Abstract
Purpose
To determine whether cigarette smoking is associated with the conversion from normoglycemia to impaired fasting glucose (IFG).
Methods
During 2003 –2004 1,455 participants (mean age 56.5, range 35–79) from the Western New York Health Study who were free of Type 2 diabetes and known cardiovascular disease at baseline (1996–2001) were reexamined (68% response rate). Incident IFG was defined as a subject whose baseline fasting plasma glucose was <100 mg/dl (normoglycemic) and between 100 and 125 mg/dl at follow-up. Prevalent IFG (n=528) was excluded.. Baseline smoking status was categorized as never, former or current.
Results
Of the 1,455 participants, 924 were normoglycemic at baseline: 101/924 converted to IFG over six- years. Compared to those who remained normoglycemic, converters to IFG were at baseline older, had a larger BMI, more likely to be hypertensive, currently smoke, and have a family history of T2DM (all P <0.05). Multivariate logistic regression demonstrated that compared to subjects who remained normoglycemic, the OR of incident IFG among former and current smokers (vs. never) was 1.68 (95% confidence interval: 0.99, 2.80) and 2.35 (95% confidence interval: 1.17, 4.72) (Ptrend =0.008), respectively.
Conclusion
Smoking was positively associated with incident IFG after accounting for several putative risk factors.
Keywords: cigarette smoking, impaired fasting glucose, epidemiology, risk factors
Type 2 diabetes mellitus (T2DM) is a formidable public health problem leading to premature morbidity and mortality and contributing to increased health care costs. Putative risk factors associated with the development of T2DM are age, ethnicity, obesity, family history of T2DM, and physical inactivity. Moreover, both the Diabetes Prevention Program and the Finish Diabetes Prevention Study have shown the strong predictive nature of impaired fasting glucose (IFG) on diabetes risk (1, 2).
Like the growing epidemic of T2DM, the number of individuals with pre-diabetes (defined as impaired fasting glucose (fasting plasma glucose ≥ 100 mg/dl and < 126 mg/dl) or impaired glucose tolerance (plasma glucose ≥ 140 and < 200 mg/dl after an oral glucose tolerance test) is also on the rise. In 2007 approximately 57 million Americans had pre-diabetes (3). The pre-diabetic period provides a prime opportunity to prevent or delay not only T2DM, but to possibly reduce the microvascular and/or macrovascular complications as well. Therefore, identification of impaired fasting glucose and its modifiable risk factors would offer the chance to intervene at an earlier point in the natural history of disease.
Cigarette smoking may be a common antecedent of both IFG and diabetes. Epidemiologic evidence accumulated over the last few decades convincingly establishes smoking as a risk factor for T2DM with a relative risk (RR) ranging from 1.5 to nearly 6.0 (4–18). There is, however, a paucity of information on the association between smoking and impaired fasting glucose. Two studies among occupational cohorts of Japanese men presented contradictory results: one prospective study reported a RR of 2.56 (95% confidence interval:1.32, 4.95) (19) whereas a cross-sectional study found no association (20). A third study, a population- based cross-sectional study of Swedish men found a null association between smoking and impaired glucose tolerance (IGT), but did not examine smoking and IFG (12).
The Western New York Follow-up Study was designed to examine the incidence of type 2 diabetes and, therefore provides a unique opportunity to prospectively examine whether smoking behavior at baseline (never, former, or current) is associated with the conversion from normoglycemia (NGT) to impaired fasting glucose in a community- based cohort that includes both men and women from Western New York
MATERIALS AND METHODS
The study design and methodology of the original Western New York Health Study have been previously published (21–23). From 1996–2001 participants were enrolled as healthy control subjects in the Western New York Health Study, a series of case-control studies of alcohol drinking patterns and risk of cardiovascular disease (CVD) and lung cancer in Erie and Niagara Counties, New York. The initial cohort of control participants was randomly selected from drivers’ license lists for those under age 65, and from the Health Care Finance Administration rolls for persons aged 65 years and older (response rate 59.5%). During 2003–2004 we conducted the first follow-up. Eligible participants for this follow-up study were at the baseline examination 35–79 years old (mean age 56.5) and without known clinical cardiovascular disease (self-reported MI, angina or revascularization surgery) or Type 2 diabetes mellitus (measured fasting plasma glucose > 125 mg/dl or self report of a physician diagnosis and taking medication) (n=2,652). Exclusion criteria were self-report of all cancers except skin cancer, and Type 1 diabetes, as well as physical or mental impairment that would prohibit the subject from completing all parts of the protocol (n=165); permanent change in residence to out-of-state (n=39), deceased (n=67), or inability to contact and determine eligibility (n=242). This left 2,139 persons eligible for the follow-up examination: 1,455 completed the full clinic protocol (68% response rate). At baseline 927 of the 1455 subjects were normoglycemic, the other 528 had prevalent IFG and therefore were not considered in this analysis. Because of missing baseline data on smoking status for three subjects the final analytic data set consisted of 924 normoglycemic subjects at baseline, 101 of who converted to IFG over the six-year follow-up.
