Abstract
Chronic obstructive pulmonary disease (COPD) is a global health problem. As understanding of pathology of COPD has increased it has been established that COPD is associated with the progressive pulmonary inflammation and destruction of lung parenchyma (emphysema) that relate to disease severity. Therefore, it is anticipated that drugs that reduce pulmonary inflammation will provide effective, disease modifying therapy for COPD. Several specific therapies are directed against the influx of inflammatory cells into the airways and lung parenchyma that occurs in COPD; these include agents directed against cytokines and chemokines. Broad-range anti-inflammatory drugs are now in phase III development for COPD; they include inhibitors of phosphodiesterase 4 (PDE4). Other drugs that inhibit cell signaling include inhibitors of p38 mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB), and phosphoinositide-3-kinase (PI3K). There is also a search for inhibitors of proteinases and matrix metalloproteinases (MMPs) to prevent lung destruction and the development of emphysema. This review highlights studies on novel or potential anti-inflammatory agents that might be considered in the development of new future therapies for COPD.
Keywords: NF-κB, MAP kinase, MMPs, cigarette smoke, cytokines, antagonists
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide with an overall prevalence in adults over 40 years currently estimated at between 9 and 10% [1]. The World Health Organization predicts that by 2020 COPD will be the 5th most prevalent disease worldwide (presently the 12th) and the 3rd most common cause of death (presently the 6th). The prevalence is estimated to be about 1% worldwide, but is about 2 times higher in Western societies, and often underestimated due to un- or misdiagnosis [2–4]. The burden of COPD for the patient is high as patients experience a poorer quality of life, suffer from comorbidites (3.7 comorbidities per patient), and direct healthcare costs 20.9 billion dollar in the USA in 2004 [5–8]. In Western countries the major cause is tobacco smoking, whereas in developing countries also indoor pollution e.g. from cooking is a cause. Other risk factors for COPD include genetic predisposition, occupational and environmental exposure, and asthma. More than 90% of patients with COPD are smokers [9], but 30%–40% of the smokers estimated to develop COPD [10] pointing at an additional risk factor for, e.g. genetic variability contributes to smoke susceptibility [11].
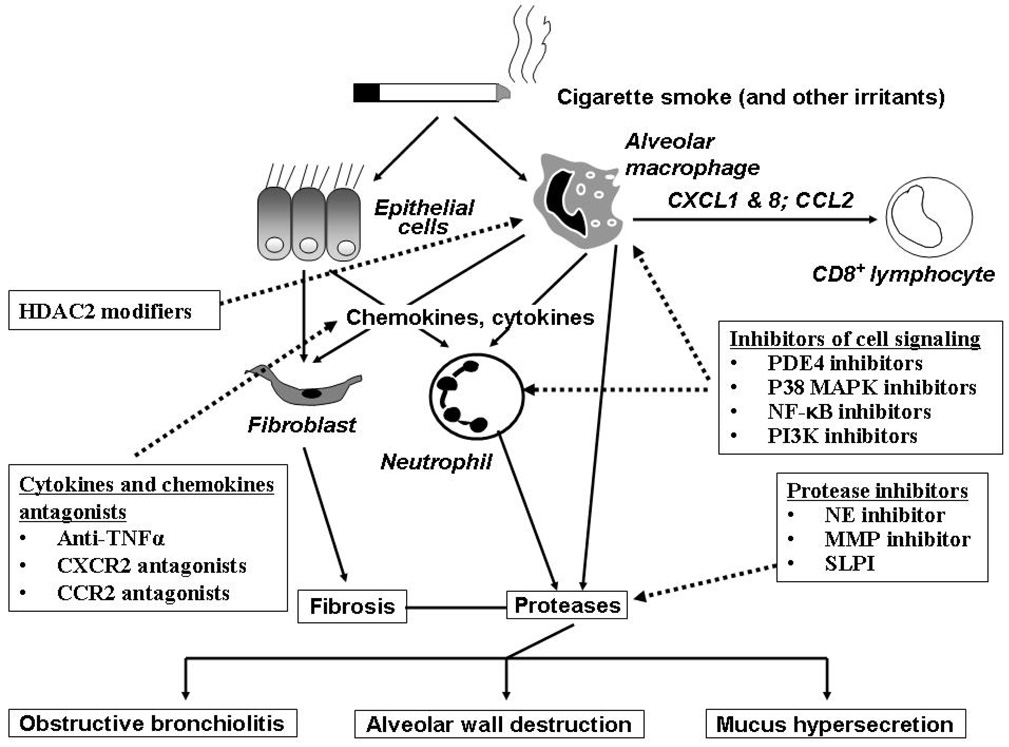
COPD is a chronic lung inflammatory disease with a systemic component characterized by chronic obstruction of lung airflow that progressively deteriorates over time and relatively insensitive to treatment with bronchodilators which provide mainly symptomatic relief by reducing hyperinflation. Classically COPD involves two major spectra of clinical or pathological presentations, chronic bronchitis and emphysema. Inflammation in COPD lungs is present in both small and large airway, alveoli and vasculature and is thought to be critical in the development of the pathology of the disease [12, 13]. Indeed the severity of inflammation is associated with disease severity as measured by spirometry [14]. In small airway inflammation, fibrosis, smooth muscle hypertrophy, globlet cell hyperplasia are present and these features increase in intensity as the disease progresses. Inflammatory cell involved in the small airways consists of CD4 and CD8+ T lymphocyte, B cell follicles, macrophages and in more severe disease neutrophils [15]. Inflammation is also present in large airways and consists of macrophages, CD8+ lymphocytes, neutrophils and plasma cells [16]. Pro-inflammatory mediators, including cytokines and chemokines are released by both structural and inflammatory cells, attracting more inflammatory cells and creating a positive inflammatory loop that enhances and maintains the inflammatory response. In addition, there is accumulation of neutrophils to the airways of smokers without any significant COPD, emphasizing the pro-inflammatory effects of cigarette smoke which is one of the risk factors for COPD. Inflammation in COPD and emerging anti-inflammatory therapy is shown in Figure 1. Interestingly, the pattern of inflammation of COPD is similar no matter what region of the lung is sampled histopathologically, albeit the marked neutrophilia seen in induced sputum is not seen in bronchial biopsies in stable disease and is distinct to that seen in bronchoalveolar lavage fluid [17]. Thus, COPD develops as a result of the participation of a variety of inflammatory cells and mediators contributed by cells and mediators that originate from both innate and adaptive immune responses.
Figure 1. Pulmonary inflammation in COPD and emerging anti-inflammatory therapy.
Exposure to cigarette smoke in susceptible individuals leads to an abnormal inflammation which appears to be self-perpetuating and is perhaps linked to infection. Alveolar macrophages and pulmonary epithelial cells release all kinds of inflammatory mediators in response to cigarette smoke which activate and stimulate migration of pulmonary inflammatory cells including neutrophils, macrophages and lymphocytes. The chronic and persistent inflammation that ensues is thought to be responsible for both the symptoms of disease and also the progressive decline in lung function that is seen in COPD patients. The loss of airway function appears to be related to the destruction of alveoli resulting in a loss of elasticity linked to increased protease activity, and obstruction and fibrosis of the small airway as a result of inflammation and mucus hyper-secretion. Emerging anti-inflammatory therapies under clinical investigation attack this chronic pulmonary inflammation via several strategies. Signaling pathway inhibitors such as PDE4 inhibitors, p38 MAPK inhibitors, NF-κB signaling inhibitors and PI3K inhibitors are in development. Reduction of pleiotropic inflammatory cytokines such as TNF-α using monoclonal antibodies that target the ligands, or soluble receptors that bind and inactivate TNF-α may also reduce the inflammatory burden in the lung. Targeting chemokines such as MCP-1 and IL-8 may reduce the influx of inflammatory cells into the lungs from the circulation by reducing the chemotactic gradient. Inhibition of protease activity in the lung may attenuate lung tissue damage and reduce the numbers of lung neutrophils. Increase HDAC2 expression restores the steroids sensitivity for treatment of COPD. Reducing the severity of inflammation in the lung may improve lung function and slow the progression of COPD.
