Abstract
Arginine vasotocin (VT), and its mammalian homologue arginine vasopressin (VP), are neuropeptides involved in the regulation of social behaviors and stress responsiveness. Previous research has demonstrated opposing effects of VT/VP on aggression in different species. However, these divergent effects were obtained in different social contexts, leading to the hypothesis that different populations of VT/VP neurons regulate behaviors in a context-dependent manner. We here use VP antagonists to block endogenous VT function in male zebra finches (Taeniopygia guttata) within a semi-natural, mixed-sex colony setting. We examine the role of VT in the regulation of aggression and courtship, and in pair bond formation and maintenance, over the course of three days. Although our results confirm previous findings, in that antagonist treatment reduces aggressive mate competition during an initial behavioral session during which males encounter novel females, we find that the treatment effects are completely reversed within hours of colony establishment, and the antagonist treatment instead facilitates aggression in later sessions. This reversal occurs as aggression shifts from mate competition to nest defense, but is not causally associated with pairing status per se. Instead, we hypothesize that these divergent effects reflect context-specific activation of hypothalamic and amygdalar VT neurons that exert opposing influences on aggression. Across contexts, effects were highly specific to aggression and the antagonist treatment clearly failed to alter latency to pair bond formation, pair bond stability, and courtship. However, VT may still potentially influence these behaviors via promiscuous oxytocin-like receptors, which are widely distributed in the zebra finch brain.
Keywords: aggression, courtship, colony, context, pair bond, social behavior, songbird, vasopressin, vasotocin, zebra finch
Introduction
Arginine vasotocin (VT) and its mammalian homologue arginine vasopressin (VP) are neuropeptides known to modulate social behaviors and stress responsiveness in virtually all vertebrate groups (Caldwell et al., 2008; De Vries and Boyle, 1998; Goodson, 2008; Goodson and Bass, 2001). However, the relationships between VT/VP and social behaviors are often not consistent across species, contexts, and behavioral phenotypes (Caldwell et al., 2008; Goodson, 2008; Greenwood et al., 2008; Semsar and Godwin, 2004). For instance, VP release into the lateral septum (LS) decreases during resident-intruder tests in aggressive, low-anxiety rats, but increases in less aggressive, high-anxiety rats (Beiderbeck et al., 2007). In songbirds, VT infusions into the LS or lateral ventricle facilitate aggression in male zebra finches (Taeniopygia guttata) during mate competition (Goodson and Adkins-Regan, 1999; Goodson et al., 2004), but inhibit territorial (resident-intruder) aggression in field sparrows (Spizella pusilla) (Goodson, 1998a) and violet-eared waxbills (Uraeginthus granatina) (Goodson, 1998b).
Given these potential context-dependent effects, the precise involvement of VT/VP in the regulation of many social behaviors remains unclear. This is particularly the case for behaviors that are expressed in the context of large social groups, since few laboratory species are both highly gregarious and readily observed in a colony environment. Zebra finches are a notably tractable, colonial species, but to date, VT effects on zebra finch behavior have been examined only in the context of short-duration tests of courtship and aggression that involve only two or three animals. We know little about the regulation of behavior in many other social contexts that are unique to group-living animals. For instance, information is lacking about the involvement of VT/VP in long-term regulation of social behaviors within mixed sex social groups in semi-natural settings. Another important question is whether endogenous VT is necessary for naturally occurring pair bond formation in birds (see Goodson et al., 2004), as is known for endogenous VP in microtine voles (Lim and Young, 2006; Wang and Aragona, 2004). Indeed, given that pair bonding is an evolutionarily labile behavior, it is particularly important to ask whether vole-like peptide functions have also evolved in support of monogamous bonding in non-microtine taxa. We attempt to address these questions in the present study.
To address these questions, it is important to keep in mind that VT/VP neurons project broadly throughout the basal forebrain and brainstem (Goodson and Bass, 2001), and also to the anterior pituitary, where VT/VP release synergizes with corticotropin-releasing factor to regulate secretion of adrenocorticotropic hormone and thereby glucocorticoids (Aguilera and Rabadan-Diehl, 2000; Baeyens and Cornett, 2006; Lightman, 2008). Furthermore, when VT/VP is released centrally, it can include both axonal and dendritic release (Bergquist and Ludwig, 2008; Landgraf and Neumann, 2004). There are thus multiple avenues by which VT/VP may be involved in the regulation of social behaviors, including via feedback from the periphery.
