Abstract
Purpose
Polymorphisms that are associated with ABCB1 expression and function may be linked to treatment efficacy and the development of neutropenia and neurotoxicity in patients with androgen independent prostate cancer (AIPC) receiving docetaxel.
Experimental Design
Patients with AIPC treated with docetaxel alone (n=23), or docetaxel and thalidomide (n=50), were genotyped for the ABCB1 1236C>T, 2677 G>T/A, and 3435 C>T alleles by direct sequencing, and diplotypes were constructed using an EM algorithm. The data were then compared to duration to onset of peripheral neuropathy, neutropenia grade, and survival after docetaxel.
Results
For patients receiving docetaxel alone, individuals carrying a diplotype consisting of the 1236C-2677G-3435C linked alleles had improved overall survival after treatment (P = 0.0017). Additionally, patients treated with docetaxel and thalidomide carrying a diplotype consisting of the 2677T-3435T haplotype had a shorter median survival (P = 0.045). After adjusting for a particular set of polymorphisms and diplotype groupings, a hazard ratio of 10.87 was found for patients carrying the 2677GG genotype versus patients carrying other genotypes (P = 0.0048) in the docetaxel and thalidomide cohort. Among both treatment arms together, individuals carrying the 2677GG genotype also had a significantly longer time to neuropathy (P = 0.035). Finally, there was a strong trend towards patients carrying the 2677TT-3435TT diplotype having higher grades of neutropenia (P = 0.053).
Conclusion
The data suggest that docetaxel-induced neuropathy, neutropenia grade, and overall survival could be linked to ABCB1 allelic variants with ensuing negative implications for docetaxel treatment in patients carrying ABCB1 variant genotypes.
Keywords: Docetaxel, polymorphisms, ABCB1, neutropenia, neuropathy, efficacy
INTRODUCTION
Taxanes are part of the larger family of anti-cancer drugs whose mechanism of action targets tubulin. Docetaxel binds to the β-tubulin subunit causing stabilization of microtubules leading to mitotic arrest and subsequent apoptosis (1, 2). Docetaxel is the standard of care for men diagnosed with androgen-independent prostate cancer (AIPC) (2). Docetaxel has been administered to men with AIPC in clinical trials where it has been found to prolong survival in men with this disease (3), although there is much inter-patient variability in overall survival following docetaxel. The major dose limiting toxicities for both drugs are the development of neutropenia and peripheral neuropathy (2).
ABCB1 (P-glycoprotein, MDR-1) is responsible for a large portion of the systemic efflux capacity toward docetaxel. Within the liver, ABCB1 is expressed in the canalicular domain of hepatocytes where it transports docetaxel into the biliary duct, resulting in drug clearance (4, 5). ABCB1 is also responsible for the transport of many drugs, including docetaxel, between bodily compartments. For instance, ABCB1 is expressed by endothelial cells of the blood-brain (6), and blood-nerve barriers (7, 8), and its expression at these sites can limit the exposure of the nervous tissue to substrate drugs by the active transport of these drugs from the nerves into the systemic circulation (9). ABCB1 is also expressed in hematopoietic precursors where it mitigates the exposure of these cells to potentially harmful substances (10, 11).
Tumor cells can also express ABCB1, resulting in the multi-drug resistant phenotype wherein ABCB1-overexpressing tumors can have limited exposure to ABCB1 substrate drugs, such as the taxanes, through efflux pathways (12). Indeed, non-drug-treated, high Gleason grade, prostate tumors have been found to have a more multi-drug resistant phenotype that is attributable to an upregulation of ABCB1 (13). This multidrug resistance most likely results in poor prognosis following docetaxel treatment (14).
ABCB1 expression and protein folding are largely influenced by three single nucleotide polymorphisms (SNPs) in the ABCB1 gene. These are the ABCB1 1236C>T, 2677G>T/A (A893S/T), and 3435C>T transitions (15). It is also likely that diplotype combinations of these three alleles are more deterministic of the efflux capacity of individuals treated with these drugs, as protein folding, expression, and functional differences are more pronounced when certain ABCB1 variants are coinherited – an increasing number of variant alleles (e.g. the 1236T-2677T-3435T haplotype) being associated typically with lower expression and also a protein that is folded with a different conformation (15–17). Recent reports by us and others have implicated commonly inherited ABCB1 variants with an increased susceptibility to the onset of peripheral neuropathy and neutropenia after paclitaxel (18), and an influence on neutrophil counts following docetaxel therapy (19). However, no previous studies have implicated these variants in association with the onset of peripheral neuropathy following docetaxel regimens, or any survival difference following taxane therapy in men with AIPC. To this end, we tested the relationship between ABCB1 genetic variants and clinical endpoints in patients treated with docetaxel alone, and with docetaxel in combination with thalidomide.
MATERIALS AND METHODS
Patients and Treatment
We evaluated ABCB1 genotypes on germline DNA obtained from 73 men with AIPC treated with either single-agent docetaxel (Aventis Pharmaceuticals, Bridgewater, NJ), administered intravenously over 1-hour at a dose of 30 mg/m2 (n=23), or with a combination of docetaxel on the same schedule and oral thalidomide at a dose of 200mg taken daily (n=50) (Celgene Corp, Warren, NJ). Inclusion and exclusion criteria, and patient characteristics have been previously published (20). Toxicity was defined by the Cancer Therapy Evaluation Program/National Cancer Institute Common Toxicity Criteria (version 2.0). The NCI Institutional Review Board approved the study and genotyping.
