value of 39 nm is determined for the hydrodynamic diameter (2RH) of this asymmetric PEO dendrimer whose molar mass is around 100 000 g ·mol−1. The click reaction was then triggered by addition of ascorbic acid and CuSO4. The morphology of the object formed upon reaction was monitored in situ by light scattering (Figure 10) and also subsequently analyzed by AFM in a tapping mode (Figure 11). Figure 11 shows a string of dendrimers attached one to another forming a sort of necklace, and the light scattering analysis indicates a dramatic increase in the size of the object formed and its nonspherical character (Figure 10).
Figure 8.
1H NMR of the Janus-type dendrimer-like with acetylene groups at one face and azido groups at the other.
Figure 9.
Dynamic light scattering of in 1 M LiCl water solution (before click chemistry).
Figure 10.
Dynamic light scattering results of after click chemistry.
Figure 11.
AFM image depicting a necklace of dendrimer-like PEOs adsorbed on mica and obtained in a tapping mode. The image is presented in height mode at a resolution of 512 × 512 pixels/line.
Conclusion
Dendrimer-like PEOs possessing orthogonal functional groups on their surface that could be further derivatized with pharmaceutically active molecules hold great promise for target biomedical applications as nanocarriers in drug or gene delivery or in molecular imaging. Advantages provided by the dendrimer-like architecture, in particular the multiplicity of its peripheral functional groups (multivalency) for surface modification, can be combined with the specific properties of PEO, including biocompatibility, water solubility, nontoxicity, and nonrecognition by the immune system (stealth effect). We have herein demonstrated that Janus-type dendrimer-like PEO-based scaffolds, bearing orthogonal functions on their surface, could be designed up to generation 6. A novel iterative and alternate divergent approach was evolved to this end, combining “living” anionic ring-opening polymerization of ethylene oxide, selective branching reactions of PEO chain ends, and postmodification of the surface of the two dendrons. In this way, the size of each generation in each dendron as well as the location of functional groups at the periphery could be finely tuned. As a proof of concept of surface modification, a fourth-generation Janus-type dendrimer-like PEO, which was selectively heterobifunctionalized with orthogonal azide and alkyne moieties, was used as a precursor for a 1,3-dipolar cycloaddition reaction (click chemistry), leading to the first example of a necklace tie-up dendrimer-like polymer. Work is in progress to conjugate molecules with bioactive properties to these multivalent Janus-type dendrimer-like PEOs in an orthogonal fashion; biological evaluation of these materials in vitro and in vivo will be also investigated. It is expected that the molecular features of the dendritic PEO scaffold will prevent the side effects due to the therapeutic or diagnostic agent and prolong the circulation time.
Scheme 6a.
a Chemical modification of the periphery of Janus-type dendrimer-like PEOs: introduction of tertiary amino groups or disulfides on one face and of geminal hydroxyls on the other.
Acknowledgment
The authors are grateful to the National Institute for Health for the financial support of this work (Program No. 5R01 RR14190-04).
References
- 1.For a review on dendrimers, see for instance: Hawker CJ. Adv. Polym. Sci. 1999;147:113. Fischer M, Vo¨gtle F. Angew. Chem., Int. Ed. 1999;38:884. doi: 10.1002/(SICI)1521-3773(19990401)38:7<884::AID-ANIE884>3.0.CO;2-K. Majoral J-P, Caminade A-M. Chem. Rev. 1999;99:845. doi: 10.1021/cr970414j. Inoue K. Prog. Polym. Sci. 2000;25:453. Fréchet JMJ, Tomalia DA. In: Dendrimers and other Dendritic Polymers. Fréchet JMJ, Tomalia DA, editors. John Wiley & Sons, Ltd; 2001. Tomalia DA. Prog. Polym. Sci. 2005;30:294. Smith DK. Chem. Commun. 2006:34. doi: 10.1039/b507416a. Boas U, Heegard PMH. Chem. Soc. Rev. 2004;33:43. doi: 10.1039/b309043b. Gitsov I, Lin C. Curr. Org. Chem. 2005;9:1025. Li JG, Meng C, Zhang XQ, Zhang L, Zhang AD. Progr. Chem. 2006;18:1157.