Context-dependent effects of VT are likely associated with different patterns of neuromodulation arising from different populations of VT neurons (Goodson, 2008). Of particular interest in relation to social behavior are the VT/VP neurons in the medial bed nucleus of the strial terminalis (BSTm), a component of the medial extended amygdala (Goodson, 2008). In songbirds, these neurons exhibit increased Fos expression, a proxy marker of neuronal activity, in the presence of positive social stimuli, and depressed activity in relation to aversive social stimuli (Goodson and Wang, 2006). VT/VP projections from the BSTm target multiple areas of the basal forebrain, including the LS, where VT/VP release regulates social recognition, agonistic communication, anxiety, and stress responses (Caldwell et al., 2008; Goodson, 2008). Whereas the BSTm neurons appear to selectively process stimuli related to affiliation, VT neurons in the paraventricular nucleus of the hypothalamus (PVN) are strongly responsive to emotional stressors (Goodson and Evans, 2004; Wotjak et al., 1996), and these neurons also project centrally and likely to the LS (Goodson and Kabelik, 2009).
The presence of multiple VT/VP populations that appear to regulate various aspects of social interactions leads us to ask the question of how endogenous VT/VP regulates initial and long-term social interactions and pair bond formation. Does a shift occur from the dominance of one neuronal population to another when mate competition decreases and nest defense increases? Given the diversity of VT/VP cell groups and their unique response profiles, it seems impossible to obtain a full and accurate view of VT/VP functions if we restrict our analyses to highly controlled and somewhat contrived behavioral tests (Ophir et al., 2008a; Ophir et al., 2008b). However, no experiments to date have examined the behavioral effects of chronic VT/VP manipulations in a semi-natural (e.g., colony) context. Therefore, we here examine aggression, courtship, and pair bond formation in a colony context over the course of three days in male zebra finches treated with either VP antagonists (VPant) or saline control. We show that while VPant manipulations primarily influence aggression, they do so in a highly dynamic manner.
Methods
Subjects
A total of 64 male and 80 female adult zebra finches were used for behavioral observations during prescreening and experimental sessions. Of these, cannulation surgeries were performed on 39 experimental male zebra finch subjects. All birds had ad libitum access to food and vitamin-enriched water, and were maintained on a 14:10 light cycle. All procedures were conducted in a humane manner and in compliance with federal and institutional guidelines.
Prescreening
Not all zebra finches are successful at pairing in a colony environment and we therefore prescreened subjects for pairing ability in order to obtain a subject population for cannulation surgeries. Zebra finches were transferred from same sex housing into colony cages in groups of four males and five females each. Colony cages were 1.2 m long (120 cm W × 40 cm H × 36 cm deep) and were supplied with plastic nest cups in each of the four corners of the cage. Food, water dishes, and burlap nesting material were placed centrally on the cage floor. Observations were conducted twice daily for three days to assess pairing status (Fig. 1). Zebra finch pair bonds are easily detected based on selective affiliation, inclusive of “clumping” (perching for periods in physical contact), following, allopreening, and co-occupation of a nest cup. Male and female groups were then separated and housed without visual access to opposite-sex individuals for at least 10 days. This duration is completely sufficient to allow for the formation of new pair bonds, since wild zebra finches typically replace mates within several days following experimental mate removal (Zann, 1996).
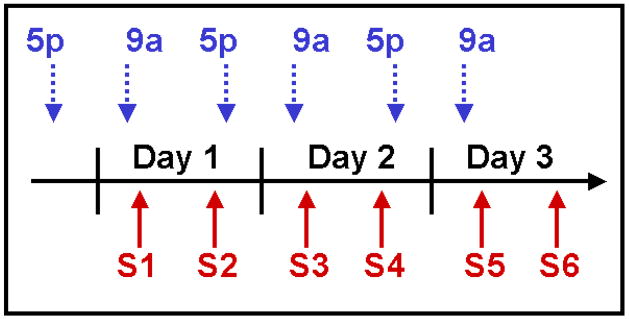
Figure 1.
A diagram depicting the timeline (horizontal arrow) of our experimental procedures. The dotted vertical arrows represent infusions of VPant or saline, either prior to daily observation sessions (approximately 9 am; 9a), or following daily observation sessions (approximately 5 pm; 5p). The vertical solid arrows represent observation sessions (S1–6). Two 10-min observations of each subject were conducted each day, one in the morning, and one in the afternoon, for a period of three days.
Surgeries
Surgeries were conducted on 39 males (from 14 colony groups) that successfully pair bonded during prescreens. Cannulation surgeries were conducted stereotaxically using isoflurane vapor anesthesia at 2–5% of a compressed air flow. Coordinates were referenced to the vascular convergence at the rostral tip of the cerebellum. A 26-gauge single guide cannula for small animals (Plastics One, Roanoke, VA) with a 4.6 mm extension beyond the pedestal was inserted 3.1 mm rostral, 1.7 mm right lateral, and 2.6 mm deep, at a 21° angle toward medial. These coordinates target the caudal portion of the lateral ventricle. The guide cannula was adhered to the skull using a combination of Nexaband S/C cyanoacrylate glue (Abbott Laboratories, North Chicago, IL) and Stoelting dental cement (Stoelting, Wood Dale, IL). A sterile cannula dummy with a wire obturator (Plastics One) was inserted into the guide cannula at all times other than during infusion procedures. Injection cannulae, but not cannula dummies, projected 1 mm beyond the length of the guide cannula. Subjects were allowed at least five days of recovery before subsequent testing.