Genotyping
DNA was extracted from plasma using a QiaBlood DNA extraction kit (Qiagen, Valencia, CA) and stored at 4°C. Genotyping was conducted via direct sequencing at three ABCB1 loci using the following PCR primers: 1236C>T F1 5’-GTTCACTTCAGTTACCCATCTCG -3’, and R1 5’-TATCCTGTCCATCAACACTGACC -3’; 2677G>T/A F1 5’-AGGCTATAGGTTCCAGGCT-3’, and R1 5’-AGAACAGTGTGAAGACAATGG-3’; and 3435C>T F1 5’-ATCTCACAGTAACTTGGCAGT-3’, and R1 5’-AACCCAAACAGGAAGTGTG-3’. A 50-µl reaction was prepared for polymerase chain reaction (PCR) amplification. The reaction consisted of 1 × PCR buffer, 2 mM of each of the four deoxynucleotidetriphosphates (dNTPs), 1.5 mM magnesium chloride, and 1 unit of Platinum Taq DNA polymerase. PCR conditions were: 94°C for 5 minutes, followed by 40 cycles of 94°C for 30 seconds, 64°C for 30 seconds, and 72°C for 30 seconds, with a final 7-minute cycle at 72°C. Direct nucleotide sequencing PCR was conducted using the Big Dye Terminator Cycle Sequencing Ready Reaction kit V1.1 on an ABI Prism 3130 xl Genetic Analyzer (Applied BioSystems, Foster City, CA). The primer sequences for these reactions were as follows: 1236C>T F2 5’-GTCAGTTCCTATATCCTGTGTCTG-3’, and R2 5’-TCCTGTCCATCAACACTGACCTG-3’; 2677G>T/A F2 5’-CCCATCATTGCAATAGCAG-3’, and R2 5’-GAACAGTGTGAAGACAATGGC-3’; and 3435C>T F2 5’-GCTGGTCCTGAAGTTGATCT-3, and R2 5’-AAACAGGAAGTGTGGCCAGAT-3’.
Pharmacokinetics of Docetaxel
Blood specimens were obtained from patients receiving docetaxel (1-hour infusion; 75 or 85 mg/m2) as described previously (21). Evaluation of docetaxel pharmacokinetics was performed using samples obtained immediately before drug infusion and at 0.5, 1, 2, 4, 6, and 24 hours after start of infusion. Concentrations of docetaxel in plasma were determined by a validated reverse-phase high-performance liquid chromatography method with UV detection (22). Pharmacokinetic parameters of docetaxel were obtained by noncompartmental analysis using WinNonlin Professional Version 5.0 (Pharsight Corporation, Mountain View, CA), model 202 (plasma data, constant infusion).
Statistical Considerations
The association between genotypes and overall survival was determined by the Kaplan-Meier method using a two-tailed log rank test (23, 24). A Cox proportional hazards model analysis was performed in order to determine whether any of the SNP or diplotype parameters were jointly statistically significant (25, 26). P-values for the survival analysis are presented without formal correction for multiple testing; however, in view of the exploratory nature of the analysis and the number of explorations performed, univariate P-values < 0.01 were considered statistically significant, while 0.01<P<0.05 would suggest trends.
The probability of development of a peripheral neuropathy during docetaxel therapy as a function of time, according to ABCB1 genotypes, was also analyzed using the Kaplan-Meier method and log-rank P-values but using formal correction of P-values to account for pooling of categories after examining results. The association between the clinical grade of neutropenia and genotypes was evaluated using a Kruskal-Wallis test for ordered columns; the association between the presence or absence of a double variant diplotype and grade of neutropenia was evaluated using an exact Cochran-Armitage test (27).
RESULTS
Genotyping
The tested variants were all in Hardy Weinberg equilibrium (P < 0.05), with the following D’ linkage values: 1236C>T-2677G>T/A D’ = 0.81; 2677G> T/A-3435C>T D’ = 0.77; and 1236C>T-3435C>T D’ = 0.63 (P < 0.001). The genotype and allele frequencies for each SNP are reported in Table I, and are similar to previously reported values. An EM algorithm was utilized to compute the most likely diplotype for each individual that consisted of two possible diplotypes (Helix Tree Software, Golden Helix, Montana), and this information was utilized in subsequent analyses (see Table II). Diplotypes were ordered to capture increasing genetic variation in the population, as it would be expected that each additional variant allele would alter protein folding and function to a greater degree based on relative synonymous codon usage, as reported by Kimchi-Sarfaty et al., where it was demonstrated that there were transport affinity and tertiary structure differences between a protein encoded by the ABCB1 1236C-2677G-3435C haplotype versus the 1236T-2677T-3435T haplotype (15) (see Table II). To explore the association between diplotype and clinical outcome measures, individual diplotypes were initially compared to overall survival, development of neuropathy, and neutropenia grade. Based on the initial exploration, diplotypes were then grouped based on statistical similarity and the p value was corrected for multiple comparisons.
Table I.
