Abstract
Infection with the obligate intracellular bacterium Chlamydia trachomatis, is controlled primarily by IFNγ and T helper type 1 (TH1) immunity. In this study we used cells from a Chlamydia specific CD4+ TCR transgenic mouse to assess the role of IFNγ in development of TH1 immunity. We show that secretion of host IFNγ or the ability of host cells to respond to secreted IFNγ is not required to initiate a TH1 immune response. Additionally, we found that antigen specific CD4+ cells that were pre-skewed towards TH1 confer protection, whereas cells pre-skewed towards T helper type 2 (TH2) cause a previously unreported exacerbation of disease leading to higher bacterial load. Chlamydia specific TH1 cells transferred into an IFNγ−/− recipient mouse demonstrate protective effects, but the same cells exacerbate bacterial burden when transferred into IFNγR−/− mice. Thus, we demonstrate that the secretion of IFNγ is necessary for protection against C. trachomatis and that in the absence of host cell IFNγR expression, both TH1 and TH2 cells lead to increased burden of C. trachomatis.
Introduction
Chlamydiae are obligate intracellular bacteria and the etiologic agent of several major human diseases. Worldwide, Chlamydia trachomatis is the most common cause of bacterial sexually transmitted disease (1). In many cases Chlamydia infection goes undetected by the host and, if left untreated, infection of women can lead to inflammatory sequelae such as pelvic inflammatory disease, ectopic pregnancy, or sterility. In addition, C. trachomatis infection of the conjunctiva induces trachoma, the leading cause of preventable blindness (1). The pathology associated with infection is caused primarily by the inflammatory response and thus a better understanding of protective versus deleterious inflammatory mediators is necessary. A thorough mechanistic understanding of Chlamydia immunity is also critical for development of an effective vaccine against this organism.
Adaptive immune protection against C. trachomatis can be demonstrated by transfer of either CD4+ or CD8+ T cells into infected lymphopenic mice (reviewed in (2)). This protection has been shown to be dependant upon secretion of IFNγ by these populations. Although the role IFNγ plays in conferring protection against C. trachomatis has been described, the role of IFNγ in priming antigen specific immunity has not been explored. Similar to lymphopenic hosts, both IFNγ−/− and IFNγR−/− mice are unable to control C. trachomatis infection (3, 4) and TH1 cells, but not TH2 cells, are protective upon transfer into recipient mice (5, 6). TH1 cell-mediated protection is thought to occur through IFNγ secretion. IFNγ can act either indirectly to augment host immunity or directly on epithelial cells, restricting Chlamydia growth through a variety of mechanisms including IDO induction, nitric oxide production, and p47 GTPase-driven effects. (7).
IFNγ is key to initiating, maintaining, and permanently establishing TH1 cell identity, and both IFNγ production and IFNγR expression are required for development of TH1-mediated immunity to C. trachomatis (3, 8, 9). Moreover the re-orientation and ligation of the IFNγ receptor on naïve T cells has been thought to be an early step required for TH1 polarization (10, 11). However, this is in direct opposition to findings by Haring et. al which demonstrated the development of Listeria-specific TH1 cells in IFNγ receptor deficient mice (12). In this report, we elucidate how IFNγ responses by host cells as well as IFNγ production by immune cells impact TH1 polarization and protection against C. trachomatis.
Contrary to previous reports, we found that the development of Chlamydia specific TH1 immunity can occur whether or not host cells express or respond to IFNγ. However, for pre-skewed cells to confer protection, the recipient host must be capable of responding to IFNγ. TH2 skewed cells induced an exacerbation of disease which was also seen when TH1 cells were transferred into a IFNγR−/− mouse. These results indicate that IFNγ is the key protective cytokine produced by TH1 cells and that other accessory TH1 immune mediators are detrimental to host immunity.
Materials and Methods
Mice
C57BL/6, B6.PL-Thy1a(CD90.1 congenic), B6.129S7-IFNgtm1Agt (IFNγ−/−), and B6.129S7-IFNgr1tm1Ag t(IFNγ receptor 1−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME). NR1 mice were described previously (13) and are maintained and cared for within the Harvard Medical School Center for Animal Resources and Comparative Medicine. All experiments were approved by Harvard's Institutional Animal Care and Use Committee.
Growth isolation and detection of bacteria
C. trachomatis serovar L2 (434/Bu) was propagated within McCoy cell monolayers grown in Eagle’s MEM (Invitrogen, Grand Island NY) supplemented with 10% FCS, 1.5 g/L sodium bicarbonate, 0.1M nonessential amino acids, and 1 mM sodium pyruvate. Infected monolayers were lifted and sonicated to disrupt the inclusion. EBs were purified by density gradient centrifugation as described (14). Aliquots were stored at −70 C in a medium containing 250 mM sucrose, 10 mM sodium phosphate, and 5 mM L-glutamic acid (SPG) and thawed immediately prior to use.
