Abstract
One of the major challenges in contemporary synthetic organic chemistry is the design and development of new tactics and strategies and their application to concise and efficient syntheses of biologically active natural products. Strategies that utilize reactions that enable the rapid assembly of the skeletal framework of such targets are thus especially attractive. In this context, we have developed novel applications of imine chemistry in Mannich and related reactions, cascade processes, and multicomponent reactions to rapidly assemble structural subunits common to diverse families of alkaloids. The practical utility of these chemistries is evidenced by their use in the execution of facile total syntheses of (±)-epilupinine (1), (±)-tashiromine (2), (−)-epimyrtine (3), and (±)-roelactamine (4) as well as other nitrogen heterocycles of potential biological interest.
INTRODUCTION
Imines and their derivatives have long been recognized as key intermediates for the synthesis of nitrogen heterocycles, especially in the arena of alkaloid synthesis.1 Over the years we have had a keen interest in developing new applications of imine chemistry that enable the facile construction of the nitrogen heterocyclic frameworks found in alkaloids and other biologically active nitrogen heterocycles.2 We have recently reported two novel strategies for the rapid synthesis of bicyclic and tricyclic nitrogen heterocycles that feature cascade and multicomponent reactions involving imines. In designing the first, we were guided by an interest to combine the reactivity modes of imines as both electrophiles and nucleophiles with the nucleophilic reactivity of allylsilanes to provide access to nitrogen heterocycles with nitrogen atoms at one of the bridgehead positions.3 In the second, we sought to develop multicomponent reactions that would provide suitably functionalized intermediates that could undergo cyclization via a number of reaction manifolds.4 Recognizing that test of any new methodology lies in its application to practical problems, we successfully applied these processes to the concise syntheses of (±)-epilupinine (1), (±)-tashiromine (2), (−)-epimyrtine (3), and (±)-roelactamine (4).
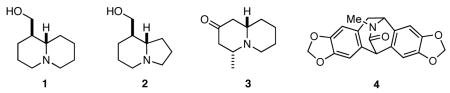
RESULTS AND DISCUSSION
Facile Route to Quinolizidines and Indolizidines via Cascade Reactions of Imines
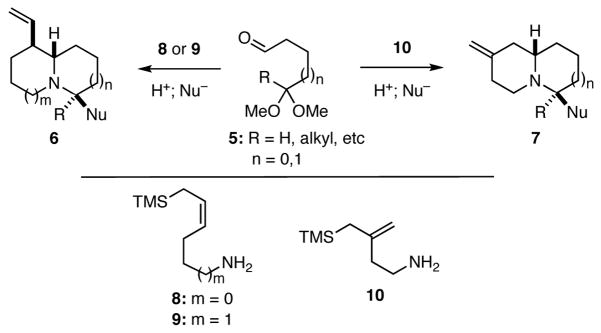
Drawing on the common knowledge that imines may serve as either electrophiles or nucleophiles in chemical reactions and that allylsilanes act as nucleophiles to add to iminium ions, we developed a plan for the synthesis of quinolizidines and indolizidines that is outlined in general terms in Scheme 1. Namely, we envisioned that monoprotected dicarbonyl compounds of the general form 5 might undergo a cascade of reactions with allylsilanes 8 or 9 in which the penultimate intermediate iminium ion is trapped with another nucleophile to give the bicyclic nitrogen heterocycles 6. Similarly, the branched allylsilane 10 could react with 5 leading to 7. The ring sizes of the products could be varied by simply altering the number of carbon atoms in the starting materials. A noteworthy feature of this approach to quinolizidines and indolizidines is that the process is convergent, allowing for the incorporation of two acyclic starting materials into a bicyclic product in one chemical operation.
Scheme 1.