Genotype and allele frequencies of the the studied variants
| Genotype Frequenciesa | Allele Frequenci | ||||||
|---|---|---|---|---|---|---|---|
| Allelic Variantc | Effectd | Ne | Wtf | Het | Var | p | |
| Docetaxel alone (N = 23)g | |||||||
| ABCB1 1236C>T | G411G | 23 | 13 (56.5) | 8 (34.8) | 2 (8.7) | 0.739 | 0. |
| ABCB1 2677G>T | A893S | 23 | 11 (47.8) | 9 (39.1) | 2 (8.7) | 0.674 | 0. |
| ABCB1 2677G>A | A893Th | 23 | 11 (47.8) | 1 (4.3) | 0 (0) | 0.674 | 0. |
| ABCB1 3435C>T | I1145I | 23 | 7 (30.4) | 10 (43.5) | 6 (26.1) | 0.522 | 0. |
| Docetaxel Plus Thalidomide (N = 50) | |||||||
| ABCB1 1236C>T | G411G | 46 | 12 (26.1) | 18 (39.1) | 16 (34.8) | 0.457 | 0. |
| ABCB1 2677G>T | A893S | 50 | 15 (30.0) | 20 (40.0) | 15 (30.0) | 0.500 | 0. |
| ABCB1 3435C>T | I1145I | 45 | 10 (22.2) | 23 (51.1) | 12 (26.6) | 0.478 | 0. |
Number represent number of patients with percentage in parenthesis; the difference in the total number of patients is due Hardy-Weinberg notation for allele frequencies (p, frequency for wild type allele and q, frequency for variant allele);
Num represents position in nucleotide sequence;
Number represents amino acid codon;
genotype data were not available in all patients as not all samples yielded sufficient DNA or PCR amplified;
WT, Homozygous wild-type allele patient; Het, Heterozygous patient; Var, Homozygous variant patient;
A single Hispanic male was also included, and his genotype was 1236C>T, unknown; 2677G>T/A, wild-type; 3435C>T, wild-type;
The 2677G>T/A polymorphism is triallelic and two d SNPs are therefore presented.
Table II.
Diplotype Groupings in the initial exploratory analysis
| - Chromosome 1 - | - Chromosome 2 - | Docetaxel Population Frequency (%) |
Docetaxel and Thalidomide Population Frequency (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Diplotype | 1236C>T | 2677G>T/A | 3435C>T | 1236C>T | 2677G>T/A | 3435C>T | ||
| Diplotype 1 | C | G | C | C | G | C | 7 (33.3) | 4 (9.5) |
| Diplotype 2 | C | G | C | * | * | * | 5 (23.8) | 10 (23.8) |
| Diplotype 3 | C | G | C | T | T | T | 4 (19.0) | 11 (26.2) |
| Diplotype 4 | T | T | T | * | * | * | 3 (14.3) | 9 (21.4) |
| Diplotype 5 | T | T | T | T | T | T | 2 (9.5) | 8 (19.0) |
| Diplotype 6 | - | G | C | - | G | C | 7 (33.3) | 6 (13.6) |
| Diplotype 7 | - | G | C | - | ♦ | ♦ | 3 (14.3) | 8 (18.2) |
| Diplotype 8 | - | G | C | - | T | T | 6 (28.6) | 14 (31.8) |
| Diplotype 9 | - | T | T | - | ♦ | ♦ | 3 (14.3) | 6 (13.6) |
| Diplotype 10 | - | T | T | - | T | T | 2 (9.5) | 10 (22.7) |
Any combination of alleles that is not mutually exclusive with another diplotype consisting of all three SNPs.
Any combination of alleles that is not mutually exclusive with another diplotype consisting of only the 2677 and 3435 SNPs.
This SNP was excluded in the Diplotypes 6–10 calculations.
Diplotypes were grouped based on whether or not the patient carried a fully wild-type or fully variant haplotype. This approach was based on a report by Kimchi-Sarfaty et al. where it was noted that protein affinity and folding was different between these haplotypes (15).
Genotype related to patient demographics
No association was found between any ABCB1 alleles or diplotypes and age, Gleason score, ECOG, body surface area, hemoglobin (g/dL), LDH (U/L), AP (U/L), or serum albumin (g/L) (P > 0.05). However, a statistically significant assocation was observed between diplotypes 6–10 and the levels of prostate specific antigen (PSA) observed prior to treatment (P = 0.0004; Table III). There was no difference in on study PSA between the two treatment arms (P = 0.83; Wilcoxon Rank Sum Test), and thus on study PSA values of patients receiving docetaxel alone, or in combination with thalidomide, were combined between treatment arms. Men carrying diplotypes 9 and 10 had higher median PSA levels (median PSA = 135.6 ng/mL) as compared to men carrying diplotypes 6–7 (median PSA = 61.65ng/mL) and diplotype 8 (25.15 ng/mL). There was also an assocation between the ABCB1 3435C>T SNP and PSA (P = 0.0002) where patients carrying the ABCB1 CT genotype had a lower PSA than individuals carrying homozygous wild-type and variant alleles (median PSA = ;see Table III). However, these data may be obscured by the fact that there were only seventeen individuals carrying homozygous wild-type alleles, while two of them had a PSA >900ng/mL. Although the 3435CC and 3435CT are statistically different from one another when individuals carrying the 3435TT genotype are compared to individuals carrying 3435CC and 3435CT, there remains a statistically significant difference (P = 0.0016) where PSA is greater in those men carrying the 3435TT genotype (median PSA = 148 ng/mL) than in pooled 3435CC and 3435CT genotypes (median PSA = 50.1 ng/mL). Thus, variant alleles in ABCB1 seem to be associated with a higher plasma level of PSA prior to receiving docetaxel-based treatment.