2. Aim of therapy
The aim of therapy for COPD is not only to treat symptoms and complications as early as possible and to improve exercise tolerance, but also to reduce the number and severity of exacerbations in order to prevent progressive deterioration of health status and lung function. Smoking cessation is still the only significant way to slow the progression of COPD even though all kinds of therapies developed. Current therapy focused mainly on reduction of symptoms using short and long acting bronchodilators either as mono-therapies or combination of long acting β2 agonist bronchodilators with inhaled corticosteroids [18, 19]. Recent TORCH studies showed that a combination of long-acting beta-agonists (salmeterol) with inhaled corticosteroids (fluticasone propionate) offers statistically significant advantages for reducing the frequency of exacerbations, and- probably most important clinically-protection against a decline in lung function [20, 21]. In addition, combined salmeterol/fluticasone propionate improves the inflammation in the airways of patients with COPD. This finding confirms the prospect of this combination therapy in current guidelines for the treatment of patients with COPD that recommend its use for those with severe COPD with frequent exacerbations but not for patients with milder disease or without frequent exacerbations. However, a caution in the use of combination therapy is urged because of the finding in the TORCH trial of an increased rate of pneumonia among all patients receiving treatment containing inhaled corticosteroids [20]. Though some recent studies using higher doses or longer duration of treatment showed reduced airway inflammation and improved quality of life, steroid treatment of patients with COPD is rather ineffective in improving the decline in lung function [22–24]. In addition, adverse effects of steroids including increased risk of hip fractures and osteoporosis, skin bruising, glaucoma, and cataract is main hurdle for its development for COPD [25]. Although emphysema may be another factor that is why the results with current anti-inflammatory therapies have been disappointing, inflammatory cells do actively participate in the pathogenesis of alveolar destruction. Therefore, the search for effective anti-inflammatory agents for COPD is emergent and this goal remains a key objective for the pharmaceutical industry.
3. Development of new anti-inflammatory agents
COPD is a chronic inflammatory disorder, thus a key question is whether novel anti-inflammatory agents can prevent or slow lung function decline characteristic of COPD that appears to be insensitive to current therapy. In addition, the relationship between inflammatory biomarker measured in sputum, bronchoalveolar lavage fluid (BALF), bronchial biopsies or blood and clinical outcomes including disease progression, disease severity, or response to therapy is limited and more work is required to understand these key relationships. Trials examining the predictive value of inflammatory biomarkers are underway but so far the only markers that show a trend towards differentiation between the different GOLD stages of disease severity are arterial oxygen tension, sputum neutrophils and IL-8, and serum TNF-α and C-reactive protein [26, 27]. The spirometric GOLD classification of severity of COPD now only includes four stages- Stage I: Mild; Stage II: Moderate; Stage III: Severe; Stage IV: Very Severe [28]. Stage 0: At Risk,”-that appeared in the 2001 report is no longer included as a stage of COPD, as there is incomplete evidence that the individuals who meet the definition of “At Risk” (chronic cough and sputum production, normal spirometry) necessarily progress on to Stage I [28]. The final part of this review focuses on the recent developments and advances in potential anti-inflammatory agents for treatment of COPD.
3.1. Inhibitors of cell signaling pathway
3.1.1. Phosphodiesterase 4 (PDE4) inhibitors
PDE4 is the predominant PDE isoenzyme in most inflammatory cells though to have a role in COPD pathogenesis and its activity is elevated in lung macrophages from COPD patients [29]. In contrast to the studies that steroids have limited anti-inflammatory efficacy in cigarette smoke models both in the mouse and guinea pig, there are increasing numbers of references documenting the in vivo efficacy of PDE4 inhibitor in animal models of COPD. There are currently five oral PDE4 inhibitors (roflumilast, Phase III from Altana; cilomilast, Phase III from GSK; GRC3886, Phase II from Glenmark; tetomilast, Phase II from Otsuka; C1393, Phase II but suspended from Merck) in clinical development for COPD.
In 24 weeks Phase multi-center III trails in COPD patients (RECORD trial), oral administration of roflumilast or cilomilast improved pre- and post-bronchodilator FEV1 [30, 31]. The health-related quality of life (SGRQ) was also improved when compared to the placebo control. In addition, exacerbation frequency was lower in drugs group than in the placebo group.
The relationship between these improvements in clinical outcome and potential anti-inflammatory activity has been investigated in a single study [32, 33]. After a 4 week treatment with roflumilast post-bronchodilator FEV1 improved by 68.7 ml compared with placebo. Treatment with roflumilast significantly reduced the absolute numbers of neutrophils and eosinophils of sputum. These were paralleled with by a reduction in IL-8 and neutrophil elastase. Although 12 weeks treatment with cilomilast had no effect on sputum neutrophils, macrophages, elastase, IL-8 or lung function; bronchial biopsies demonstrated that cilomilast treatment was associated with significant reductions in CD8+ T lymphocyte and CD68+ cells. The results showed that related outcomes observed in longer term trials could be due, at least in part, to anti-inflammatory activity of drugs.
In an attempt to reduce the potential for systemic side effects and to administer relatively higher doses to the lung, inhaled PDE4 inhibitors are being developed. GSK842470 (AWD-12-281) was licensed from Elbion and reached Phase II for asthma and COPD but there have been unconfirmed reports that it had no advantage over oral PDE4 inhibitors. This compound no longer appears on GSK’s pipeline but remains in development for rhinitis by Elbion. Currently, GSK (Phase I) and Pfizer (Phase II) are reported to have inhaled PDE4 inhibitors in clinical development for COPD. In addition, other step might overcome the limitation of side effects. Vomiting seems likely to be due to inhibition of a particular subtype of PDE4. At least four human PDE4 genes have been identified, and each has several splice variants [34]. Thus, subtype-selective inhibitors could be developed that preserve the anti-inflammatory effect, while having less propensity to cause side-effects. PDE4D seems to be particularly important in nausea and vomiting and is expressed in the chemosensitive trigger zone in the brainstem [35]. In mice, deletion of the gene for PDE4D prevents a behavioral equivalent of emesis [36]. This isoenzyme seems to be less important in anti-inflammatory effects, and targeted gene-disruption studies in mice have shown that PDE4B is more important than PDE4D in inflammatory cells [37]. PDE4B selective inhibitors might therefore have a greater therapeutic ratio and be effective anti-inflammatory drugs.
3.1.2. p38 mitogen-activated protein kinase (MAPK) inhibitors
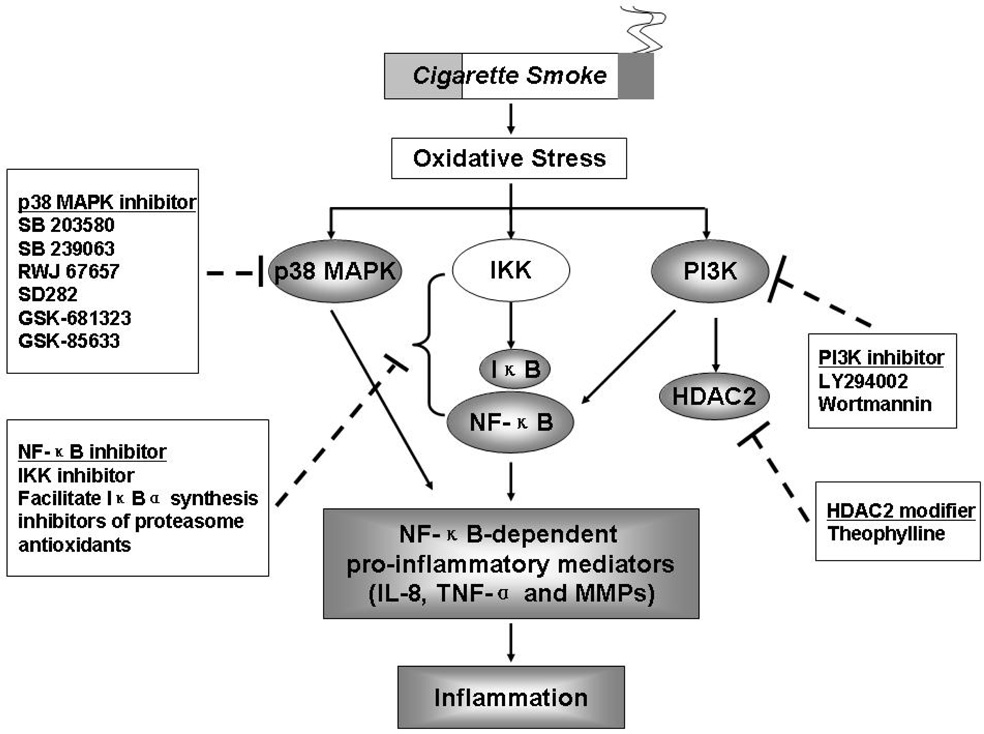
MAPK play a key role in chronic inflammation and several complex enzyme cascades have now been defined [38]. One of these, the p38 MAPK pathway, is activated by cellular stress and regulates the expression of wide variety of inflammatory cytokines that include IL-8, TNF-α and MMPs [39] (Figure 2). Small molecule inhibitors of p38 MAPK, such as SB 203580, SB 239063 and RWJ 67657 having broad range of anti-inflammatory effects have been developed [40]. Administration of SB203580 has beneficial effects in animal disease models such as collagen-induced arthritis and endotoxin-induced septic shock [41]. SB203580 also inhibits cytokine production and proliferation that has been induced by mitogen stimulation or T-cell receptor ligation, with or without CD28-mediated co-stimulation [42–44]. p38 MAPK has also been shown to upregulate cytokine production by several independent mechanisms, including direct phosphorylation of transcription factors, and direct or indirect stabilization and increased translation of mRNAs containing 3% untranslated region adenylate/uridylate-rich elements (AREs) by phosphorylation of ARE-binding proteins [45–47]. These observations have attracted interest in p38 MAPK as a molecular target in the treatment of inflammatory human diseases. Studies in healthy volunteers given p38α/p38β inhibitors found reductions in pro-inflammatory cytokine secretion from ex-vivo LPS-stimulated peripheral-blood mononuclear cells [48], and decreased LPS-induced pro-inflammatory cytokine production, neutrophil and endothelial-cell activation in vivo. SB239063 on the other hand reduces neutrophil infiltration and the concentrations of IL-6 and MMP-9 in BALF of rats after endotoxin inhalation, suggesting its potential as an anti-inflammatory agent in COPD [49].