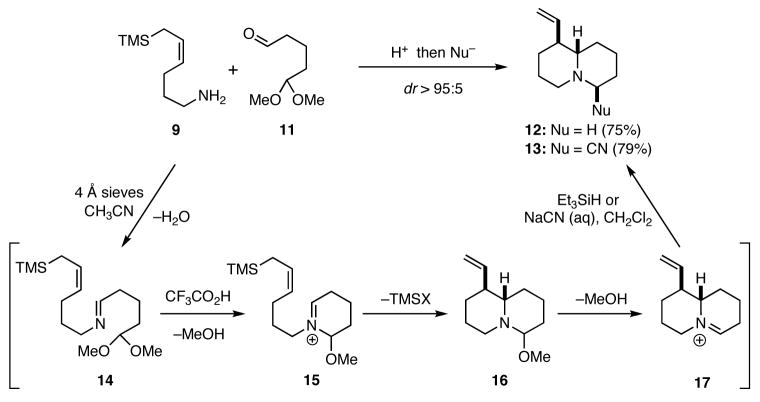
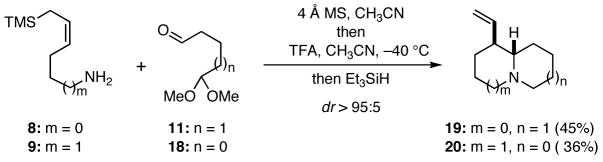
In order to probe the viability of this approach to the synthesis of quinolizidines, we first condensed the known amino allylsilane 95 with the known monoprotected dialdehyde 116 to provide the imine 14 (Scheme 2). Further transformation of 14 into the putative iminium ion 17, which presumably proceeded via 15 and 16, was best achieved by the action of trifluoroacetic acid at −40 °C. Termination of the cascade using triethylsilane or cyanide ion as a trapping agent for 17 furnished 12 or 13, respectively, in excellent overall yield. In order to explore the scope of this chemistry, the monoprotected dialdehyde 11 and the linear amino allylsilane 87 were allowed to react, and the reaction was quenched with triethylsilane to give 19. Similarly, application of this sequence to 9 and the dialdehyde monoacetal 186 led to 20. These later two reactions were not optimized. Several preliminary experiments to extend this cascade sequence to the synthesis of pyrrolizidines were not successful.
Scheme 2.
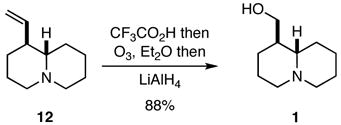
As noted previously, the true test of any new strategy or methodology is whether it may be applied to preparing targets of interest, and it did not escape attention that compounds 12 and 20 might be readily transformed into naturally occurring quinolizidines and indolizidines. Indeed, compound 12 was a known intermediate in a previous synthesis of (±)-epilupinine (1),8,9 but we developed an improved protocol for effecting this conversion that involved the ozonolysis of the trifluoroacetate salt of 12 followed by hydride reduction of the intermediate ozonide (Eq. 1). Similarly, compound 20 was converted into the natural product (±)-tashiromine (2) (Eq. 2).10
 |
(1) |
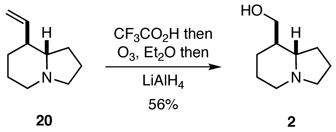 |
(2) |
We then wished to explore cascade processes involving branched amino allylsilanes and keto aldehyde monoacetals. In the event, condensation of the known branched amino allylsilane 1011 with the monoprotected dialdehyde 11 followed by sequential treatment of the imine thus generated in situ with TFA and then aqueous NaCN furnished an epimeric mixture (88:12) of the aminonitriles 23 in excellent yield (Scheme 4). We were also pleased to discover that keto aldehyde monoacetals were also suitable reaction partners. For example, 21 and 22 were each condensed with the allylsilane 10, and the intermediate imines were treated with trifluoroacetic acid to initiate cyclization; termination of the cascade by the addition of cyanide ion afforded the aminonitriles 24 and 25.
Scheme 4.
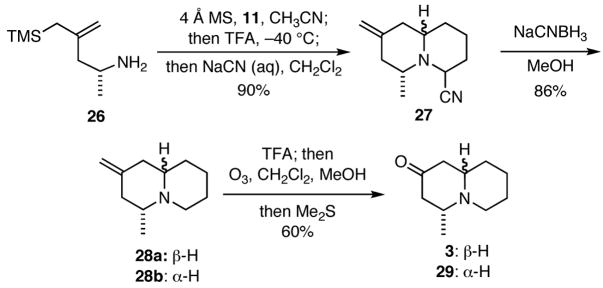
In order to illustrate the utility of this process, it was applied to a concise, enantioselective synthesis of the quinolizidine alkaloid (−)-epimyrtine (3) (Scheme 5). Thus, the known amino allylsilane 2612 was condensed with the dialdehyde monoacetal 11 to give an imine that was then treated sequentially with trifluoroacetic acid and aqueous NaCN to give 27, reduction of which with NaCNBH3 provided a mixture (95:5) of epimeric quinolizidines 28a,b. Ozonolysis of the exocyclic olefin in the trifluoroacetate salt of 28a,b gave an inseparable mixture (95:5) of (−)-epimyrtine (3) and (+)-myrtine (29).13
Scheme 5.