Table 3.
Summary of analyses conducted comparing genotype or haplotype to PSA, survival, peripheral neuropa and neutropenia following docetaxel.
| Genotype or Haplotype | Treatment | Effect | P Value |
|---|---|---|---|
| Endpoint - PSA Prior to Study | |||
| 1236C>T | Combined arms | none | NS* |
| 2677G>T/A | Combined arms | none | NS* |
| 3435C>T | Combined arms | Lower PSA with ABCB1 3435CT genotype | 0.0001* |
| Diplotype 1–3 vs. 4–5 | Combined arms | none | NS* |
| Diplotype 6–7 vs. 8 vs. 9–10 | Combined arms | Higher PSA with diplotypes 9-10 | 0.0004* |
| Endpoint - Overall Survival | |||
| 1236C>T | Doc. | none | NS** |
| Doc. & Thal. | Lower survival with CT, TT genotypes vs. CC | 0.0028** | |
| 2677G>T/A | Doc. | none | NS** |
| Doc. & Thal. | Lower survival with GT, GA, TT genotypes vs.GG | 0.018** | |
| 3435C>T | Doc. | none | NS** |
| Doc. & Thal. | none | NS** | |
| Diplotype 1–3 vs 4–5 | Doc. | Lower survival with Diplotypes 4–5 | 0.0017*** |
| Doc. & Thal. | Lower survival with Diplotypes 4–5 | 0.045*** | |
| Diplotype 6–7 vs 8–10 | Doc. | none | NS*** |
| Doc. & Thal. | Lower survival with Diplotypes 8–10 | 0.04*** | |
| Endpoint - Peripheral Neuropathy | |||
| 1236C>T | Doc. | none | NS** |
| Doc. & Thal. | none | NS** | |
| 2677G>T/A | Doc. | none | NS** |
| Doc. & Thal. | More rapid neuropathy onset with 2677GT, | 0.0070** | |
| 3435C>T | Doc. | none | NS** |
| Doc. & Thal. | none | NS** | |
| Diplotype 1–3 vs 4–5 | Doc. | none | NS** |
| Doc. & Thal. | none | NS** | |
| Diplotype 6–7 vs 8–10 | Doc. | none | NS** |
| Doc. & Thal. | none | NS** | |
| Endpoint - Neutropenia | |||
| 1236C>T | Doc. | none | NS**** |
| Doc. & Thal. | none | NS**** | |
| 2677G>T/A | Doc. | none | NS**** |
| Doc. & Thal. | none | NS**** | |
| 3435C>T | Doc. | none | NS**** |
| Doc. & Thal. | none | NS**** | |
| Diplotype 1–3 vs. 4–5 | Doc. | none | NS**** |
| Doc. & Thal. | none | NS**** | |
| Diplotypes 6–9 vs. 10 | Doc. | No association, although only a single individual with diplotype 10 was observed. | NS**** |
| Doc. & Thal. | Diplotype 10 was associated with neutropenia | 0.049***** | |
| Combined arms | Combined group trended towards an association with neutropenia |
0.053***** | |
Kruskal-Wallis Test or Wilcoxon Rank-Sum test as appropriate
Log-Rank test
Cox model
Fisher's Exact Test
Cochran-Armitage Trend Test
Genotype Related to Survival
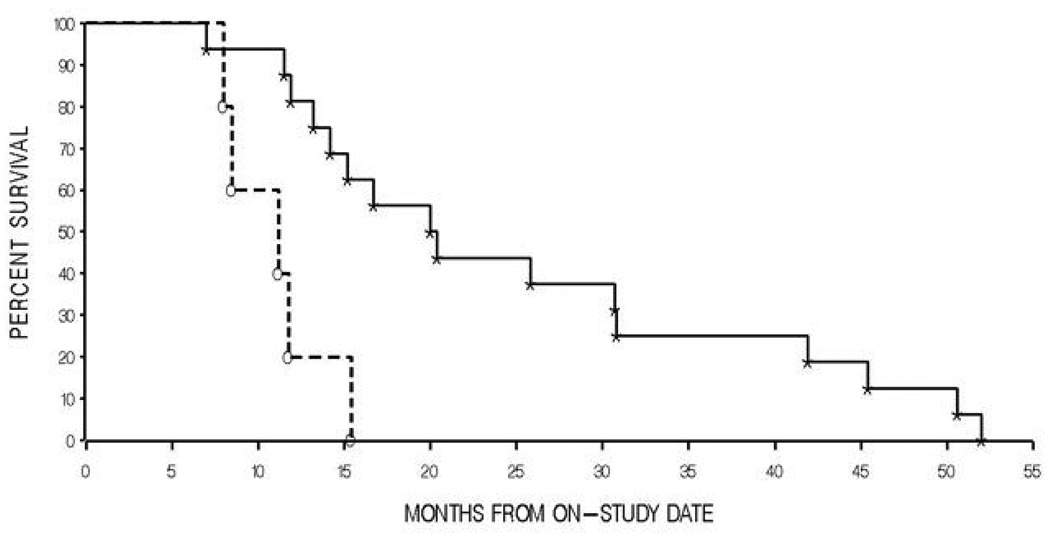
Initial Kaplan-Meier analyses revealed that none of the SNPs alone was associated with a significant difference in overall survival following treatment with single-agent docetaxel (all P > 0.05, Table III). Within the cohort treated with both docetaxel and thalidomide, both the ABCB1 1236C>T, and the 2677G>T/A polymorphisms were associated with survival (P = 0.0028, and P = 0.018 respectively, Table III). The ABCB1 3435C>T polymorphism was not associated with survival in the docetaxel and