Following all experimental procedures, birds were infused with 1 μl of ink, euthanized by isoflurane overdose, perfused with 0.1 M phosphate buffered saline followed by 4% paraformaldehyde, and their brains were sectioned on a cryostat at 40 μm. Six males showed no ink in the lateral ventricle and were therefore excluded from analyses. Of the 33 remaining males, 16 were in the VPant group and 17 were in the saline control group.
Antagonists and Infusions
Infusions were either of vehicle (0.9 % NaCl) or VPant. Treatments within colonies were counterbalanced as much as possible. Of the 14 colonies (mean cannulated subjects per colony = 2.36) that contained at least one cannulated male, 2 colonies had no VPant males (these colonies contained a total of 1 and 2 cannulated subjects), 8 had one VPant male, and 4 had two VPant males. Likewise, 2 colonies had no saline males (these colonies contained a total of 1 and 2 cannulated subjects), 7 had one saline male, and 5 had two saline males.
The VPant infusions were delivered as a cocktail containing a combination of the specific V1a antagonist ([β-Mercapto-β, β-cyclopentamethylenepropionyl1, O-Me- Tyr2, Arg8]-Vasopressin), also known as Manning compound (V2255, Sigma-Aldrich, St. Louis, MO) and the less specific V1 antagonist ([deamino-Pen1, O-Me-Tyr2, Arg8]-Vasopressin, V1880, Sigma-Aldrich). Each antagonist was infused at a dose of 250 ng (consistent with Goodson et al., 2004), and both were delivered together in 1 ul of 0.9% NaCl. The decision to employ an antagonist cocktail was based on the fact that multiple V1-like receptors have been cloned in birds (Baeyens and Cornett, 2006) and their distributions have not been fully described.
Animals received their first infusion on the afternoon prior to the first day of behavioral observations (Fig. 1). Each morning thereafter (for three test days), infusions were administered approximately 1 hr after lights-on and observations were initiated 40 min later. On days 1 and 2, a second infusion was given following afternoon behavioral observations in order to maintain high levels of VPant exposure. VP antagonists, including the V1a antagonist used here, have been shown to remain physiologically active for at least 24 hours (Bosch and Neumann, 2008).
Behavioral Observations
Forty minutes following the first infusion on day 1, subjects were moved from same-sex housing into colony cages with nests and burlap, as during the prescreening. Subjects then remained in these cages for the duration of the experiment. Colonies consisted of the same groups of four males as in the prescreening (thus a mix of cannulated and unmanipulated males, since not all males paired in prescreening) and five novel females. Ten-min focal observations of individual subjects commenced immediately after the colony groups were formed, and were conducted twice daily for a total of three days, once per morning (9:30 am – 12:00 pm) and once per afternoon (2 pm – 5 pm).
Observations were conducted from behind a curtain “blind” and were dictated by voice onto a digital recorder for later transcription. Behaviors recorded include aggressive behaviors (chases, threats, beak fences, and pecks), courtship behaviors (directed songs and dances), affiliative and partner-directed behaviors (greets, follows, clumping, allopreening, and mounting), and various other behaviors associated with arousal and maintenance (beak wipes, undirected song, eating, drinking, and autogrooming). These behaviors were scored as previously described (Goodson et al., 1999). We here present aggressive behaviors as an aggregate count of chases, threats, beak fences, and pecks—either to males, females, or to both sexes. We also report directed songs as a measure of courtship, as well as an aggregate score of arousal and maintenance behaviors that includes instances of eating, drinking, and beak wiping. Instances of other behaviors were too infrequent for statistical analyses.
Statistical Analyses
Analyses of session 1 (mate competition)
We present behavioral data as behavioral units per minute of time off of the nest, as done previously (Goodson et al., 1999). Data for the first morning session (session 1) were analyzed separately in order to focus on aggressive competition over mates, which previous studies have shown to be influenced by VT (Goodson and Adkins-Regan, 1999; Goodson et al., 2004). Mate competition then declines dramatically over the course of the first day in the test environment, since many subjects pair and begin nesting almost immediately. Session 1 data (aggression, directed songs, and arousal behaviors) were analyzed using unequal variances t-tests, either to compare pharmacological treatments among unpaired males, or to compare unpaired and paired males regardless of pharmacological treatment. To determine posthoc differences among all groups, we ran a one-way ANOVA with student’s t-tests comparisons across all groups.
Repeated measures analyses (pre- versus post-pairing) for sessions 2 to 6
For the remaining observations (sessions 2 to 6), the effects of drug treatment and pairing status on behavioral data (aggression, directed songs, and general arousal) were analyzed using a two-way ANOVA in a repeated measures design. Behavior scores for each subject were averaged for all sessions in which that individual was unpaired and for all sessions in which they were paired. This analysis is restricted to individuals for whom both unpaired and paired data are available during sessions 2 to 6. Because not all individuals paired, and some individuals were paired continuously during these sessions, this analysis could only be conducted on a subsample of 14 individuals.