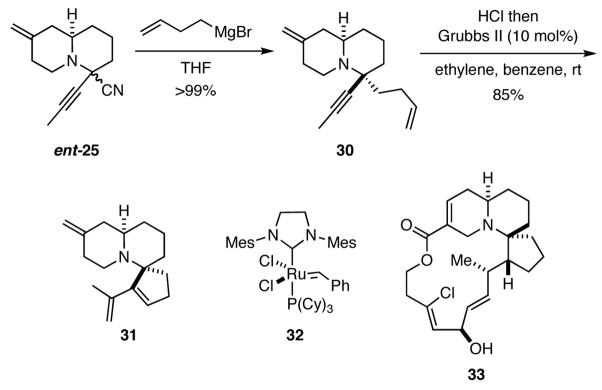
The potential utility of the α-aminonitriles formed in sequences depicted in Scheme 4 did not escape our attention. For example, α-aminonitriles may be deprotonated with strong bases to generate stabilized carbanions that may undergo reactions with a number of different electrophiles, and they may also serve as iminium ion equivalents in Bruylants and related processes.14 For example, the utility of the α-aminonitrile 25 is exemplified by its facile conversion to 31, which possess the tricyclic core of halichlorine (33).15 Accordingly, ent-25 was allowed to react with 3-butenylmagnesium bromide to deliver quinolizidine 30 as a single diastereomer in virtually quantitative yield (Scheme 6). The hydrochloride salt of 30 underwent facile enyne ring-closing metathesis in the presence of Grubbs II catalyst (32) to provide 31. The synthesis of 31 is remarkable for its brevity, only three steps from acyclic starting materials, and its efficiency (64% overall yield).
Scheme 6.
Imines and Diversity-Oriented Synthesis
Diversity-oriented synthesis (DOS) and various manifestations thereof constitute areas of considerable importance at the interface of the fields of organic synthesis and chemical biology.16 One of the critical challenges in DOS is the efficient generation of collections of functionally and stereochemically diverse small molecules, especially those possessing skeletons found in natural products or drug-like molecules. One attractive and powerful method that has recently emerged for generating such collections of molecules comprises using multicomponent reactions (MCRs)17 to create suitably substituted intermediates that may be readily transformed by cyclizations and refunctionalizations that lead to further increases in molecular complexity and diversity.4
Some years ago we discovered a multicomponent reaction that featured vinylogous Mannich reactions of electron rich dienes with N-acyl iminium ions generated in situ by N-acylation of imines to give adducts that were readily transformed into a number of complex alkaloids representing different families.1f In the context of DOS, we envisioned that a related four-component process involving combination of an amine 34, an aldehyde 35, and an acylating agent 36 might generate a reactive N-acyl iminium ion that could be subsequently trapped with a nucleophile 37 to give a functionalized amide 38 (Scheme 7). A variant of this protocol would entail addition of the nucleophile to an intermediate imine, followed by N-acylation to furnish 38.18 This experimental flexibility together with the ready availability of numerous reactants 34–37 allows for the incorporation of high levels of functional and structural diversity in the products 38, so that a number of different cyclizations might be subsequently performed to generate an array of heterocyclic scaffolds in only a few steps from commercially available starting materials.
Scheme 7.