Flow cytometry
Tissues were mechanically disaggregated and immediately stained for activation markers or stimulated for five hours with 50 ng/ml PMA (Alexis Biochemical) and 500 ng/ml ionomycin (Calbiochem) in the presence of brefeldin A (GolgiStop, BD Biosciences) to determine intra-cellular cytokine staining. Cells were pre-incubated with anti-FcRγ (Bio X-Cell) before staining with anti-CD4 Pacific Blue (Biolegend) and anti-CD90.1 peridinin chlorophyll-a protein (BD Bioscience). For activation marker analysis we examined anti-CD44 phycoerythrin-cychrome 7 (Biolegend) anti-CD62L allophycocyanin-Alexa 750 (Ebioscience) and anti-CD25 allophycocyanin (BD Bioscience). For intracellular staining the following antibodies were used: anti-IFNγ phycoerythrin or Alexa 700, anti-IL2 phycoerythrin or allophycocyanin, anti-IL4 phycoerythrin or allophycocyanin, anti-IL10 phycoerythrin, anti-IL17 phycoerythrin or Alexa 647, and anti-TNFα phycoerythrin or phycoerythrin-cychrome 7 (BD Biosciences). Cells were permeablized with the Cytofix/Cytoperm Plus kit according to manufacturers instructions (BD Bioscience). Data were collected on a modified FACSCalibur (Cytek Development) or an LSRII (BD Bioscience) and analyzed using Flow Jo (Tree Star Industries).
Transfer of NR1 cells, infection of mice, and preparation of tissue
Prior to transfer, NR1 cells were isolated from peripheral lymphoid tissues and labeled with 5 µM CFSE (CFDA,SE, Invitrogen) in serum free media. Recipient mice were injected intravenously with either 106 or 107 C. trachomatis (Cta1133–152 )specific CD4+ T cells (13). To infect the genital tract, mice were treated with 2.5 mg of medroxyprogestrone acetate subcutaneously and then infected one week later in the uterine horns with 106 IFU of C. trachomatis L2. Five days post infection lymph nodes, spleen, and uterus were collected. The uterus was digested with 1 mg/ml of type XI collagenase (Sigma) and 50 Kunitz/ml of DNase (Sigma) for 45 min at 37 C. Single cell suspensions were prepared for staining via mechanical disaggregation.
Skewing of NR1 cells
CD4+ T cells were purified from NR1 mice using a mouse CD4 negative isolation kit (Dynal, Invitrogen) per the manufacturers directions. The T cells were cultured in RPMI-1640 (Invitrogen) supplemented with 10% FCS, L-glutamine, HEPES, 50 µM 2-mercaptoethanol, 50 U/ml penicillin, and 50 ug/ml streptomycin. To stimulate the T cells, irradiated feeder splenocytes were pulsed with 5 µM of Cta1133–152 peptide and cocultured with the CD4-enriched NR1 cells at a stimulator to T cell ratio of 4:1. The following conditions were used for polarization: for TH1 polarization the T cells were incubated with 10 ng/ml of IL-12 (Peprotech) and 10 µg/ml of anti-IL4 (Biolegend); for TH2 polarization the T cells were incubated with 10 ng/ml of IL-4 (Peprotech), 10 µg/ml of anti-IL12/23 (Biolegend), and 10 µg/ml of anti-IFNγ (Biolegend); for TH17 polarization the T cells were incubated with 30 ng/ml of IL-6 (Peprotech), 10 ng/ml of TGFβ (Peprotech), and 10µg/ml of anti-IFNγ (Biolegend); for non-specific polarization TH0, T cells were incubated with peptide alone. Cells were stimulated for five to seven days prior to CFSE labeling and transfer into naïve CD90.2+ host mice.
In vivo activation and protection assay
NR1 cells were transferred into mice and twenty-four hours later mice were either challenged with 107 IFU of C. trachomatis L2 intravenously or 106 IFU instilled in the uterus. For activation experiments, tissues were harvested five days after infection whereas for protection experiments spleens were harvested three days after infection. To assess the protective capacity of the skewed cells, spleens from infected mice were homogenized, sonicated, diluted, and applied to McCoy cell monolayers. Inclusions were counted by immunofluorescence microscopy 30 hours after infection.
In vitro protection assay
NR1 cells were skewed as detailed above for 5 days and centrifuged. The supernatants were applied to semi-confluent monolayers of SV-40 large T antigen immortalized C57BL/6 MEF cells (MEF-TAg - a kind gift from Richard Flavell) for 18 hours. The supernatants were then removed and the tissue culture wells were washed with SPG. C. trachomatis was applied at an MOI of 0.5:1, or 1000 IFU/well, and centrifuged at 1928 × g for 1 h at 37 C. After centrifugation the SPG was replaced with DMEM medium. After 30 h cells were fixed and stained to enumerate inclusions.
Statistical analysis
All groups were evaluated for statistical significance through the use of unpaired two-tailed t tests. Where it appeared necessary to highlight significant differences between data points, the level of significance is depicted as * = p<0.05, ** = p<0.01, and *** = p<0.005.