Repeated measures analyses (pre- versus post-pairing) for all sessions
Additionally, we examined the effects of VPant treatment on behaviors across all six observation sessions, collapsing data according to pairing status, in order to assess the average role that VT plays in unpaired versus paired individuals across the entire observation period. As above, we used a two-way ANOVA in a repeated measures design. This analysis allowed for inclusion of the 24 individuals for whom both unpaired and paired data are available for sessions 1 to 6.
Analyses of pairing
Finally, we examined the effect of drug treatment on latency to pair bond formation using a Wilcoxon Rank-Sums test, the effect of drug treatment on total number of sessions during which an individual was paired using a t-test, the effect of drug treatment on the number of instances of divorce/polygamy per number of pair bonds using a Chi-Square test, and the effects of aggression within session 1 on latency to pairing (session number of first scored pairing) and total number of paired sessions using Spearman’s rho correlation.
Data that did not meet assumptions of normality or homoschedasticity were log transformed in order to meet these assumptions for parametric analyses, though we present untransformed data in figures for easier interpretation. One male in the VPant group did not leave the nest during the two sessions in which he was paired, and thus was eliminated from behavioral analyses involving those sessions.
Results
As shown descriptively in Fig. 2, aggression levels appeared to decrease across sessions, and tended to be higher when males were pair-bonded than when unpaired. We analyzed these data in several ways in order to disentangle the effects of pairing, phases of colony establishment (initial mate competition versus the later period of intensive nest establishment), and drug treatment.
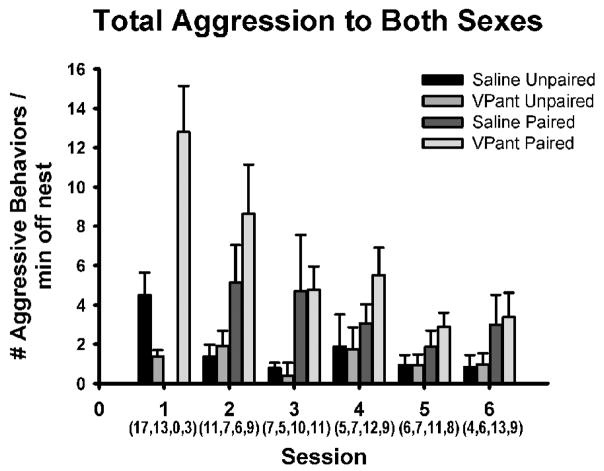
Figure 2.
A descriptive presentation of total aggression levels (chases, beak fences, pecks, threats) per minute off the nest as exhibited by unpaired and paired male zebra finches, treated either with VP antagonists (VPant) or with saline, toward male and female conspecifics, across six observation sessions. Aggression levels tended to be higher in paired individuals and to decline over time. Two sessions were conducted per day. The pattern shown here is virtually identical if the data are subdivided into male- and female-directed aggression, or are not corrected for time on the nest (see Results). Sample sizes are denoted in parentheses below session number. Data are shown as mean ± S.E.M. Because some males changed between unpaired and paired status, and were examined across sessions, the data must be subdivided for statistical analyses, as shown in Fig. 3.
Session 1
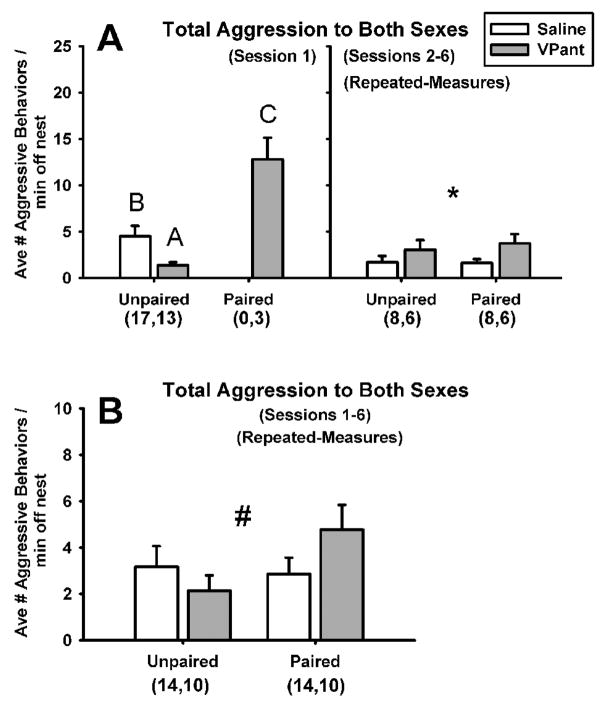
First, we conducted separate analyses for session 1, during which experimental males were competing over novel females. Given that observations were initiated immediately after male groups were introduced to the colony cage, session 1 data were collected within 45 min of colony formation. Because three individuals in the VPant treatment paired within this first session, we first compared pharmacological treatments solely within unpaired males, and then paired versus unpaired subjects regardless of treatment (Fig. 3A, left panel). We detected an effect of drug treatment on total aggression in unpaired males (t=2.97, n=30, p=0.006), with VPant males exhibiting less total aggression than saline males. Furthermore, an effect of pairing status was also present (t=7.26, n-30, p<0.0001), with the three paired males in session 1 exhibiting higher aggression levels than unpaired males. Despite showing very rapid pairing, the pair bonds of these three males were stable for the duration of the study, and such rapid pairing is not unusual in this species (Zann, 1996). The posthoc one-way ANOVA revealed that all three groups (unpaired saline, unpaired VPant, paired VPant) were significantly different from one another (F(2,30)=10.61, p=0.0003, all groups differing at α=0.05 in student’s t comparisons).
