Abstract
Manganese (Mn) is an essential trace element, but overexposure is characterized by Parkinson’s like symptoms in extreme cases. Previous studies have shown Mn accumulation is exacerbated by dietary iron deficiency (ID) and disturbances in norepinephrine (NE) have been reported. Because behaviors associated with Mn neurotoxicity are complex, the goal of this study was to examine the effects of Mn exposure and ID-associated Mn accumulation on NE uptake in synaptosomes, extracellular NE concentrations, and expression of NE transport and receptor proteins. Sprague-Dawley rats were assigned to four dietary groups: control (CN; 35 mg Fe/kg diet), iron-deficient (ID; 6 mg Fe/kg diet), CN with Mn exposure (via the drinking water; 1 g Mn/L) (CNMn), and ID with Mn (IDMn). 3H-NE uptake decreased significantly (R=−0.753, p=0.001) with increased Mn concentration in the locus coeruleus, while decreased Fe was associated with decreased uptake of 3H-NE in the caudate putamen (R=0.436, p=0.033) and locus coeruleus (R=0.86; p<0.001). Extracellular concentrations of NE in the caudate putamen were significantly decreased in response to Mn exposure and ID (p<0.001). A diverse response of Mn exposure and ID was observed on mRNA and protein expression of NE transporter (NET) and α2 adrenergic receptor. For example, elevated brain Mn and decreased Fe caused an approximate 50% decrease in NET and α2 adrenergic receptor protein expression in several brain regions, with reductions in mRNA expression also observed. These data suggest that Mn exposure results in a decrease in NE uptake and extracellular NE concentrations via altered expression of transport and receptor proteins.
Keywords: rat, norepinephrine, manganese, brain, microdialysis
1. Introduction
An essential trace element and a cofactor for several enzymes (Hurley and Keen, 1987), manganese (Mn) is responsible for proper immune function, regulation of metabolism, reproduction, digestion, bone growth, and blood clotting (see review by Aschner et al., 2005). However, neurotoxicity is known to result from exposure to high concentrations of Mn. Known as manganism, Mn neurotoxicity is associated with the accumulation of Mn in iron-rich, dopaminergic regions of the brain, specifically areas of the basal ganglia (Aschner et al., 2005). Initially, manganism is characterized by a psychiatric disorder resembling schizophrenia, an anxiety disorder which may involve the noradrenergic system (Yamamoto and Hornykiewicz, 2004), and shares similarities with several clinical disorders, in particular Parkinson’s disease (Pal et al., 1999). Most often, Mn neurotoxicity arises from chronic occupational exposure of welders, miners, and steel workers to high levels of airborne Mn particulates (Pal et al., 1999; Mergler et al., 1994), though cases from exposure to contaminated drinking water have been reported as well (Wasserman et al., 2006; Kondakis et al., 1989).
It has become clear that iron deficiency (ID) is a risk factor for Mn accumulation (Davis et al., 1992; Erikson et al., 2002; Finley, 1999; Kwik-Uribe et al., 2000). Affecting more than 2 billion individuals worldwide (WHO/UNICEF/UNU, 2006), ID is associated with alterations in cognition and behavior (Beard, 2001), potentially disturbing neurochemistry via the facilitation of Mn accumulation in the brain, as iron (Fe) status may affect absorption of Mn, regardless of Mn concentrations in the body (Chandra and Shukla, 1976; Shukla et al., 1976). Manganese most likely competes with Fe for transport via divalent metal transporter 1 (DMT-1) (Gunshin et al., 1997; Aschner et al., 2005), a transporter of various divalent metals. Increased expression of DMT-1 mRNA in the brain has been shown in cases of ID (Gunshin et al., 2001; Burdo et al., 1999), and this protein has recently become of interest as a potential transporter for Mn across the blood-brain barrier (Garrick et al., 2003; Roth and Garrick, 2003). In the blood, the majority of Mn is bound to albumin, with a small fraction bound to transferrin (Aschner et al., 2005). While most research has focused on the effects of Mn neurotoxicity on the metabolism of dopamine (DA) due to locomotor effects, alterations in the biology of other neurotransmitters such as norepinephrine (NE) have been noted (Table 1) (Autissier et al., 1982; Seth and Chandra, 1984; Struve et al., 2007).
Table 1.
Summary of studies examining the effects of Mn exposure on NE biology.
| Reference | Dose & Route | Effect |
|---|---|---|
| Struve et al., 2007 | 1.5 mg Mn/m3 via inhalation | Marginal decrease in NE content in the caudate of rhesus monkeys |
| Kontur and Fechter, 1988 | 25 or 50 µg Mn/g/day via gavage | No effects on rat brain NE |
| Chandra et al., 1984 | 100, 500, or 1000 µM Mn | Decreased uptake of NE in rat synaptosomes |
| Seth and Chandra, 1984 | 10 mg Mn/kg | Increased NE |
| Autissier et al., 1982 | 1 mg Mn/100 g/day via intraperitoneal injection | Increased NE in rat brain stem and hypothalamus |
| Lai et al., 1982 | 1.5 µM Mn | Decreased uptake of NE in rat synaptosomes |
| Chandra and Shukla, 1981a | 1 mg Mn/ml via drinking water | Increased turnover of rat brain NE |
| Chandra and Shukla, 1981b | 1 mg Mn/ml via drinking water | Initial increase in rat striatal NE |
| No change from 180 to 240 days | ||
| Decreased NE from 300 to 360 days | ||
| Deskin et al., 1981 | 1, 10, or 20 µg Mn/g via gavage | No effects on rat striatal NE |
| Shukla et al., 1980 | 15 mg Mn/kg via intraperitoneal injection | Increased NE in whole brain of rats |
| Chandra et al., 1979 | 3 µg Mn/ml via drinking water | Increased NE in mouse striatum |
| Mustafa and Chandra, 1971 | 400 mg Mn/kg via intrathecal injection | Decreased NE in whole brain of rabbits |
References, dosing regimen, and brief results are given for previous studies of the effect of Mn exposure on NE biology.
Norepinephrine, a neuromodulatory neurotransmitter derived from DA, exerts its effects via G-protein linked receptors and is associated with motivational behaviors and alertness, as well as locomotion and autonomic functions (Troadec et al., 2001). The locus coeruleus, a small area of the pons, is a major nucleus of NE expression, containing approximately half of the noradrenergic neurons in the brain (Andrade and Aghajanian, 1984). Projecting fibers throughout the forebrain and cerebellum, the locus coeruleus plays a role in mediating stress and anxiety, modulation of the nigrostriatal pathway, and is affected by neuronal loss during idiopathic Parkinson’s disease (Marien et al., 2004). Experimental lesioning of the locus coeruleus has been shown to exacerbate the pathology and symptomology in animal models of Parkinson’s disease (Rommelfanger and Weinshenker, 2007).
A recent study by Struve et al., (2007) found a marginal increase in NE tissue concentrations following Mn exposure via inhalation in rhesus monkeys. In earlier studies, varying alterations of tissue NE concentrations were identified in animal models of Mn exposure (see Table 1 for summary). Manganese has also been shown to inhibit NE uptake in a dose-dependent manner in synaptosomes isolated from the forebrain of Wistar rats (Lai et al., 1982), which could potentially lead to alterations in extracellular NE concentrations. A more recent study (Beard et al., 2006) examined the effects of Fe depletion on the expression of NET in PC12 cells and rat brain tissue. Those cells treated with desferrioxamine, an iron-chelating agent, exhibited a dose-dependent decrease in 3H-NE uptake that correlated with a decrease in NET protein levels. Attenuated NET protein expression in the brain during ID has been reported as well (Burhans et al., 2005); however, the exact role of NET during Mn exposure and ID still remains to be fully elucidated.
Based upon previous data from our lab showing alterations in GABA biology as a result of dietary Mn exposure and ID (Anderson et al., 2007; 2008), coupled with the knowledge that behaviors associated with Mn neurotoxicity may relate to NE metabolism and gaps remaining concerning the effects of Mn exposure on NE biology, we hypothesize that dietary Mn exposure and ID-associated Mn accumulation could potentially lead to a decrease in NE uptake, leading to changes in the concentration of extracellular NE, most likely due to altered NE transporter and/or receptor expression. To examine this hypothesis, we developed this study: 1.) to establish the effects of dietary Mn exposure and ID-associated Mn accumulation on 3H-NE uptake and in the neuromodulatory extracellular concentrations of NE; and 2.) to examine alterations in the protein and mRNA expression of the transport and receptor proteins of NE resulting from dietary Mn exposure and ID, which may provide insight into neurodegenerative processes where metal toxicity is implicated.