thalidomide treated cohort (P > 0.05, Table III). Those patients carrying the 1236CC genotype survived significantly longer than individuals carrying the 1236CT and 1236TT genotype (45.1 months vs. 20.1 months), while patients carrying the 2677GG genotype also had improved overall survival when compared to individuals carrying genotypes consisting of the 2677T or 2677A alleles (52.4 months vs. 21.4 months) (Table III). However, a similar exploratory analysis was conducted in the docetaxel cohort with diplotype groupings (based on all 3 SNPs) that indicated a diplotype grouping consisting of diplotypes 4 and 5 had a significantly lower median overall survival (9.8 months) as compared to the diplotype grouping consisting of Diplotypes 1, 2, and 3 (20.0 months; P = 0.0017; Figure 1, Table III). The same analysis was conducted in the docetaxel and thalidomide cohort, where the diplotype grouping of diplotypes 4 and 5 again showed a significantly lower median overall survival (24.0 months) as compared to diplotypes 1, 2, and 3 combined (41.9 months; P = 0.045; Table III).
Figure 1.
Kaplan-Meier curve of survival following initiation of docetaxel therapy in men with androgen independent prostate cancer versus ABCB1 diplotypes 1–5 (* - Diplotypes 1–3; o- Diplotypes 4, and 5). P = 0.0017 by a two-tailed log-rank test.
Cox model analyses were performed individually within each of the two treatment arms because of the different parameters worthy of exploration in the models. For the docetaxel arm the aforementioned pooling of diplotypes was the sole significant parameter that was associated with survival (HR = 5.85, 95%CI = 1.71 – 20.10; P = 0.0049, Table III). However, for patients treated with docetaxel and thalidomide, the grouped 1236C>T and 2677G>T/A SNPs along with the diplotype grouping (6), (7) vs. (8), (9), (10) were included in an initial Cox model. By backward elimination, the 1236C>T SNP was removed, leaving the 2677G>T transition (HR = 10.87, 95%CI = 2.07 – 57.06; P = 0.0048) and the aforementioned diplotype grouping (HR = 5.13, 95%CI = 1.07 – 24.33; P = 0.04, Table III) as parameters that are jointly associated with overall survival in a final Cox model.
Genotype Related to Docetaxel-Induced Toxicity
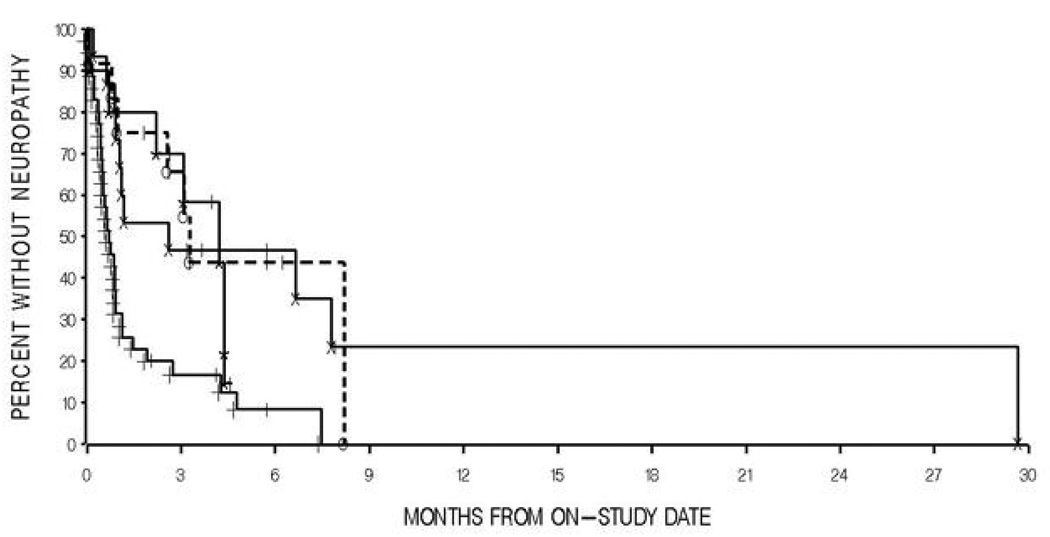
Patients treated with docetaxel alone showed no difference in time to development of neuropathy in a comparison of patients carrying the wild type ABCB1 2677G allele to those patients with at least one variant allele (P = 0.68, Figure 2, Table III). However, patients wild type for the ABCB1 2677 allele developed neuropathy significantly slower (1.9 months to onset) than those with at least one variant allele (0.7 months to onset) when examining patients treated with both docetaxel and thalidomide (P = 0.0070, Figure 2, Table III). No other ABCB1 allele or diplotype was associated with peripheral neuropathy (P > 0.05, Table III).
