Abstract
Background
Participant attrition from randomized controlled trials reduces the statistical power of the study and can potentially introduce bias. Early identification of potential causes of attrition can help reduce patient attrition. We performed secondary analyses of two trials involving cancer patients.
Purpose
To identify predictors of attrition during two early phases, i.e. from consent to screening (Phase-1), and from screening to intake interview (Phase-2) in two clinical trials.
Methods
Cancer patients undergoing chemotherapy were asked to enroll in one of two clinical trials. In each trial the benefits of a cognitive behavioral intervention were compared with a psycho-educational intervention to assist patient to manage cancer and treatment related symptoms. Following consent patients were screened for their symptoms’ severity to determine their eligibility.
Results
Of the 885 consenters 785 completed screening and of the 782 eligible for participation, 713 completed intake interview. In the first phase, longer delays between consent and first contact attempt, lower levels of patient education, minority race and prolonged duration of screening increased the likelihood of dropping out with a significantly stronger effect on minorities than white patients. In the second phase, low education, being a minority, longer screening delays and impact of symptom severity on enjoyment of life significantly increased probability of attrition.
Limitations
Participant reported causes of attrition were not modeled; however exclusion of patients who died during the time period of this research meant that most patients leaving the study made a conscious decision to do so. 4
Conclusions
To assure preservation of external validity, the time between consent and randomization into the arms of a trial must be held to a minimum. Delays between contacts, and run in time, that may include screening patients to assure they will benefit from a trial, must be balanced against rates of attrition. Compressing intervals between contacts is particularly important to retain minorities.
Keywords: Attrition, early attrition, randomized controlled trials, clinical trials, study design
Introduction
Recruitment and retention into clinical trials of cancer patients who are undergoing chemotherapy represents a significant challenge [1, 2]. The recruitment of patients representative of the target population is necessary for generalization of the findings (external validity), whereas their retention, particularly after randomization, is perhaps more important as high attrition can reduce the trial’s statistical power and also pose serious threat to its internal validity [3]. Patient attrition from clinical trials, defined as attrition at any time following consent to participate, has therefore been an area of interest for researchers. Multiple studies, have examined attrition following randomization and during participation in the trial [4–10], prompting testing of various interventions to reduce attrition with varying success rates [11]. However, patients are at risk of attrition as soon as they consent to participate, but attrition in the time window from consent to completion of the baseline interview and randomization (marking formal participation) has not been examined. It is attrition during this time frame i.e. from consent to intake into one or the other arms of a trial that is the focus of this paper.
Studying attrition during this early stage of a clinical trial is important as factors influencing patients to drop out before randomization may be different from factors affect attrition after randomization. Additionally, prior to randomization, the logistical activities for the data collection are more intense, therefore identified modifiable factors, at this stage can be easily tailored to trial design so as to minimize attrition. In the research reported here, we performed secondary analyses of the data from two of our completed trials that targeted reduction in symptom severity of cancer and cancer-treatment related symptoms in patients undergoing chemotherapy. Our trials included a process where patients were screened to assess their level of symptom severity and potential for benefit from the trials. We therefore focused on attrition during two early phases of the trials; 1) from time of consent to completion of screening of patients for the severity of their symptoms, and eligibility to continue participation (Phase-1); and 2) from completion of screening to the intake (baseline) interview and randomization i.e. the formal entry into the trial (Phase-2).
The original purpose of each of the two trials was to compare the effect of a cognitive behavioral intervention with a psycho-educational intervention on reducing cancer patients’ symptom severity. The findings from the original research are described in detail elsewhere [12]. Briefly, there were no differences between the arms in symptom severity at the end of intervention. The trials were powered to detect the effect size of .28 between the groups; however the observed effect size for group differences in symptom severity was virtually zero. All groups produced clinically significant improvement over baseline, with effect sizes exceeding .5. This secondary analysis of the logistical and patient characteristics associated with attrition from the early phases of these trials is guided by the following research questions. (1) Are the patients who delay making first study contact after consent, more likely to drop out? (2) Once contacted, does the time, in number of days, taken to complete the screening process affect participants’ probability of dropping out before completing the intake interview i.e. the initiation of the trial? And (3) Does patient characteristics, such as age, sex and race moderate the effect of these time factors on probability of attrition?