2. Results
Plasma manganese and iron
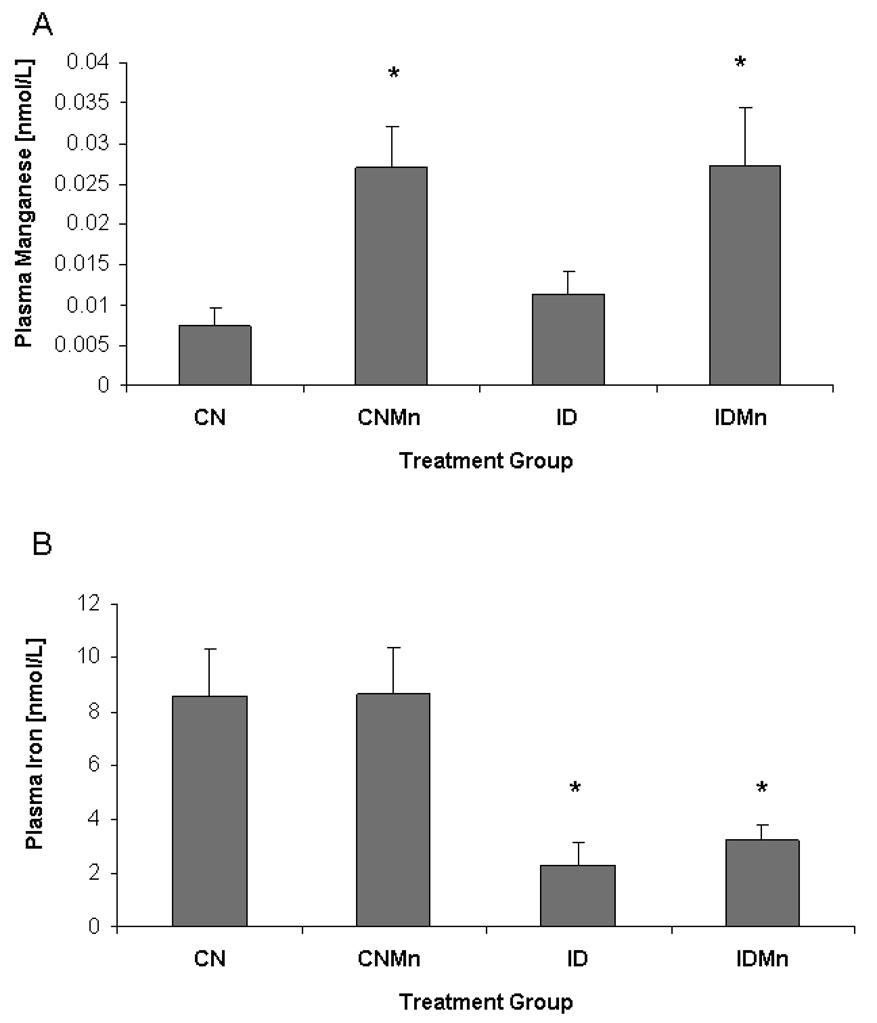
Plasma Mn concentrations were significantly increased in those animals receiving Mn supplementation versus those animals receiving deionized water alone (p=0.02) (Figure 1A), as previously reported (Anderson et al., 2008). A significant decrease in plasma Fe concentrations was observed in animals receiving the ID diet versus the CN diet (p=0.007) (Figure 1B).
Figure 1.
Plasma metal concentrations at six weeks. (A) Plasma manganese concentrations expressed as nmol/L were significantly increased in those animals receiving manganese supplementation versus those animals receiving deionized water alone (p=0.02) (n=24). (B) A significant decrease in plasma iron concentration was observed in animals receiving the ID diet versus the CN diet (p=0.007).
Brain manganese and iron
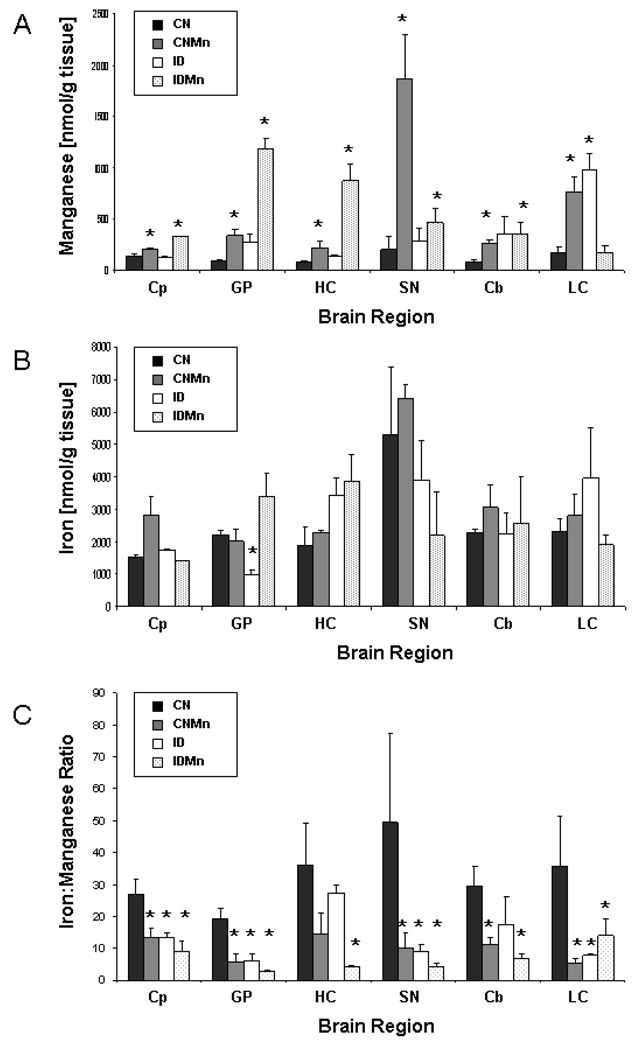
As previously reported, Mn concentration was significantly increased in the Mn-exposure group versus those animals that did not receive Mn supplementation in all brain regions (p<0.05) (Figure 2A). A heterogeneous response was observed in the brain in regard to Fe levels, with a general decrease in Fe concentration in those animals receiving the ID diet versus animals receiving the CN diet, though the only significant decrease was seen in the globus pallidus (p<0.05) (Figure 2B). The Fe:Mn ratio is reported in Figure 2C, illustrating a clear effect of Mn exposure on Fe homeostasis.
Figure 2.
Brain metal concentrations at six weeks. Overall, Mn exposure caused a significant increase in brain regional Mn concentrations versus CN. Mean concentrations ± SEM are shown for manganese (A) and iron (B) for caudate putamen (Cp), globus pallidus (GP), hippocampus (HC), substantia nigra (SN), cerebellum (Cb), and locus coeruleus (LC). The Fe:Mn ratio is also reported, illustrating the impact of Mn exposure on Fe homeostasis (C). CN is represented in black (n=6), CNMn in gray (n=6), ID in white (n=6), and IDMn in dotted area (n=6). Asterisks (*) indicate a statistically significant difference from CN according to Dunnett’s post-hoc analysis.
In Vitro Studies
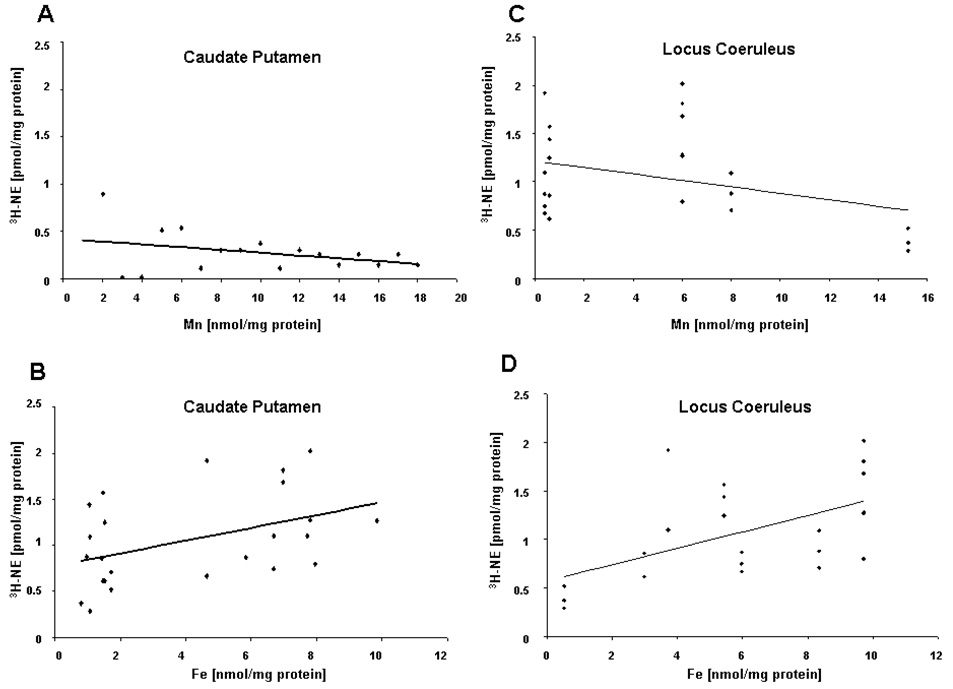
Iron level was associated with a significant decrease in 3H-NE uptake in synaptosomes isolated from the caudate putamen after six weeks of dietary intervention (R=0.436; p=0.033) (Figure 3B). No significant association was observed between Mn exposure and 3H-NE uptake in the caudate putamen (Figure 3A). Increased Mn concentration was associated with a significant decrease in 3H-NE uptake in synaptosomes isolated from the locus coeruleus at six weeks (R=−0.753; p=0.001) (Figure 3C). Iron level was associated with a significant decrease in 3H-NE uptake in the locus ceruleus (R=0.765; p<0.001) (Figure 3D).