Figure 3.
(A) Total aggression levels per minute off of the nest exhibited by male zebra finches toward males and females, depicted separately for session 1 (left panel), when males were competing for mates, and during sessions 2 to 6 (right panel), when many subjects had paired and were nesting. Data for session 1 include all subjects, whereas data for sessions 2–6 are restricted to males for whom both unpaired and paired data are available (analyzed in a repeated-measures design). In session 1, paired males exhibited more aggression than unpaired males (p<0.0001), and within unpaired individuals, VPant treatment resulted in a decrease in aggression relative to treatment with saline (p=0.006). Letters above bars indicate groups differing at α=0.05 in posthoc analyses. In sessions 2–6, VPant treatment produced an increase in aggression levels relative to saline treatment (* denotes main effect of drug treatment at p=0.04). (B) Total aggression levels to males and females presented for sessions 1 to 6 combined, depicted separately for trials during which subjects were unpaired versus pair-bonded (# designates a significant interaction between drug treatment and pairing status at p=0.04). Sample sizes are denoted in parentheses under pairing status. Data are shown as mean ± S.E.M.
To verify the validity of the above results, we also conducted analyses that included as covariates either the percent of males within a colony cage that received VPant infusions or the percent of handled individuals (those receiving infusions of either VPant or saline). These analyses controlled for treatment group composition within a given colony. The results of analyses including these variables (data not shown) were completely in accordance with the findings reported above, and maintained either the significance or nonsignificance of any given comparison.
Furthermore, in order to compare the above data to studies that examine solely male-male aggressive encounters, we conducted analyses examining only aggression exhibited toward males. The pattern of aggression data appeared extremely similar to those above (mean + S.E.M.: saline unpaired = 3.87 ± 1.10, VPant unpaired = 1.09 ± 0.35, VPant paired = 9.78 ± 3.28). We again found a significant effect of both drug (t=2.53, n=30, p=0.017) and pairing status (t=5.00, n=30, p=0.004). The pattern of results for aggression directed solely to females was likewise similar (mean + S.E.M.: saline unpaired = 0.51 ± 0.12, VPant unpaired = 0.22 ± 0.06, VPant paired = 1.67 ± 0.59), although significant only for pairing status (t=4.08, n=30, p=0.03), and not drug treatment (t=1.58, n=30, p=0.13).
Consistent with previous results (Goodson et al., 2004), no effects of drug treatment were observed on courtship behavior, as assessed by the number of directed songs performed to females (t=1.25, n=30, p=0.22), or on behaviors reflecting general arousal and maintenance (t=0.67, n=30, p=0.51).
Sessions 2 to 6
We next analyzed combined data for sessions 2 to 6, when many males had paired and began nesting. Regardless of pairing status, repeated measures ANOVA showed that VPant subjects now exhibited higher levels of aggression than control subjects (F(1,12)=5.30, p=0.04; Fig. 3A, right panel), a complete reversal of the effect observed in session 1 (within session 1, aggression levels for this same subset of males was 4.78+1.82 (mean + S.E.M.) for the 8 saline males and only 1.27+0.37 for the 6 VPant males). We did not find a significant effect of pairing status on aggression (F(1,12)=1.26, p=0.28) in this subset of individuals, or an interaction between pairing status and drug treatment (F(1,12)=0.00, p=1.0).
The inclusion of percent of VPant males or percent of handled males as a covariate again failed to alter the above results (data not shown). Also, separate analyses for aggression solely toward males (data not shown) or solely toward females (data not shown) resulted in highly similar data patterns, though nonsignificant effects of treatment on aggression (p=0.16 and p=0.07 respectively). No effects of pharmacological treatment were observed on courtship behavior (F(1,13)=0.09, p=0.77) or general arousal and maintenance (F(1,12)=0.43, p=0.52).
All sessions
Because we were also interested in examining the overall effects of endogenous VT release across all time points within the colony setting, we examined the combined data for sessions 1 to 6. This analysis revealed a significant interaction, with VPant treatment reducing aggression in unpaired males while conversely increasing aggression in paired males relative to saline-treated control individuals (F(1,22)=4.59, p=0.04, Fig. 3B). No main effects of drug treatment (F(1,22)=2.96, p=0.10) or pairing status (F(1,22)=0.02, p=0.88) were observed.