Figure 2. Activation of p38 MAPK, NF-κB and PI3K pathway in COPD and possible therapeutic targets.
Cigarette smoke-mediated oxidative stress can result in activation of various kinases [p38 mitogen-activated protein kinase (p38 MAPK), IκB kinase (IKK) and phosphoinositide 3-kinase (PI3K)] leading to release of pro-inflammatory mediators by activating transcription factor NF-κB. A reversible PI3K inhibitor, LY294002, can restore defective HDAC2 expression and activity (possibly restores steroid efficacy) which were shown in the lungs of patients with COPD. Therefore, these kinases would be possibly therapeutic targets although these inhibitors/modifiers of above-mentioned kinases have not yet been proven by appropriate randomized placebo trials.
The potential therapeutic utility of p38 MAPK inhibition in respiratory disease has been supported by data generated in a range of pulmonary inflammatory models in vivo including LPS induced pulmonary neutrophilia [50], bleomycin induced fibrosis [51], and antigen induced eosinophilia [52]. A recent study demonstrated the efficacy of p38α MAPK inhibitor, SD282, in a mouse model of cigarette smoke-induced inflammation. In this model, SD-282 inhibited cigarette smoke induced pulmonary neutrophilia and macrophage recruitment. Although a number of oral p38 MAPK inhibitors are in clinical development for arthritis and cancer only two compounds are currently in development for COPD. GSK-681323 is currently in a 4 week Phase II trial where the efficacy outcome measures include lung function, sputum and serum biomarkers, including C-reactive protein. GSK-85633 is in Phase I. Although it is likely that such a broad spectrum anti-inflammatory drug might ensue some toxicity or impair natural immune responses, but inhalation route might be a feasible therapeutic approach.
3.1.3. Nuclear factor-κB (NF-κB) inhibitors
Studies with inhibitor of κB (IκB)α mutants [53, 54] gave the first evidence that NF-κB pathway could be specifically inhibited. Signal-induced phosphorylation and degradation of cytoplasmic IκBα is required for NF-κB pathway activation. However, an IκBα protein with mutations at serine-32 and 36 is not phosphorylated by IKK (IκBα kinase) and therefore not degraded by the proteasome. This IκBα mutant or super-repressor exerts its negative effect by sequestering NF-κB in the cytoplasm and thus prevents the induction of specific NF-κB target genes.
There are several possible approaches to inhibition of NF-kB, including gene transfer of IκB, inhibitors of IκB kinases (IKK), NF-κB-inducing kinase and IκB ubiquitin ligase, which regulate the activity of NF-κB, and inhibit the degradation of IκB [55] (Figure 2). The most promising approach may be the inhibition of IKK-2 by small molecule inhibitors [56], which suppress the release of inflammatory cytokines and chemokines from alveolar macrophages [57] whereas alveolar macrophages are resistant to the anti-inflammatory actions of corticosteroids. However, it is of concern that long-term inhibition of IKK-2 may result in immunosuppressive side effect while IKK-1 inhibitor may be another approach to inhibit the inflammation response in animal models (authors’ unpublished observation). In addition, alternative modulation of pathways of NF-κB activation via kinases other than IKK might be a more safer approach in inflammatory disease and would have less potential effect on innate and adaptive immune responses [58].
Glucocorticoids, such as dexamethasone and prednisone, are widely used for their anti-inflammatory and immunosuppressive properties. Dexamethasone induces the synthesis of IκBα mRNA in glucocorticoid receptor-expressing Jurkat cells [59] and in monocytic cells [60], therefore resulting in the cytoplasmic retention of p65 due to binding of IκBα to the pre-existing NF-κB complexes [60]. Retention of NF-κB in an inactive cytoplasmic complex thus down regulates expression of pro-inflammatory genes.
Another novel way whereby NF-κB activity may be regulated is by the use of inhibitors of proteasome function, which can reduce the degradation of IκB and thus prevent NF-κB activation [53, 54]. A series of peptide aldehydes such as MG101, MG132, and MG115, make up a family of agents that inhibit the protease activity of the proteasome. Lactacystin, another class of proteasome inhibitor, blocks proteolytic activity by acylating a threonine residue in one of the key proteasome subunits. Furthermore, a group of boronic acid peptides, including PS-341, are extremely potent inhibitors of proteasome function [61], thus inhibiting activation of the NF-κB pathway. It is also possible that inhibitors of the ubiquitin ligase that mediates IκB ubiquitination may be a useful target in preventing proteasome degradation of IκB. Thus, a wide variety of potential inhibitors of proteasome function may have a therapeutic role in anti- NF-κB pathway dependent strategies.
Certain natural polyphenolic antioxidants/products such as flavonoids quercetin, resveratrol, and myricetin are also known to mediate their anti-inflammatory properties through down regulation of the NF-κB pathway [62, 63]. For example, resveratrol, which is found in red wine, can inhibit NF-κB activity and induce apoptosis in transformed cells, which may reduce mortality from coronary heart diseases, certain cancers and inflammatory diseases [62]. Resveratrol has strong inhibitory effects on iNOS expression and NO generation in activated macrophages [63]. Since treatment of macrophages with resveratrol blocks LPS-induced phosphorylation and degradation of IκBα to decrease NF-κB DNA binding activity, is suggestive of the fact that its anti-inflammatory effects may be due at least in part to the inhibition of NF-κB-dependent NO synthesis [63]. Thus several of the biological activities of flavonoids may be mediated by their inhibition of the NF-κB pathway. Indeed, flavanoid resveratrol inhibits inflammatory cytokine release from macrophages isolated from COPD patients [57] However, the clinical utility of resveratrol in patients with COPD is not known.
3.1.4. Phosphoinositide 3-kinases (PI3K) inhibitor
The PI3K are a family of proteins that catalyze the phosphorylation of the 3-OH position of phosphoinositides and generate lipids that control a wide variety of intracellular signaling pathways. Recent studies suggested that numerous components of the PI3K pathway play a crucial role in the expression and activation of inflammatory mediators, inflammatory cell recruitment, immune cell function and airway remodeling as well as corticosteroid insensitivity in chronic inflammatory respiratory disease such as asthma [64–67] (Figure 2).
Although no reports on the effect of PI3K inhibitors on experimental models of COPD have been published, the data suggesting a potential role of PI3K in the pathogenesis of COPD is now accumulating. PI3K is important in the activation of macrophage and neutrophils, which are key players in COPD inflammation [68]. Moreover, MMP-9 degrades extracellular matrix components (particularly elastin) and is related to the pathogenesis of pulmonary emphysema. MMP-9 is present in low quantities in the healthy adult lung but much more abundant in COPD and the inappropriate expression of MMP-9 is thought to contribute to the pathogenesis of COPD [69]. MMP-9 expression whether stimulated by platelet activating factor or fibronectin, probably through an action on NF-κB, is also regulated by PI3K signaling pathways [70].