Results
Chlamydia specific TH1 immunity does not require host IFNγ receptor expression or the ability of host cells to produce IFNγ
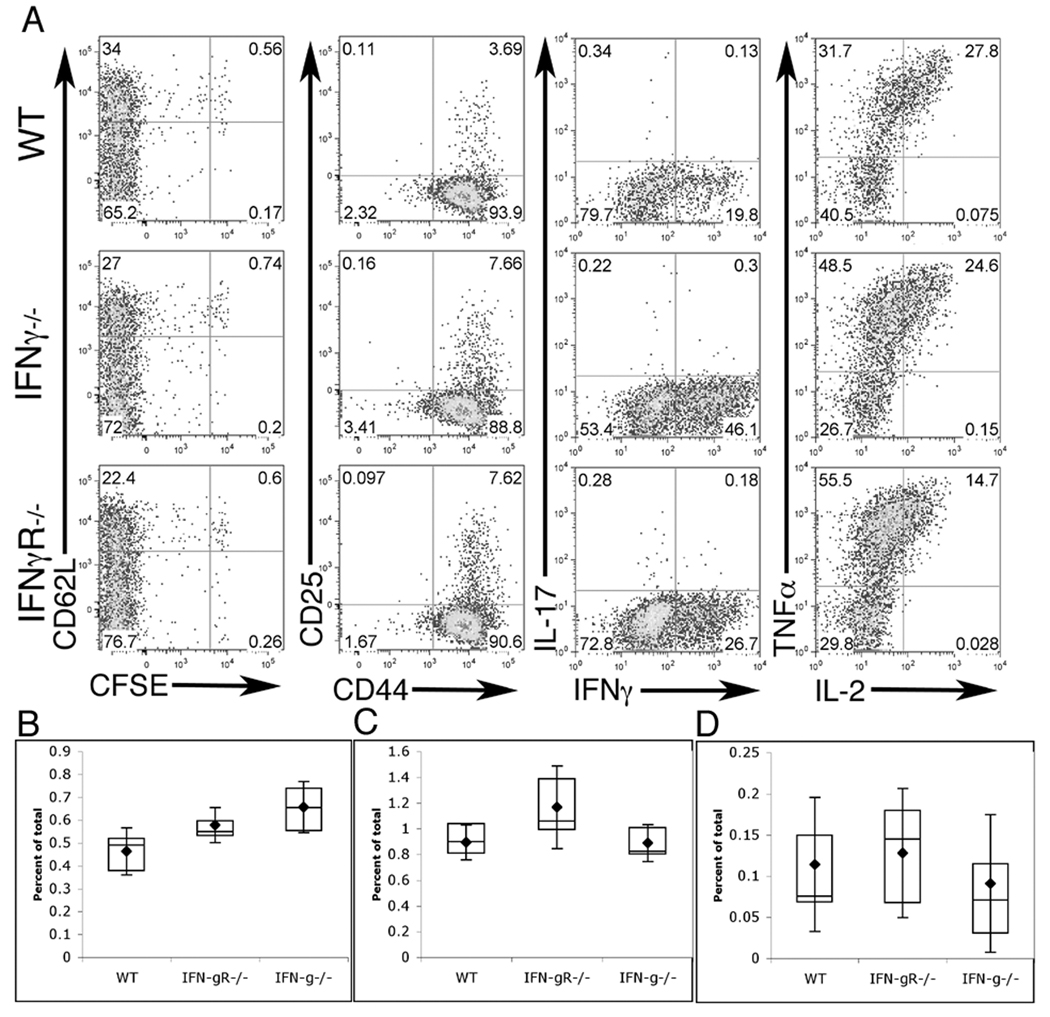
IFNγ is the hallmark cytokine of the TH1-type immune response. Clearance of C. trachomatis pivotally rests on the ability of the host to mount a TH1 response. Additionally, IFNγ signaling in the host antigen presenting cells is required to facilitate T cell skewing towards TH1 (15). Therefore, we tested both the impact of host-derived IFNγ production as well as the requirement for host responsiveness to IFNγ in the generation of a Chlamydia-specific T cell response. To follow CD4+ T cell development, naive CFSE labeled CD90.1+ Chlamydia-specific NR1 TCR transgenic cells were transferred into CD90.2+ mice that were wild type, IFNγ−/−, or IFNγR−/−. Mice were then challenged in the uterus with 106 IFU of C. trachomatis L2 and five days later the spleen, draining (iliac) lymph nodes, and uterus were examined for NR1 cell activation and cytokine secretion. As shown in Figure 1A, regardless of whether the recipient animal could produce or respond to IFNγ, the antigen specific NR1 cells were able to proliferate, down regulate CD62L and up regulate CD25 and CD44. Moreover, these cells were able to produce robust amounts of IFNγ, IL-2 and TNFα. However, the NR1 cells did not produce a significant amount of IL-17, IL-4, or IL-10 (Figure 1A and data not shown). NR1 cells accumulated to a greater extent in the draining lymph nodes of mice deficient in IFNγ and IFNγR (Figure 1B). However, cells accumulated to an equivalent extent in the spleen and uterus (Figure 1 C,D). These data demonstrate that in order to mount a TH1 immune response, the host need not produce or respond to IFNγ.
Figure 1. CD4+ T cells specific for C. trachomatis can be stimulated in IFNγ−/− or IFNγR−/− mice.