Figure 2.
Kaplan-Meier curve of percent without neuropathy following initiation of docetaxel or docetaxel and thalidomide treatment in men with androgen independent prostate cancer versus ABCB1 2677 genotypes(* - Docetaxel-treated, 2677 GT; o-Docetaxel-treated, 2677,GT, GA, TT; X – Docetaxel and thalidomide-treated, 2677GG; + - Docetaxel and thalidomide-treated, 2677 GT, GA, TT). P = 0.68 for docetaxel alone and P = 0.0070 for docetaxel and thalidomide, by two-tailed log-rank tests.
Patients carrying diplotype 10 (carrying only variant alleles at both the 2677 and 3435 SNPs) were also marginally more likely to experience higher grade neutropenia while on study as compared to the rest of the population (P = 0.053; Cochran-Armitage Trend Test, Table III). Overall, 3 of 13 patients carrying diplotype 10 experienced a clinically significant grade 3 neutropenia while on study whereas only 1 of 60 total patients carrying all of the other possible diplotypes experienced similar toxicity. This association is concentrated in those patients receiving both docetaxel and thalidomide, with a similar magnitude of significance (P = 0.049, Table III). There is no such association found in the patients who received docetaxel alone (P = 1.00), or with any other genotype or diplotype (Table III). This observation is similar to a previous report where it was found that Diplotype 10 is associated with a significantly lower neutrophil count at nadir in patients treated with paclitaxel (18). It is notable that this same strong relationship can be found when utilizing less robust clinical grading systems such as were used in this study.
Pharmacokinetic analysis
To address the question of whether or not ABCB1 genetic variation is associated with docetaxel plasma concentrations, pharmacokinetic data from 22 patients treated with docetaxel (21) were analyzed as a function of ABCB1 1236C>T, 2677G>T/A, and 3435C>T genotypes and diplotypes. There was no statistically significant effect of genotype, either alone or in combination, on the clearance of docetaxel for any allele tested (P > 0.34; Kruskal-Wallis Test; Supplemental Table I). The pharmacokinetics of thalidomide were not evaluated given that thalidomide is not a substrate of ABCB1 (28), and therefore would not be expected to demonstrate inter-individual variability based on ABCB1 genotypes.
DISCUSSION
We found that ABCB1 allelic variation is associated with clinical endpoints including overall survival, peripheral neuropathy onset, and neutropenia development following docetaxel-based regimens, but is not associated with docetaxel pharmacokinetics. Our results suggest that variant alleles that are associated with lowered ABCB1 expression (17) and altered function (29) result in a clinical phenotype that has reduced docetaxel efficacy and also increased toxicity in men with AIPC. It is possible that expression of ABCB1 outside of the liver is responsible for these findings, as polymorphic variants in ABCB1 can modulate the exposure of ABCB1 substrates in tumor cells where ABCB1 is highly upregulated (13), in blood-tissue barriers (7), and in hematopoietic cells (30).
The current literature regarding the pharmacokinetics of docetaxel and its structural analogue paclitaxel in relation to ABCB1 genotypes is controversial, some finding no relationship between genotypes and pharmacokinetics and others finding such relationships, as reviewed by Marsh et al. (31). Some have found that the 1236T polymorphism is associated with decreased docetaxel clearance in Caucasian patients, although when other ABCB1 alleles were combined in a haplotype, there was no association (32). Our data suggest that none of the aforementioned ABCB1 alleles or diplotypes are associated with docetaxel pharmacokinetics, supporting the findings of most other studies in this regard (31).
Others have noted response differences in patients with ovarian cancer treated with paclitaxel. Originally, it was found that individuals carrying ABCB1 2677G>T/A variants had a moderately significant improved progression free survival after paclitaxel therapy (33). However, a follow-up study in a larger patient population was unable to replicate their observations (34), and a later study found that individuals carrying 2677G>T/A variants actually had a 2.5 times higher risk of death following paclitaxel therapy (35). It is therefore possible that alterations in dosage, schedule, patient selection, and concomitant medications can alter the relationship between survival after taxane treatment and ABCB1 genotype status. Our approach is unique to the above studies in that we used very conservative exploratory statistics and an approach that takes coinheritance of SNPs into account, as has been recommended by some (31).
Given the findings of the above studies, it is interesting that we also found the ABCB1 2677G>T/A polymorphism to be strongly associated with survival in men with AIPC receiving docetaxel, as this is the only non-synonymous polymorphism of the three studied variants. The corresponding ABCB1 893Ser and 893Thr transitions efflux certain drugs, such as vincristine, with much higher maximal transport rates than the wild-type 893Ala, despite similar protein levels (29). In the context of the present study, these data could suggest that patients carrying ABCB1 2677 variant alleles have an altered tumor disposition toward the taxanes, perhaps due to a more efficient transport of the taxanes out of tumor tissues where ABCB1 is found highly expressed.