Patients with COPD and severe asthma do not respond well to corticosteroids though corticosteroids are very effective in controlling mild to moderate asthma. Ito and coworkers [71] reported a reduction in corepressor, histone deacetylases 2 (HDAC2) expression and total HDAC activity in COPD patients. By overexpression and knockdown of HDAC2 demonstrated that HDAC2 is a prerequisite molecule for corticosteroid action in airway macrophages and reduction of HDAC2 is one of the causes of corticosteroid insensitivity in COPD [72]. In vitro experiments have also shown that oxidative stress raised by hydrogen peroxide reduced HDAC2 expression and in preliminary experiments LY294002 (a reversible PI3K inhibitor) restored defective HDAC2 expression and activity in these cells. Therefore, PI3K inhibitors will be more efficacious in more severe steroid-insensitive asthma and in COPD where corticosteroids are of limited effectiveness and no alternative therapy is available.
In addition, non-specific PI3K inhibitors exhibit some compound specific toxicity and possess off-target effects [wortmannin (an irreversible inhibitor): myosin light chain kinase inhibition, LY294002: casein kinase-2 inhibition]. As reviewed by Ito and colleague [73], most inflammatory cells relevant to asthma and COPD are controlled by type I PI3Ks, especially PI3Kδ and γ. Thus, selective PI3Kγ and/or PI3Kδ inhibitors might have relevant anti-inflammatory activity in COPD.
3.2. Cytokine and chemokine antagonists
In patients with COPD, protein and/or mRNA levels of different pro-inflammatory cytokines and chemokines have been found to be increased compared with subjects without COPD. Among these, TNFα or TNFR levels, soluble IL-1 receptor antagonist (sIL-1Ra), CCL2 (monocyte chemoattractant protein 1, MCP-1) and its receptor CCR2, CCL3 (macrophage inflammatory protein 1α, MIP-1α) and CCL4 (MIP-1β) and their receptor CCR5, CXCL8 (IL-8), and CXCL10 (interferon-inducible protein 10, IP-10) can be discerned as pro-inflammatory factors. In addition to the inflammatory effects, recent studies provided more evidence that cytokines and chemokines are also involved in tissue remodelling apart from growth factors, pointing to cytokine-driven effects of inflammatory cells on epithelial wound repair [74].
Several recent reviews point to the development of novel antagonists of cytokines, chemokines or their receptors [74–76]. These molecules may reduce gene expression, impair production or secretion of mature proteins, antagonize binding of cytokines and chemokines to their receptors or inhibit receptor signal transduction. Antibodies and solubilized receptors like TNFR often scavenge solubilized cytokines and chemokines, or prevent binding of these proteins to their receptors. Small molecules 1) prevent binding of cytokines and chemokines to their receptors by non-activating mimicry of cytokines or chemokines, or 2) prevent intracellular signal transduction activation, or 3) interfere with gene expression and translation by direct inhibition of transcription factors (like IKK2 inhibition) or mRNA binding via small interference (si) RNA or antisense mRNA.
3.2.1. TNFα and receptors antagonists
Concentration of TNFα and soluble TNFα receptor are increased in the sputum of COPD patients [77, 78]. Experimental animal models show that TNFα over-expression induces the pathological changes similar to emphysema and pulmonary fibrosis. COPD patients with cachexia have also increased release of TNFα and soluble TNFα receptor in circulation, which may be a factor contributing to the weight loss in patients with the disease [79]. Therefore, several drugs have been developed to reduce TNFα levels, of which some have been approved by e.g. the Federal Drug Administration for treatment of RA, ankylosing spondylitis, Crohn’s disease, or psoriasis. These approved drugs include etanercept (soluble human TNFR2), infliximab (chimeric human/mouse IgG1 antibody against TNFα), and adalumimab (human IgG1 antibody against TNFα). Many others are being developed in order to enhance efficacy, reduce side effects due to frequent subcutaneous injection, increase bioavailability or protection to proteolytic degradation by coupling to polyethylene glycol chains, or reduce immunogenicity by humanization of antibodies or designing small molecules. In contrast to treatment of refractory asthma, recent clinical phase II trials demonstrated that in general COPD patients do not benefit from treatment with infliximab in terms of quality of life, lung function or physical endurance [80,81]. Patients with moderate to severe asthma may benefit from treatment with either infliximab or etanercept. However, as pointed out in several reviews [76, 82] infliximab is just one of the many antagonists of TNFα and its receptors and its effect may need longer treatment instead 6 weeks of treatment. Three infusions of infliximab over 6 weeks reduced the number of exacerbations as well as sputum levels of TNFα, IL-6, CXCL8 and CXCL10 but not peak expiratory flow or inflammatory cell count in sputum of patients with moderate asthma [83]. Other studies demonstrated that twice-weekly treatment with etanercept during 10 to 12 weeks improved the bronchial hyperresponsiveness (BHR, expressed as PC20), post-bronchodilator FEV1 and the quality of life of patients with refractory, severe asthmatic patients [84, 85]. Treatment of asthmatics with marimastat, an inhibitor of TNFα and MMP activation, also reduced BHR but failed to significantly reduce sputum inflammatory cell numbers, asthma symptoms, FEV1 or bronchodilator use [86]. Future studies should sort out whether long-term treatment or treatment with different TNFα antagonists are beneficial to all COPD patients or only a specific phenotype/population of COPD patients [80,82].
3.2.2. CXCL1, CXCL8 and receptors antagonists
CXCL1 (GROα) and CXCL8 (IL-8) are produced by both structural and inflammatory cells including macrophages, and are chemotactic and activate inflammatory cells while IL-8 also induces neutrophils to degranulate and causes an oxidative burst. Both bind to their receptor CXCR2 whereas IL-8 binds also to CXCR1. Both receptors are expressed on neutrophils while CXCR2 is also expressed on other inflammatory cells including a subset of CD8+ T cells, mast cells and macrophages.
Concentration of IL-8 is very high in the sputum of patients with COPD and is correlated with the disease severity [77, 87]. Blocking antibodies to IL-8 and related chemokines inhibit certain types of neutrophilic inflammation in animals [88] and reduce the chemotactic response of neutrophils to sputum from COPD patients [89, 90]. Several CXCR2 and IL-8 antagonists are available, some of which were in clinical trial for COPD [76]. Updated information shows that either the testing of these drugs is discontinued (like the antibody ABX-IL-8 against human IL-8) or is not to be found in the public domain. Hence, little is known yet on treatment of patients with COPD with IL-8 or CXCR2 antagonists. The small molecule CXCR2 antagonist SB-656933 (by GSK) has recently been demonstrated to inhibit the IL-8-induced expression of CD11b molecules on peripheral blood neutrophils from COPD patients [91]. The antagonist was mentioned to enter clinical trial studies for COPD in 2005, but is not so in GSK’s pipeline of 2006/2007. Data from these studies have not yet been published. SB-265610 is a small molecule inhibiting CXCR2. Studies demonstrated that hyperoxia in newborn rats led to pulmonary inflammation by neutrophils and the formation of ROS and RNS mediating impaired lung development and lipid peroxidation [92, 93]. Treatment with SB-265610 reduced airway neutrophilia, radical formation, lipid peroxidation and protein nitration, as well as improved conservation of lung development and lung function. This reflects to the importance of reducing neutrophilia in order to reduce reactive species formation, lipid peroxidation or nitration, and tissue destruction or alterations. Data from other studies supported the effectiveness of IL-8 or CXCR2 antagonists in reducing neutrophilia in vivo in rodents and inhibition of neutrophil activation and degranulation in vitro [76, 94]. These data point to the potential need for development of novel antagonists of CXCR1, CXCR2 or their ligands CXCL1 and IL-8. Recent studies showed that novel thiazolopyrimidine, cyclobutenedione (e.g. SCH 527123), or imidazolylpyrimidine CXCR2 antagonists had a good oral bioavailability in rats with reasonable pharmacokinetics (half life of at least 1.2h) [95–97], and inhibition of CXCL1- or IL-8-induced chemotaxis of cells [95, 96].
3.2.3. CCL2 (MCP-1) and CCR2 antagonists
Patients with chronic bronchitis have higher CCL2 in the BALF than healthy non-smokers [98]. In bronchial biopsy specimens, CCL2 mRNA bronchiolar epithelium expression, including the expression of its receptor (CCR2 in macrophages), was also increased in COPD patients when compared to subjects without COPD [99]. In addition, the level of CCL2 in sputum samples of smoking COPD patients were also increased when compared to non-smokers and healthy smokers [100]. Furthermore, CCL2 levels in the exhaled breath condensate from COPD patients correlated with the degree of airflow obstruction as measured by FEV1/FVC ratio [101]. These data support a major role for CCL2 and CCR2 in airway remodeling and inflammation directly or via macrophages. Antagonists of CCR2 or CCL2 may, therefore, be an attractive approach to therapeutic treatment of COPD.