Figure 3.
Effect of dietary treatment on uptake of 3H-NE. 3H-NE uptake decreased with increased Mn concentration in the locus coeruleus, with decreased Fe associated with decreased uptake of 3H-NE in the caudate putamen and locus coeruleus. Correlational analysis of 3H-NE uptake in the caudate putamen versus (A) synaptosomal Mn concentration and (B) synaptosomal Fe concentration (R=0.436; p=0.033). Correlation analysis of 3H-NE uptake in the locus coeruleus versus (C) synaptosomal Mn concentration (R=−0.753; p=0.001) and (D) synaptosomal Fe concentration (R=0.86; p<0.001) (n=24).
Microdialysis Studies
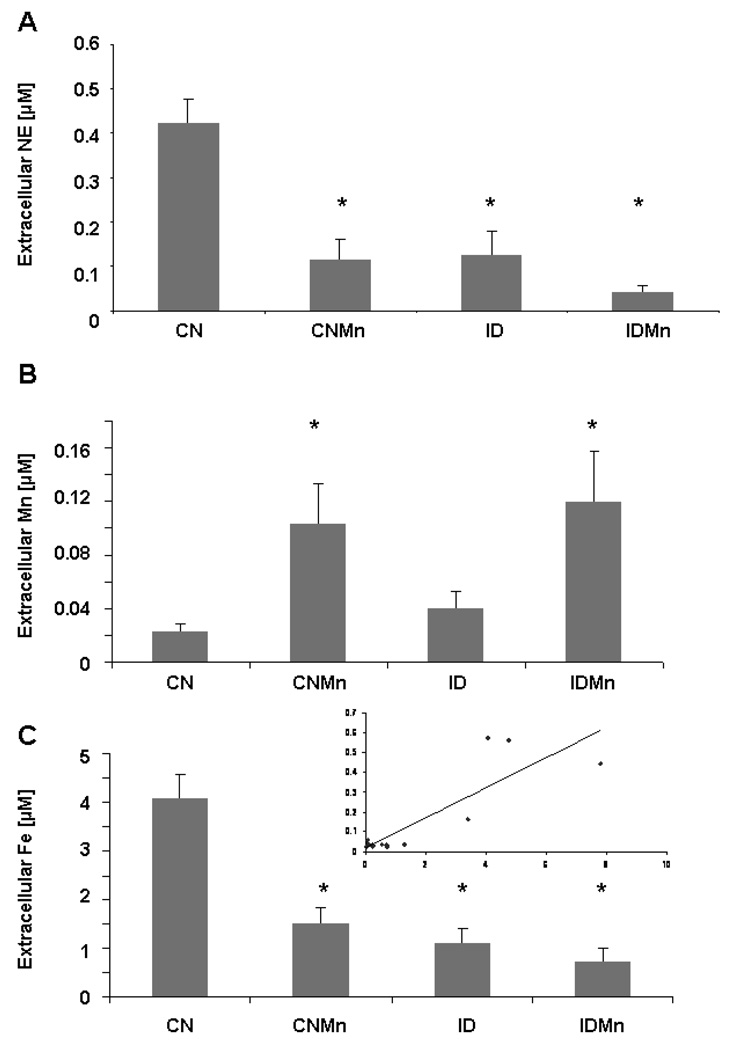
Extracellular concentrations of NE were significantly decreased in response to Mn exposure and ID in the caudate putamen versus CN (p<0.001) (Figure 4A). In the dialysate samples, Mn concentrations were significantly increased in CNMn and IDMn versus CN (p<0.05) (Figure 4B). Extracellular concentration of Fe was significantly decreased in all dietary treatments versus CN (p<0.05) (Figure 4C). A significant correlation was observed between extracellular NE and Fe concentrations, with decreased NE associated with decreased Fe (R=0.86; p<0.001) (Figure 4C inset). No significant correlation was observed between extracellular NE and Mn concentrations.
Figure 4.
Microdialysate analysis. Extracellular concentrations of NE in the caudate putamen were significantly decreased in response to Mn exposure and ID. Mean concentrations ± SEM are shown for (A) extracellular NE, (B) manganese, and (C) iron in microdialysate samples from the caudate putamen after six weeks of dietary treatment (n=24). Inset: Correlational analysis of extracellular NE versus extracellular Fe (R=0.86; p<0.001). *p<0.001
Western Blot Analysis
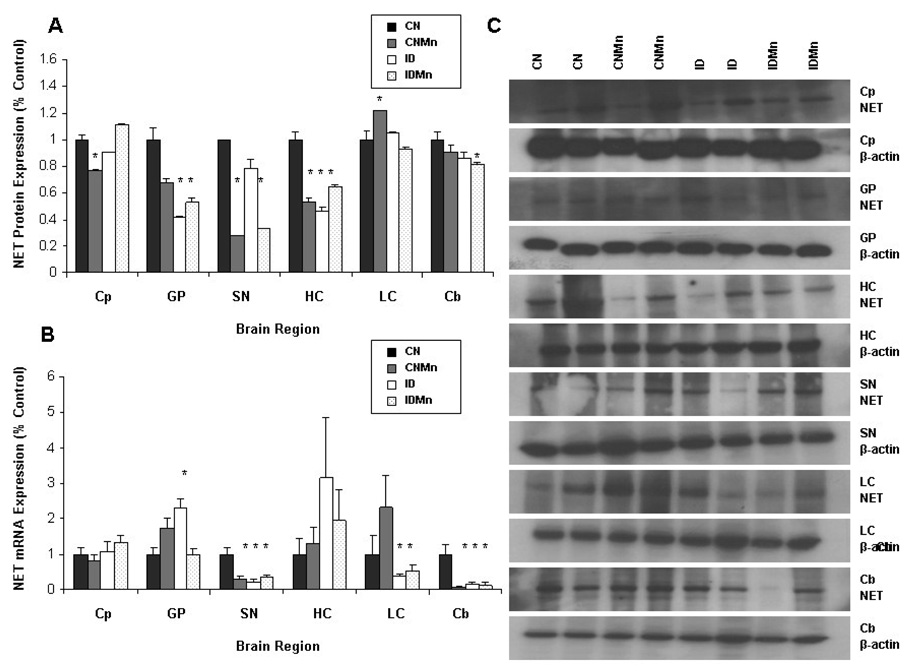
Elevated brain Mn and decreased Fe caused an approximate 50% decrease in NET protein expression in the globus pallidus, hippocampus, substantia nigra, and locus ceruleus (Figure 5A). NET protein expression was significantly decreased in the globus pallidus (CNMn 33%); ID 59%; IDMn 47%) substantia nigra (CNMn 73%; IDMn 68%), hippocampus (CNMn 48%; ID 55%; IDMn 36%), and locus coeruleus (IDMn 43%) versus CN (p<0.05) (Figure 5A). No significant change in NET protein expression was observed in the caudate putamen or cerebellum (Figure 5A). The dietary treatment had no effect on β-actin protein levels. Representative blots for NET and β-actin for each region are shown, with each band representing an individual animal (Figure 5C).
Figure 5.