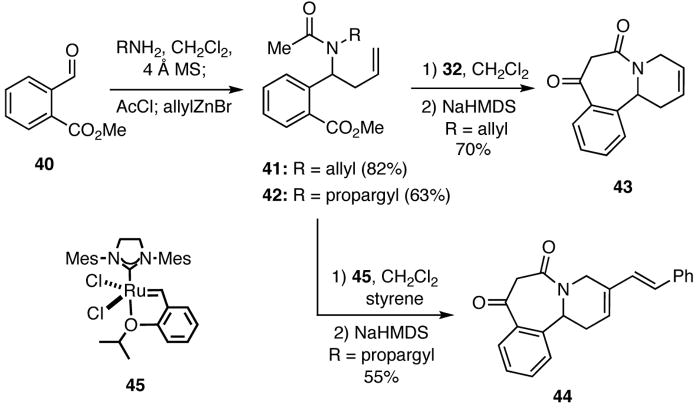
The question at this juncture was whether we could in fact marshal such reactions to rapidly assemble heterocyclic systems that would be of interest for DOS. Toward this end, we focused on combining unsaturated amines, aryl aldehydes, simple acid chlorides, and allylic and π-nucleophiles to prepare adducts that could be further transformed by cyclizations involving RCM, Dieckmann and Heck reactions, and Diels-Alder and dipolar cycloadditions. For example, condensation of methyl 2-formylbenzoate (40) with allyl and propargylamine provided intermediate imines that were treated sequentially with acetyl chloride and allylzinc bromide to furnish adducts 41 and 42 in a one pot operation (Scheme 8). Compound 41 was converted into the benzazepine 43 via a RCM using Grubbs catalyst 32 followed by a Dieckmann cyclization. In a related process, 42 and styrene, which served as a fifth component in the process, underwent an enyne RCM/CM cascade upon treatment with the Hoveyda-Grubbs catalyst (45) to give an intermediate that was cyclized by a Dieckmann condensation to give 44. These two examples nicely illustrate how simply changing one of the inputs in the initial multicomponent process allows for differential processing of the adducts into targets of varying complexity and structure.
Scheme 8.
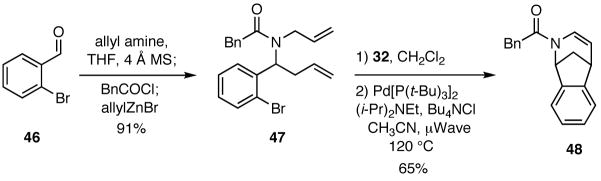
Varying the functionality on the aldehyde component enables access to other cyclization manifolds and hence other heterocyclic systems. Illustrative of this feature is the use of 2-bromobenzaldehyde (48) in a four-component process to furnish substrates that are amenable to Heck cyclizations (Scheme 9). The tertiary amide 47 thus formed was subjected to a two step sequence of cyclizations involving a RCM followed by a Heck reaction to give 46.
Scheme 9.
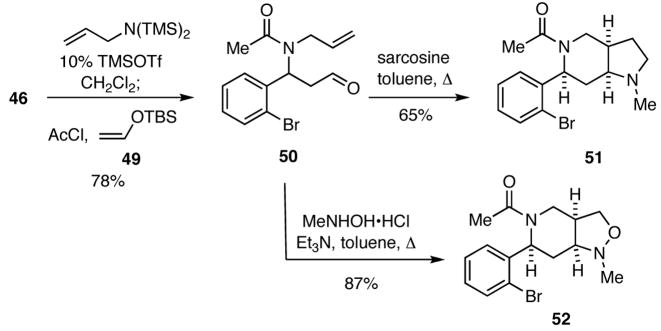
Another aspect of this strategy for DOS involves the use of silyl enol ethers as inputs in the initial four-component process to generate aldehydes that can then be employed as precursors of 1,3-diploles that may undergo intramolecular [3+2] dipolar cycloadditions with proximal double or triple bonds to generate diverse heterocyclic scaffolds. For example, reaction of the bromoaldehyde 46 with bis(trimethylsilyl)allylamine in the presence of catalytic amounts of TMSOTf, followed by treatment of the thus obtained imine in situ with acetyl chloride and the enol ether 49 delivered the adduct 50 (Scheme 10). When 50 was condensed with sarcosine in refluxing toluene, the intermediate azomethine ylide readily underwent a [3+2] cycloaddition to give 51, Similarly, reaction of 50 with N-methylhydroxylamine under the same conditions generated a nitrone that underwent facile [3+2] cycloaddition to provide 52.
Scheme 10.