At baseline, compared to persons who refused, participants were less likely to smoke and more likely to have completed high school. There were no significant differences in fasting glucose concentrations, BMI or sex ratio (data not presented). The mean follow-up time was 5.9 years (SD = 0.8 yrs) (median =6.0 years). The protocol was approved by the University at Buffalo Health Science Institutional Review Board and all participants provided written informed consent prior to participation.
Study Protocol
At both the baseline and follow-up examinations all participants underwent a standardized clinical examination that included resting blood pressure, measures of height, weight, and waist girth according to standardized protocols (24, 25). Body mass index was calculated as weight in kilograms divided by height in meters squared. Sagital girth (the distance between the subject’s back and the front of the subject’s abdomen) was measured with the subject in a supine position using a Holtain-Khan Abdominal Caliper. Three measurements were taken and recorded to the nearest 0.1 cm and the average of the last two used: for accuracy it was confirmed that all three measurements were within 0.5 cm of each other. Hypertension was defined as a resting systolic pressure ≥140 mmHg or diastolic pressure ≥90 mmHg (both were measured three times and the average of the last two used) and/or taking antihypertensive medications. At both exams study participants were asked to provide a fasting (at least 10 hrs overnight) blood sample and refrain from smoking and vigorous physical activity for 24 hours prior to their appointment and their adherence was validated at each exam prior to their blood draw. Several standardized questionnaires were administered including physical activity (Stanford 7-Day Recall)(26), general health and well-being, personal and family health history, medication use, and socioeconomic status. Current alcohol consumption was defined as reported use of any type of alcoholic beverage in the 30 days preceding the interview. A family history of Type 2 diabetes was defined as a positive report in a first degree relative. Participants were instructed to bring all medications to the clinic visits permitting identification of oral medications as well as insulin use.
Baseline smoking status was ascertained by asking participants if they smoked more than 100 cigarettes in their lifetime. Participants who answered “no” were considered “never smokers” and people who smoked >100 cigarettes but did not currently smoke were categorized as “former smokers.” Persons who had not quit were defined as “current smokers.” Lifetime total pack-years was assessed by asking participants about the number of packs of cigarettes smoked per day in each decade of life up to their current age. Total lifetime pack-years equals the number of packs smoked per day multiplied by the number of years smoked in each decade of life summed across all decades. Lifetime total pack-years is a measure of smoking intensity, combining duration and frequency. Lifetime pack-years was examined separately among current and former smokers: each smoking category was dichotomized based upon the median number of pack-years smoked (23 and 12 pack-years, respectively). The primary endpoint of interest was incident IFG defined as a subject with normal fasting plasma glucose (FPG)< 100 mg/dl at the baseline examination and FPG ≥ 100 mg/dl and ≤ 125 mg/dl at the follow-up visit (n=101). Because of the small number subjects who converted from normoglycemia (the at risk population identified) to Type 2 diabetes at follow-up (n=6), incident Type 2 diabetes is not herein reported.
Laboratory Methods
Fasting glucose concentrations were determined by the glucose oxidase method (Beckman instruments, Fullerton, CA). The interassay coefficient of variation (CV) was below 5%.
Statistical Procedures
Outcome measures were compared between subjects who progressed to IFG and those who remained normoglycemic (NGT). Categorical variables were compared with χ2 tests and continuous variables by t tests or analysis of variance. The cumulative incidence of IFG was calculated as the number of subjects who converted from normoglycemia to IFG over the follow-up period divided by the population at risk over the follow-up period (101/924). Logistic regression was utilized to estimate the Odds Ratio (OR) (95% CI) of smoking status (former or current vs. never) on incident IFG after adjustment for age (continuous) sex (male vs. female), sagittal girth (continuous), and family history of T2DM (no/yes). Fasting glucose (continuous) was also included to account for potential residual confounding (Model 1). In Model 2 hypertension (no/yes) was considered in addition to the covariates included in Model 1. The OR (95% CI) of IFG is shown for each category of smoking status compared to never smokers. Additionally, the OR of incident IFG was estimated according to the median lifetime total pack -years smoked within each smoking category. The p-value for trend was calculated using categories of pack-years as a score variable. Likelihood ratio tests were conducted to test for statistical interactions. No significant effect modification was noted. The population attributable risk percent of IFG due to cigarette smoking was calculated using Levin’s equation: P e (RR−1)/Pe (RR−1) +1 × 100 where Pe is the prevalence of the exposure (former and current smoking) in the cohort (a+b/T) and where RR is a/a+b divided by c/c+d. (27, 28). All statistical tests were two-sided and a p-value <0.05 was considered statistically significant. Analyses were carried out using the SPSS for Windows version 14.0 (Chicago, IL).