Methods
Patients were recruited from two comprehensive cancer centers, one community cancer oncology program, and six hospital affiliated community oncology centers in the Mid-West. The Institutional Review Boards of each center approved the trials. To be eligible to participate in the two original trials, patients had to meet the following requirements: 1) 21 years of age or older, 2) a diagnosis of a solid tumor cancer or non-Hodgkin’s lymphoma, 3) undergoing a course of chemotherapy, 4) able to speak and read English, without hearing deficits that would prevent them from using a telephone, and 5) have a touchtone telephone. Patients meeting these criteria had the studies explained to them and were invited to sign an informed consent. Following consent, enrollment data were entered into a secure web-based data collection system. Prior to trial assignment patients were screened to assess the severity of 13 symptoms known to be related to cancer and its treatment. All patients were called twice weekly for up to six weeks, by an automated voice response system (AVR), at a time and telephone number of the patients choice. Patients who completed the six week screening but never scored a 2 or higher (range 0–10) on any of the 13 symptoms received a letter thanking them for their participation. These patients were released since they did not qualify for further participation. For analytic purposes these patients who never reached threshold were not considered drop outs in the Phase-1 analysis. They were however not included in Phase-2 analysis. Patients scoring 2 or higher on severity at any contact for at least one of the 13 cancer related symptoms were eligible for entry into one of the two trials. Patients entering study I were randomized to receive a 6 contact eight-week long cognitive behavioral intervention either from a nurse or from a non-nurse coach. Those in study II were randomized to either the same nurse delivered intervention as in study I, or to information and self-care strategies delivered via an AVR system.
Measures
Since this research examines attrition up to the intake interview, a limited number of variables were available for analysis. Information on patients’ age, sex, site and stage of cancer were obtained from the patients’ medical records. Educational attainment, marital status and race were determined in a brief interview following consent. Age of the patient was recorded in one year intervals. Education, initially a five level variable was collapsed into three categories; less than high school, high school, and more than high school. Race of patients was recorded per US Bureau of Census into one of the following categories white, black or African American, American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander. For analytic purposes however, we grouped all non-whites into one category, dichotomizing the race variable into whites and minorities (all non-whites).
Two time frames were measured in number of days, (1) time from consent to first contact attempt, and (2) time taken to complete screening, taken from the first screening call to the last one. The first duration was primarily determined by the patients themselves as they were asked to state a date and time of their choice at which they would like to receive the screening call. The second duration depended on ability of screeners to make contact with the patient and on the symptoms being at severity of 2 or higher.
During screening patients reported their symptoms severity using an 11 point scale (0 symptom not present to 10 worst imaginable). When a symptom was present patients were also asked to rate how much the symptom’s severity interfered with their daily general activity, mood, working, walking, relationships and enjoyment of life. Interference was measured on the same 11 point scale as symptom severity.
Data Analyses
The research questions examined patient attrition in two different time spans (from consent to end of screening; and from screening to intake interview) and thus required two separate logistic regression models. In the first model the outcome was failure to complete symptom screening. Screening was considered complete if 1) a patient was found eligible to continue participation at any of the calls or 2) if he/she completed all 12 screening calls without exceeding the threshold of 2 or higher on severity for any of the 13 symptoms. All other patients were considered drop-outs. The explanatory variables included the variables of interest, i.e. time in days from consent to first screening attempt and screening duration. Also included were race [6, 13] and education [14–16], the two variables that are frequently reported in literature to be associated with attrition.
At the next phase the analysis of attrition was restricted to those who completed screening and were eligible for further participation. The outcome of interest in the second analysis was whether the patient completed the baseline interview. Patients not completing the baseline interview were labeled dropouts. Since the protocol required completion of baseline interview within 30 days following screening, 3 patients who died within 30 days following their completion of screening were excluded from this analysis. Also excluded from analysis were 3 patients who completed 12 screening calls but never went above threshold and thus were ineligible to continue the study. The explanatory variables included screening duration in number of days, race, education, and symptoms interference with enjoyment of life. The interference data were collected during screening and were thus available for analysis in second model only. The poor health and poor perceived quality of life are known factors associated with attrition [10, 17], the interference with enjoyment of life in this analysis serves as a proxy variable for depression or at least a pre-depression state that can significantly impact a person’s perceived quality of life in this analysis. Interactions among variables were also tested but only the significant ones were retained in the final models. All analyses were performed using SAS1 software version 9.1.