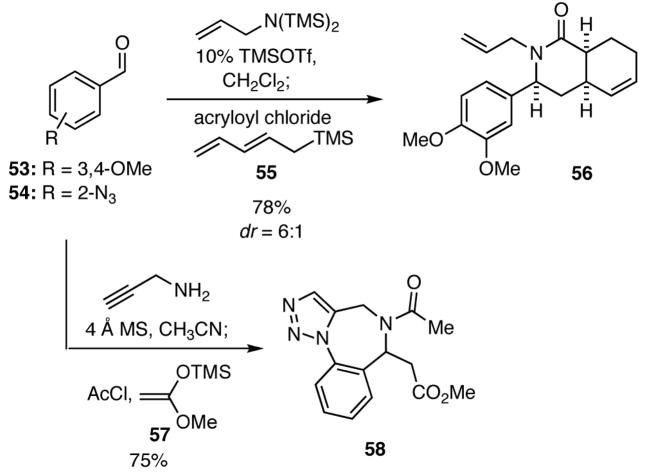
The intramolecular Diels-Alder (IMDA) reaction is another powerful reaction for the synthesis of complex molecules. Hence, we turned our attention to using inputs in the four-component process that would provide substrates capable of undergoing such transformations. In the event, we found that when the pentadienylsilane 55 and acryloyl chloride were added to the imine generated in situ upon condensation of the benzaldehyde 53 with bis(trimethylsilyl)allylamine in the presence of TMSOTf, the resulting product underwent spontaneous [4+2] cyclization to give 56 (Scheme 11). In a related cascade reaction, condensation of 2-azidobenzaldehyde (54) with propargyl amine followed by treatment of the intermediate imine with acetyl chloride and the ketene acetal 57 delivered the triazole 58 via a [3+2] dipolar cycloaddition. A noteworthy aspect of these constructions is that complex heterocyclic systems are rapidly generated in simple one-pot operations.
Scheme 11.
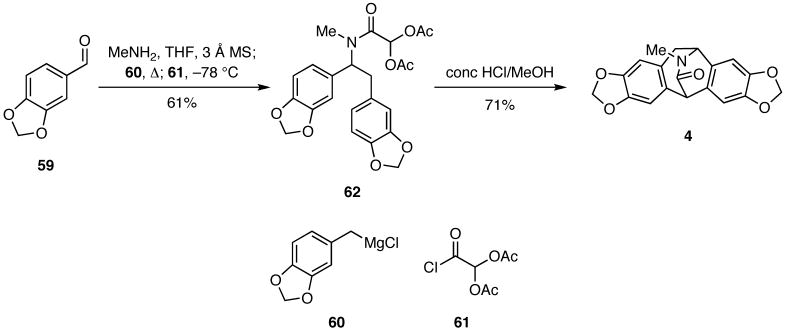
Inasmuch as we are always interested in applications of novel strategies for heterocycle synthesis to natural products, we queried whether this novel strategy for DOS, might be exploited to prepare complex alkaloids. We thus envisioned that a four-component reaction might be exploited to assemble 62 and analogs thereof, which would nicely serve as precursors of the benzanulated azabicyclo[3.2.2]nonane core present in the isopavine alkaloids.19 Gratifyingly, we discovered that condensation of piperonal (59) with methylamine gave an intermediate imine that was sequentially treated in situ with the Grignard reagent 60 and then the acid chloride 61 to provide 62 (Scheme 12). When 62 was treated with aqueous acid, it underwent facile cyclization to give (±)-roelactamine (4), an alkaloid that was isolated in 1992 from Roemeria refracta DC.20
Scheme 12.
CONCLUSIONS
We have thus described several novel applications of imine chemistry. The first of these featured a variety of related cascade reactions in which iminium ions were generated and trapped by allylsilanes and other nucleophiles such as cyanide ion and hydride donors to give functionalized quinolizidines and indolizidines in a one-pot process involving the formation of two rings and four new bonds from simple acyclic starting materials. The importance and practical utility of this new entry to polycyclic nitrogen heterocycles was demonstrated by its application to the facile syntheses of several quinolizidine and indolizidine alkaloids as well as the tricyclic core of the more complex alkaloid halichlorine. The second advance was the design and development of a novel approach for DOS that features a one pot process in which four components are combined to give functionalized adducts that may be transformed via various cyclization manifolds into a diverse collection of functionalized heterocyclic scaffolds that may be further diversified. The utility of this strategy for DOS was exemplified by its application to the synthesis of a isopavine alkaloid. Further applications of these processes to the preparation of targets of biological interest are under active investigation, and the results will be reported in due course.
Scheme 3.