RESULTS
At baseline there were 924 normoglycemic subjects: 101 progressed to IFG over an average follow-up of 5.9 ± 0.8 years (median 6.0 years). The cumulative incidence of IFG was 10.9%. (Table 1) At baseline, compared to participants who remained normoglycemic converters to IFG were older (58.1 vs. 54.8 yrs, P=0.006) had a larger mean BMI (28.1 vs. 26.6 kg/m2, P=0.004), waist circumference (92.0 vs. 86.1 cm) and sagittal girth (21.3 vs. 19.8 cm) (P=<0.001 for both). Converters to IFG had a higher fasting glucose than those who remained normoglycemic (94.2 mg/dl vs. 90.3 mg/dl, P <0.001). Converters to IFG were more likely than non-converters to currently smoke at baseline (16.8%) vs. 11.4%) and less likely to have never smoked (36.6 % vs. 50.3%) (P=0.032). Moreover, converters to IFG smoked longer (25.6 vs. 22.3 years, P=0.05) and more pack-years (15.3 vs. 9.2, P=0.01) compared to subjects who remained normoglycemic. A family history of T2DM was more prevalent among converters to IFG than subjects who remained normoglycemic (40.9% vs. 30.2%, P=0.036) as well as hypertension (36.7% vs. 21.2%, P=0.001).
Table 1.
Mean (standard deviation) or N(%) of Baseline Characteristics According to Conversion Status at Follow-up among Participants in the Western New York Health Study (1996–2004).
| Normoglycemic n=823 | Impaired Fasting Glucose n=101 | P-value | |
|---|---|---|---|
| Mean(SD) | |||
| Age (years) | 54.8(11.2) | 58.1 (11.1) | 0.006 |
| BMI (kg/m2) | 26.6(4.8) | 28.1(5.1) | 0.004 |
| Waist (cm) | 86.1(13.1) | 92.0 (13.1) | <0.001 |
| Sagittal girth (cm) | 19.8(3.3) | 21.3 (3.2) | <0.001 |
| Fasting glucose (mg/dl) | 90.3 (5.6) | 94.2(4.4) | <0.001 |
| ‘ Physical activity | 1.4(1.7) | 1.4 (2.0) | 0.95 |
| Lifetime total pack-years | 9.2 (15.3) | 15.3 (22.8) | 0.01 |
| Duration of smoking (years) | 22.3(13.9) | 25.6 (15.0) | 0.05 |
| N (%) | |||
| Male | 275 (33.4) | 45 (44.6) | 0.034 |
| White, non-Hispanic | 779 (94.7) | 96 (96.0) | 0.55 |
| Formal education ≥ 12 years | 534 (64.9) | 61 (604) | 0.37 |
| Family history of T2DM | 239 (30.2) | 38 (40.9) | 0.036 |
| !Hypertension | 171 (21.2) | 36 (36.7) | 0.001 |
| Current drinking | 572 (70.2) | 63 (62.4) | 0.11 |
| Smoking: | 0.027 | ||
| Never | 414 (50.3) | 37 (36.6) | |
| Former | 315 (38.3) | 47 (46.5) | |
| Current | 94 (11.4) | 17 (16.8) | |
Physical activity in hrs/day ≥ moderate activity.
Hypertension defined as SBP≥140 or DBP≥90 and/or taking anti-hypertensive medication
Table 2 presents the mean (sd) or percentage of baseline characteristics by smoking status. Current smokers were younger (P=0.019) and had a smaller BMI (P=0.003) and waist circumference (P=0.001) compared to never and former smokers. Former smokers had the largest mean sagittal girth (P<0.001). There was no significant difference in FPG or physical activity. The lifetime total pack-years smoked was 70% greater among current smokers compared to former smokers and the duration of smoking was twice as long among current vs. former smokers (P<0.001 for both). Men were more likely to smoke than women, but a greater proportion of current smokers were female (P=0.039). Current smokers were less educated, more likely to be a minority, and female. Never smokers were least likely to report current alcohol consumption (P=0.04).
Table 2.