The humanized monoclonal antibody MLN1202 (by Millennium) against CCR2 is currently in phase II for treatment of chronic inflammatory diseases like multiple sclerosis and artherosclerosis. Clinical data from these studies have not yet been published. Other inhibitors in clinical trial include the small molecule CCR2 inhibitors CCX915 (by ChemoCentryx) for multiple sclerosis (phase I), INCB3284 (by Incyte/Pfizer) which is in phase IIa for treatment of RA, and the monoclonal antibody ABN912 against CCL2 (by Novartis) for COPD (phase I) and RA (phase II). Recently, patients with RA did not clinically and immunohistologically respond to a 2-week treatment with ABN912 [102]. Serum levels of CCL2 increased clearly after treatment (doses higher than 1 mg/kg body weight), and chemotactic complexes of the antibody with CCL2 were formed. This may hamper the treatment of patients with COPD; however these results have to be awaited. Several other compounds based on butyramides or γ-aminoamides were recently developed as specific CCR2 antagonists with a good oral bioavailablity in rats [103, 104]. Others are in preclinical phase, including INCB8696, JNJ-27553292, SKL-2841 and INCB3344. INCB3344 is a rodent CCR2 small molecule antagonist [105]. The compound was shown to inhibit macrophage influx in a mouse model for delayed hypersensitivity.
3.3. Anti-proteinases
3.3.1. Neutrophil elastase inhibitor
Neutrophil elastase is a serine protease that is synthesized in the neutrophils and secreted following neutrophils activation. One of the major actions of neutrophil elastase is its ability to degrade matrix proteins. Neutrophil elastase increases MMPs activity by directly activating MMPs such as MMP-9 [106] and by inactivating the endogenous MMP inhibitor, tissue inhibitor of matrix metalloproteinase (TIMP) [107], thus potentially enhancing the role of MMPs in COPD. It also has a pro-inflammatory role in COPD pathogenesis [108, 109]. In the absence of neutrophil elastase, neutrophils had difficulty escaping the capillary to the lung interstitium and alveolar space [109]. This was not due to an inability of neutrophils to penetrate basement membranes in the absence of NE [110]. NE is required to dissociate the CD11/CD18-ICAM-1 complex tethering the neutrophil to the endothelium [111]. Similarly, marked suppression of macrophage accumulation in the lungs in neutrophil elastase-deficient mice exposed to cigarette smoke [109]. Moreover, neutrophil elastase has also been reported to stimulate mucin secretion and modulate apoptosis of human lung epithelial cells. Therefore, neutrophil elastase may play a role in emphysema and lung remodeling through matrix degradation and by inducing apoptosis.
In cigarette smoke models the neutrophil elastase knockout mice is protected from emphysema development and this effect is accompanied by an inhibition of both neutrophils and macrophages recruitment [109]. Treatment with α-1-anti-trypsin inhibited emphysema development and reduced pulmonary neutrophils and macrophages recruitment [112, 113]. The role of α-1-antitrypsin replacement therapy in slowing the progression of α1-antitrypsin-deficiency related emphysema seems likely, but has not yet been proven by appropriate randomized placebo trials. Similarly, a small molecule neutrophil elastase inhibitor, ZD0892, reduced cigarette smoke induced emphysema and pulmonary cells recruitment in a guinea pig model [114]. These studies suggest inhibition of neutrophil elastase can be anti-inflammatory in addition to preventing emphysema development.
The search for potent, safe oral inhibitors of neutrophil elastase has been going on for over 20 years and many of the compounds that progressed into clinical development failed because of poor PK and low therapeutic index. Subsequently, tripeptidyl trifluoromethyl ketones were developed that had a better oral profile, although these molecules were not fully optimized for oral delivery [115] and monocyclic beta lactam neutrophil elastase inhibitor were also identified [116]. More recently, both GSK (GSK-311366) and ONO Pharmaceuticals (ONO6818) have had oral compounds in Phase I/II. However, they failed in early clinical trials and thus far no oral elastase inhibitors have been evaluated fully in Phase II COPD trials. The AstraZeneca protease inhibitor AZD3342 (thought to be an elastase inhibitor) is assessed in Phase II studies and Bayer has an inhibitor in Phase I.
Protein inhibitors of neutrophil elastase administered via nebulisation are also being pursued and supportive data has been obtained for inhaled recombinant α1-antitypsin in mouse cigarette smoke models. The probability of inhaled combination products consisting of elastase inhibitors with bronchodilators is low. However, the opportunity for safe long acting inhaled elastase inhibitors with low systemic bioavailability that are suitable for combination with bronchodilators is being pursued by Argenta Discovery.
3.3.2. MMPs inhibitor
MMPs have been suggested from recent studies as the major proteolytic enzymes involved in the pathogenesis of COPD. Increased levels of MMP-1 and MMP-9 have been detected in bronchoalveolar lavage fluid of patients with emphysema [117]. As compared with healthy subjects, patients with COPD have a marked increase in expression and activity of MMP-2, MMP-9 and MT1-MMP in their lung parenchyma [118] and increased gelatinolytic activity linked to MMP-2 and MMP-9 in their sputum [119, 120]. In BALF of smokers with emphysema, an increase of collagenolytic activity, probably due to elevated levels of MMP-8, is measured when compared to smokers without emphysema indicating that injury to the collagen network of the lung is also a part of the disease. When studied ex vivo, alveolar macrophages from patients with emphysema express more MMP-1 and MMP-9 than macrophages from healthy subjects suggesting that macrophages are the main cellular source of MMP-1 and MMP-9 in COPD [121]. As it has a potent elastolytic activity, MMP-12 is thought to play a determinant role in development of COPD and emphysema. Experimental data show that mice exposed to cigarette smoke express more MMP-12 mRNA than non-exposed mice [122]. MMP-12 is also markedly increased in induced sputum from patients with stable COPD compared with controls [123]. MMP-12 gene deletion in mice protects from the development of emphysema after long term exposure to cigarette smoke [124]. Alveolar macrophages were considered to be the unique source of MMP-12 (macrophage metalloelastase). Taken together, these elements suggest that MMP-12 could be seriously considered as a potential target for COPD treatment [125]. Indeed, some reports establish the potential interest for a MMP inhibition-based approach in COPD, based on results obtained in animal models. Orally bioavailable synthetic MMP inhibitors were showed to decrease the rapidity of alveolar destruction in murine and Guinea Pig models of tobacco smoke exposure. Although non-specific MMP inhibitors, such as marimastat, seem to have substantial musculoskeletal side-effect [126], these adverse effects could be reduced by increasing the selectivity for specific MMP or by targeting delivery to lung parenchyma. Interestingly, many MMP inhibitors may actually improve the progression of emphysema but perhaps are not so potent in preventing airway inflammation. Many potential drugs such as BMS-561392 and GW3333, which can inhibit MMPs and TNF-α converting enzyme, are still in preclinical development or in clinical trial phases for diseases other than COPD. In addition, some antibiotics such as minocycline and roxithromycin have MMPs inhibitory effect and improve the MMPs/TIMP imbalance as well. Therefore, these drugs may be other candidates to improve the progression of COPD.
3.4 HDACs modifiers
NF-κB being a pro-inflammatory transcription factor, on activation binds to specific recognition sequence motifs in DNA and subsequently interacts with a multitude of co-activator molecules, such as cAMP-response-element-binding protein (CREB), CREB-binding protein (CBP), p300 and p300/CBP-associated factor (pCAF). These co-activators act as molecular switches of transcription and acetylate histones by their intrinsic histone acetyltransferase (HAT) activity [127, 128]. Acetylation of core histones allow DNA unwinding and thus access to wide variety of transcription factors [127]. The transcriptional process can be reversibly switched off by deacetylation of acetylated histones, thus rewinding the exposed DNA, consequently leading to gene silencing. The deacetylation of acetylated histones is brought about by HDACs, which act as co-repressors, in association with other co-repressor proteins that are concomitantly recruited. Ito and co-workers have shown a role for histone acetylation and deacetylation in IL-1β-induced TNFαrelease in alveolar macrophages derived from cigarette smokers [129]. The activity and expression of HDAC, a transcriptional corepressor, was decreased in COPD due to oxidative stress and consequently cytokine transcription was increased [71]. Furthermore, both cigarette smoke/H2O2 and TNFα caused an increase in histone acetylation (HAT activity) leading to IL-8 expression in monocytes and alveolar epithelial cells both in vitro and in vivo in rat lungs [130–132]. Since all inflammatory processes involve acetylation and deacetylation of histones (chromatin remodeling) [133], HDACs therefore are attractive targets for anti-inflammatory therapies.