It is also notable that efficacy is decreased while toxicity is increased in patients carrying variant alleles. This is surprising because tumor cells and normal cells would be expected to have either increased or decreased exposure based on ABCB1 genotype status, resulting in increased efficacy along with increased toxicity and vice versa. However, as advanced prostate tumor cells already overexpress ABCB1 as compared to normal tissue (13), it is possible that protein efflux efficiency differences brought on by these polymorphisms are more deterministic of the efflux phenotype rather than expression differences. Thus, if the variant allele is responsible for lower expression (via transcriptional and translational differences) and also increased efflux of the drug (via protein folding differences), it is likely that the drug efflux efficiency is more important when the protein is already upregulated, as ABCB1 has been shown to be in non-drug-treated prostate tumors. By contrast, in tissues where ABCB1 is expressed at low basal levels (e.g. endothelial and hematopoietic cells) it is likely that these polymorphisms influence drug penetration by altering expression. This hypothesis is borne out by our clinical data, and warrants validation in future investigations.
An alternative explanation for the lowered survival in men carrying variant alleles could be that those men had a greater tumor burden prior to treatment. This is supported by our data showing that men with variant alleles had a higher PSA prior to receiving docetaxel. There is also biological precedent for this hypothesis, given that the expression and function of ABCB1 is important to modulating intracellular levels of dihydrotestosterone (DHT) through efflux pathways. Inhibition, or lack of ABCB1 expression, results in accumulation of DHT and subsequent increases in PSA through AR signaling mechanisms (36). Thus, variant alleles may be involved in a tumor phenotype in which DHT accumulates during the androgen-dependant stage of prostate cancer, thereby driving AR signaling even in the presence of androgen deprivation therapy. We have already shown that active transport of testosterone, and potentially also testosterone metabolites, may be an important determinant of ADT failure by conferring tumor cells the ability to scavenge low levels of testosterone (37, 38). It is therefore also possible that tumor burden following ADT is also influenced by transport mechanisms through ABCB1, and docetaxel efflux from tumor cells may be not truly be associated with docetaxel efficacy.
It is interesting to note that there is a significant increase in time to development of neuropathy when comparing ABCB1 2677 genotype for the docetaxel and thalidomide trial but not when comparing the same allele in the docetaxel-alone trial. Others have already demonstrated that thalidomide is not transported by ABCB1, and thus variances in this gene should have no bearing on presence of, and subsequent time to neuropathy development due to, thalidomide (28). However, the toxicity of thalidomide has also been well-established (39). We propose that treatment by thalidomide exposes the patient to a certain threshold level of toxicity, and subsequent treatment by docetaxel confers an excess level of toxicity that causes a difference in time to neuropathy development. The influence of ABCB1 SNPs on neuropathy development may therefore be very small in those patients treated with docetaxel alone.
This study is limited by a small sample size. However, it should be noted that, given the high variant allele frequencies of these polymorphisms, the statistical power to detect associations with these SNPs and clinical endpoints is greatly improved. Furthermore, we used very conservative statistical methods to evaluate these relationships to reduce the chances of spurious findings. This study was also done specifically to explore and report potential genotypes and diplotypes which may be useful to consider in determining the prognosis for survival in patients with androgen independent prostate cancer. We have not attempted to determine the significance of these genetic findings in the context of other published prognostic parameters, since the number of patients in our trial was very small. We would hope that larger, multi-institutional trials would consider evaluating these parameters in similar studies with greater numbers of patients in order to more definitively determine their role in evaluating prognosis.
In summary, we have identified a patient subset with lowered docetaxel treatment efficacy and also increased toxicity. It is essential that similar studies in larger patient cohorts be investigated in a similar fashion to verify these findings. In addition, as newer treatment options for AIPC become available, taxane treatment should eventually be reconsidered in patients carrying ABCB1 variant alleles.
Supplementary Material
Acknowledgments
We would like to thank Dr. Romano Danesi for his helpful comments in writing this manuscript.
Financial Support
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Bethesda, Md.