- 2.For a review on dendrigraft polymers, see: Teerstra SJ, Gauthier M. Prog. Polym. Sci. 2004;29:277. Gauthier MJ. Polym. Sci, Part A: Polym. Chem. 2007;45:3803.
- 3.For a review on dendronized polymers, see: Schlu¨ter AD. Top. Curr. Chem. 1998;197:165. Schlu¨ter AD, Rabe JR. Angew. Chem., Int. Ed. 2000;39:864. Frauenrath H. Prog. Polym. Sci. 2005;30:325. Zhang A. Progr. Chem. 2005;17:157.
- 4.For a recent highlight on dendrimer-like polymers, see: Taton D, Feng X, Gnanou Y. New J. Chem. 2007;31:157.
- 5.For a review on dendrimers used in catalysis, see: Astruc D, Chardac F. Chem. Rev. 2001;101:2991. doi: 10.1021/cr010323t. Mery D, Astruc D. Coord. Chem. Rev. 2006;250:1965. van de Coevering R, Klein Gebbink RJM, van Koten G. Prog. Polym. Sci. 2005;30:474. Hwang S-H, Shreiner CD, Moorefield CN, New kome GR. New J. Chem. 2007;31:1192. Andre´s R, de Jesus E, Flores JC. New J. Chem. 2007;31:1161. Caminade A-M, Servin P, Laurent R, Majoral J-P. Chem. Soc. Rev. 2008;1:56. doi: 10.1039/b606569b. Helms B, Fréchet JM. J. Adv. Synth. Catal. 2006;348:1125.
- 6.For a highlight or a review on applications of dendrimers in light harvesting and energy and electron transfer, see: Fréchet JM. J. J. Polym. Sci., Part A: Polym. Chem. 2003;41:3713. Jiang D-L, Aida T. Prog. Polym. Sci. 2005;30:403. Ceroni P, Bergamini G, Marchioni F, Balzani V. Prog. Polym. Sci. 2005;30:453. Mongin O, Pla-Quintana A, Terenziani F, Drouin D, Le Droum-aguet C, Caminade A-M, Majoral J-P, Blanchard-Desce M. New J. Chem. 2007;31:1354.
- 7.(a) Zimmerman SC, Wendland MS, Rakow NA, Zharov I, Suslick KS. Nature. 2002;418:399. doi: 10.1038/nature00877. [DOI] [PubMed] [Google Scholar]; (b) Ong W, Gómez-Kaifer M, Kaifer AE. Chem. Commun. 2004:1677. doi: 10.1039/b401186d. [DOI] [PubMed] [Google Scholar]
- 8.For applications of dendrimers as contrast agents, see: Langereis S, Dirksen A, Hackeng TM, Van Genderen MHP, Meijer EW. New J.Chem. 2007;31:1152.
- 9.For applications of dendrimers in drug or gene delivery, see: Stiriba S-E, Frey H, Haag R. Angew. Chem., Int. Ed. 2002;41:1329. doi: 10.1002/1521-3773(20020415)41:8<1329::aid-anie1329>3.0.co;2-p. Gillies ER, Fre`ıchet JM. J. Drug Discovery Today. 2005;10:35. doi: 10.1016/S1359-6446(04)03276-3. Aulenta F, Hayes W, Rannard S. S. Eur. Polym. J. 2003;39:1741. Dufés C, Uchegbu IF, Scha¨tzlein AG. Adv. Drug Delivery Rev. 2005;57:2177. doi: 10.1016/j.addr.2005.09.017. Krishna TR, Parent M, Werts MHV, Moreaux L, Gmouh S, Charpak S, Caminade A-M, Majoral J-P, Blanchard-Desce M. Angew. Chem., Int. Ed. 2006;45:4645. doi: 10.1002/anie.200601246.
- 10.For references on dendrimers for biomedical applications, see refs 7 to 9 and also: Slany M, Caminade A-M, Majoral J-P. Tetrahedron Lett. 1996;37:9053. Esfand R, Tomalia DA. Drug Discovery Today. 2001;6:427. doi: 10.1016/s1359-6446(01)01757-3. Svenson S, Tomalia DA. Adv. Drug Delivery Rev. 2005;57:2106. doi: 10.1016/j.addr.2005.09.018. Cloninger MJ. Curr. Opin. Chem. Biol. 2002;6:742. doi: 10.1016/s1367-5931(02)00400-3.