Effect of dietary treatment on NET protein and mRNA expression. Overall, Mn exposure and ID lead to a decrease in NET protein and mRNA expression. Mean expression as percentage of control ± SEM for NET (A) protein and (B) mRNA relative to β-actin are shown for caudate putamen (Cp), globus pallidus (GP), hippocampus (HC), substantia nigra (SN), locus coeruleus (LC), and cerebellum (Cb) (n=24). CN is represented in black, CNMn in gray, ID in white, and IDMn in dotted area. (C) Representative blots for NET and β-actin for each region are shown, with each band representing an individual animal. *p<0.05 according to Dunnett’s post-hoc analysis
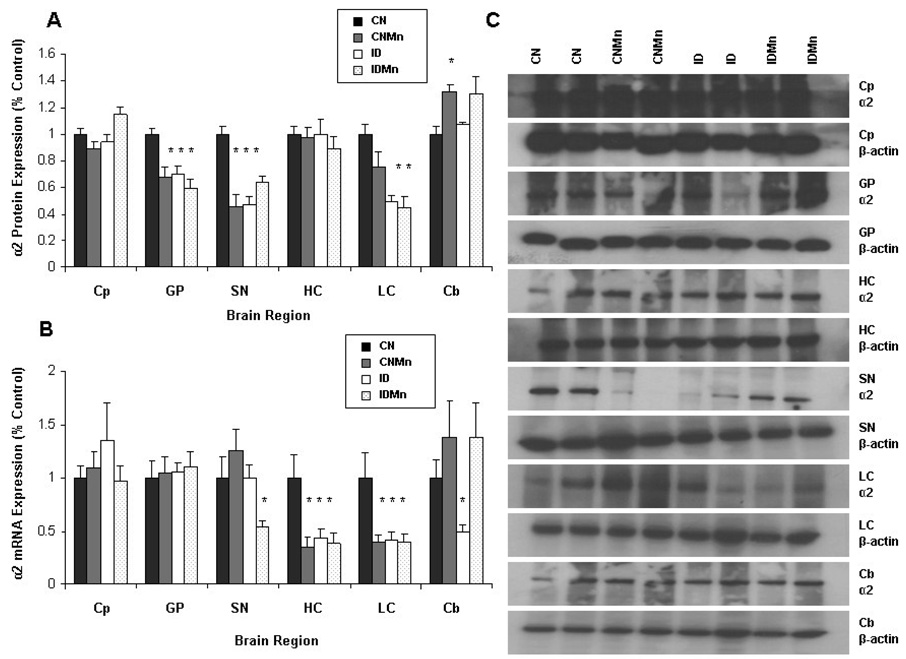
Iron deficiency and Mn exposure were associated with a decrease in protein expression of α2 adrenergic receptor in the globus pallidus (CNMn 33%; ID 30%; and IDMn 41%), the substantia nigra (CNMn 55%; ID 54%; and IDMn 37%), and the locus coeruleus (ID 51%; IDMn 56%) versus CN (p<0.05) (Figure 6A). No significant change in α2 adrenergic receptor protein expression was observed in the caudate putamen or cerebellum (Figure 6A). The dietary treatment had no effect on β-actin protein levels. Representative blots for α2 adrenergic receptor and β-actin for each region are shown, with each band representing an individual animal (Figure 6C).
Figure 6.
Effect of dietary treatment on α2 receptor protein and mRNA expression. Overall, Mn exposure and ID lead to a decrease in α2 receptor protein and mRNA expression. Mean expression as percentage of control ± SEM for α2 receptor (A) protein and (B) mRNA relative to β-actin are shown for caudate putamen (Cp), globus pallidus (GP), hippocampus (HC), substantia nigra (SN), locus coeruleus (LC) and cerebellum (Cb) (n=24). CN is represented in black, CNMn in gray, ID in white, and IDMn in dotted area. (C) Representative blots for α2 receptor and β-actin for each region are shown, with each band representing an individual animal. *p<0.05 according to Dunnett’s post-hoc analysis
Quantitative PCR analysis
Increased Mn and ID significantly decreased mRNA expression of NET in the substantia nigra (CNMn 61%; ID 72%; IDMn 55%; p<0.05) and cerebellum (CNMn 93%; ID 86%; IDMn 87%; p<0.05) (Figure 5B). In the locus coeruleus, an increase in mRNA expression of NET was observed in CNMn (130%), while a significant decrease was observed in ID (64%) and IDMn (49%) (p<0.05) (Figure 5B). Expression of NET mRNA was significantly increased in the globus pallidus (CNMn 73%; ID 127%; p<0.05) (Figure 5B). No significant change in NET mRNA expression was observed in the hippocampus. The dietary treatment had no effect on β-actin mRNA levels.
Iron deficiency and Mn exposure were associated with a decrease in mRNA expression of α2 adrenergic receptor in the hippocampus (CNMn 63%; ID 57%; IDMn 62%; p<0.05), locus coeruleus (CNMn 61%; ID 60%; IDMn 61%; p<0.003), and substantia nigra (IDMn 47%; p<0.05) (Figure 6B). Iron deficiency was associated with a significant decrease in α2 adrenergic receptor mRNA expression in the cerebellum (ID 51%; p<0.05) (Figure 6B). Expression of α2 adrenergic receptor mRNA was not significantly affected in the caudate putamen or globus pallidus. The dietary treatment had no effect on β-actin mRNA levels.
3. Discussion
While previous studies have examined the effects of Mn exposure and ID on DA and GABA biology (Anderson et al., 2007; 2008), little research had been conducted to investigate these effects on NE biology. This is the first study to date to probe both of these paradigms with regard to the noradrenergic system. Using synaptosomes isolated from the caudate putamen and locus coeruleus, a significant correlation was observed between synaptosomal Fe concentration and 3H-NE uptake after six weeks of dietary intervention (Figure 3), while a significant inverse correlation was seen between synaptosomal Mn concentration and 3H-NE uptake in synaptosomes isolated from the locus ceruleus (Figure 3). These data demonstrate that Fe and Mn are inversely related in terms of neurochemistry (i.e., low Fe and high Mn associated with attenuated NE uptake), with the effect being brain region dependent. Exposure to Mn and decreased Fe significantly decreased extracellular concentrations of NE in the caudate putamen (Figure 4A), with a significant correlation observed between extracellular concentration of NE and Fe (Figure 4C inset), but not Mn. In addition, dietary Mn exposure was shown to alter expression of NE receptor (Figure 6) and transport (Figure 5) proteins and mRNA in vivo, with a varied effect observed across the brain regions examined. These data suggest that altered levels of NE due to Mn exposure and reduced Fe levels may be the result of changes in expression of transport and receptor proteins in the locus coeruleus and basal ganglia, leading to perturbations in extracellular concentrations of NE in the caudate putamen.
As in earlier studies, our dietary protocol led to an increase in systemic levels of Mn, as evidenced by increased plasma Mn concentrations (Figure 1A). In general, Fe was depleted in those animals receiving the ID diet (Figure 2B); however, the only statistical difference in Fe levels in the brain was observed in the globus pallidus, as reported before (Anderson et al., 2008) and similar to a previous study from our lab (Erikson et al., 2004). This varied response in Fe levels has been observed in our previous studies at both four weeks (Anderson et al., 2007) and six weeks (Anderson et al., 2008; 2007) of dietary exposure, with Fe levels varying between and within regions. Further, when the Fe:Mn ratio is examined, a clear reduction in brain Fe in these animals is apparent, with the ID diet causing a significant decrease in the Fe:Mn ratio in most regions (Figure 2C).
Based on our previous studies (Anderson et al., 2008; 2007), we set out to directly examine the effects of Mn exposure and ID by measuring 3H-NE uptake in synaptosomes. Synaptosomes were chosen due to their simplicity and separation from other interacting neuronal systems in order to provide direct interpretation of results (Whittaker, 1993). Decreased uptake of 3H-NE was observed after six weeks of dietary treatment in synaptosomes isolated from the caudate putamen and the locus coeruleus, though the effects were region dependent (Figure 3). Conversely, reduced Fe levels were associated with a decrease in 3H-NE uptake in the caudate putamen and locus coeruleus. This inverse relationship between Mn and Fe has been reported in our previous study with regard to 3H-GABA uptake (Anderson et al., 2007). The differential response observed in the present study may be an effect of time, as these results represent a snapshot of the ongoing neurochemical changes occurring over the course of the dietary intervention. For example, in our previous study, 3H-GABA uptake was affected by Mn exposure and ID differently from four weeks to six weeks of dietary intervention, with major effects observed during week six, leading to the premise of the current study examining the effects of Mn exposure and ID on NE uptake at six weeks. Manganese itself has been shown to affect the uptake of NE in a dose-dependent manner in rat forebrain synaptosomes, with selectivity for inhibition of NE uptake versus that of DA or GABA (Lai et al., 1982), and in rat whole brain synaptosomes as well (Chandra et al., 1984). These effects on uptake of NE in the caudate putamen and locus coeruleus imply altered extracellular concentrations of NE, which could in turn affect the functioning of the nigrostriatal pathway and behaviors associated with the noradrenergic system (Figure 7).
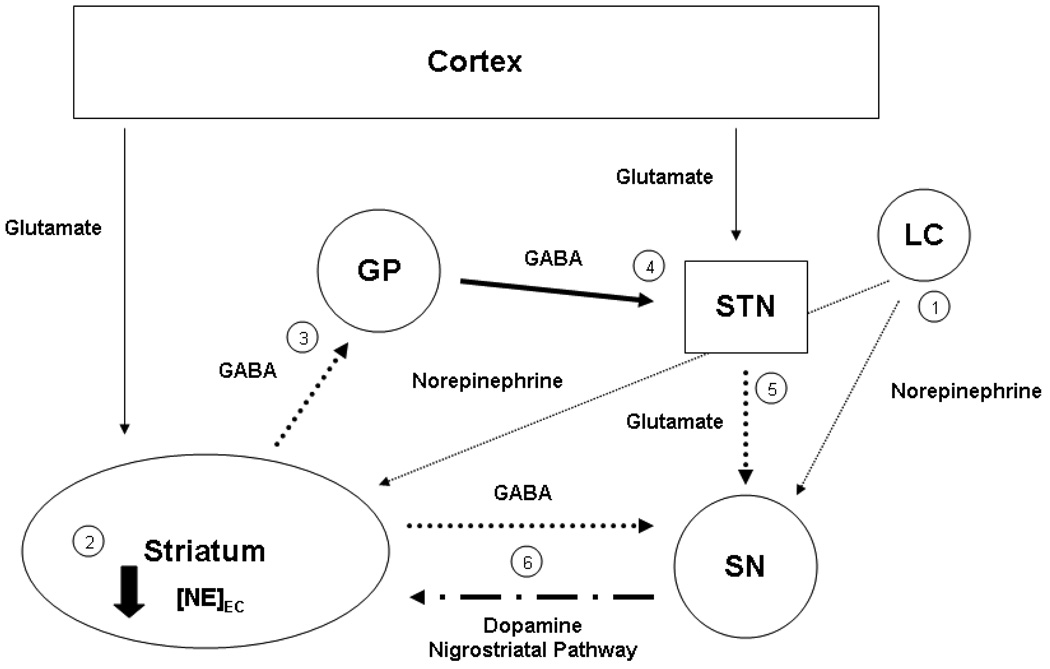
Figure 7.