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
References
- 1.Hagisawa S, Mikami T, Sato Y. Docetaxel-induced apoptosis in the mitotic phase: electron microscopic and cytochemical studies of human leukemia cells. 1999;32:167–174. doi: 10.1007/s007950050024. [DOI] [PubMed] [Google Scholar]
- 2.Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere) Cancer Treat Rev. 1993;19:351–386. doi: 10.1016/0305-7372(93)90010-o. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Shirakawa K, Takara K, Tanigawara Y, et al. Interaction of docetaxel ("Taxotere") with human P-glycoprotein. Jpn J Cancer Res. 1999;90:1380–1386. doi: 10.1111/j.1349-7006.1999.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordon-Cardo C, O'Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito T, Zhang ZJ, Ohtsubo T, et al. Homozygous disruption of the mdrla P-glycoprotein gene affects blood-nerve barrier function in mice administered with neurotoxic drugs. Acta Otolaryngol. 2001;121:735–742. doi: 10.1080/00016480152583683. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Zhang ZJ, Shibamori Y, et al. P-glycoprotein expression in capillary endothelial cells of the 7th and 8th nerves of guinea pig in relation to blood-nerve barrier sites. Neurosci Lett. 1997;232:41–44. doi: 10.1016/s0304-3940(97)00574-0. [DOI] [PubMed] [Google Scholar]
- 9.Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 10.Drach D, Zhao S, Drach J, et al. Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype. Blood. 1992;80:2729–2734. [PubMed] [Google Scholar]
- 11.Fruehauf S, Wermann K, Buss EC, et al. Protection of hematopoietic stem cells from chemotherapy-induced toxicity by multidrug-resistance 1 gene transfer. Recent Results Cancer Res. 1998;144:93–115. doi: 10.1007/978-3-642-46836-0_12. [DOI] [PubMed] [Google Scholar]
- 12.Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43:553–576. doi: 10.2165/00003088-200443090-00001. [DOI] [PubMed] [Google Scholar]
- 13.Bhangal G, Halford S, Wang J, Roylance R, Shah R, Waxman J. Expression of the multidrug resistance gene in human prostate cancer. 2000;5:118–121. doi: 10.1016/s1078-1439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 15.Kimchi-Sarfaty C, Oh JM, Kim IW. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song P, Lamba JK, Zhang L, et al. G2677T and C3435T genotype and haplotype are associated with hepatic ABCB1 (MDR1) expression. J Clin Pharmacol. 2006;46:373–379. doi: 10.1177/0091270005284387. [DOI] [PubMed] [Google Scholar]
- 18.Sissung TM, Mross K, Steinberg SM, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42:2893–2896. doi: 10.1016/j.ejca.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran A, Jullien V, Alexandre J, et al. Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79:570–580. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 21.Louwerens M, Smorenburg C, Sparreboom A, Loos WJ, Verweij J, de Wit R. Phase I pharmacokinetic and sequence finding study of the combination of docetaxel and methotrexate in patients with solid tumours. Eur J Cancer. 2002;38:497–504. doi: 10.1016/s0959-8049(01)00386-0. [DOI] [PubMed] [Google Scholar]
- 22.Loos WJ, Verweij J, Nooter K, Stoter G, Sparreboom A. Sensitive determination of docetaxel in human plasma by liquid-liquid extraction and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;693:437–441. doi: 10.1016/s0378-4347(97)00089-3. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan E, Meier P. Non-Parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 25.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34:187–202. [Google Scholar]
- 26.Matthews D, Farewell V. Using and understanding medical statistics. 3rd ed. Basel: Karger; 1996. [Google Scholar]
- 27.Agresti A. Catagorical Data Analysis. New York: John Wiley and Sons, Inc.; 1990. [Google Scholar]
- 28.Zimmermann C, Gutmann H, Drewe J. Thalidomide does not interact with P-glycoprotein. Cancer Chemother Pharmacol. 2006;57:599–606. doi: 10.1007/s00280-005-0087-3. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer M, Roots I, Gerloff T. In-vitro transport characteristics discriminate wild-type ABCB1 (MDR1) from ALA893SER and ALA893THR polymorphisms. Pharmacogenet Genomics. 2006;16:855–861. doi: 10.1097/01.fpc.0000230113.03710.34. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 31.Marsh S, McLeod HL. Pharmacogenetics and oncology treatment for breast cancer. Expert Opin Pharmacother. 2007;8:119–127. doi: 10.1517/14656566.8.2.119. [DOI] [PubMed] [Google Scholar]
- 32.Bosch TM, Huitema AD, Doodeman VD, et al. Pharmacogenetic screening of CYP3A and ABCB1 in relation to population pharmacokinetics of docetaxel. Clin Cancer Res. 2006;12:5786–5793. doi: 10.1158/1078-0432.CCR-05-2649. [DOI] [PubMed] [Google Scholar]
- 33.Green H, Soderkvist P, Rosenberg P, Horvath G, Peterson C. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12:854–859. doi: 10.1158/1078-0432.CCR-05-0950. [DOI] [PubMed] [Google Scholar]
- 34.Marsh S, King CR, McLeod HL, Paul J, Gifford G, Brown R. ABCB1 2677G>T/A genotype and paclitaxel pharmacogenetics in ovarian cancer. Clin Cancer Res. 2006;12:4127. doi: 10.1158/1078-0432.CCR-06-0461. author reply -9. [DOI] [PubMed] [Google Scholar]
- 35.Ludwig AH, Kupryjanczyk J. Does MDR-1 G2677T/A polymorphism really associate with ovarian cancer response to paclitaxel chemotherapy? Clin Cancer Res. 2006;12:6204. doi: 10.1158/1078-0432.CCR-06-1374. [DOI] [PubMed] [Google Scholar]
- 36.Fedoruk MN, Gimenez-Bonafe P, Guns ES, Mayer LD, Nelson CC. P-glycoprotein increases the efflux of the androgen dihydrotestosterone and reduces androgen responsive gene activity in prostate tumor cells. Prostate. 2004;59:77–90. doi: 10.1002/pros.10354. [DOI] [PubMed] [Google Scholar]
- 37.Hamada A, Sissung T, Price D, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in Caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-4118. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharifi N, Hamada A, Sissung T, et al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. British Journal of Urology International. 2008 doi: 10.1111/j.1464-410X.2008.07629.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molloy FM, Floeter MK, Syed NA, et al. Thalidomide neuropathy in patients treated for metastatic prostate cancer. Muscle Nerve. 2001;24:1050–1057. doi: 10.1002/mus.1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.