Glucocorticoid suppression of inflammatory genes requires recruitment of HDAC2 to the transcription activation complex by the glucocorticoid receptor [129, 134]. This results in deacetylation of histones and a decrease in inflammatory gene transcription. A reduced level of HDAC2 was associated with increased proinflammatory response and reduced responsiveness to glucocorticoids in alveolar macrophages obtained from smokers [129–132, 134]. Culpitt and co-workers have shown that cigarette smoke solution stimulated release of IL-8 and GM-CSF, which was not inhibited by dexamethasone, in alveolar macrophages obtained from patients with COPD compared to that of smokers [135]. They suggested that the lack of efficacy of corticosteroids in COPD might be due to steroid insensitivity of macrophages in the respiratory tract. Recent study showed that overexpression of HDAC2 in glucocorticoid-insensitive alveolar macrophages from patients with COPD is able to restore glucocorticoid sensitivity [72]. Mechanisms study demonstrated that HDAC2 acts by deacetylating glucocorticoid receptor, thereby enabling p65-NF-κB association and subsequent attenuation of proinflammatory gene transcription. Thus, the cigarette smoke/oxidant-mediated reduction in HDAC2 levels and activity in alveolar epithelial cells and macrophages will not only increase inflammatory gene expression but will also cause a decrease in glucocorticoid function in patients with COPD.
Consequently, a potential means by which to treat COPD would be to increase HDAC2 expression and activity such that steroids regain their anti-inflammatory activity. Co-incubation of cells with NAC and H2O2 protects HDAC2 from down regulation and reduction of specific activity [131]. In addition, it has been reported that theophylline and PI3K inhibitor (LY294002) have a similar effect in lung macrophage cells, increasing HDAC2 expression and re-sensitizing the cells to steroids [73, 136]. Alternative means of upregulating HDAC2 activity would be of great interest for potential combination therapies for steroid-insensitive COPD.
4. Conclusion
After smoking cessation inflammation still goes on which may hamper tissue repair. Hence, an effective treatment of patients with COPD not only needs stopping exposure to toxic compounds like smoke, but also needs attenuation of excessive inflammation. Several anti-inflammatory mechanisms are being investigated in COPD patients and the data generated within next 5 years will indicate if significant anti-inflammatory activity can be achieved in COPD patients and whether this results in improved clinical outcomes. Oral drugs have the advantage of improved compliance over inhaled agents and may reduce the chronic systemic inflammation present in COPD patients in addition to reducing the inflammation within the lungs. Inhaled therapies may have the advantage of targeting pulmonary inflammation while have low systemic exposure and improved therapeutic index. In addition, development of specific isoform inhibitors of inflammatory signaling pathway is also way to reduce the adverse effect of broad spectrum inhibitor. Another approach is to enhance negative homeostatic signals such as anti-inflammatory IL-10 would counteract the ongoing airway inflammation in COPD based on the finding showing that IL-10 level in sputum is reduced in COPD and smoker [137]. Furthermore, combination of drugs can be explored with the aim of enhancing the efficacy of mono-therapy and reducing the side action, e.g. HDAC2 modifiers restore the steroids sensitivity to COPD.
ACKNOWLEDGEMENT
Supported by the NIH R01-HL085613 and NIEHS ES-01247.
ABBREVIATIONS
- BALF
Bronchoalveolar lavage fluid
- BHR
Bronchial hyperresponsiveness
- CBP
CREB-binding protein
- COPD
Chronic obstructive pulmonary disease
- CREB
cAMP-response-element-binding protein
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GR
Glucocorticoid receptor
- HAT
Histone acetyltransferase
- HDAC
Histone deacetylase
- IκB
Inhibitor of Κb
- IKK
IκBα kinase
- MAPK
Mitogen-activated protein kinase
- MMPs
Matrix metalloproteinases
- NF-κB
Nuclear factor-κB
- PDE4
Phosphodiesterase 4
- PI3K
Phosphoinositide-3-kinase
- TIMP
Tissue inhibitor of matrix metalloproteinase
References
- 1.Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg A, Bjerg-Backlund A, Ronmark E, et al. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100:264–272. doi: 10.1016/j.rmed.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 5.Hoogendoorn M, Feenstra TL, Rutten-van Molken MP. [Projections of future resource use and the costs of asthma and COPD in the Netherlands] Ned Tijdschr Geneeskd. 2006;150:1243–1250. [PubMed] [Google Scholar]
- 6.Hynninen KM, Breitve MH, Wiborg AB, et al. Psychological characteristics of patients with chronic obstructive pulmonary disease: a review. J Psychosom Res. 2005;59:429–443. doi: 10.1016/j.jpsychores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Jones PW. Health status: what does it mean for payers and patients? Proc Am Thorac Soc. 2006;3:222–226. doi: 10.1513/pats.200512-126SF. [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 9.Snider GL. Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annu Rev Med. 1989;40:411–429. doi: 10.1146/annurev.me.40.020189.002211. [DOI] [PubMed] [Google Scholar]
- 10.Lokke A, Lange P, Scharling H, et al. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampsonas F, Karkoulias K, Kaparianos A, et al. Genetics of chronic obstructive pulmonary disease, beyond a1-antitrypsin deficiency. Curr Med Chem. 2006;13:2857–2873. doi: 10.2174/092986706778521922. [DOI] [PubMed] [Google Scholar]
- 12.Voelkel NF, Cool CD. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:28s–32s. doi: 10.1183/09031936.03.00000503. [DOI] [PubMed] [Google Scholar]
- 13.Jeffery PK. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest. 2000;117:251S–260S. doi: 10.1378/chest.117.5_suppl_1.251s. [DOI] [PubMed] [Google Scholar]
- 14.Turato G, Zuin R, Miniati M, et al. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med. 2002;166:105–110. doi: 10.1164/rccm.2111084. [DOI] [PubMed] [Google Scholar]
- 15.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 16.Molfino NA, Jeffery PK. Chronic obstructive pulmonary disease: Histopathology, inflammation and potential therapies. Pulm Pharmacol Ther. 2006 doi: 10.1016/j.pupt.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Rutgers SR, Timens W, Kaufmann HF, et al. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J. 2000;15:109–115. doi: 10.1183/09031936.00.15110900. [DOI] [PubMed] [Google Scholar]
- 18.Barnes NC, Qiu YS, Pavord ID, et al. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;173:736–743. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- 19.Hattotuwa KL, Gizycki MJ, Ansari TW, et al. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–1596. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- 20.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 21.Rabe KF. Treating COPD--the TORCH trial, P values, and the Dodo. N Engl J Med. 2007;356:851–854. doi: 10.1056/NEJMe068307. [DOI] [PubMed] [Google Scholar]
- 22.Gartlehner G, Hansen RA, Carson SS, et al. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes. Ann Fam Med. 2006;4:253–262. doi: 10.1370/afm.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;25:1084–1106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- 24.Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med. 2005;5:3. doi: 10.1186/1471-2466-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD. A critical review. Chest. 1997;111:732–743. doi: 10.1378/chest.111.3.732. [DOI] [PubMed] [Google Scholar]
- 26.Franciosi LG, Page CP, Celli BR, et al. Markers of exacerbation severity in chronic obstructive pulmonary disease. Respir Res. 2006;7:74. doi: 10.1186/1465-9921-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldonyte R, Eriksson S, Piitulainen E, et al. Analysis of systemic biomarkers in COPD patients. Copd. 2004;1:155–164. doi: 10.1081/copd-120030828. [DOI] [PubMed] [Google Scholar]