- 11.(a) Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr J. Med. Chem. 2005;48:5892. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]; (b) Majoros IJ, Myc A, Thomas TP, Mehta CB, Baker JR., Jr Biomacromolecules. 2006;7:572. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 12.Gillies ER, Fre´chet JMJ. J. Am. Chem. Soc. 2002;124:14137. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 13.Hawker CJ, Fre´chet JMJ. Macromolecules. 1990;23:4726. [Google Scholar]
- 14.Maraval V, Maraval A, Spataro G, Caminade A-M, Majoral J-P, Ha; Kim D, Knoll W. New J. Chem. 2006;30:1731. [Google Scholar]
- 15.Weil T, Wiesler UM, Herrmann A, Bauer R, Hofkens J, De Schryver FC, Klaus Mullen KJ. Am. Chem. Soc. 2001;123:8101. doi: 10.1021/ja010579g. [DOI] [PubMed] [Google Scholar]
- 16.Martin IK, Twyman LJ. Tetrahedron Lett. 2001;42:1119. [Google Scholar]
- 17.Wu P, Malkoch M, Hunt JN, Vestberg R, Kaltgrad E, Finn MG, Fokin VV, Sharpless KB, Hawker CJ. Chem. Commun. 2005:5775. doi: 10.1039/b512021g. [DOI] [PubMed] [Google Scholar]
- 18.Dirksen A, Meijer EW, Adriens W, Hackeng T. Chem. Commun. 2006:1667. doi: 10.1039/b600286b. [DOI] [PubMed] [Google Scholar]
- 19.Lukin O, Gramlich V, Kandre R, Zhun I, Felder T, Schalley CA, Dolgonos G. J. Am. Chem. Soc. 2006;128:8964. doi: 10.1021/ja061606b. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y-C, Wu T-F, Jiang L, Deng J-G, Liu H, Zhu J, Jiang Y-Z. J. Org. Chem. 2005;70:1006. doi: 10.1021/jo048317v. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin AP, Lam SS, Freìchet JMJ. J. Am. Chem. Soc. 2007;129:6994. doi: 10.1021/ja071530z. [DOI] [PubMed] [Google Scholar]
- 22.Maraval V, Laurent R, Donnadieu B, Mauzac M, Caminade A-M, Majoral J-P. J. Am. Chem. Soc. 2000;122:2499. [Google Scholar]
- 23.(a) Lim J, Simanek EE. Mol. Pharmaceutics. 2005;2:273. doi: 10.1021/mp050030e. [DOI] [PubMed] [Google Scholar]; (b) Zhang W, Nowlan DT, Thomson LM, Lackowski WM, Simanek EE. J. Am. Chem. Soc. 2001;123:8914. doi: 10.1021/ja0041369. [DOI] [PubMed] [Google Scholar]
- 24.Nierengarten J-F, Eckert J-F, Rio Y, Carreon MdP, Gallani J-L, Guillon DJ. Am. Chem. Soc. 2001;123:9743. doi: 10.1021/ja010155m. [DOI] [PubMed] [Google Scholar]; (b) Zhang S, Rio Y, Cardinali F, Bourgogne C, Gallani J-L, Nierengarten J-F. J. Org. Chem. 2003;68:9787. doi: 10.1021/jo035040a. [DOI] [PubMed] [Google Scholar]
- 25.Bury I, Donnio B, Gallani J-L, Guillon D. Langmuir. 2007;23:619. doi: 10.1021/la062066z. [DOI] [PubMed] [Google Scholar]
- 26.Saez IM, Goodby JW. Chem.—Eur. J. 2003;9:4869. doi: 10.1002/chem.200305100. [DOI] [PubMed] [Google Scholar]
- 27.Kimoto A, Cho J-S, Higuchi M, Yamamoto K. Macromolecules. 2004;37:5531. [Google Scholar]
- 28.Ropponen J, Nummelin S, Rissanen K. Org. Lett. 2004;6:2495. doi: 10.1021/ol049555f. [DOI] [PubMed] [Google Scholar]
- 29.Percec V, Imam MR, Bera TK, Balagurusamy VSK, Peterca M, Heiney PA. Angew. Chem., Int. Ed. 2005;44:4739. doi: 10.1002/anie.200501254. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Ambade AV, Vutukuri DR, Thayumanavan S. J. Am. Chem. Soc. 2006;128:14760. doi: 10.1021/ja065625x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal P, Yoon K, Weck M. Chem.—Eur. J. 2007;13:8801. doi: 10.1002/chem.200700129. [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, MacKay JA, Fre`ıchet JMJ, Szoka FC. Nat. Biotechnol. 2002;54:459. [Google Scholar]; (b) Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fre`ıchet JMJ, Dy ED, Szoka FC. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16649. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.For reviews on PEGylation reaction of protein and peptide, see: Roberts MJ, Bentley MD, Harris JM. AdV. Drug DeliVery ReV. 2002;54:459. doi: 10.1016/s0169-409x(02)00022-4. Pasut G, Veronese FM. AdV. Polym. Sci. 2006;192:95.