We found no difference between VPant and control males in terms of latency to formation of the first pair bond, total number of pair-bonded sessions, the probability of pairing, or the probability of divorcing or entering into a polygamous relationship with two or more females (p>0.05 for all, Table 1). Furthermore, no relationship was found between total aggression exhibited during session 1 and either latency to pairing (rs=−0.16, n=27, p=0.42) or total number of paired sessions (rs=0.14, n=27, p=0.49; data not shown).
Table 1.
The effects of drug treatment on several aspects of pair bond formation. The latency to pair bond formation is presented as mean session number (± S.E.M) during which the first pairing was observed, excluding males who never paired. The total number of paired sessions analysis includes all subjects. The analysis of pairing probability also includes all subjects. The analysis of pairing irregularities examines the probability of divorces from a pair bond, as well as polygamous relationships with multiple females, and excludes males that never formed a pair bond. None of these measures of pair bond formation was affected by VPant treatment.
| 1st Paired Session | Mean | S.E.M. | n | Χ2 | df | p |
|---|---|---|---|---|---|---|
| saline | 3.07 | 0.34 | 14 | 2.51 | 1 | 0.11 |
| VPant | 2.46 | 0.42 | 13 | |||
| # Paired Sessions | Mean | S.E.M. | t | df | p | |
|
| ||||||
| saline | 3.06 | 0.49 | 17 | 0.09 | 31 | 0.93 |
| VPant | 3.12 | 0.55 | 16 | |||
| Pairing Success | None | Paired | Χ2 | df | p | |
|
| ||||||
| saline | 3 | 14 | 17 | 0.007 | 1 | 0.93 |
| VPant | 3 | 13 | 16 | |||
| Divorces/Polygamy | Yes | No | Χ2 | df | p | |
|
| ||||||
| saline | 4 | 10 | 14 | 0.003 | 1 | 0.74 |
| VPant | 3 | 10 | 13 | |||
Discussion
Our findings demonstrate that whereas endogenous VT facilitates aggression in a social context that involves competition over access to potential new mates, it instead acts to suppress aggression during later sessions, perhaps in relation to the establishment of stable social groups or the defense of nests. This switch from facilitation to suppression may be due to a change in the relative activation of neuronal populations from which VT is released. Blocking VT receptors did not, however, affect pair bond formation or maintenance. This suggests that VT release is not necessary for pair bonding in this species, at least not via V1-like VT receptors, although it is possible that VT may still act via promiscuous oxytocin-like receptors that are present in songbirds and also bind VT, but which do not appear to be blocked by VPant treatment (Leung et al., 2009). Nevertheless, the actions of VT on aggression may still impact aspects of pairing. For instance, the facilitation of aggressive competition over mates by VT may be adaptive for obtaining high quality mates, while the inhibitory effects of VT on aggression in later sessions may benefit parental care.
Consistent with previous results (Goodson et al., 2004), we did not observe an effect of VPant treatment on directed courtship singing to females. We also found no effect of VPant treatment on general arousal and maintenance behaviors. These results suggest that arousal was not affected by the drug treatment, and that the behavioral effects of VPant are highly specific to certain social behaviors, such as mate competition and other forms of aggression. Furthermore, despite our use of a V1 antagonist that could potentially bind to V1b-like receptors, which are present in the pituitary (Baeyens and Cornett, 2006), the observed effects are consistent with those obtained in previous studies of zebra finches and territorial songbirds using a selective V1a antagonist (Goodson and Adkins-Regan, 1999; Goodson and Kabelik, 2009; Goodson et al., 2004), suggesting a central site of action.
Context-Dependent Effects of VT on Aggression
In the present study, we observed both an initial stimulatory and later inhibitory effect of endogenous VT on aggression. The initial enhancement of aggression by endogenous VT, as indicated by the inhibitory influence of VPant treatment, is similar to that observed previously in zebra finches using a mate competition paradigm (Goodson and Adkins-Regan, 1999; Goodson et al., 2004). However, this effect is transient and apparently only observed in the context of mate competition. In support of this claim, the three males that formed stable pair bonds during session 1, and thus ceased competing for mates, all exhibited very high levels of aggression despite being in the VPant group. Pairing status itself did not underlie this switch, because all individuals, whether pair-bonded or not, exhibited a facilitation of aggression with VPant treatment in sessions 2 to 6, when social and dominance relationships had presumably stabilized, and mate competition had declined. Instead, during sessions 2 to 6, many pair bonds were established, and aggression appeared to shift from being focused on potential mates to being focused on defense of nests (for a discussion of nest defense, see Zann, 1996). It is important to note that both paired and unpaired males defended nests in the present experiments. Therefore, endogenous VT appears to exert opposing effects on aggression depending on social context, leading to a facilitation of aggression during mate competition, and an inhibition of aggression during nest defense.