Results
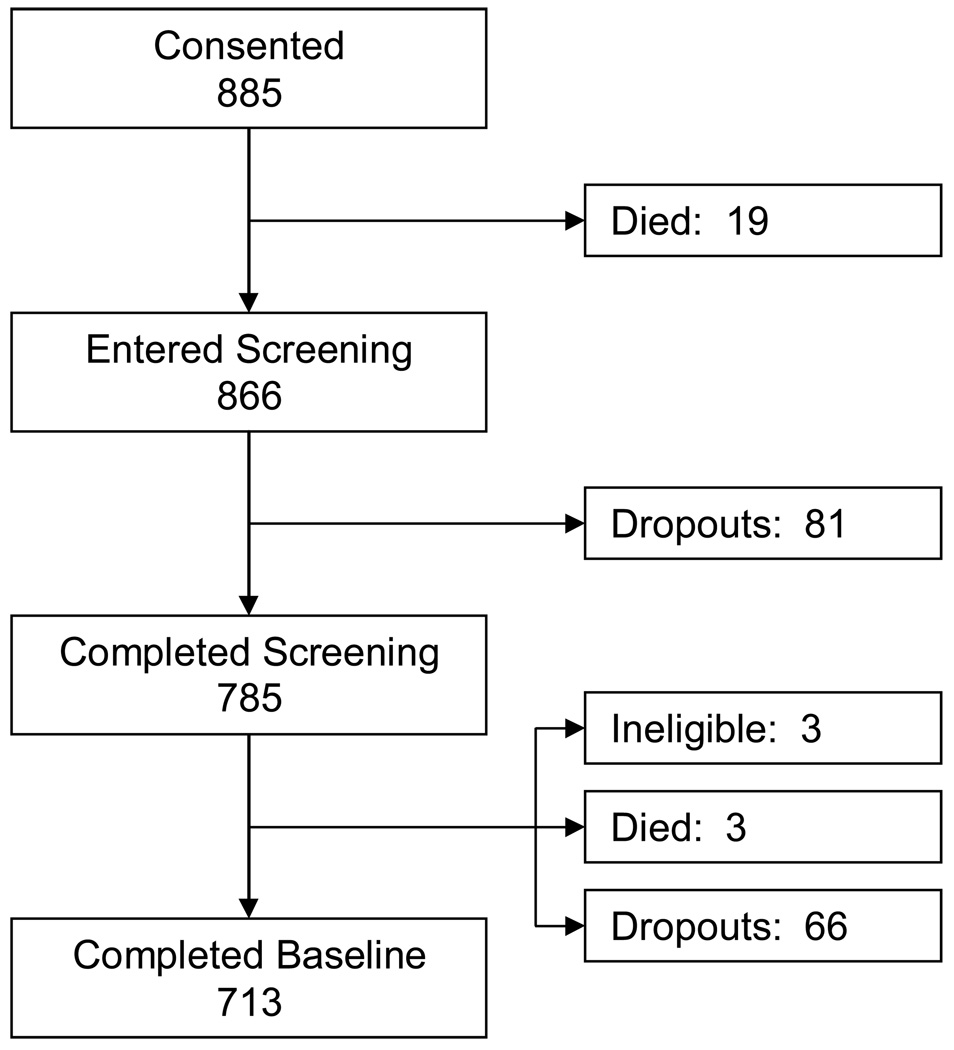
Figure 1 summarizes the flow of patients from consent, through symptom screening (eligibility), to baseline interview. Table 1 summarizes the basic socio-demographic and selected clinical information available for the purpose of describing the sample. Average age of patients in our sample was about 60 years. There were more females than males (70% vs. 30%), and the racial distribution reflected the distribution in the source population. The majority of patients were married and 64% had more than a high school education.
Figure 1.
Flowchart showing progress of patients through various stages of the study.
Table 1.