NE biology during Mn overload and reduced Fe. This simple schematic of the basal ganglia represents the potential cause and consequences of the decreased extracellular NE concentrations in the striatum (caudate putamen) due to alterations of Mn and Fe status observed in the current study. (1) Altered expression of NE transport and receptor proteins and/or neuronal loss in the locus coeruleus (LC) would lead to a decrease in NE release (thin dotted line), (2) decreasing extracellular NE concentrations in the striatum, reducing the activity of the GABA striatopallidal projection neurons (3) (dotted line). This reduction in activity would (4) increase the GABAergic inhibitory firing from the globus pallidus (GP) to the subthalamic nucleus (STN) (heavy black line), in turn (5) decreasing the excitatory glutamatergic firing from this region to the substantia nigra (SN) (dotted line). (6) Decreased glutamatergic excitation in the SN, along with decreased GABAergic inhibition from the striatonigral projection neurons (dotted line), decreased adrenergic activity from the LC (1) (thin dotted line), and decreased protein expression of NET and α2 adrenergic receptor, would lead to a dysregulation of dopaminergic firing to the Cp along the nigrostriatal pathway (alternating line).
Extracellular concentrations of NE in the caudate putamen were significantly decreased in response to Mn exposure and decreased Fe (Figure 4A). Extracellular metal concentrations were similar to those observed in our previous microdialysis study (Anderson et al., 2008). A correlation between extracellular NE and Fe concentrations was also observed, suggesting that lowered Fe levels (due to both dietary deficiency and Mn-exposure) affect the concentration of extracellular NE. This is not surprising given that Fe is involved in the synthesis of norepinephrine through the activity of tyrosine hydroxylase (Beard et al., 1994). Additionally, Fe status and brain Mn levels affect brain levels of copper (Cu) (Garcia et al., 2007; Erikson et al., 2004), which may adversely impact conversion of dopamine to norepinephrine via the Cu-dependent enzyme dopamine β-hydroxylase (Pyatskowit and Prohaska, 2007) in the locus coeruleus (Zecca et al., 2004).
With no alterations in protein and mRNA expression of NET observed in the caudate putamen, and given the fact that little if any NE is synthesized in this region, these changes in NE concentration are most likely the result of alterations in NE biology in the locus coeruleus and the ventral medullary nuclei, major loci of adrenergic activity in the brain that play a neuromodulatory role in the caudate putamen and the nigrostriatal pathway (Marien et al., 2004). Data supporting direct innervation of the caudate putamen by the locus coeruleus is mixed (Berridge and Waterhouse, 2003). Even without direct innervation, NE may affect the caudate putamen in a more paracrine fashion via extra-synaptic release from surrounding regions (Marien et al., 2004). Still, the involvement of the locus coeruleus in modulating activity of the nigrostriatal pathway is clear. The locus coeruleus is important for the facilitation and maintenance of the nigrostriatal dopaminergic pathway, a role supported by anatomical, electrophysiological, neurochemical, and behavioral studies in animals (see review by Marien et al., 2004). This pathway is not only affected in Parkinson’s disease, but in manganese neurotoxicity as well. Degeneration of the locus coeruleus may precede and potentially surpass dopaminergic degeneration in the substantia nigra (Rommelfanger and Weinshenker, 2007), as both regions share anatomical and biochemical similarities (Zecca et al., 2004), and depletion of NE in the substantia nigra by greater than 80% is a hallmark of idiopathic Parkinson disease (Marien et al., 2004), a condition similar to Mn neurotoxicity. The attenuated levels of NE observed in the caudate putamen may result from neuronal loss in the locus coeruleus and/or from perturbations in NET and receptor expression, not only in the locus coeruleus but in other regions of the basal ganglia, affecting the nigrostriatal pathway and functioning of the caudate putamen (Figure 7).
ID has been shown to affect both NET (Beard et al., 2006; Burhans et al. 2005) and DAT density and function (Erikson et al., 2000; 2001), while Mn exposure and ID alter GAT-1 protein and mRNA expression (Anderson et al., 2008). DAT, GAT-1, and NET are all members of the solute carrier-6 (SLC-6) transporter family, sharing similar amino acid sequences and pump mechanisms (Gether et al., 2006; Mandela and Ordway, 2006), leading to the possibility of similar effects of Mn exposure on NET as those demonstrated with respect to DAT and GAT-1. Indeed, NET protein expression was significantly decreased (Figure 5A), and may result from a decrease in mRNA expression in some regions (Figure 5B).
While Mn is not known to have specific actions on the transcription of NET, Mn has been shown to bind to various forms of DNA structure, leading to conformational changes that might potentially affect the efficiency of gene transcription (Hazell et al., 2003; Kennedy and Bryant, 1986). Some shared transcription factors of both DAT and NET (Burhans et al., 2005) are influenced by cellular Fe levels, with ID leading to a potential decrease in protein expression (Bianco et al., 2008; Ruiz et al., 2000; Kramer-Stickland et al., 1999). Protein expression of NET may also be attenuated through post-translational processes, such as recycling and degradation, mediated by regulatory kinases that may be affected by excess Mn, a scenario that may be occurring in the hippocampus in this instance. Protein kinase C (PKC) is known to regulate NET via phosphorylation, causing internalization of these proteins, degradation, and recycling (Mandela and Ordway, 2006). An acute Mn treatment (300 µM) has been shown to activate caspase-3 leading to activation of PKCδ in N27 mesenchephalic cells (Latchoumycandane et al., 2005). A similar response was seen in those cells incubated for 72 hours with a lower dose of Mn (50 µM), replicating chronic exposure. This 300 µM dose represents a physiologically relevant dose similar to that achieved in the current study. Also, increased expression of PKC-β1 has been reported in the caudate putamen during ID (Bianco et al., 2008), indicating further potential for PKC signal transduction. Decreases in both mRNA and protein expression of NET might indicate neuronal loss as a result of Mn exposure (Sloot et al., 1994), via caspase-mediated apoptosis (Latchoumycandane et al., 2005). In addition, necrotic neuronal loss may be occurring through a caspase-independent pathway as well (Roth et al., 2000).
An increase in NET mRNA was observed in the globus pallidus in response to Mn exposure and the ID diet (Figure 5B). This increase in NET mRNA in the globus pallidus may be a compensatory mechanism in response to the decrease seen in NET protein expression in that region. In the locus coeruleus, a varied effect of Mn exposure and ID on NET mRNA expression was observed, with an increase in CNMn animals and a decrease in ID and IDMn animals (Figure 5B). This varied response is most likely indicative of a region in which a variety of mechanisms are occurring simultaneously, such as compensation for NET protein loss through degradation pathways, effects on gene transcription, and/or neuronal loss. Action of NE at pre-synaptic α2 adrenergic receptors, which regulate NE activity and NET expression (Marien et al., 2004), may also be involved in this region, and the others examined.
Expression of NET may be affected by the action of pre-synaptic autoreceptors, which can play a part in regulating expression of the protein (Mandela and Ordway, 2006; Zahniser and Doolen, 2001). Decreases in these autoreceptors (specifically α2 adrenergic receptor) could result in a general decrease of NET density in that region. As anticipated, α2 adrenergic receptor protein and mRNA expression were decreased (Figure 6A and 6B) in regions in which NET protein expression was also lowered (Figure 5A). These losses in α2 adrenergic receptor expression would lead to dysregulation of the noradrenergic system in the locus coeruleus and alterations in NE synthesis and release (Marien et al., 2004). Lesions of the locus coeruleus and chronic attenuated levels of NE have been shown to affect DA concentrations in the caudate putamen using knockout mouse models of the α2 adrenergic receptor (Marien et al., 2004). Dysfunction of the adrenergic system in the locus coeruleus could further exacerbate the dysregulation of the nigrostriatal pathway observed during Mn neurotoxicity, leading to perturbations in motor activity (Figure 7).
