Cellular activities are mediated by proteins, which often undergo conformational changes to regulate binding and enzymatic activities that direct signaling pathways. Hence, it is important to develop and apply tools to characterize dynamic changes in protein conformation. A variety of approaches are available, including those based on NMR, 1 FRET, 2 and plasmonics.3,4 Plasmonic approaches are particularly useful because they provide an intense signal that does not bleach and allow non-destructive measurement over long periods of time. In addition to acting as probes of molecular interactions, plasmonic devices have significant potential as nanoscale optical switches, waveguides, light sources, microscopes and lithographic tools.3,4 Herein, we demonstrate a plasmonic switching device based on the calcium-induced conformational changes of calmodulin. The extinction maximum (λmax) of a Localized Surface Plasmon Resonance (LSPR) sensor functionalized with calmodulin reversibly shifts by 2-3 nm in response to changes in Ca2+ concentration, creating a unique on/off switch and providing information about the dynamics and structure of the protein. A high resolution (HR)-LSPR spectrometer with a wavelength resolution (3σ) of 1.5×10-2 nm was used to detect the calcium-modulated wavelength shifts (supporting information).
The LSPR is a unique nanoscale phenomenon that gives rise to an intense extinction and scattering spectrum in noble metal nanoparticles that is highly dependent on the local refractive index at the nanoparticle surface. Biomolecule adsorption to a nanoparticle alters the local refractive index, causing shifts in the extinction maximum (λmax). The magnitude and direction of these shifts provide detailed information about the packing density of the adsorbed species.5 LSPR sensors can be used to characterize and detect a wide variety of biological events, including DNA hybridization,6 carbohydrate-protein binding,7 and antigen-antibody interactions.8,9
A commercially established technology based on propagating SPR in thin Au films can sense changes in refractive index up to 1 μm away from the sensor surface. Such SPR sensors have been used to detect protein conformational changes due to denaturation.10 11, However, the relatively large sensing region of propagating SPR sensors gives rise to interference from bulk refractive index changes. In contrast, the LSPR sensing region in Ag nanoparticles is confined to a thin (25 - 30 nm) shell around the nanoparticle.12 As a result, LSPR sensors possess 40-fold greater spatial resolution normal to the sensor surface, allowing improved detection of low molecular weight binding events. Monitoring LSPR changes in real-time provides information about the dynamics of binding events and protein folding, with much less interference from bulk refractive index changes.
Despite the superior spatial resolution of LSPR sensors, low S/N ratios have presented a challenge. However, developments in instrumentation and data analysis have dramatically improved the resolution of LSPR sensors enabling real-time detection in solution. For example, Höök et. al. developed a sensor capable of measuring LSPR shifts of less than 5×10-4 nm to detect binding to nanohole films.13 This level of sensitivity is comparable to propagating SPR sensors in terms of shift/molecule/area. Using single nanoparticles, LSPR spectroscopy has the potential to surpass the sensitivity of SPR.14
In this work we use an HR-LSPR spectrometer to detect binding and conformational changes in real-time with a standard deviation in LSPR wavelength of 5×10-3 nm for nanosphere lithography (NSL)-fabricated Ag nanoprism arrays incubated in solution. While this noise level is 10-fold higher than reported by Höök, in part because nanoholes absorb more light than NSL arrays, the shift per molecule is higher for NSL arrays due to their shorter electromagnetic field decay length,6 resulting in a similar overall sensitivity per molecule. This noise level corresponds to a S/N ratio of 800 for typical (MW ~ 60 kDa) protein binding events, facilitating measurement of binding rates.
To demonstrate the capabilities of our real-time, HR-LSPR spectrometer, we characterized the calcium-dependent conformational change in calmodulin (CaM), a protein that mediates many cellular responses to the second messenger calcium ion. CaM binds Ca2+ with dissociation constants of order 1.0 μM.15 Crystal structures show differences in the conformation and shape of the protein in its Ca2+-bound and unbound states, and previous work has demonstrated CaM gels that change shape,16 making it an ideal protein to test both the real-time and wavelength resolution of our LSPR spectrometer in response to small, reversible refractive index changes.