Summary statistics of the demographic and select clinical characteristics of the consenting patients.
| Variables | n (%) |
|---|---|
| Age (Mean 57.6, SD 12.2) | |
| ≤30 | 13 (1.5) |
| 31–40 | 52 (6.0) |
| 41–50 | 175 (20.2) |
| 51–60 | 276 (31.9) |
| 61–70 | 221 (25.5) |
| 71–80 | 105 (12.1) |
| 81–90 | 22 (2.5) |
| >90 | 2 (0.2) |
| Gender | |
| Male | 261 (30.1) |
| Female | 605 (69.9) |
| Race1 | |
| American Indian, Alaskan | 2 (0.2) |
| Asian | 5 (0.6) |
| African American | 101 (11.7) |
| Hawaiian, Pacific Islanders | 20 (2.3) |
| White | 738 (85.2) |
| Ethnicity | |
| Hispanic or Lation | 15 (1.73) |
| Non-Hispanic or Latino | 851 (98.27) |
| Marital Status | |
| Married | 566 (65.4) |
| Separated | 13 (1.5) |
| Divorced | 126 (14.6) |
| Widowed | 76 (8.8) |
| Never Married | 85 (9.8) |
| Education | |
| Some High School | 61 (7.1) |
| Completed High School | 248 (28.7) |
| Some College | 232 (26.8) |
| Completed College | 196 (22.7) |
| Completed Graduate School | 128 (14.8) |
| Work Status | |
| Full-time | 214 (24.8) |
| Part-time | 82 (9.5) |
| Self-employed | 16 (1.9) |
| Not employed | 98 (11.3) |
| Retired | 281 (35.5) |
| Disabled | 137 (15.9) |
| Homemaker | 36 (4.2) |
| Cancer Site | |
| Breast | 290 (33.5) |
| Colon | 90 (10.4) |
| Lung (Non-small cell) | 142 (16.4) |
| Lung (Small cell) | 41 (4.7) |
| Prostate | 27 (3.1) |
| Kidney | 10 (1.2) |
| Ovarian | 47 (5.4) |
| Pancreas | 26 (3) |
| Non-Hodgkins Lymphoma | 43 (5) |
| Endometrial | 6 (0.7) |
| Other | 144 (16.6) |
| Metastatic cancer | |
| Yes | 474 (54.7) |
| No | 392 (45.3) |
Minorities comprise of all races except white
Phase-1 (consent to screening completion)
The first research question examined the effect of the elapsed time expressed in days between the signing of consent and the first screening call. The mean interval was 7 days with a standard deviation of 5.6 days. The Phase-1 model is summarized in table 2. Among the two spans of interest, the time interval, in number of days, from consent to the first screening call was found to be nearly significantly (p=0.07) associated with the likelihood of attrition (odds ratio (OR) 1.04, 95% confidence interval (CI) of odds ratio 1.00 – 1.10).
Table 2.
Final logistic regression model of attrition from consent to screening, with parameter estimates and adjusted odds ratios with their 95% confidence intervals
| Parameter Estimate (Standard error) | p-value | Adjusted Odds ratio | 95% Confidence Interval of Odds ratio | |
|---|---|---|---|---|