In conclusion, the alterations observed in protein and mRNA expression of NE receptor (Figure 6) and transport proteins (Figure 5) were not universal, with a diverse effect of Mn exposure and ID-associated Mn accumulation on these two indices not only from region to region, but, in some cases, within the same region (such as the locus coeruleus), consistent with the effects on the GABAergic system that we have previously reported (Anderson et al., 2008; 2007). The alterations in NE biology observed in this study, including attenuated uptake (Figure 3), reductions in extracellular concentrations (Figure 4), and effects on NE transporter (Figure 5) and receptor (Figure 6) protein and gene expression, could have profound effects on the functioning of the locus coeruleus causing dysregulation of the nigrostriatal pathway leading to behavioral alterations such as reduced motor activity, impaired cognition, and increased anxiety (Meyer and Quenzer, 2005; Beard et al., 2006). Furthermore, the novel results of this study illustrate the complex mechanisms at play during Mn neurotoxicity and ID. Whether our findings in this study are due to either Mn exposure or ID alone is difficult to say, given that these two pathologies may be concurrent. When examining these data in conjunction with our previous studies (Erikson et al., 2002; Anderson et Al., 2008; 2007), it is clear that the neurobiological changes that are related to extracellular neurotransmitter concentrations are due to the lowered Fe levels (Figure 4C; Anderson et al., 2008), whereas those changes related to tissue neurotransmitter biology are due to increased Mn levels in our current experimental model (Figure 3C; Erikson et al., 2002; Anderson et al., 2007; 2008). It is also quite possible that increased Mn and decreased Fe levels due to Mn exposure and ID may be affecting concentrations of other metals, particularly Cu (Garcia et al., 2007) which would have a significant impact on NE biology (Pyatskowit and Prohaska, 2007). Thus, future studies that tease apart the effects of the alterations of these metals on neurochemical functioning are critical in order to develop effective modalities not only for the treatment of vulnerable populations (e.g., Mn-exposed workers, ID individuals), but also for understanding the etiology of neurodegenerative diseases where brain metal imbalances are implicated (e.g., Parkinson’s Disease, Huntington’s Disease, Alzheimer’s Disease).
4. Experimental Procedure
Animals
Male weanling Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) (n=48 for synaptosome studies; n=24 for microdialysis studies; n=24 for Western and PCR analysis) were randomly divided into four dietary treatment groups as in previous studies (Anderson et al., 2008; 2007): control (CN; 35 mg Fe/kg, 10 mg Mn/kg diet & d.i. water); control Mn-exposed (CNMn; control diet & 1 g Mn (as MnCl2)/L d.i. water); iron-deficient (ID; 4 mg Fe/kg, 10 mg Mn/kg diet & d.i. water); and iron-deficient/Mn-exposed (IDMn; ID diet & 1 g Mn/L d.i. water). Diets were obtained from Bio-Serv (Frenchtown, NJ) and certified for metal content. Rats had free access to food and water 24 hr/day, with the lights off between 1800 and 600 h and room temperature maintained at 25 ± 1° C. The University of North Carolina at Greensboro Animal Care and Use Committee approved all of the animal procedures.
In vitro studies
Synaptosomes were utilized to indirectly assess the effect of ID and Mn exposure on disturbances in NE biology by measuring 3H-NE uptake, following our previously described (Anderson et al., 2007) modified method from Cotman et al. (1981). After six weeks of dietary treatment, brain regions (caudate putamen and locus coeruleus) from two rats per treatment group were pooled for each individual experiment. Briefly, tissue was homogenized using a Teflon/glass homogenizer in 20 volumes of ice-cold 0.32 M sucrose-HEPES, pH 7.4. The homogenate was then centrifuged at 2000 × g for 10 minutes at 4° C. The supernatant was removed and centrifuged at 20, 000 × g for 15 minutes at 4° C. The pellet was resuspended in 10 ml fresh ice cold Krebs-Ringer-HEPES (KRH) buffer [118.4 mM NaCl, 1.18 mM MgSO4, 4.7 mM KCl, 1.2 mM KH2PO4, 10.0 mM HEPES, 5.6 mM dextrose (pH 7.4)]. An aliquot of 200 µl of the synaptosomes was placed in a tube with 50 µl KRH buffer. A 10 µl aliquot of 3H-NE (50 nM at a specific activity of 10.9 Ci/mmol) (NEN, Boston, MA) was added to each tube and tubes were incubated for 15 minutes at 37° C. The reaction was stopped by adding 5 mL ice-cold KRH buffer to each tube, followed by rapid filtration through a GF/F Whatman fiberglass filter on a Millipore sampling manifold. Filters were washed twice with ice cold KRH, placed in scintillation vials with 5 ml scintillation cocktail, and counted on a Beckman LS scintillation counter (Beckman, Fullerton, CA). An additional set of tubes, one for each treatment group, was incubated on ice (approximately 4° C) to determine non-specific uptake. Protein analysis was performed utilizing the bicinchoninic acid (BCA) method (Pierce, Rockford, IL) to determine total protein of the synaptosome fraction.
Stereotaxic Surgery
After five weeks of dietary treatment and one week prior to microdialysis experiments, rats were anesthetized with ketamine-HCl (80 mg/kg) and xylazine (12 mg/kg) (IDMn dosage: ketamine-HCl 60 mg/kg; xylazine 8 mg/kg) and maintained on a heating pad at 37° C. The heads of the rats were shaved and wiped with a 5% povidone-iodine solution to reduce risk of infection. Sterile instruments and gloves were used throughout the surgical procedure. The rats were secured in the stereotaxic frame and an incision was made perpendicular to the bregma. A guide cannula (CMA/12, CMA Microdialysis, Acton, MA) was implanted into the caudate putamen using the following coordinates: 2.4 mm lateral to the midline, 7.5 mm anterior to the lambda. The cannula was lowered to a depth of 2.5 mm, positioning it in the medial area of the caudate putamen (Paxinos and Watson, 1998). Anchoring screws were utilized to maintain the position of the cannula before being cemented into place using dental adhesive. Animals were given 0.9% sterile saline (0.5 ml/kg body weight, i.p.) to reduce fluid lost while under anesthesia and to aid in recovery time. Animals were also given the xylazine reversal agent Antisedan (Atapimazole) (0.1 mg/kg body weight, i.p.) (Allivet, Hialeah, FL), to reduce recovery time. Animals were returned to shoebox cages with Tek-Fresh bedding (Harlan, Indianapolis, IN) and monitored daily until microdialysis experiments began.
Microdialysis
During week six of the dietary protocol, a microdialysis probe (CMA/12 Elite, CMA Microdialysis, Acton, MA) was inserted into the guide cannula and the rat was perfused with artificial cerebral spinal fluid (aCSF) (155 mM Na+, 0.83 mM Mg2+, 2.9 mM K+, 132.76 mM Cl−, 1.1 mM Ca+, pH 7.4) for one hour at a flow rate of 1 µl/min. After perfusion, the flow rate was adjusted to 0.5 µl/min and 30 minute fractions were collected in microtubes for a total of four and a half hours (9 samples per rat) in a refrigerated fraction collector (CMA Microdialysis, Acton MA). Samples were stored at −80° C until analysis of the dialysate fraction. To quantify levels of NE from the microdialysate, fractions were analyzed using capillary electrophoresis with laser induced fluorescence detection (CE-LIF) (Biorad Biofocus 2000, Hercules, CA, with 488 nm diode laser/590 nm emission filter). Rats were then returned to their home cage, and the following day were sacrificed, brains removed, and probe placement verified post mortem. Brains were dissected into six regions (caudate putamen, globus pallidus, substantia nigra, hippocampus, locus coeruleus, and cerebellum) for metal, protein, and mRNA analyses (see below). The same individual dissected all brain regions, using brain atlas coordinates (Paxinos and Watson, 1998) and a stainless steel rat brain matrix for coronal sectioning. Regions were selected based on the known heterogeneous accumulation of metals in response to alterations in dietary Fe and Mn levels (Anderson et al., 2008; 2007; Erikson et al., 2002) and the density of adrenergic neurons.