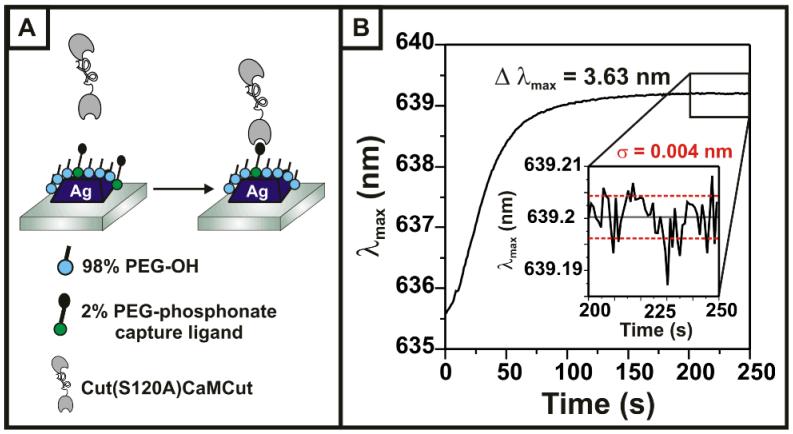
To immobilize calmodulin, we created a protein construct consisting of a CaM domain sandwiched between two cutinase domains, with the N-terminal cutinase rendered inactive. The active C-terminal cutinase reacts with a phosphonate ligand on the monolayer to give a covalent, site-specific attachment to the nanoparticle.17 The design of this construct confers two benefits: 1) CaM is uniformly oriented at the surface, with the cutinase acting as a spacer molecule to reduce substrate interference and more closely approximate a solution-phase protein, and 2) the N-terminal cutinase domain provides an additional mass of 22 kDa, creating a more significant change in the overall protein packing density when treated with calcium. The λmax of the LSPR sensor was monitored in real-time as CutCaMCut bound to the SAM, demonstrating a shift of 3.63 nm in aqueous buffer and a noise level of 4×10-3 nm (Figure 1B). Spectra measured in a N2 environment before and after this immobilization step show an increase in the extinction intensity and a Δλmax of + 26 nm, verifying that CutCaMCut is bound to the surface (S.I.).
Figure 1.
(A) Schematic representation of the binding of CutCaMCut fusion protein to a phosphonate functionalized Ag nanoparticle sensor; (B) plot of changes in λmax in real-time as CutCaMCut binds to the sensor surface in solution. Inset: Closeup after the reaction is complete, showing a noise level of 4×10-3 nm averaged over 50 seconds.
We next tested whether the HR-LSPR spectrometer could detect the conformational changes of calmodulin that accompany Ca2+ binding. The λmax was monitored while exposing the sensor to alternating cycles of 2 mM CaCl2 followed by 2 mM EGTA in 20 mM Tris buffer at pH 7.4 (Figure 2A). We expected that as calmodulin opened or closed in response to Ca2+, the λmax of the LSPR sensor would reversibly shift as the center-of-mass of the calmodulin localizes further into and out of the LSPR sensing region. Indeed, the λmax shifted to the red in the presence of Ca2+ (open conformation) and to the blue in response to Ca2+ chelation by EGTA (closed conformation). With each alternating buffer cycle, the λmax shifted an average of 2.197 ± 0.176 nm after the initial incubation (Figure 2B). Exponential fits to the data with a correction for linear baseline drift reveal an opening rate of ~0.034 s-1 and a closing rate of ~0.127 s-1 (Figure 2C). Furthermore, two controls were performed to verify that the LSPR shifts observed were the result of conformational changes. In the first, Ag nanoparticles with a phosphonate-terminated SAM and no calmodulin on the surface were subjected to CaCl2 and EGTA cycles. In the second, Ag nanoparticles functionalized with the non-calcium sensitive cutinase-cutinse construct were also exposed to consecutive CaCl2 and EGTA cycles. Both controls showed a small continuous baseline drift due to solvent annealing, but minimal Ca2+-modulated changes in λmax, verifying that the observed extinction shifts result from calmodulin conformational changes and not refractive index changes in the bulk media (S.I.).
Figure 2.
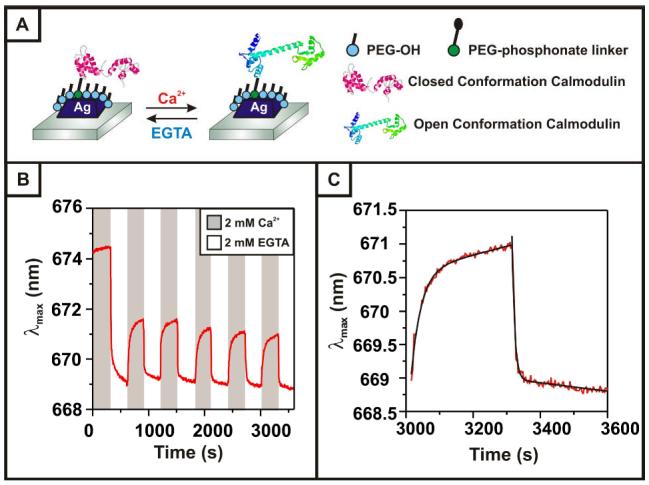
(A) Schematic of the experimental setup for conformational cycling of CutCaMCut immobilized on the LSPR nanosensor. (B) Plot of changes in the extinction maximum (1 Hz collection) of the sensor as the buffer is cycled between 2 mM CaCl2 and 2 mM EGTA, a calcium chelator. (C) Closeup of one CaCl2/EGTA cycle, with an exponential fit of λmax = λmax(0) + Aexp(-t/τ) +B*t (black) to the experimental data (red), where τ(open)=30 s, τ(close)=8 s, and B<0.0013 nm/s.