| Number of days from consent to first screening call | 0.043 (0.024) | 0.07 | 1.04 | 1.00 – 1.10 |
| Education (Ref: > High school) | ||||
| < high school | 0.849 (0.433) | 0.05 | 2.34 | 1.00 – 5.46 |
| High school | 0.305 (0.291) | 0.29 | 1.36 | 0.77 – 2.40 |
| Screening duration | 0.098 (0.015) | <0.01 | -- | -- |
| Minorities (Ref: Whites) | -0.831 (0.635) | 0.19 | -- | -- |
| Interaction between screening duration and race | 0.108 (0.044) | 0.01 | -- | -- |
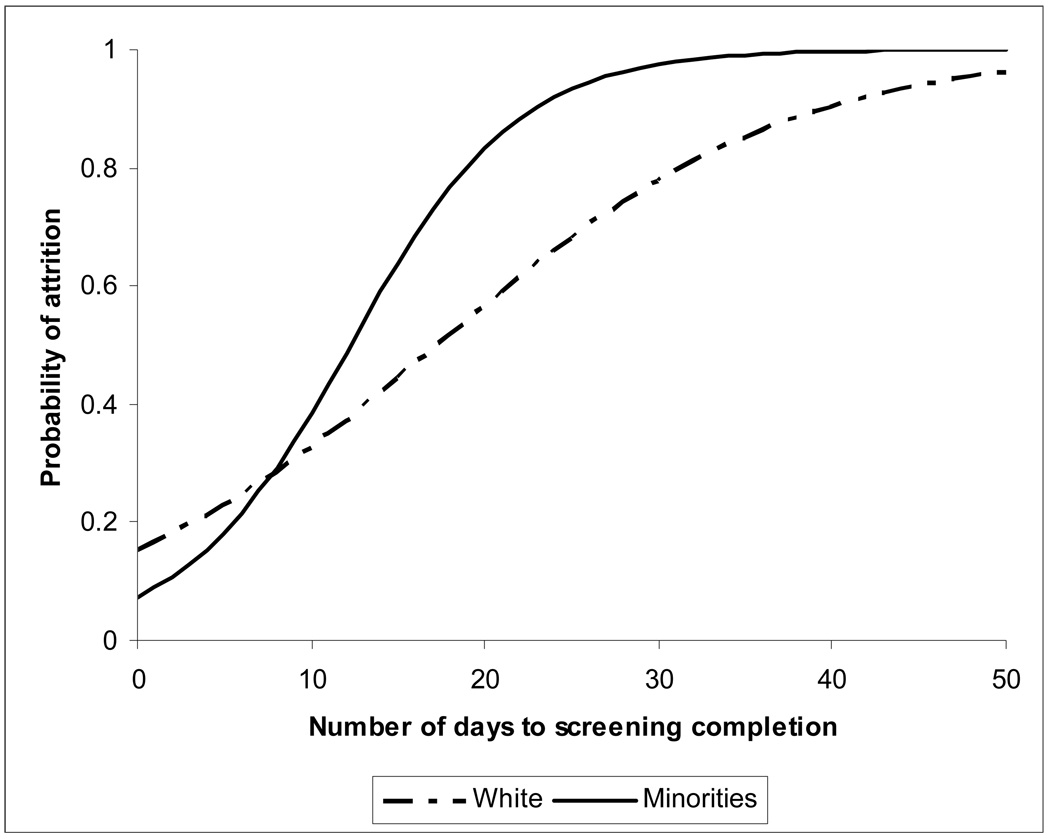
The other time span of interest, screening duration showed a significant interaction with the race variable, which means that screening duration affected minority patients differently than it affected white patients. The moderating effect of time by race is explained with the help of the following example. The predicted probability of attrition among majority (white) patients who took five days to complete screening was 0.23 (log odds 0.494), and the probability of attrition for the same time period for minorities was 0.18 (log odds 0.204). As seen in Figure 2 the two curves (for majority and minority patients) were not parallel. The probability of attrition among whites who took 15 days to complete screening was 0.44 (log odds 1.482) and that among minorities was 0.64 (log odds 2.275). Thus, while all patients were more likely to dropout as the screening intervals increased, the rise in probability was differential by race, with attrition among minorities rising at a higher rate (Figure 2).
Figure 2.
Probability of attrition predicted from the logistic model for attrition from consent to first contact, by race of the patient.
Phase 2 (from screening completion to randomization)
The second question sought to examine the effect of time lapsed from completion of screening to the intake interview. For these analyses only patients retained following screening were included. The phase 2 logistic model is summarized in Table 3. Above and beyond the effect of other covariates in the model, the duration of screening increases the chance of patients’ attrition prior to the intake interview (OR 1.05, 95% CI 1.01 – 1.09). Patients reporting a higher interference with enjoyment of life were more likely to drop out of the study than those reporting lower interference (OR 1.11, 95% CI 1.02 – 1.20). As with the previous model, interactions were tested after defining the main effects model, but none were found significant and thus were not included in the final model.
Table 3.