- 28.Rabe KF, Hurd S, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of COPD - 2006 Update. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 29.Barber R, Baillie GS, Bergmann R, et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- 30.Rabe KF, Bateman ED, O'Donnell D, et al. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 31.Rennard SI, Schachter N, Strek M, et al. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;129:56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Grootendorst DC, Gauw SA, Sterk PJ. Treatment with PDE4 inhibitor roflumilast reduces sputum neutrophil and eosinophil numbers in patients with COPD. Proc Am Thoracic Soc. 2005;2:A543. doi: 10.1136/thx.2006.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamble E, Grootendorst DC, Brightling CE, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:976–982. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- 34.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamontagne S, Meadows E, Luk P, et al. Localization of phosphodiesterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain Res. 2001;920:84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- 36.Robichaud A, Stamatiou PB, Jin SL, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 39.Meja KK, Seldon PM, Nasuhara Y, et al. p38 MAP kinase and MKK-1 co-operate in the generation of GM-CSF from LPS-stimulated human monocytes by an NF-kappa B-independent mechanism. Br J Pharmacol. 2000;131:1143–1153. doi: 10.1038/sj.bjp.0703684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 41.Lee JC, Kassis S, Kumar S, et al. p38 mitogen-activated protein kinase inhibitors--mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 42.Haeryfar SM, Hoskin DW. Selective pharmacological inhibitors reveal differences between Thy-1- and T cell receptor-mediated signal transduction in mouse T lymphocytes. Int Immunopharmacol. 2001;1:689–698. doi: 10.1016/s1567-5769(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Salojin KV, Gao JX, et al. p38 mitogen-activated protein kinase mediates signal integration of TCR/CD28 costimulation in primary murine T cells. J Immunol. 1999;162:3819–3829. [PubMed] [Google Scholar]
- 44.Rincon M, Enslen H, Raingeaud J, et al. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. Embo J. 1998;17:2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitti E, Iakovleva T, Brook M, et al. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol. 2006;26:2399–2407. doi: 10.1128/MCB.26.6.2399-2407.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briata P, Forcales SV, Ponassi M, et al. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol Cell. 2005;20:891–903. doi: 10.1016/j.molcel.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Dean JL, Sully G, Clark AR, et al. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Parasrampuria DA, de Boer P, Desai-Krieger D, et al. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657, a specific p38 mitogen-activated protein kinase inhibitor: a first-in-human study. J Clin Pharmacol. 2003;43:406–413. doi: 10.1177/0091270002250615. [DOI] [PubMed] [Google Scholar]
- 49.Underwood DC, Osborn RR, Bochnowicz S, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L895–L902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- 50.Haddad EB, Birrell M, McCluskie K, et al. Role of p38 MAP kinase in LPS-induced airway inflammation in the rat. Br J Pharmacol. 2001;132:1715–1724. doi: 10.1038/sj.bjp.0704022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuoka H, Arai T, Mori M, et al. A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;283:L103–L112. doi: 10.1152/ajplung.00187.2001. [DOI] [PubMed] [Google Scholar]
- 52.Underwood DC, Osborn RR, Kotzer CJ, et al. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther. 2000;293:281–288. [PubMed] [Google Scholar]
- 53.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 54.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 55.Delhase M, Li N, Karin M. Kinase regulation in inflammatory response. Nature. 2000;406:367–368. doi: 10.1038/35019154. [DOI] [PubMed] [Google Scholar]
- 56.Castro AC, Dang LC, Soucy F, et al. Novel IKK inhibitors: beta-carbolines. Bioorg Med Chem Lett. 2003;13:2419–2422. doi: 10.1016/s0960-894x(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 57.Jazrawi E, Cosio BG, Barnes PJ, et al. Inhibition of IKK2 and JNK differentially regulates GM-CSF and IL-8 release in epithelial cells and alveolar macrophages. Am J Respir Crit Care Med. 2003;167:A798. [Google Scholar]
- 58.Nasuhara Y, Adcock IM, Catley M, et al. Differential IkappaB kinase activation and IkappaBalpha degradation by interleukin-1beta and tumor necrosis factor-alpha in human U937 monocytic cells. Evidence for additional regulatory steps in kappaB-dependent transcription. J Biol Chem. 1999;274:19965–19972. doi: 10.1074/jbc.274.28.19965. [DOI] [PubMed] [Google Scholar]
- 59.Auphan N, DiDonato JA, Rosette C, et al. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 60.Scheinman RI, Cogswell PC, Lofquist AK, et al. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 61.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 62.Holmes-McNary M, Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 63.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezeamuzie CI, Sukumaran J, Philips E. Effect of wortmannin on human eosinophil responses in vitro and on bronchial inflammation and airway hyperresponsiveness in Guinea pigs in vivo. Am J Respir Crit Care Med. 2001;164:1633–1639. doi: 10.1164/ajrccm.164.9.2101104. [DOI] [PubMed] [Google Scholar]
- 65.Duan W, Aguinaldo DatilesAM, Leung BP, et al. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol. 2005;5:495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Lee KS, Kim SR, Park SJ, et al. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) reduces vascular endothelial growth factor expression in allergen-induced airway inflammation. Mol Pharmacol. 2006;69:1829–1839. doi: 10.1124/mol.106.022228. [DOI] [PubMed] [Google Scholar]
- 67.Kwak YG, Song CH, Yi HK, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–1092. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas MJ, Smith A, Head DH, et al. Airway inflammation: chemokine-induced neutrophilia and the class I phosphoinositide 3-kinases. Eur J Immunol. 2005;35:1283–1291. doi: 10.1002/eji.200425634. [DOI] [PubMed] [Google Scholar]
- 69.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 70.Ko HM, Kang JH, Choi JH, et al. Platelet-activating factor induces matrix metalloproteinase-9 expression through Ca(2+)- or PI3K–dependent signaling pathway in a human vascular endothelial cell line. FEBS Lett. 2005;579:6451–6458. doi: 10.1016/j.febslet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 71.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 72.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ito K, Caramori G, Adcock IM. Therapeutic potential of PI3K inhibitors in inflammatory respiratory disease. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.111674. [DOI] [PubMed] [Google Scholar]
- 74.De Boer WI, Alagappan VK, Sharma HS. Molecular mechanisms in chronic obstructive pulmonary disease: potential targets for therapy. Cell Biochem Biophys. 2007 doi: 10.1385/cbb:47:1:131. [DOI] [PubMed] [Google Scholar]
- 75.Chung KF. Cytokines as targets in chronic obstructive pulmonary disease. Curr Drug Targets. 2006;7:675–681. doi: 10.2174/138945006777435263. [DOI] [PubMed] [Google Scholar]
- 76.de Boer WI. Perspectives for cytokine antagonist therapy in COPD. Drug Discov Today. 2005;10:93–106. doi: 10.1016/S1359-6446(04)03300-8. [DOI] [PubMed] [Google Scholar]
- 77.Keatings VM, Collins PD, Scott DM, et al. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 78.Vernooy JH, Kucukaycan M, Jacobs JA, et al. Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am J Respir Crit Care Med. 2002;166:1218–1224. doi: 10.1164/rccm.2202023. [DOI] [PubMed] [Google Scholar]
- 79.Takabatake N, Nakamura H, Abe S, et al. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 80.Rennard SI, Fogarty C, Kelsen S, The safety, efficacy of infliximab in moderate to severe chronic obstructive pulmonary diseaseAm, et al. J. Respir Crit Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 81.van der Vaart H, Koeter GH, Postma DS, et al. First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:465–469. doi: 10.1164/rccm.200501-147OC. [DOI] [PubMed] [Google Scholar]
- 82.Antoniu SA. Infliximab for chronic obstructive pulmonary disease: towards a more specific inflammation targeting? Expert Opin Investig Drugs. 2006;15:181–184. doi: 10.1517/13543784.15.2.181. [DOI] [PubMed] [Google Scholar]
- 83.Erin EM, Leaker BR, Nicholson GC, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006;174:753–762. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 84.Berry MA, Hargadon B, Shelley M, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 85.Howarth PH, Babu KS, Arshad HS, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60:1012–1018. doi: 10.1136/thx.2005.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruce C, Thomas PS. The effect of marimastat, a metalloprotease inhibitor, on allergen-induced asthmatic hyper-reactivity. Toxicol Appl Pharmacol. 2005;205:126–132. doi: 10.1016/j.taap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Yamamoto C, Yoneda T, Yoshikawa M, et al. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112:505–510. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- 88.Yang XD, Corvalan JR, Wang P, et al. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease states. J Leukoc Biol. 1999;66:401–410. doi: 10.1002/jlb.66.3.401. [DOI] [PubMed] [Google Scholar]
- 89.Hill AT, Bayley D, Stockley RA. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am J Respir Crit Care Med. 1999;160:893–898. doi: 10.1164/ajrccm.160.3.9901091. [DOI] [PubMed] [Google Scholar]