IFNγ−/−, IFNγR−/−, or WT C57BL/6 mice were injected with naïve NR1 cells and challenged the following day with C. trachomatis. On day 5 post-infection flow cytometry was used to analyze cells from the uterus, draining lymph node, and spleen (A). Cells were assessed for activation markers (left two panels) or restimulated for 5 hours with PMA/ionomoycin and assessed for intra-cellular cytokine staining (right two panels). Flow cytometry data were first gated on live, CD4+, CD90.1+ cells and are representative of three independent experiments. Percentage of total live cells was calculated for draining lymph node (B), spleen (C), and uterus (D). Statistical analysis performed via Student’s T test, * = p<0.05, ** = p<0.01.
Only TH1 cells accumulate in mice infected with C. trachomatis
To explore the impact of pre-skewing of NR1 transgenic cells, we preactivated NR1 cells under conditions designed to skew the cells towards a TH1, TH2, TH17, or TH0 phenotype. After 7 days of stimulation the cells were assessed for cytokine production to confirm their skewed phenotype (Figure 2A, B). For each phenotype, a total of 106 skewed cells were transferred into recipient mice. The following day the recipient mice were challenged in the uterus with 106 IFU of C. trachomatis. Five days after challenge we found that only TH1 cells were able to accumulate in the uterus, draining lymph node, and spleen (Figure 2C, D, E). Despite differences in cellular accumulation, all cells showed a similar level of CFSE dilution and activation marker expression (data not shown). However, following transfer and subsequent infection with C. trachomatis, IFNγ was being produced by all skewed groups to a similar extent, despite the cytokine secretion pattern observed prior to transfer (Fig 2F). Thus, only NR1 cells which are polarized towards the IFNγ-secreting TH1 phenotype are able to persist following infection with C. trachomatis.
Figure 2. TH1 cells preferentially survive following C. trachomatis infection.
CD4+ cells purified from naïve NR1 mice were stimulated for 5–7 d in conditions that left the cells unskewed or which skewed the cells towards TH1, TH2, or TH17. Cells were assessed for their cytokine profile via simultaneous stain (A - polyvariate plot) or via singular stains with phycoerythrin conjugated antibodies (B). Shown is mean percentage of NR1 cells or Mean Fluorescence intensity (MFI) in bold with standard deviation in italics. Skewed cells were injected into naïve mice prior to infection with C. trachomatis. Five days post-infection, the percentage of NR1 cells that were skewed was assessed in the uterus (C), lymph node (D), and spleen (E). Cells were restimulated for five hours with PMA/Ionomycin and stained for intracellular cytokines (F). The gates shown were pre-set on CD4+, CD90.1+ cells, and data are representative of four independent experiments. Statistical analysis of the box-and-whisker plots was conducted using a Student’s T test, * = p<0.05, ** = p<0.01, and *** = p<0.005.
TH1 cells confer protection against C. trachomatis
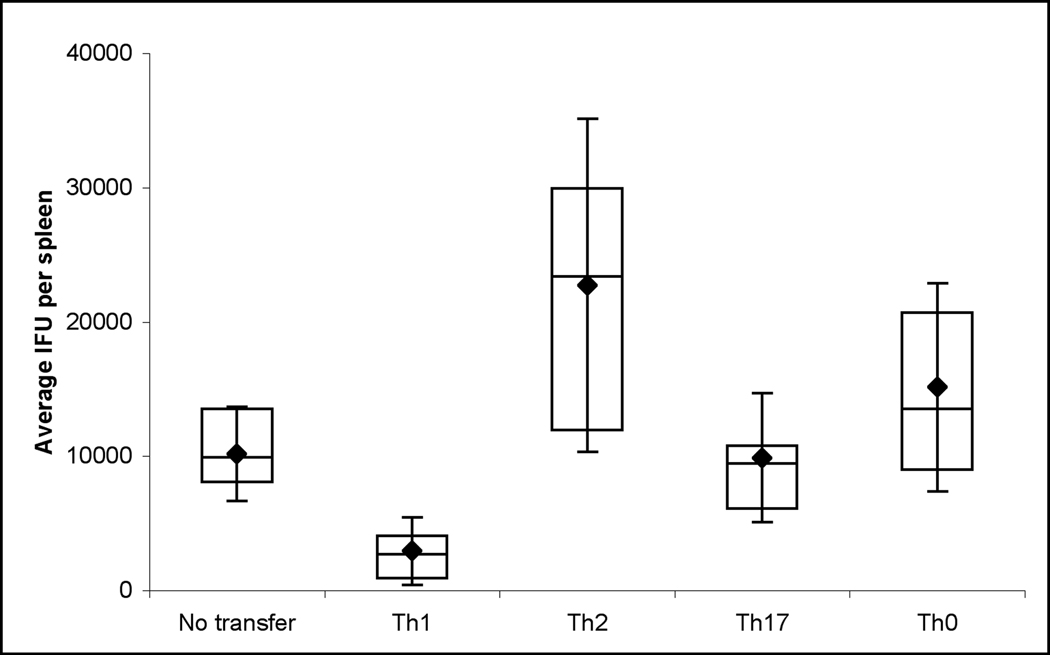
At early time points after infection, the clearance of C. trachomatis is primarily mediated by the innate immune response. However, if pre-activated antigen specific cells that are secreting IFNγ are transferred into naive mice, early protection can also be observed. For example, others have reported that the transfer of TH1 clones into recipient mice confers protection against Chlamydia muridarum (6, 16). In order to compare the protective effect of unskewed cells with cells skewed towards TH1, TH2, TH17, we adoptively transferred 107 NR1 cells of each phenotype into naive B6 mice. Mice were then challenged intravenously with 107 IFU of C. trachomatis L2 and assessed three days later for the number of IFU in the spleen. Consistent with published results (16), TH1 skewed cells are capable of conferring protection (p<0.001) (Figure 3). Interestingly, TH2 cells were found to exacerbate infection. Mice receiving the TH2 cells had 2-fold higher IFU in the spleen than naïve recipients, and ten-fold more IFU than recipients that had received TH1 cells (Figure 3). Mice receiving the TH17 cells had IFU in the spleen comparable to mice that did not receive a transfer, whereas mice receiving the TH0 cells had significantly higher recoverable IFU then naïve mice. Transfer of 107 naïve Chlamydia-specific cells also did not impart protection (data not shown). From these data we concluded that the presence of TH2 skewed Chlamydia antigen specific cells is deleterious to the host’s clearance of C. trachomatis.