Final logistic regression model of attrition from screening to baseline interview, with parameter estimates and adjusted odds ratios with their 95% confidence intervals.
| Parameter Estimate (Standard error) | p-value | Adjusted Odds ratio | 95% Confidence Interval of Odds ratio | |
|---|---|---|---|---|
| Screening duration | 0.052 (0.020) | 0.01 | 1.05 | 1.01 – 1.09 |
| Minorities (Ref: Whites) | 0.624 (0.322) | 0.05 | 1.87 | 0.99 – 3.51 |
| Education (Ref: > High school) | ||||
| < High school | 0.886 (0.431) | 0.04 | 2.43 | 1.04 – 5.65 |
| High School | 0.366 (0.291) | 0.21 | 1.44 | 0.82 – 2.55 |
| Impact of symptoms’ severity on enjoyment of life | 0.101 (0.041) | 0.01 | 1.11 | 1.02 – 1.20 |
Discussion
We identified an important factor associated with increased probability of participants dropping out of a clinical trial: a delay in initiation and increased duration of screening is associated with decreased willingness to participate after enrollment in clinical trials. In this trial the delay in contacting the patient after consent, was determined primarily by the patient and was not due to delays on part of the research team, since patients were asked to state a day and time of their choice for research team to call for symptom screening. Thus, patients who defer participation for a longer period are more likely to drop before completion. This may be a passive resistance to participation by those who find it difficult to refuse outright. It is possible that this effect may be due to the fact that patients for whom it took longer to make the first screening call were also more severely ill and felt a need to delay their screening and eventually dropped out. However this is unlikely since a comparison of the means of total symptom severity score and interference with enjoyment of life scores between patients who were above and below the mean contact time (7 days) showed no difference (Severity: above mean 51.42, below mean 51.03, p = 0.87 and Interference: above mean 3.95, below mean 3.91, p = 0.86). This shows that both groups of patients were similar in terms of disease severity and perceived impact of their disease on their lives suggesting that these are not explanations for differences in attrition.
Another time span associated with higher attrition was the duration of screening. Longer time taken to complete the screening process increased the probability of attrition of all patients, but for minority patients elapsed time had a greater impact than for white patients (Figure 2). This moderating effect of delay on race suggests a greater susceptibility of minorities to drop out when recruitment or screening process takes longer to complete. Historically, minority patients have been less likely to participate in clinical trials [18], therefore the concerns about the external validity of the findings from the trials led to a 1993 National Institutes of Health’s (NIH) revitalization act (Public law 103-43), which resulted in increased recruitment of women and minority patients in research, particularly phase III clinical trials [19]. However increased recruitment does not guarantee increased retention and thus patients who may have been initially reluctant to participate but consented because of focused efforts by study teams to recruit them, dropped out in early stages of the study. Our data support this explanation, as 70% of the patients completed screening within two days after initiation of screening. However when these numbers were examined separately by race, only 57% of the minorities as compared to 73% of white patients completed screening within two days. The delays experienced in completion of screening were primarily due to inability of study personnel to contact patients rather than because patient failed to score at least one symptom above threshold. The average duration of screening was also significantly longer for minority patients (5.5 vs. 3.1, p < 0.01). Therefore the minority patients were harder to contact during screening, and with the fact that were more likely to drop out, reflects a ‘mechanism’ by which minority patient quit clinical trials before completion.
It can be argued that patients who completed screening within a shorter duration were more symptomatic, whereas those who took longer to complete screening may have been less symptomatic and may have had less interest in the trial. This is however, seems an unlikely explanation for higher attrition among patients who took longer to complete screening because the time taken to complete screening was mainly determined by time taken to reach the patients, i.e. when calls to patients went unanswered, and not because the patient was reached but had not crossed the threshold. Therefore it is probably not true that those who completed screening faster were more symptomatic. Additionally, even if this were true it would not explain differential attrition by race for screening duration in days.
Ethnically, very few patients in our sample were Hispanic and almost all white patients (98.1%) were non-Hispanic. Differences in attrition were therefore found by race only.
The race and educational attainment of patients played a role in patient attrition in both phases of the study (from consent to screening initiation and from screening to the intake interview). Therefore minorities and the less educated patients continued to be more likely to drop-out throughout the study, and the effect of race and education on attrition persists across the trajectory from consent to beginning the intake interview. Since most of the patients who were found eligible to continue in the study had their eligibility established within 3 days of the first screening call, some patients who didn’t intend to continue with the trial didn’t have enough time to make their intentions known. Therefore, they dropped out at the next phase of our observation. The role of education and race in attrition as found in our study is consistent with findings of other researchers [14–16].