In addition to demonstrating reproducible Ca2+-dependent plasmonic modulations, the HR-LSPR nanosensor provides information about the orientation and dynamics of the CutCaMCut system. Using the LSPR response model,9 the red shift upon Ca2+ exposure indicates that CutCaMCut reorients in a way that results in a higher overall packing density close to the nanoparticle surface. In addition, analysis of the real-time modulations reveals a closing rate ~ 4x faster than the opening rate. Published rates of conformational changes for solution-phase calmodulin reflect both a faster and a more closely matched time scale between opening and closing rates. This discrepancy may be the result of the surface-bound nature of the protein and/or the presence of the fused cutinase domains. More detailed investigations are underway to determine the effect of calcium ion concentration on the rate of conformational changes.
These results mark the first time that a LSPR sensor has exhibited reversible spectral modulations in response to the changing conformation of a label-free protein. We have demonstrated that the calcium induced change in λmax of approximately 2.2 nm is far greater than the noise level (S/N ~ 5×102), label-free, and can be measured over long periods of time because the particles do not bleach. In addition, the combination of reversibility, high S/N ratio, and long-term stability of this real-time HR-LSPR sensor make it a valuable tool for studies of protein orientation and structure, with potential future applications in drug discovery and disease research.
Supplementary Material
Acknowledgment
This research was supported by the National Science Foundation (Grants EEC-0647560, CHE-0414554, DMR-0520513, and BES-0507036), the National Cancer Institute (1 U54 CA119341-01), a Ruth L. Kirschstein National Research Service Award (5 F32 GM077020) to J.N.A., a George W. Beadle Postdoctoral Fellowship to Y.L., and a Ryan Fellowship to W.P.H.
Footnotes
Supporting Information Available: Experimental details and data not shown in the manuscript are available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Ishima R, Torchia DA. Nature Structural Biology. 2000;7:740–743. doi: 10.1038/78963. [DOI] [PubMed] [Google Scholar]
- 2.Heyduk T. Current Opinion in Biotechnology. 2002;13:292–296. doi: 10.1016/s0958-1669(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 3.Atwater HA. The Promise of Plasmonics. Sci. Am. 2007;296(4):56–63. [PubMed] [Google Scholar]
- 4.Ozbay E. Science. 5758. Vol. 311. Washington, DC, United States: 2006. pp. 189–193. [DOI] [PubMed] [Google Scholar]
- 5.Willets KA, Van Duyne RP. Annu. Rev. Phys. Chem. 2007;58:267–297. doi: 10.1146/annurev.physchem.58.032806.104607. [DOI] [PubMed] [Google Scholar]
- 6.Sonnichsen C, Reinhard B, Liphardt J, Alivasatos AP. Nature Biotechnology. 2005;23:741–745. doi: 10.1038/nbt1100. [DOI] [PubMed] [Google Scholar]
- 7.Yonzon CR, Jeoung E, Zou S, Schatz GC, Mrksich M, Van Duyne RP. J. Am. Chem. Soc. 2004;126:12669–12676. doi: 10.1021/ja047118q. [DOI] [PubMed] [Google Scholar]
- 8.Riboh J, Haes A, McFarland AD, Ranjit C, Van Duyne RP. J. Phys. Chem. B. 2003;107:1772–1780. [Google Scholar]
- 9.Haes AJ, Chang L, Klein WL, Van Duyne RP. J. Am. Chem. Soc. 2005;127:2264–2271. doi: 10.1021/ja044087q. [DOI] [PubMed] [Google Scholar]
- 10.Chah S, Kumar C, Hammond M, Zare RN. Anal. Chem. 2004;76:2112–2117. doi: 10.1021/ac035416k. [DOI] [PubMed] [Google Scholar]
- 11.Kang T, Hong S, Choi I, Jun Sung J, Kim Y, Hahn J, Yi J. J. Am. Chem. Soc. 2006;128:12870–12878. doi: 10.1021/ja0632198. [DOI] [PubMed] [Google Scholar]
- 12.Haes AJ, Zhou S, Schatz GC, Van Duyne RP. J. Phys.Chem. B. 2004;108:109–116. [Google Scholar]
- 13.Dahlin AB, Tegenfeldt JO, Hook F. Anal. Chem. 2006;78:4416–4423. doi: 10.1021/ac0601967. [DOI] [PubMed] [Google Scholar]
- 14.McFarland AD, Van Duyne RP. Nano Lett. 2003;3:1057–1062. [Google Scholar]
- 15.Linse S, Helmersson A, Forsen S. J. Bio. Chem. 1991;266:8050–8054. [PubMed] [Google Scholar]
- 16.Murphy WL, Dillmore WS, Modica J, Mrksich M. Angew Chem. 2007;46:3066–3069. doi: 10.1002/anie.200604808. [DOI] [PubMed] [Google Scholar]
- 17.Hodneland CD, Lee Y, Min D, Mrksich M. PNAS. 2002;99:5048. doi: 10.1073/pnas.072685299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.