Figure 3. TH1 cells protect, while TH2 cells exacerbate infection with C. trachomatis.
Skewed NR1+ cells were adoptively transferred into mice followed by infection with 107 IFU C. trachomatis i.v.. Three days post infection spleens were assessed for recoverable IFU. Data is representative of three independent experiments. Statistical analysis of box-and-whisker plots was done via Student’s T test. By pair wise analysis, no transfer vs TH17 was not significant, no transfer vs TH0 = p<0.05, all other groups were significant greater then p < 0.01.
Response to IFNγ, but not production of endogenous IFNγ, is required for protective immunity
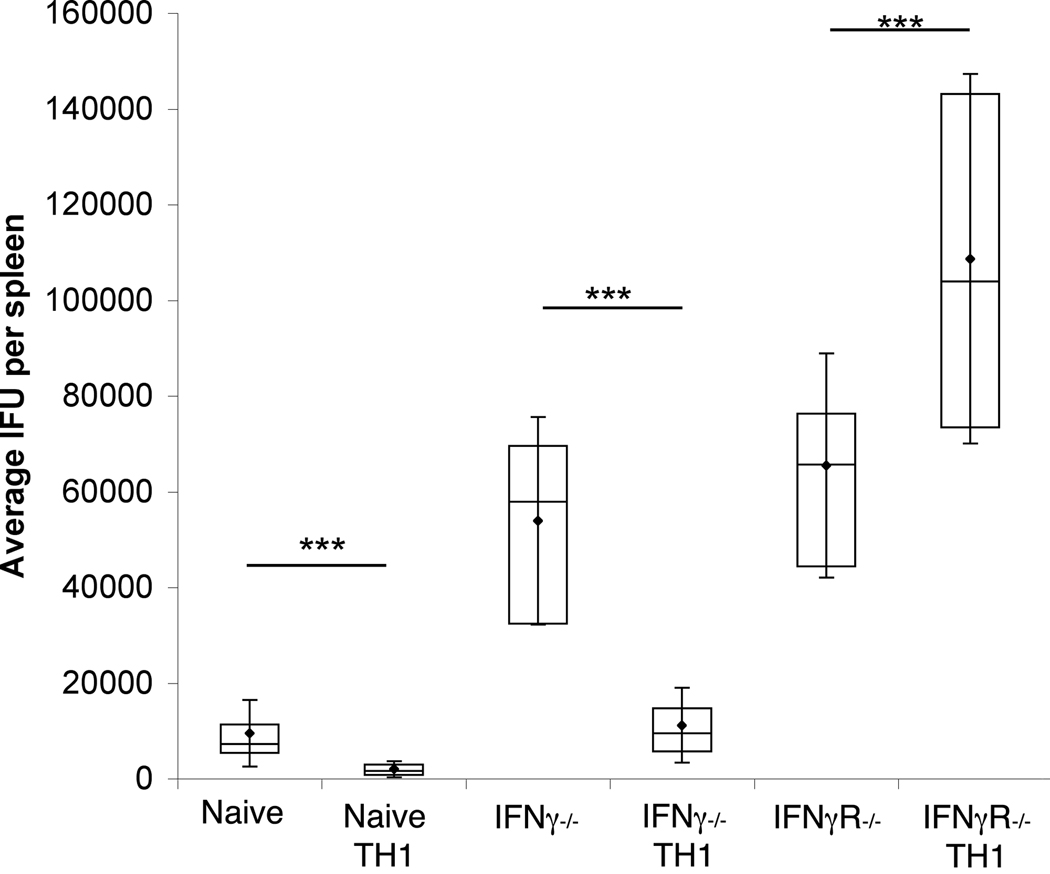
One possible mechanism by which TH1 cells may protect against C. trachomatis infection is by inducing endogenous IFNγ production. To test the requirement for host derived IFNγ, in TH1-mediated protective immunity, IFNγ−/− mice were injected with 107 pre-skewed cells and challenged the next day with 107 IFU of C. trachomatis. As shown in figure 4, despite higher bacterial burden in the IFNγ−/− mice, TH1 skewed NR1 cells were able to confer protection. Thus, the protective capacity of TH1 cells does not require endogenous production of IFNγ.