Patients reporting a higher impact of their symptoms on their enjoyment of life were found to be more likely to drop out. The symptom interference with enjoyment of life was highly correlated with the total severity reported by patients calculated as the sum of severity over all symptoms (ρ = 0.66, p-value < 0.01), therefore the addition of the severity variable to the model did not effect the significance of enjoyment of life variable indicating that interference with enjoyment of life had an independent effect on attrition that was above and beyond any influence of symptom severity on attrition. The enjoyment of life may be thought of as a proxy variable for depression or at least a pre-depression state [20]. The significant effect of interference with the enjoyment of life on attrition is therefore not surprising as depression is one of the most consistently reported factors associated with attrition in contemporary literature [21, 22].
One limitation of this research was that we were unable to establish if the delay in attempting to conduct the baseline interview after completion of screening had an effect on attrition (just as the delay in initiating screening contact had). Since attrition at this step was defined as failure to complete baseline interview the time from completion of screening to baseline interview could not be determined for the drop-outs.
The very nature of the trial and the intervention can be a strong determinant of a trial participant’s decision to continue or drop out.. Our trials are no exception and some of the participants may certainly have perceived the intervention more beneficial to them than others, influencing their decision to stay in or drop out of the study. However, the research reported here applies to the period of the study before any of the participants were delivered any intervention; therefore we do not expect the trials context to be factor influencing participants’ likelihood of dropping out at this early stage in the trial.
Conclusions
Most factors associated with attrition at the early phases of our trials were primarily un-modifiable. However, important associated factors were identified that help researchers identify potential drop outs early in the trial. Patients, who delay initiating contact with the research team, after they have consented to participate, were found to be more likely to drop out. Attempts to convince patients to reduce this time may be of benefit. If such patients start participating early, keeping them engaged may improve their chances of continued participation. This seems especially true for minority patients. When a trial requires screening for symptoms, completion of screening in a timely manner will be dependent on many factors beyond investigator’s control. However any delays, especially when unrelated to patient’s morbidity, should alert study personnel to a potential increase in the rate of attrition, especially among minority patients. Additional methods of contacting patient such as by mail, and/or attempting to reach patient via a family member may be of benefit.
Acknowledgements
National Cancer Institute, Automated Telephone Monitoring for Symptom Management (#RO1 CA030724), Charles Given, PI, and National Cancer Institute, Family Home Care for Cancer: A Community Based Model (#RO1 CA79280), Barbara Given, PI. and In Affiliation with Walther Cancer Institute, Indianapolis, Indiana.
Footnotes
SAS software. Copyright, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Contributor Information
Azfar-e-Alam Siddiqi, Michigan State University, 500 A West Fee Hall, East Lansing, MI 48824, United States of America, Phone: (517) 432-8355, E-mail: azfar.siddiqi@ht.msu.edu.
Alla Sikorskii, Michigan State University, B515A West Fee Hall, East Lansing, MI 48824, United States of America, Phone: 517-353-5231, E-mail: alla.sikorskii@hc.msu.edu.
Charles W. Given, Michigan State University, B108 Clinical Center, Family Practice, East Lansing, MI 48824, United States of America, Phone: 517-353-0851 x420 E-mail: givenc@msu.edu.
Barbara Given, Michigan State University, B515B West Fee Hall, Family Care Study, East Lansing, MI 48824, United States of America, Phone: 517-353-0306, E-mail: Barb.Given@hc.msu.edu.