CE-LIF analysis
A protocol by Chen et al. (2001) allowing for detection of amino acids and biogenic amines at nanomolar concentrations modified to accommodate the needs of our previous study (Anderson et al., 2008) was utilized in the current study as well. The advantages of applying CE analysis to neuroactive compounds include minimal required sample volumes, speed of analysis, and high separation efficiency (Powell and Ewing, 2005). Briefly, on the day of sample analysis, 5 µl of microdialysate sample were derivatized at 40° C by the addition to 100 nmol ATTOTAG™ FQ fluorogenic reagent (Molecular Probes, Eugene, OR) and 10 µL of a 10 mM borate (Fisher, Fair Lawn, NJ)/ 25 mM KCN (Fluka) solution (pH 9.18). The total sample volume was adjusted to 20 µl using HPLC grade methanol (G.J. Chemical Company, Newark, NJ). After a minimum reaction time of 90 min., 1 µl of an FQ derivatized homoserine (Sigma, St.Louis, MO) internal standard solution was added to the derivatized microdialysate sample and analyzed. CE-LIF conditions leading to high efficiency peaks for NE samples were 10 kV for 10 min with sample injections at 10 psi/sec. Uncoated silica capillary (Polymicro, Arizona) with an i.d. of 25 µm, o.d. of 361 µm, and effective/total lengths of 25.4/30.0 cm was used. The run buffer was 15 mM sodium borate (Fisher), pH 9.0, with 45 mM sodium dodecyl sulfate (Pierce, Rockford, IL), 5 mM sodium cholate (Anatrace, Maumee, OH), and 4% (v/v) 2-propanol (Fisher). Three replicates were analyzed for each sample, with a calibration curve for NE constructed each day of sample analysis using three points with a concentration range of 5 µM to 10 µM NE. Norepinephrine (Sigma) and homoserine standard solutions used for construction of calibration curves were prepared in aCSF with the same composition as that used in the microdialysis studies. To verify that NE content in microdialysate samples fell within the sensitivity range of the method, a calibration curve was constructed by serial dilution of derivatized standards until such time as no discernable peak was obtained for NE. From this, the limit of detection for NE, defined as 3σ/m, where σ represents the standard deviation of the background and m represents the slope or sensitivity of the calibration curve, was determined to be 59 nM for this method, with a linear dynamic range of 2.93 decades. The ratio of NE peak height to homoserine peak height for each sample was used to determine the concentration of NE based on the calibration curve response.
Protein extraction
Protein was extracted from the brain tissue samples for Western blot analysis. Tissue samples were sonicated in 500 µl of RIPA lysis buffer (1% Nonidet 40, 1% SDS, 0.5% sodium deoxycholate, 1 mM NaF, 2 mM β-glycerolphosphate, 1 mM sodium orthovanadate, and 1X protease inhibitor cocktail (Sigma, St. Louis, MO) in 1X PBS) on ice until completely homogenized. Homogenates were incubated on ice for 20 minutes before being centrifuged at 10,000 × g for 20 minutes at 4° C. Supernatant was then transferred to new tubes and total protein concentration determined by BCA assay (Pierce, Rockford, IL) before proceeding with Western analysis.
Western blot analysis
Western blot analysis was conducted to examine the effects of the dietary treatment on expression of NET and α2 adrenergic receptor in vivo. The α2 adrenergic receptor was chosen due to its role in regulating adrenergic activity and expression of NET (Marien et al., 2004). Protein samples (20 µg) were combined with 4X LDS sample buffer (Invitrogen, Carlsbad, CA) containing 5% β-mercaptoethanol and heated at 70° C in a heat block for 10 minutes. Samples were then loaded onto a 4–12% Bis-Tris pre-cast mini gel (Invitrogen, Carlsbad, CA) and electrophoretically separated under denaturing conditions in 1X MOPS buffer containing 1% antioxidant (Invitrogen, Carlsbad, CA). Proteins were transferred to a PVDF membrane (Millipore, Billerica, MA) before blocking with 5% BSA. Membranes were probed overnight at 4° C with primary antibody (rabbit polyclonal anti-NET; rabbit polyclonal anti-α2 adrenergic receptor; Chemicon, Temecula, CA) (mouse monoclonal anti-β-actin; Santa Cruz Biotech, Santa Cruz, CA) for the protein of interest in 5% BSA. Membranes were rinsed in 1X TBST (10 mM Tris, pH 7.4, 150 mM NaCl, 0.05% Tween 20) and probed for 2 hours at room temperature with an HRP-conjugated secondary antibody (goat anti-rabbit; Chemicon, Temecula, CA) (goat anti-mouse; Santa Cruz Biotech, Santa Cruz, CA) in 5% BSA. Membranes were then rinsed several times in 1X TBST before incubation in ECL solution (Perkin Elmer, Waltham, MA) and exposure to radiographic film (Pierce, Rockford, IL). Membranes were probed for β-actin to verify equal loading and for image analysis. This housekeeping protein was chosen for comparison over GAPDH, which has been shown to be affected by Mn concentrations (Hazell, 2002). Films were analyzed using image analysis software (Image J, NIH, Bethesda, MD), with the amount of the target protein from each sample standardized to the amount of β-actin from the same sample.
RNA isolation and cDNA synthesis
Total RNA was isolated from brain regions for quantitative PCR analysis. Tissue samples were stored in 1 ml of RNAlater® solution (Ambion Inc., Austin, TX) and stored at −20° C until analysis. Total RNA was isolated utilizing the ToTALLY RNA™ kit (Ambion Inc., Austin, TX) following manufacturer’s instructions. RNA concentration and purity were determined by spectrophotometric analysis before carrying out cDNA synthesis. Synthesis of cDNA from total RNA was performed using the High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA) following manufacturer’s instructions.
Quantitative PCR
Quantitative real-time PCR analysis was utilized to determine the effects of the dietary protocol on the mRNA expression of NET and α2 adrenergic receptor. Triplicate aliquots of cDNA were analyzed on 96-well plates using expression assays for the genes of interest obtained from Applied Biosystems (Foster City, CA). Values of cDNA expression were normalized relative to the expression of β-actin analyzed from the same sample on the same plate and reported as percent of control.
Metal analysis
Tissue Mn and Fe concentrations were measured with graphite furnace atomic absorption spectrometry (Varian AA240, Varian, Inc., USA). Blood samples were collected at the end of the experiment into heparinized tubes, cooled to 4° C, and centrifuged for 15 minutes to separate cells from plasma. Plasma was frozen at −80° C until analyzed for Mn and Fe. Equal volumes of plasma and 0.5% Triton-X were vortexed for 30 s before being centrifuged at 12,000 × g for 10 minutes. The supernatant was removed and an aliquot of 50 µl brought to 1 ml total volume in 2% nitric acid and analyzed for Mn and Fe content. Brain regions (caudate putamen, globus pallidus, substantia nigra, hippocampus, locus coeruleus, and cerebellum) were digested in ultrapure nitric acid (1:10 w/v dilution) for 48–72 hours in a sand bath (60° C). A 50 µl aliquot of digested tissue was brought to 1 ml total volume with 2% nitric acid for analysis. The unused fraction of synaptosomes from each dietary treatment was centrifuged at 2000 × g for 10 minutes at 4° C. The supernatant was removed and the pellet was digested in 500 µl ultra-pure nitric acid for 48 hours. A 100 µl aliquot was brought up to 1 ml total volume with 2% nitric acid for analysis. Bovine liver (NBS Standard Reference Material, USDC, Washington, DC) (10 µg Mn/g; 184 µg Fe/g) was digested in ultrapure nitric acid and used as an internal standard for analysis (final concentration 5 µg Mn/L; 92 µg Fe/L).
Statistical analysis
Statistical analyses were conducted using SPSS v14. Data were examined for normality of distribution using a one-sample Kolmogorov-Smirnov test and for the presence of outliers by boxplot analysis. Data were analyzed using analysis of variance, with Dunnett’s post-hoc analysis conducted to assess difference from controls when p<0.05. Pearson’s correlational analyses were conducted to examine relationships between: 1) 3H-NE uptake and Mn/Fe concentrations; and 2) extracellular concentrations of NE, Mn, and Fe.