Figure 4. IFNγ receptor expression but not production of endogenous IFNγ is required for host protection.
NR1+ cells skewed toward the TH1 phenotype were transferred into naïve IFNγ−/−, IFNγR−/−, or wild type mice. Mice were then challenged with 107 IFU of C. trachomatis. Three days after infection the spleens were assessed for C. trachomatis IFU. Data are representative of two independent experiments. Statistical analysis of box-and-whisker plots was conducted using a Student’s T test, * = p<0.05, ** = p<0.01, and *** = p<0.005.
To determine whether it was IFNγ alone that was responsible for bacterial clearance, or whether other TH1 mediators were also contributing to the clearance of C. trachomatis, we tested the ability of IFNγR−/− mice to clear infection after receiving TH1 skewed NR1 cells. After transfer of the cells and challenge of the mice with C. trachomatis, we found that IFNγR−/− mice were not only unprotected, but the presence of the NR1 TH1 cells significantly exacerbated bacterial burden when they were unable to produce IFNγ. The lack of IFNγ production by the TH1 cells resulted in nearly two fold higher numbers of recoverable IFU (figure 4). Bacterial burden increase was not due to deficit of TH1 cell infiltration into the uterus as NR1 cells were readily recruited to the site of infection in an IFNγR−/− mouse (data not shown). Thus, the protective effect of NR1 TH1 cells requires that the host be able to respond to IFNγ being produced by the NR1 cells, and moreover, non-IFNγ cytokines produced by the TH1 NR1 appear to be deleterious to host immunity.
Production of IFNγ is directly protective while TH2 and non-gamma TH1 cytokines do not impact innate epithelial immunity
To determine if the TH1 cytokines which exacerbate bacterial burden in the IFNγR−/− mice were having a direct impact on epithelial cell control of Chlamydia infection, we developed an in vitro assay to measure the effects of cytokines on the growth of C. trachomatis within cultured cells. NR1 cells were stimulated and skewed as previously described. After 7 days of culture under conditions that skew to each phenotype, we harvested the supernatant from the skewed cells and applied it to monolayers of C57BL/6 MEF-TAg cells. The following day, the cells were infected with C. trachomatis L2. We found that supernatants from TH1 and TH0 activated NR1 cells were able to enhance epithelial cell control of C. trachomatis infection (Figure 5). In contrast, supernatants from TH2 and TH17 cultures did not enhance epithelial cell control of C. trachomatis, nor did they exacerbate bacterial burden (Figure 5 and data not shown). In addition, the MEF-TAg cell control of C. trachomatis seen following treatment of the cells with the TH1 supernatant is entirely dependent upon IFNγ since neutralization of the IFNγ completely blocked the protective effect of the TH1 supernatant.
Figure 5. Of the cytokines secreted by TH1 cells, IFNγ alone is able to limit C. trachomatis infection in epithelial cells.
NR1+ cells were skewed for 7 days. Supernatants were then collected and applied to B6-Mef-TAg cells overnight. Wells were infected the next day with 1000 IFU of C. trachomatis and incubated for an additional 30 h before enumeration of inclusions.
Discussion
In this study we demonstrate that Chlamydia specific TH1 cells can differentiate in the absence of host production of IFNγ or ability to respond to IFNγ. Furthermore we show that the presence of a pre-skewed TH2 immune response, or a TH1 immune response where the host is unable to respond to IFNγ, causes an increase in bacterial burden. We confirm that IFNγ is the key mediator of the protective TH1 response to C. trachomatis.
Previously it had been shown that protective immunity against Chlamydia infection requires the production of endogenous IFNγ and the ability to respond to this cytokine (3, 8) (reviewed in (7)). Mice deficient in IFNγ generally develop TH2 immunity when challenged with pathogens that would normally engender a TH1 response (17, 18). Therefore, we sought to determine the importance of host-derived IFNγ in the induction of TH1 immunity. We demonstrate that naïve wild type Chlamydia specific CD4+ T cells are primed, proliferate, and secrete TH1 cytokines when transferred into wild type, IFNγ−/−, or IFNγR−/− mice. However, recent models of Listeria monocytogenes infection suggest it is possible that the IFNγ produced by the transferred T cells might act in an autocrine manner to promote TH1 immunity (19). To address this possibility Harring et al. infected IFNγR1/2−/− mice with L. monocytogenes and demonstrated the development of an endogenous TH1 response in the absence of IFNγ signaling (12). Our findings using a C. trachomatis model support the IFNγ-independent nature of TH1 cell development.