References
- 1.Jordhoy MS, Kaasa S, Fayers P, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med. 1999 June 1;13(4):299–310. doi: 10.1191/026921699668963873. [DOI] [PubMed] [Google Scholar]
- 2.Young AF, Powers JR, Bell SL. Attrition in longitudinal studies: who do you lose? Aust N Z J Public Health. 2006 Aug;30(4):353–361. doi: 10.1111/j.1467-842x.2006.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 3.Twisk J, de Vente W. Attrition in longitudinal studies. How to deal with missing data. J Clin Epidemiol. 2002 Apr;55(4):329–337. doi: 10.1016/s0895-4356(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AR, Mokuau N, Hughes C, Tortolero-Luna G, Risendal B, Ho RCS, et al. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000 Nov;10(8 Suppl):S22–S34. doi: 10.1016/s1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 5.Neumark DE, Stommel M, Given CW, Given BA. Research design and subject characteristics predicting nonparticipation in a panel survey of older families with cancer. Nurs Res. 2001 Nov–Dec;50(6):363–368. doi: 10.1097/00006199-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihelic AH, Crimmins EM. Loss to folow-up in a sample of Americans 70 years of age and older: The LSOA 1984–1990. J Gerontol B Psychol Sci Soc Sci. 1997 Jan;52(1):S37–S48. doi: 10.1093/geronb/52b.1.s37. [DOI] [PubMed] [Google Scholar]
- 8.Morrison TC, Wahlgren DR, Hovell MF, Zakarian J, Burkham-Kreitner S, Hofstetter CR, et al. Tracking and follow-up of 16,915 adolescents: minimizing attrition bias. Control Clin Trials. 1997 Oct;18(5):383–396. doi: 10.1016/s0197-2456(97)00025-1. [DOI] [PubMed] [Google Scholar]
- 9.Snow WM, Connett JE, Sharma S, Murray RP. Predictors of attendance and dropout at the Lung Health Study 11-year follow-up. Contemp Clin Trials. 2007 Jan;28(1):25–32. doi: 10.1016/j.cct.2006.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson AJ, 3rd, Webber IL. Attrition in a longitudinal study of an aged population. Exp Aging Res. 1976 Sep;2(5):367–387. doi: 10.1080/03610737608257996. [DOI] [PubMed] [Google Scholar]
- 11.Mapstone J, Elbourne D, Roberts I. Strategies to improve recruitment to research studies. Cochrane Database Syst Rev. 2007;2:MR000013. doi: 10.1002/14651858.MR000013.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Sikorskii A, Given CW, Given B, Jeon S, Decker V, Decker D, et al. Symptom Management for Cancer Patients: A Trial Comparing Two Multimodal Interventions. J Pain Symptom Manage. 2007;34(3):253. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Given CW, Given BA, Coyle BW. Prediction of patient attrition from experimental behavioral interventions. Nurs Res. 1985 Sep–Oct;34(5):293–298. [PubMed] [Google Scholar]
- 14.Agosti V, Nunes E, Ocepeck-Welikson K. Patient factors related to early attrition from an outpatient cocaine research clinic. The American journal of drug and alcohol abuse. 1996 Feb;22(1):29–39. doi: 10.3109/00952999609001643. [DOI] [PubMed] [Google Scholar]
- 15.Chen A. Noncompliance in Community Psychiatry: A Review of Clinical Interventions. Hosp Community Psychiatry. 1991 March 1;42(3):282–287. doi: 10.1176/ps.42.3.282. [DOI] [PubMed] [Google Scholar]
- 16.Vendetti J, McRee B, Miller M, Christiansen K, Herrell J. Correlates of pre-treatment drop-out among persons with marijuana dependence. Addiction. 2002;97(s1):125–134. doi: 10.1046/j.1360-0443.97.s01.8.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolinsky FD, Armbrecht ES, Wyrwich KW. Rethinking functional limitation pathways. Gerontologist. 2000 Apr;40(2):137–146. doi: 10.1093/geront/40.2.137. [DOI] [PubMed] [Google Scholar]
- 18.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst. 1995 Dec 6;87(23):1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 19.Cabral DN, Napoles-Springer AM, Miike R, McMillan A, Sison JD, Wrensch MR, et al. Population- and Community-based Recruitment of African Americans and Latinos: The San Francisco Bay Area Lung Cancer Study. Am J Epidemiol. 2003 August 1;158(3):272–279. doi: 10.1093/aje/kwg138. [DOI] [PubMed] [Google Scholar]
- 20.Bottomley A. Depression in cancer patients: a literature review. Eur J Cancer Care (Engl) 1998 Sep;7(3):181–191. doi: 10.1046/j.1365-2354.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- 21.Davis L, Evans S, Fishman B, Haley A, Spielman LA. Predictors of attrition in HIV-positive subjects with peripheral neuropathic pain. AIDS Care. 2004 Apr;16(3):395–402. doi: 10.1080/09540120410001665394. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Peragallo N, DeForge B. Predictors of participation in an HIV risk reduction intervention for socially deprived Latino women: a cross sectional cohort study. Int J Nurs Stud. 2006 Jul;43(5):527–534. doi: 10.1016/j.ijnurstu.2005.07.005. [DOI] [PubMed] [Google Scholar]