While it is probable that aggression involving mate competition is modulated by VT release from neurons in the BSTm, since these neurons exhibit increased Fos expression during such encounters (Goodson and Wang, 2006), aggression associated with nest defense may instead be primarily modulated by VT release from hypothalamic PVN neurons, which are associated with stress and anxiety (Goodson and Evans, 2004; Wotjak et al., 1996). In male song sparrows (Melospiza melodia), the percentage of these PVN neurons that colocalize Fos is negatively correlated with aggressive response to a simulated territorial challenge (Goodson and Kabelik, 2009). Furthermore, homologous parvocellular preoptic area neurons in several fish species exhibit higher levels of VT mRNA or immunoreactivity in subordinate/non-territorial/low aggression male phenotypes (Greenwood et al., 2008; Grober et al., 2002; Larson et al., 2006; Lema, 2006; Miranda et al., 2003; but see Dewan et al., 2008), and VT release from these parvocellular neurons (via a feedback loop through the periphery) inhibits approach behaviors (Thompson et al., 2008). Therefore, VT neurons in the PVN likely inhibit aggression. If this multiple VT population hypothesis is correct, then the switch from aggression induction to inhibition by VT during the present study may be the result of a shift in predominance of VT release from the BSTm during session 1 to release from hypothalamic PVN neurons during later sessions. However, additional factors may also be involved in this switch, including interactions between VT and changes in circulating levels of the steroid hormone corticosterone. Such interactions regulate the switch between facilitation and inhibition of reproductive clasping behaviors in male newts (Rose et al., 1995).
Additionally, VP neurons in the anterior hypothalamus increase their Fos expression during territorial encounters in hamsters and voles and release of VP in this area promotes offensive aggression in hamsters (Delville et al., 1996; Ferris et al., 1997; Gobrogge et al., 2007). However, homologous neurons in C57BL/6 mice (J. M. Ho, G. E. Demas, J. L. Goodson, unpublished) and topographically similar neurons in song sparrows (Goodson and Evans, 2004; Goodson and Kabelik, 2009) fail to exhibit similar Fos responses. The evidence therefore suggests that the primary VT regulators of territorial aggression in songbirds are the VT neurons in the PVN. These neurons almost certainly exert inhibitory effects on aggression, since VT infusions inhibit territorial (resident-intruder) aggression in multiple species (Goodson, 1998a; Goodson, 1998b) and VT-Fos colocalization in the PVN correlates negatively with aggression (Goodson and Kabelik, 2009).
Resolution of Past Discrepancies
The observed switch from facilitation to inhibition of aggression in this study clearly demonstrates that VT exerts context-dependent effects on aggression, perhaps due to a shift in the relative activity levels of distinct VT neuron populations. The contextual differences observed here show strong parallels to putative species differences. Thus, central VT infusions have been shown to decrease territorial aggression in field sparrows (Goodson, 1998a) and violet-eared waxbills (Goodson, 1998b), whereas VT increases aggressive competition over mates in zebra finches (Goodson and Adkins-Regan, 1999; Goodson et al., 2004). These findings have been interpreted as species-specific effects of VT on aggression, but the confound of context is obvious in hindsight, and recent data from violet-eared waxbills demonstrate that aggressive mate competition is facilitated by VT in that species, as it is in zebra finches (Goodson and Kabelik, 2009).
We now show that endogenous VT also inhibits aggression in zebra finches, but only during later testing sessions when much of the fighting is centered on nest defense (see Zann, 1996 for a discussion of nest defense). Thus, resident-intruder aggression in territorial species and nest defense aggression in zebra finches are likely both inhibited by VT (although it should be noted that we are not able to separately analyze “nest defense” from other aggression in our relatively small colony cages). Aggression in both nest defense and territorial contexts may be inhibited by the activation of stress-responsive hypothalamic VT neurons, as addressed above. VT neurons in the BSTm are likely not responsible for the regulation of territorial aggression, since territorial species do not show increased Fos activity within these neurons during same-sex (Goodson and Wang, 2006) or territorial interactions (Goodson and Kabelik, 2009), although the absence of a Fos response during these encounters is not definitive proof of noninvolvement. However, the notion that VT neurons in the BSTm regulate territorial aggression is further opposed by the fact that territorial estrildid species express only about a tenth the number of VT neurons in the BSTm as do highly colonial species (Goodson and Wang, 2006). In contrast, VT neurons in the BSTm of male zebra finches increase their Fos activity during positive social interactions, including mate competition for access to an opposite-sex individual (barring intense subjugation), and these neurons may thus generally facilitate aggression in the context of appetitive, goal-oriented social behavior (Goodson et al., 2009; Goodson and Wang, 2006).