Transfer of Chlamydia muridarum specific clonal T cell lines producing either TH1 or TH2 cytokine profiles has previously demonstrated protective capacity by only the TH1 cell line (6, 16). Hawkins et. al. demonstrated that their TH2 clone did not confer protection or accumulate in the genital tract. However they saw equivalent accumulation of TH1 and TH2 C. muridarum specific clones in the draining lymph nodes and other peripheral tissues. In agreement with this, our data suggests that TH2 cells do not protect against Chlamydia infection, moreover we find that they exacerbate bacterial load. We also observed that while TH1 cells accumulate in the genital tract and peripheral lymphoid organs, TH2, TH17, and TH0 cells were eliminated from the host. In addition, we found that a large proportion of the skewed cells which remain in the animals now expressed IFNγ, regardless of how they were skewed prior to transfer. The ability of skewed cells to switch from one T-helper profile to another has been extensively examined and found not to occur once cells are strongly polarized (20). Therefore, we believe the accumulation of IFNγ producing cells in the TH2, TH17, and TH0 populations is an enrichment for those cells which were producing IFNγ prior to adoptive transfer. Chlamydia infection is highly polarizing, eliciting IFNγ secretion from innate (NK, DC) as well as adaptive immune cells. IFNγ signaling of naïve T cells causes a down regulation of IFNγR2 and an up regulation of IL-12Rβ2 (21, 22). Thus, early in activation IFNγ helps reinforce the TH1 bias of the immune response. However, constitutive IFNγ signaling is highly toxic to CD4+ cells (23, 24). As a result, the non-TH1 skewed cells that express IFNγR2 are susceptible to apoptosis (25) (26). Moreover, when expression of IFNγR2 is forced, it confers a reduction in TH1 cytokine secretion (27). When Chlamydia specific TH2 cells were transferred to IFNγ−/− mice we found accumulation of TH2 cells in the uterus was equivalent to TH1 (data not shown). Thus, in addition to promoting TH1 development via up regulating IL-12R expression, IFNγ induces a blockade of non-TH1 Chlamydia specific cells.
This cross-regulation works in both directions. The adoptive transfer of Chlamydia specific cells pre-skewed towards TH2 directly interferes with the development of the endogenous TH1 immune response by altering the cytokine milieu of the host (28). In our model the time from infection to assessment of IFU is too short for the TH2 cells to influence endogenous adaptive immunity. In addition, we demonstrated that TH2 cytokines did not exacerbate bacterial load in an in vitro epithelial cell infection model. Therefore we would propose that the supernatant from TH2 cells has a negative impact on the innate immune response and leads to reduced ability to clear bacteria. This is consistent with a recent report showing IL-4-mediated inhibition of Mycobacterium clearance from macrophages (29).
A recent report by Li et al. demonstrated that following infection with C. muridarum the transferred polarized CD4+ cells are able to confer protection in IFNγ−/− but not IFNγR−/− mice (30). We confirm and extend these findings using a model of C. trachomatis infection C. trachomatis clearance is highly dependent on IFNγ production, whereas C. muridarum has been shown to be capable of evading IFNγ-mediated effects in mice (4, 9, 31). We have found that when mice are infected in the uterus with C. trachomatis, some inflammation-induced pathologies associated with human disease manifest in the murine genital tract (DCG and MNS, unpublished observations). Previously we reported that transfer of Chlamydia specific CD8+ T cells were capable of conferring protection only if the host was IFNγ competent (32). Unlike the CD8+ T cells, we show here that transfer of TH1-skewed CD4+ cells were fully capable of conferring protection to a naïve or IFNγ−/− host challenged with Chlamydia. In contrast to the findings of Li et al., we observed that transferred TH1 cells exacerbate disease in IFNγR−/− mice (30). This highlights the critical nature of IFNγ in conferring protection and suggests that other TH1 cell factors (either secreted or surface bound) are deleterious to the host following Chlamydia challenge. Several non-IFNγ TH1 cytokines, such as IFNα, IFNβ, IL-1, and TNFα have been implicated in causing inflammation leading to both increased bacterial dissemination and enhanced pathology (4, 33–36). Limiting the action of these non-IFNγ factors might be beneficial in promoting C. trachomatis clearance and minimizing inflammation-induced pathology.
Collectively these data demonstrate the critical role of IFNγ in the protective immune response to C. trachomatis. Chlamydia-specific CD4+ T cells alone are capable of establishing protective immunity, highlighting the promise of T cell based vaccines targeting this bacterium. Clearly vaccine candidates are most promising when designed to elicit protection while minimizing the expression of deleterious cytokines during vaccination or subsequent infection.