Acknowledgments
This research was supported by NIEHS Grant #ES013791-01 (KME), Wake Forest University (CLC and TLW), and North Carolina Biotechnology Center Award #2007-BRG-1253 (CLC and TLW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JG, Fordahl SC, Cooney PT, Erikson KM. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. NeuroToxicology. 2008;29:1044–1053. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JG, Cooney PT, Erikson KM. Brain manganese accumulation is inversely related to GABA uptake in male and female rats. Tox. Sci. 2007;95:188–195. doi: 10.1093/toxsci/kfl130. [DOI] [PubMed] [Google Scholar]
- Andrade R, Aghajanian GK. Locus coeruleus activity in vitro: intrinsic regulation by a calcium-dependent potassium conductance but not α2-adrenoceptors. J Neurosci. 1984;4:161–170. doi: 10.1523/JNEUROSCI.04-01-00161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit. Rev. Tox. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Autissier N, Rochette L, Dumas P, Beley A, Loireau A, Bralet J. Dopamine and norepinephrine turnover in various regions of the rat brain after chronic manganese chloride administration. Toxicology. 1982;24:175–182. doi: 10.1016/0300-483x(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Jones BC. Cellular iron concentrations directly affect the expression levels of norepinephrine transporter in PC12 cells and rat brain tissue. Brain Res. 2006;1092:47–58. doi: 10.1016/j.brainres.2006.03.071. [DOI] [PubMed] [Google Scholar]
- Beard JL. Iron biology in immune function, muscle metabolism, and neuronal functioning. J Nutr. 2001;131:568s–579s. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Chen Q, Connor JR, Jones BC. Altered monoamine metabolism in caudate putamen of iron-deficient rats. Pharmacol. Biochem. Behav. 1994;48:621–624. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–215. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Martin J, Menzies SL, Dolan KG, Romano MA, Fletcher RJ, Garrick MD, Garrick LM, Connor JR. Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience. 1999;93:1189–1196. doi: 10.1016/s0306-4522(99)00207-9. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr. Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Murthy RC, Husain T, Bansal SK. Effect of interaction of heavy metals on (Na+-K+) ATPase and the uptake of 3H-DA and 3H-NA in rat brain synaptosomes. Acta Pharmacol. et Toxicol. 1984;54:210–213. doi: 10.1111/j.1600-0773.1984.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS. Effect of manganese on synthesis of brain catecholamines in growing rats. Pharmacol. et Toxicol. 1981a;48:349–354. doi: 10.1111/j.1600-0773.1981.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS. Concentrations of striatal catecholamines in rats given manganese chloride through drinking water. J. Neurochem. 1981b;36:683–687. doi: 10.1111/j.1471-4159.1981.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS, Saxena DK. Manganese-induced behavioral dysfunction an its neurochemical mechanism in growing mice. J. Neurochem. 1979;33:1217–1221. doi: 10.1111/j.1471-4159.1979.tb05267.x. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS. Role of iron deficiency in inducing susceptibility to manganese toxicity. Arch. Toxicol. 1976;35:319–323. doi: 10.1007/BF00570272. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wu J, Baker GB, Parent M, Dovichi NJ. Application of capillary electrophoresis with laser-induced fluorescence detection to the determinations of biogenic amines and amino acids in brain microdialysate and homogenate samples. J Chromatography A. 2001;914:293–298. doi: 10.1016/s0021-9673(01)00539-8. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Foster AC, Lanhorn T. An overview of glutamate as a neurotransmitter. Adv. Biochem. Psychopharmacol. 1981;27:1–27. [PubMed] [Google Scholar]
- Davis C, Wolf T, Greger J. Varying levels of manganese and iron affect absorption and gut endogenous losses of manganese by rats. J Nutr. 1992;122:1300–1308. doi: 10.1093/jn/122.6.1300. [DOI] [PubMed] [Google Scholar]
- Deskin R, Bursian SJ, Edens FW. Neurochemical alterations induced by manganese chloride in neonatal rats. Neurotoxicology. 1981;2:65–73. [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M. Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J. Nutr Biochem. 2004;15:335–341. doi: 10.1016/j.jnutbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biolog. Trace Elem. Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol. Biochem. Behav. 2001;69:409–418. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J. Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Expósito I, Del Arco A, Segovia G, Mora F. Endogenous dopamine increases extracellular concentrations of glutamate and GABA in striatum of the freely moving rat: involvement of D1 and D2 dopamine receptors. Neurochem. Res. 1999;24:849–856. doi: 10.1023/a:1020901929419. [DOI] [PubMed] [Google Scholar]
- Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- Galindo A, Del Arco A, Mora F. Endogenous GABA potentiates the potassium-induced release of dopamine in striatum of the freely moving rat: a microdialysis study. Brain Res. Bull. 1999;50:209–214. doi: 10.1016/s0361-9230(99)00199-9. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Tox. Sci. 2007;95:205–214. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. BioMetals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson EM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmcol. Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Normandin L, Nguyen B, Kennedy G. Upregulation of ‘peripheral-type’ benzodiazepine receptors in the globus pallidus in a sub-acute rat model of manganese neurotoxicity. Neurosci. Lett. 2003;349:13–16. doi: 10.1016/s0304-3940(03)00649-9. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem. Int. 2002;41:271–277. doi: 10.1016/s0197-0186(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Hurley LS, Keen CL. Manganese. In: Underwood E, Mertz W, editors. Trace Elements in Human Health and Animal Nutrition. New York, NY: Academic Press; 1987. pp. 185–223. [Google Scholar]
- Kennedy SD, Bryant RG. Manganese-deoxyribonucleic acid binding modes. Nuclear magnetic relaxation dispersion results. Biophys. J. 1986;50:669–676. doi: 10.1016/S0006-3495(86)83507-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch. Environ. Health. 1989;44:175–178. doi: 10.1080/00039896.1989.9935883. [DOI] [PubMed] [Google Scholar]
- Kontur PJ, Fechter LD. Brain regional manganese levels and monoamine metabolism in manganese-treated neonatal rats. Neurotoxicol. Teratol. 1988;10:295–303. doi: 10.1016/0892-0362(88)90031-1. [DOI] [PubMed] [Google Scholar]
- Kramer-Stickland K, Edmonds A, Bair WB, III, Bowden GT. Inhibitory effects of deferoxamine on UVB-induced AP-1 transactivation. Carcinogenesis. 1999;20:2137–2142. doi: 10.1093/carcin/20.11.2137. [DOI] [PubMed] [Google Scholar]
- Kwik-Uribe CL, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice alter brain iron concentrations and behavior despite postnatal iron supplementation. J. Nutr. 2000;130:2040–2048. doi: 10.1093/jn/130.8.2040. [DOI] [PubMed] [Google Scholar]
- Lai JCK, Lim L, Davison AN. Effects of Cd2+, Mn2+, and Al3+ on rat brain synaptosomal uptake of noradrenalin and serotonin. J. Inorg. Biochem. 1982;17:215–225. doi: 10.1016/s0162-0134(00)80100-2. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cδ is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exper. Therap. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J. Neurochem. 2006;97:310–333. doi: 10.1111/j.1471-4159.2006.03717.x. [DOI] [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res. Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, Tardif R, Smargiassi A, Martin L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Quenzer LF. Psychopharmacology: drugs, the brain, and behavior. Sunderland MA: Sinauer Associates; 2005. pp. 132–137. [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press Inc; 1998. [Google Scholar]
- Powell PR, Ewing AG. Recent advances in the application of capillary electrophoresis to neuroscience. Anal. Bioanal. Chem. 2005;382:581–591. doi: 10.1007/s00216-005-3075-x. [DOI] [PubMed] [Google Scholar]
- Pyatskowit JW, Prohaska JR. Rodent brain and heart catecholamine levels are altered by different models of copper deficiency. Comp. Biochem. Physiol. Part C. 2007;145:275–281. doi: 10.1016/j.cbpc.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson’s disease. Biochem. Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem. Physiol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Roth JA, Feng L, Walowitz J, Browne RW. Manganese-induced rat pheochromocytoma (PC12) cell death is independent of caspase activation. J. Neurosci. Res. 2000;61:162–171. doi: 10.1002/1097-4547(20000715)61:2<162::AID-JNR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ruiz IG, de la Torre P, Diaz T, Esteban E, Morillas JD, Muñoz-Yagüe T, Solís-Herruzo JA. Sp family of transcription factors is involved in iron-induced collagen alpha (I) gene expression. DNA Cell Biol. 2000;19:167–178. doi: 10.1089/104454900314555. [DOI] [PubMed] [Google Scholar]
- Seth PK, Chandra SV. Neurotransmitters and neurotransmitter receptors in developing and adult rats during manganese poisoning. NeuroToxicology. 1984;5:67–76. [PubMed] [Google Scholar]
- Shukla GS, Chandra SV, Seth PK. Effect of manganese on the levels of DNA, RNA, DNAse and RNAse in cerebrum, cerebellum and rest of the brain regions of rat. Acta Pharmacol. Toxicol. (Copenhagen) 1976;39:562–569. doi: 10.1111/j.1600-0773.1976.tb03206.x. [DOI] [PubMed] [Google Scholar]
- Sloot WN, van der Sluijs-Gelling AJ, Gramsbergen JBP. Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus. J. Neurochem. 1994;62:205–216. doi: 10.1046/j.1471-4159.1994.62010205.x. [DOI] [PubMed] [Google Scholar]
- Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Amer. J. Indust. Med. 2007;50:772–778. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, Michel PP. Noradrenalin provides long-term protection of dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001;79:200–210. doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- Vidal L, Alfonso M, Campos F, Faro LRF, Cervantes RC, Durán R. Effects of manganese on extracellular levels of dopamine in rat striatum: an analysis in vivo by brain microdialysis. Neurochem. Res. 2005;30:1147–1154. doi: 10.1007/s11064-005-7775-6. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Persp. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker VP. Thirty years of synaptosome research. J. Neurocytol. 1993;22:735–742. doi: 10.1007/BF01181319. [DOI] [PubMed] [Google Scholar]
- WHO/UNICEF/UNU. Iron deficiency anaemia: assessment, prevention and control. Geneva: World Health Organization; A guide for programme managers. 2006
- Yamamoto K, Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:913–922. doi: 10.1016/j.pnpbp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Doolen S. Chronic and acute regulation of Na+/Cl− -dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Therap. 2001;92:21–55. doi: 10.1016/s0163-7258(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M, Giaveri G, Arosio P, Santambrogio P, Fariello RG, Karatekin E, Kleinman MH, Turro N, Hornykiewicz O, Zucca FA. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. PNAS. 2004;101:9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]