Acknowledgments
A number of outstanding students and postdoctoral associates have contributed to our applications of imine chemistry to alkaloid synthesis over the years in which we have been involved, and I am particularly grateful to them for their hard work and creative contributions. These include in alphabetical order: Shawn Amorde, Dr. Rodrigo Andrade, Brigette Benage, Scott Bur, Dr. Kevin Chen, Cameron Clark, Jeff Corbett, Jason Deck, Dr. Alex Deiters, Dr. Christopher Dockendorff, Dr. Todd Eary, Tsung-hao (Bob) Fu, Dr. Andrew Fryer, Leo Geraci, Dr. Betty Jane Goh, Dr. Brian Gulledge, Dr. Richard Heidebrecht, Jr., Dr. James Hunter, Dr. Masayuki Ito, Ivan Jewett, Dr. Andrew Judd, Dr. Spiros Liras, Dr. Rainer Machauer, Dr. Michael Mortimore, Christopher Neipp, Andreas Reichelt, Alex Rudolph, Suvi Simila, James Sunderhaus, Dr. Binh Vu, and Dr. David Washburn. We thank the National Institutes of Health, the Robert A. Welch Foundation, Pfizer, Inc., Merck Research Laboratories, and Boehringer Ingelheim Pharmaceuticals for their generous support of our research programs. We are also grateful to Dr. Richard Pederson (Materia, Inc.) for catalyst support.
References
- 1.For reviews of N-alkyl iminium ions, see: Blumenkopf TA, Overman LE. Chem Rev. 1986;86:857.Kleinman EF, Volkmann RA. In: Comp Org Syn. Trost BM, editor. 1991. p. 975.Royer J, Bonin M, Micouin L. Chem Rev. 2004;104:2311. doi: 10.1021/cr020083x.For reviews of N-acyl iminium ions, see: Speckamp WN, Moolenaar MJ. Tetrahedron. 2000;56:3817.Maryanoff BE, Zhang HC, Cohen JH, Turchi IJ, Maryanoff CA. Chem Rev. 2004;104:1431. doi: 10.1021/cr0306182.For a review of vinylogous Mannich reactions, see: Martin SF. Acc Chem Res. 2002;35:895. doi: 10.1021/ar950230w.
- 2.For selected examples, see: Martin SF, Benage B, Geraci LS, Hunter JE, Mortimore M. J Am Chem Soc. 1991;113:6161.Martin SF, Clark CC, Corbett JW. J Org Chem. 1995;60:3236.Ito M, Clark CC, Mortimore M, Goh JB, Martin SF. J Am Chem Soc. 2001;123:8003. doi: 10.1021/ja010935v.Liras S, Lynch CL, Fryer AM, Vu BT, Martin SF. J Am Chem Soc. 2001;123:5918. doi: 10.1021/ja010577w.Reichelt A, Bur SK, Martin SF. Tetrahedron. 2002;58:6323.Deiters A, Chen K, Eary CT, Martin SF. J Am Chem Soc. 2003;125:4541. doi: 10.1021/ja0296024.Martin SF, Neipp CE. J Org Chem. 2003;68:8867. doi: 10.1021/jo0349936.Washburn DG, Heidebrecht RW, Jr, Martin SF. Org Lett. 2003;5:3523. doi: 10.1021/ol0354066.Rudolph AC, Machauer R, Martin SF. Tetrahedron Lett. 2004;45:4895.Simila STM, Martin SF. J Org Chem. 2007;72:5342. doi: 10.1021/jo070732a.
- 3.For a preliminary account of this work, see: Amorde SM, Judd AS, Martin SF. Org Lett. 2005:2031. doi: 10.1021/ol050544b.
- 4.For a preliminary account of this work, see: Sunderhaus JD, Dockendorff C, Martin SF. Org Lett. 2007;9:4223. doi: 10.1021/ol7018357. See also references therein for related strategies for rapid assembly of functionalized heterocycles.
- 5.Wasserman HH, Vu CB, Cook JD. Tetrahedron. 1992;48:2101. [Google Scholar]
- 6.Schreiber SL, Claus RE, Reagan J. Tetrahedron Lett. 1982;23:3867. [Google Scholar]
- 7.Mooiweer HH, Hiemstra H, Fortgens HP, Speckamp WN. Tetrahedron Lett. 1987;28:3285. [Google Scholar]
- 8.Pandey G, Reddy GD, Chakrabarti D. J Chem Soc, Perkin Trans. 1;1996:219–24. [Google Scholar]
- 9.For some other syntheses of epilupinine, see: Comins DL, Brown JD. Tetrahedron Lett. 1986;27:2219.Morley C, Knight DW, Share AC. J Chem Soc Perkin Trans 1. 1994:2903.Naidu BN, West FG. Tetrahedron. 1997;53:16565.Mangeney P, Hamon L, Raussou S, Urbain N, Alexakis A. Tetrahedron. 1998;54:10349.Ma S, Ni B. Chem Eur J. 2004;10:3286. doi: 10.1002/chem.200305581.