VT and Pair Bonds
VP and the related neuropeptide oxytocin are known to facilitate pair bond formation in the monogamous prairie vole (Microtus ochrogaster), in a largely sex-specific manner (Lim and Young, 2006; Wang and Aragona, 2004). In males, central VP infusions are sufficient to induce partner preferences in the absence of mating (Winslow et al., 1993) and VP release into the ventral pallidum is necessary for natural pair bonding (Lim and Young, 2004). Furthermore, experimental upregulation of pallidal V1a receptors in promiscuous meadow voles (M. pennsylvanicus) is sufficient to promote monogamous-like partner preferences (Lim et al., 2004). However, we previously found that VT infusions do not similarly induce partner preferences following overnight cohabitation in zebra finches (Goodson et al., 2004), although the relevance of endogenous VT for natural pair bond formation remained untested. We here tested the relevance of endogenous VT, but found no effects of VPant treatment on natural pair bond formation or maintenance. We also found that aggression levels, which are affected by VT release, were uncorrelated with latency to pair bond formation or the total number of paired sessions. Notably, however, our power to detect an effect of mate competition on pairing was low, given that an excess of females was available. Indeed, an excess of females was provided in order to quantify pairing effects without the confound of VT’s effects on aggression. Thus, the present results strongly support the conclusion that V1-like VT receptors are not involved in pair bond formation or stability in this species, although the potential involvement of OT-like receptors remain to be addressed.
Conclusions
Central VT release acts generally to modulate aggression, but social context plays a large role in determining the direction of effects. Central VT release enhances aggression in unpaired males during aggressive competition for mates, whereas it inhibits aggression in other social contexts when most males (paired or not) attempt to defend nests. Nest defense is similar in many ways to the response of territorial songbirds to intruders, and both appear to be similarly inhibited by endogenous VT. Additionally, while blockade of VT receptors does not affect pair bond formation or maintenance in this species, its effects on aggressive mate competition may potentially facilitate the acquisition of higher quality mates.
Acknowledgments
We would like to thank Sara E. Schrock for assistance with behavioral trials and tissue sectioning, and two anonymous reviewers for comments on this manuscript. This study was funded by NIMH grant RO1 MH 62656 to J.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Baeyens DA, Cornett LE. The cloned avian neurohypophysial hormone receptors. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:12–19. doi: 10.1016/j.cbpb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M. Dendritic transmitter release: A comparison of two model systems. J Neuroendocrinol. 2008;20:677–686. doi: 10.1111/j.1365-2826.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee H, Macbeth AH, Young WS., III Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav. 1996;60:25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Dewan AK, Maruska KP, Tricas TC. Arginine vasotocin neuronal phenotypes among congeneric territorial and shoaling reef butterflyfishes: species, sex and reproductive season comparisons. J Neuroendocrinol. 2008;20:1382–1394. doi: 10.1111/j.1365-2826.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998a;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998b;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;101:167–180. [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic Limbic Networks and Social Diversity in Vertebrates: From Neural Context to Neuropeptides. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.05.007. invited review, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Biol Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober MS, George AA, Watkins KK, Carneiro LA, Oliveira RF. Forebrain AVT and courtship in a fish with male alternative reproductive tactics. Brain Res Bull. 2002;57:423–425. doi: 10.1016/s0361-9230(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Larson ET, O’Malley DM, Melloni RH., Jr Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res. 2006;167:94–102. doi: 10.1016/j.bbr.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Lema SC. Population divergence in plasticity of the AVT system and its association with aggressive behaviors in a Death Valley pupfish. Horm Behav. 2006;50:183–193. doi: 10.1016/j.yhbeh.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural distribution of nonapeptide binding sites in two species of songbird. J Comp Neurol. 2009;513:197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Lightman SL. The neuroendocrinology of stress: A never ending story. J Neuroendocrinol. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Oliveira RF, Carneiro LA, Santos RS, Grober MS. Neurochemical correlates of male polymorphism and alternative reproductive tactics in the Azorean rock-pool blenny, Parablennius parvicornis. Gen Comp Endocrinol. 2003;132:183–189. doi: 10.1016/s0016-6480(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Campbell P, Hanna K, Phelps SM. Field tests of cis-regulatory variation at the prairie vole avpr1a locus: Association with V1aR abundance but not sexual or social fidelity. Horm Behav. 2008a;54:694–702. doi: 10.1016/j.yhbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc Natl Acad Sci U S A. 2008b;105:1249–1254. doi: 10.1073/pnas.0709116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Kinnaird JR, Moore FL. Neurophysiological effects of vasotocin and corticosterone on medullary neurons: implications for hormonal control of amphibian courtship behavior. Neuroendocrinology. 1995;62:406–417. doi: 10.1159/000127030. [DOI] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm Behav. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC, Bhalla R, George KC, Beth EH. A primitive social circuit: vasotocin-substance P interactions modulate social behavior through a peripheral feedback mechanism in goldfish. Eur J Neurosci. 2008;27:2285–2293. doi: 10.1111/j.1460-9568.2008.06210.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci. 1996;16:7725–7732. doi: 10.1523/JNEUROSCI.16-23-07725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]