- 34.Feng XS, Taton D, Chaikof EL, Gnanou Y. J. Am. Chem. Soc. 2005;127:10956. doi: 10.1021/ja0509432. [DOI] [PubMed] [Google Scholar]; (b) Van Renterghem LM, Feng X, Taton D, Gnanou Y, Du Prez FE. Macromolecules. 2005;38:10609. [Google Scholar]
- 35.Rele SM, Cui W, Wang L, Hou S, Barr-Zarse B, Taton D, Gnanou Y, Esko JD, Chaikof EL. J. Am. Chem. Soc. 2005;127:10132. doi: 10.1021/ja0511974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng XS, Taton D, Borsali R, Chaikof EL, Gnanou Y. J. Am. Chem. Soc. 2006;128:11551. doi: 10.1021/ja0631605. [DOI] [PubMed] [Google Scholar]
- 37.Feng XS, Taton D, Chaikof EL, Gnanou Y. Biomacromolecules. 2007;8:2374. doi: 10.1021/bm070146r. [DOI] [PubMed] [Google Scholar]
- 38.Tomalia DA, Huang B, Swanson DR, Brothers HM, Klimash JW. Tetrahedron. 2003;59:3799–3813. [Google Scholar]; (b) DeMattei CR, Huang BH, Tomalia DA. Nano Lett. 2004;4:771–777. [Google Scholar]
- 39.Wu P, Feldman AK, Anne K, Nugent, Hawker CJ, Scheel A, Voit B, Pyun J, Fre´chet JMJ, Sharpless KB, Fokin VV. Angew. Chem., Int. Ed. 2004;43:3928. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 40.For a recent study by AFM on block copolymers composed of a dendron-type block, see: Pyun J, Tang C, Kowalewski T, Fréchet JMJ, Hawker CJ. Macromolecules. 2005;38:2674.
- 41.See for instance: Percec V, Johanson G, Ungar G, Zhou J. J. Am. Chem. Soc. 1996;118:9855. Balagurusamy VSK, Ungar G, Percec V, Johanson G. J. Am. Chem. Soc. 1997;119:1539. Percec V, Cho W-D, Ungar G, Yeardley DJP. J. Am. Chem. Soc. 2001;123:1302. Percec V, Dulcey AE, Peterca M, Adelman P, Samant R, Balagurusamy VSK, Heiney PA. J. Am. Chem. Soc. 2007;129:5992. doi: 10.1021/ja071088k.
- 42.For recent reviews on the use of 1,3-dipolar cycloaddition in macromolecular synthesis, see: Binder WH, Sachsenhofer R. Macromol. Rapid Commun. 2007;28:15. Evans RE. Aust. J. Chem. 2007;60:384. Lutz J-F. Angew. Chem., Int. Ed. 2007;46:1018. doi: 10.1002/anie.200604050.
- 43.For a review on the synthesis and hazards of azido derivatives, see: Brase S, Gil C, Knepper K, Zimmermann V. Angew. Chem., Int. Ed. 2005;44:5188. doi: 10.1002/anie.200400657.