- 10.For representative syntheses of tashiromine, see: Olivier D, Bellec C, Fargeau-Bellassoued MC, Lhommet G. Heterocycles. 2001;55:1689.Bates RW, Boonsombat J. J Chem Soc Perkin Trans 1. 2001:654.Dieter RK, Watson R. Tetrahedron Lett. 2002;43:7725.
- 11.(a) Gramain JC, Remuson R. Tetrahedron Lett. 1985;26:327. [Google Scholar]; (b) Burfeindt J, Patz M, Mueller M, Mayr H. J Am Chem Soc. 1998;120:3629. [Google Scholar]
- 12.Monfray J, Gelas-Mialhe Y, Gramain JC, Remuson R. Tetrahedron Lett. 2003;44:5785. [Google Scholar]
- 13.For syntheses of epimyrtine, see: Slosse P, Hootele C. Tetrahedron. 1981;37:4287.Gardette D, Gelas-Mialhe Y, Gramain JC, Perrin B, Remuson R. Tetrahedron: Asymmetry. 1998;9:1823.Davis FA, Zhang Y, Anilkumar G. J Org Chem. 2003;68:8061. doi: 10.1021/jo030208d.
- 14.Enders D, Shilvock JP. Chem Soc Rev. 2000;29:359–373. [Google Scholar]
- 15.For representative syntheses of halichlorine, see: Trauner D, Schwartz JB, Danishefsky SJ. Angew Chem Int Ed. 1999;38:3542. doi: 10.1002/(sici)1521-3773(19991203)38:23<3542::aid-anie3542>3.0.co;2-i.Christie HS, Heathcock CH. Proc Natl Acad Sci USA. 2004;101:12079. doi: 10.1073/pnas.0403887101.Matsumura Y, Aoyagi S, Kibayashi C. Org Lett. 2004;6:965. doi: 10.1021/ol0301431.Zhang HL, Zhao G, Ding Y, Wu B. J Org Chem. 2005;70:4954. doi: 10.1021/jo047882v.Andrade R, Martin SF. Org Lett. 2005;7:5733. doi: 10.1021/ol0525009.
- 16.(a) Burke MD, Schreiber SL. Angew Chem, Int Ed. 2004;43:46. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]; (b) Spring DR. Org Biomol Chem. 2003;1:3867. doi: 10.1039/b310752n. [DOI] [PubMed] [Google Scholar]; (c) Strausberg RL, Schreiber SL. Science. 2003;300:294. doi: 10.1126/science.1083395. [DOI] [PubMed] [Google Scholar]
- 17.For example, see: Marcaccini S, Torroba T. In: Multicomponent Reactions. Zhu J, Bienayme H, editors. Wiley-VCH; Weinheim: 2005. p. 33.Dömling A. Chem Rev. 2006;106:17. doi: 10.1021/cr0505728.
- 18.For a related MCR see: Yin Y, Zhao G, Li G-L. Tetrahedron. 2005;61:12042.
- 19.For selected syntheses of isopavine alkaloids, see: Gozler B. In: The Alkaloids. Brossi A, editor. Vol. 31. Academic Press; New York: 1987. p. 343.Gottlieb L, Meyers AI. J Org Chem. 1990;55:5659.Carrillo L, Badía D, Domínguez E, Vicario JL, Tellitu I. J Org Chem. 1997;62:6716.Shinohara T, Takeda A, Toda J, Sano T. Heterocycles. 1998;48:981.Dragoli DR, Burdett MT, Ellman JA. J Am Chem Soc. 2001;123:10127. doi: 10.1021/ja016349j.Tambar UK, Enber DC, Stoltz BM. J Am Chem Soc. 2006;128:11752. doi: 10.1021/ja0651815.
- 20.Gözler B, Önür MA, Bilir S, Hesse M. Helv Chem Acta. 1992;75:260. [Google Scholar]