References
- 1.Starnbach MN, Roan NR. Conquering sexually transmitted diseases. Nat Rev Immunol. 2008;8:313–317. doi: 10.1038/nri2272. [DOI] [PubMed] [Google Scholar]
- 2.Roan NR, Starnbach MN. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008;10:9–19. doi: 10.1111/j.1462-5822.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 3.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry LL, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell HD. Differential Sensitivity of Distinct Chlamydia trachomatis Isolates to IFN-{gamma}-Mediated Inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 5.Cain TK, Rank RG. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins RA, Rank RG, Kelly KA. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect Immun. 2002;70:5132–5139. doi: 10.1128/IAI.70.9.5132-5139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. The role of IFN-gamma in the outcome of chlamydial infection. Curr Opin Immunol. 2002;14:444–451. doi: 10.1016/s0952-7915(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 8.Jupelli M, Guentzel MN, Meier PA, Zhong G, Murthy AK, Arulanandam BP. Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice. J Immunol. 2008;180:4148–4155. doi: 10.4049/jimmunol.180.6.4148. [DOI] [PubMed] [Google Scholar]
- 9.Ito JI, Lyons JM. Role of Gamma Interferon in Controlling Murine Chlamydial Genital Tract Infection. Infect. Immun. 1999;67:5518–5521. doi: 10.1128/iai.67.10.5518-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado RA, Irvine DJ, Schreiber R, Glimcher LH. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature. 2004;431:527–532. doi: 10.1038/nature02916. [DOI] [PubMed] [Google Scholar]
- 11.Whitmire JK, Benning N, Whitton JL. Cutting edge: early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J Immunol. 2005;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- 12.Haring JS, Badovinac VP, Olson MR, Varga SM, Harty JT. In vivo generation of pathogen-specific Th1 cells in the absence of the IFN-gamma receptor. J Immunol. 2005;175:3117–3122. doi: 10.4049/jimmunol.175.5.3117. [DOI] [PubMed] [Google Scholar]
- 13.Roan NR, Gierahn TM, Higgins DE, Starnbach MN. Monitoring the T cell response to genital tract infection. Proc Natl Acad Sci U S A. 2006;103:12069–12074. doi: 10.1073/pnas.0603866103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard L, Orenstein NS, King NW. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974;27:102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson LM, Scott P. STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J Immunol. 2007;178:7259–7266. doi: 10.4049/jimmunol.178.11.7259. [DOI] [PubMed] [Google Scholar]
- 16.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 17.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J. Exp. Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakil AE, Wang ZE, Ryan JC, Fowell DJ, Locksley RM. Interferon gamma derived from CD4(+) T cells is sufficient to mediate T helper cell type 1 development. J Exp Med. 1998;188:1651–1656. doi: 10.1084/jem.188.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 21.Foulds KE, Rotte MJ, Paley MA, Singh B, Douek DC, Hill BJ, O'Shea JJ, Watford WT, Seder RA, Wu C-Y. IFN-{gamma} Mediates the Death of Th1 Cells in a Paracrine Manner. J Immunol. 2008;180:842–849. doi: 10.4049/jimmunol.180.2.842. [DOI] [PubMed] [Google Scholar]
- 22.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the Interleukin (IL)-12R beta 2 Subunit Expression in Developing T Helper 1 (Th1) and Th2 Cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalton DK, Haynes L, Chu C-Q, Swain SL, Wittmer S. Interferon {gamma} Eliminates Responding CD4 T Cells during Mycobacterial Infection by Inducing Apoptosis of Activated CD4 T Cells. J. Exp. Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon {gamma} Is Required for Activation-induced Death of T Lymphocytes. J. Exp. Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pernis A, Gupta S, Gollob KJ, Garfein E, Coffman RL, Schindler C, Rothman P. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Apilado R, Coleman J, Ben-Sasson S, Tsang S, Hu-Li J, Paul WE, Huang H. Interferon {gamma} Stabilizes the T Helper Cell Type 1 Phenotype. J. Exp. Med. 2001;194:165–172. doi: 10.1084/jem.194.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tau GZ, von der Weid T, Lu B, Cowan S, Kvatyuk M, Pernis A, Cattoretti G, Braunstein NS, Coffman RL, Rothman PB. Interferon gamma signaling alters the function of T helper type 1 cells. J Exp Med. 2000;192:977–986. doi: 10.1084/jem.192.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Current Opinion in Immunology. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 29.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T Helper 2 Cytokines Inhibit Autophagic Control of Intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Murthy AK, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection. J Immunol. 2008;180:3375–3382. doi: 10.4049/jimmunol.180.5.3375. [DOI] [PubMed] [Google Scholar]
- 31.Coers J, Bernstein-Hanley I, Grotsky D, Parvanova I, Howard JC, Taylor GA, Dietrich WF, Starnbach MN. Chlamydia muridarum evades growth restriction by the IFN-gamma-inducible host resistance factor Irgb10. J Immunol. 2008;180:6237–6245. doi: 10.4049/jimmunol.180.9.6237. [DOI] [PubMed] [Google Scholar]
- 32.Lampe MF, Wilson CB, Bevan MJ, Starnbach MN. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect Immun. 1998;66:5457–5461. doi: 10.1128/iai.66.11.5457-5461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu HY, Fan AG, Joyee S, Wang X, Han H, Bai L, Jiao N, Van Rooijen, Yang X. Type I IFNs Enhance Susceptibility to Chlamydia muridarum Lung Infection by Enhancing Apoptosis of Local Macrophages. J Immunol. 2008;181:2092–2102. doi: 10.4049/jimmunol.181.3.2092. [DOI] [PubMed] [Google Scholar]
- 34.Nagarajan UM, Prantner D, Sikes JD, Andrews CW, Jr, Goodwin AM, Nagarajan S, Darville T. Type I IFN signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 2008 doi: 10.1128/IAI.00629-08. IAI.00629–00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hvid M, Baczynska A, Deleuran B, Fedder J, Knudsen HJ, Christiansen G, Birkelund S. Interleukin-1 is the initiator of Fallopian tube destruction during Chlamydia trachomatis infection. Cell Microbiol. 2007;9:2795–2803. doi: 10.1111/j.1462-5822.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 36.Williams DM, Magee DM, Bonewald LF, Smith JG, Bleicker CA, Byrne GI, Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect. Immun. 1990;58:1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]