- 90.Beeh KM, Kornmann O, Buhl R, et al. Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B4. Chest. 2003;123:1240–1247. doi: 10.1378/chest.123.4.1240. [DOI] [PubMed] [Google Scholar]
- 91.Nicholson GC, Tennant RC, Carpenter DC, et al. A novel flow cytometric assay of human whole blood neutrophil and monocyte CD11b levels: upregulation by chemokines is related to receptor expression, comparison with neutrophil shape change, and effects of a chemokine receptor (CXCR2) antagonist. Pulm Pharmacol Ther. 2007;20:52–59. doi: 10.1016/j.pupt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Liao L, Ning Q, Li Y, et al. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res. 2006;60:299–303. doi: 10.1203/01.pdr.0000233058.08200.d6. [DOI] [PubMed] [Google Scholar]
- 93.Auten RL, Richardson RM, White JR, et al. Nonpeptide CXCR2 antagonist prevents neutrophil accumulation in hyperoxia-exposed newborn rats. J Pharmacol Exp Ther. 2001;299:90–95. [PubMed] [Google Scholar]
- 94.De Boer WI. Cytokines and therapy in COPD: a promising combination? Chest. 2002;121:209S–218S. doi: 10.1378/chest.121.5_suppl.209s. [DOI] [PubMed] [Google Scholar]
- 95.Baxter A, Cooper A, Kinchin E, et al. Hit-to-Lead studies: the discovery of potent, orally bioavailable thiazolopyrimidine CXCR2 receptor antagonists. Bioorg Med Chem Lett. 2006;16:960–963. doi: 10.1016/j.bmcl.2005.10.091. [DOI] [PubMed] [Google Scholar]
- 96.Dwyer MP, Yu Y, Chao J, et al. Discovery of 2-Hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): A Potent, Orally Bioavailable CXCR2/CXCR1 Receptor Antagonist. J Med Chem. 2006;49:7603–7606. doi: 10.1021/jm0609622. [DOI] [PubMed] [Google Scholar]
- 97.Ho KK, Auld DS, Bohnstedt AC, et al. Imidazolylpyrimidine based CXCR2 chemokine receptor antagonists. Bioorg Med Chem Lett. 2006;16:2724–2728. doi: 10.1016/j.bmcl.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 98.Capelli A, Di Stefano A, Gnemmi I, et al. Increased MCP-1 and MIP-1beta in bronchoalveolar lavage fluid of chronic bronchitics. Eur Respir J. 1999;14:160–165. doi: 10.1034/j.1399-3003.1999.14a27.x. [DOI] [PubMed] [Google Scholar]
- 99.de Boer WI, Sont JK, van Schadewijk A, et al. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J Pathol. 2000;190:619–626. doi: 10.1002/(SICI)1096-9896(200004)190:5<619::AID-PATH555>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 100.Traves SL, Culpitt SV, Russell RE, et al. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax. 2002;57:590–595. doi: 10.1136/thorax.57.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ko FW, Lau CY, Leung TF, et al. Exhaled breath condensate levels of 8-isoprostane, growth related oncogene alpha and monocyte chemoattractant protein-1 in patients with chronic obstructive pulmonary disease. Respir Med. 2006;100:630–638. doi: 10.1016/j.rmed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 102.Haringman JJ, Gerlag DM, Smeets TJ, et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2387–2392. doi: 10.1002/art.21975. [DOI] [PubMed] [Google Scholar]
- 103.Butora G, Morriello GJ, Kothandaraman S, et al. 4-Amino-2-alkyl-butyramides as small molecule CCR2 antagonists with favorable pharmacokinetic properties. Bioorg Med Chem Lett. 2006;16:4715–4722. doi: 10.1016/j.bmcl.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 104.Pasternak A, Marino D, Vicario PP, et al. Novel, orally bioavailable gamma-aminoamide CC chemokine receptor 2 (CCR2) antagonists. J Med Chem. 2006;49:4801–4804. doi: 10.1021/jm060439n. [DOI] [PubMed] [Google Scholar]
- 105.Brodmerkel CM, Huber R, Covington M, et al. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005;175:5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- 106.Ferry G, Lonchampt M, Pennel L, et al. Activation of MMP-9 by neutrophil elastase in an in vivo model of acute lung injury. FEBS Lett. 1997;402:111–115. doi: 10.1016/s0014-5793(96)01508-6. [DOI] [PubMed] [Google Scholar]
- 107.Okada Y, Watanabe S, Nakanishi I, et al. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988;229:157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- 108.Chughtai B, O'Riordan TG. Potential role of inhibitors of neutrophil elastase in treating diseases of the airway. J Aerosol Med. 2004;17:289–298. doi: 10.1089/jam.2004.17.289. [DOI] [PubMed] [Google Scholar]
- 109.Shapiro SD, Goldstein NM, Houghton AM, et al. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacIvor DM, Shapiro SD, Pham CT, et al. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–4293. [PubMed] [Google Scholar]
- 111.Loike JD, Sodeik B, Cao L, et al. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci U S A. 1991;88:1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pemberton PA, Kobayashi D, Wilk BJ, et al. Inhaled recombinant alpha 1-antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. Copd. 2006;3:101–108. doi: 10.1080/15412550600651248. [DOI] [PubMed] [Google Scholar]
- 113.Churg A, Wang RD, Xie C, et al. alpha-1-Antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2003;168:199–207. doi: 10.1164/rccm.200302-203OC. [DOI] [PubMed] [Google Scholar]
- 114.Wright JL, Farmer SG, Churg A. Synthetic serine elastase inhibitor reduces cigarette smoke-induced emphysema in guinea pigs. Am J Respir Crit Care Med. 2002;166:954–960. doi: 10.1164/rccm.200202-098OC. [DOI] [PubMed] [Google Scholar]
- 115.Edwards PD, Andisik DW, Bryant CA, et al. Discovery and biological activity of orally active peptidyl trifluoromethyl ketone inhibitors of human neutrophil elastase. J Med Chem. 1997;40:1876–1885. doi: 10.1021/jm960819g. [DOI] [PubMed] [Google Scholar]
- 116.Vincent SH, Painter SK, Luffer-Atlas D, et al. Orally active inhibitors of human leukocyte elastase. II. Disposition of L-694,458 in rats and rhesus monkeys. Drug Metab Dispos. 1997;25:932–939. [PubMed] [Google Scholar]
- 117.Finlay GA, Russell KJ, McMahon KJ, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax. 1997;52:502–506. doi: 10.1136/thx.52.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohnishi K, Takagi M, Kurokawa Y, et al. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest. 1998;78:1077–1087. [PubMed] [Google Scholar]
- 119.Cataldo D, Munaut C, Noel A, et al. MMP-2- and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 2000;123:259–267. doi: 10.1159/000024452. [DOI] [PubMed] [Google Scholar]
- 120.Vignola AM, Riccobono L, Mirabella A, et al. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998;158:1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- 121.Finlay GA, O'Driscoll LR, Russell KJ, et al. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997;156:240–247. doi: 10.1164/ajrccm.156.1.9612018. [DOI] [PubMed] [Google Scholar]
- 122.Bracke K, Cataldo D, Maes T, et al. Matrix metalloproteinase-12 and cathepsin D expression in pulmonary macrophages and dendritic cells of cigarette smoke-exposed mice. Int Arch Allergy Immunol. 2005;138:169–179. doi: 10.1159/000088439. [DOI] [PubMed] [Google Scholar]
- 123.Demedts IK, Morel-Montero A, Lebecque S, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61:196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hautamaki RD, Kobayashi DK, Senior RM, et al. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 125.Nenan S, Lagente V, Planquois JM, et al. Metalloelastase (MMP-12) induced inflammatory response in mice airways: effects of dexamethasone, rolipram and marimastat. Eur J Pharmacol. 2007;559:75–81. doi: 10.1016/j.ejphar.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 126.Belvisi MG, Bottomley KM. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): a therapeutic role for inhibitors of MMPs? Inflamm Res. 2003;52:95–100. doi: 10.1007/s000110300020. [DOI] [PubMed] [Google Scholar]
- 127.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 128.Ogryzko VV, Schiltz RL, Russanova V, et al. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 129.Ito K, Lim S, Caramori G, et al. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. Faseb J. 2001;15:1110–1112. [PubMed] [Google Scholar]
- 130.Marwick JA, Kirkham PA, Stevenson CS, et al. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 131.Moodie FM, Marwick JA, Anderson CS, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. Faseb J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 132.Rahman I, Gilmour PS, Jimenez LA, et al. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002;234–235:239–248. [PubMed] [Google Scholar]
- 133.Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27:413–426. doi: 10.1183/09031936.06.00125404. [DOI] [PubMed] [Google Scholar]
- 134.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 135.Culpitt SV, Rogers DF, Shah P, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- 136.Cosio BG, Tsaprouni L, Ito K, et al. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med. 2004;200:689–695. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takanashi S, Hasegawa Y, Kanehira Y, et al. Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J. 1999;14:309–314. doi: 10.1034/j.1399-3003.1999.14b12.x. [DOI] [PubMed] [Google Scholar]