Baseline Characteristics by Smoking Status
| Never (n=451) | Former (n=362) | Current (n=111) | P-value | |
|---|---|---|---|---|
| Baseline characteristic | Mean (SD) | |||
| Age (years) | 54.5 (11.3) | 56.4 (11.0) | 53.5 (11.2) | 0.019 |
| BMI (kg/m2) | 26.5 (4.6) | 27.4 (5.1) | 25.9 (4.8) | 0.003 |
| Waist (cm) | 85.5 (12.8) | 88.7 (13.7) | 85.1 (13.0) | 0.001 |
| Sagital girth (cm) | 19.6 (3.1) | 20.6 (3.4) | 19.7 (3.3) | <0.001 |
| Fasting glucose (mg/dl) | 90.4 (5.72) | 91.0 (5.5) | 91.0 (5.5) | 0.31 |
| Physical Activity (hrs) | 1.0 (1.3) | 1.0 (1.4) | 1.3 (1.8) | 0.11 |
| Lifetime total number packs | 0.0 (0.0) | 6027 (6493) | 10216 (6665) | <0.001 |
| Lifetime total pack-years | 0.0 (0.0) | 16.5 (17.8) | 28.0 (18.3) | <0.001 |
| Smoking duration (years) | 0.0 (0.0) | 18.7 (12.1) | 36.0 (11.5) | <0.001 |
|
| ||||
| % | ||||
| Male | 30.6 | 39.0 | 36.9 | 0.039 |
| Whites | 95.6 | 95.9 | 88.3 | 0.004 |
| Education >12 years | 67.6 | 58.0 | 56.0 | <0.001 |
| Family history of diabetes | 31.5 | 30.3 | 34.3 | 0.74 |
| Hypertension | 21.5 | 24.7 | 22.2 | 0.55 |
| Current drinker | 65.6 | 73.9 | 69.4 | 0.04 |
Table includes only those who were normoglycemic at baseline. HTN 140/90 and/or medication Some variables have missing data.
Physical Activity- hours per day in >= moderate activity
Table 3 shows the OR (95% confidence interval) of developing IFG compared to remaining normoglycemic over the follow-up period according to baseline smoking status. In model 1, adjusted for age (continuous), FPG (continuous), sagittal girth (continuous), family history of T2DM (yes vs. no) and sex (male vs. female) compared to never smokers the OR of IFG among former smokers was 1.54 (95% CI: 0.93, 2.60) and among current smokers it was 2.36 (95% CI 1.20, 2.46, Ptrend=0.01). With the addition of hypertension (Model 2) the OR wasslightly heightened among former smokers OR 1.68 (95% CI: 0.99, 2.80), but unchanged for current smokers OR 2.35 (95% CI: 1.17, 4.72, Ptrend=0.008). Inclusion of alcohol consumption in the models had no material effect on the ORs.
Table 3.
Multivariate Adjusted OR (95%CI) of Incident IFG
| Model 1 | Model 2 | |
|---|---|---|
| Never | 1.0 | 1.0 |
| Former | 1.54 (0.93, 2.60) | 1.68 (0.99, 2.80) |
| Current | 2.36 (1.20, 4.60) | 2.35 (1.17, 4.72) |
| P trend | 0.01 | 0.008 |
Model 1 age (years), family history (yes/no), glucose (mg/dl), sex, sagittal girth (cm)
Model 2, model 1 plus HTN (yes/no)
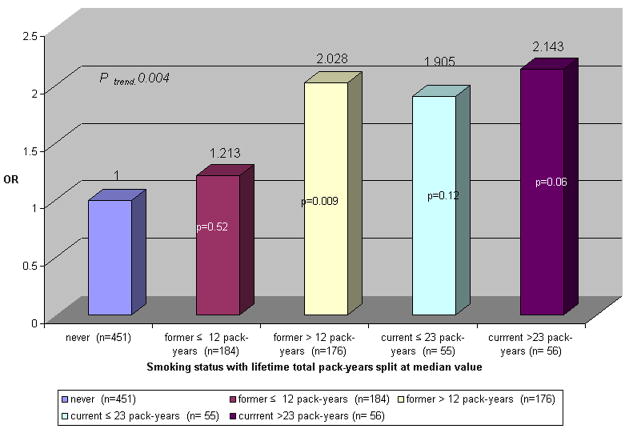
We assessed the effects of duration and intensity (total lifetime pack-years) on the development of IFG. Figure 1 shows the OR of IFG among former and current smokers compared to never smokers stratified by their respective median number of pack-years smoked (12 and 23 pack-years, respectively). Compared to never smokers, the OR of IFG among former smokers with < 12 pack-years was 1.21 (95% CI :0.67, 2.19) and 2.03 (95% CI :1.19,3.44) with ≥ 12. Compared to never smokers, among current smokers with < 23 pack-years the OR of IFG was 1.91 (95% CI: 0.84, 4.33) and ≥ 23 the OR was 2.14 (95% CI :0.97,4.71) (P trend =0.004)
FIGURE 1.
OR of IFG by baseline smoking status with lifetime total pack-years compared to never smokers
DISCUSSION
This community- based prospective study of middle-aged and older men and women from Erie and Niagara Counties in NY is among the first to show that smoking was associated with an increased risk of incident IFG over a 5.9 year follow-up period. The elevated risk extended to both former and current smokers: former smokers carried about a 60% excess risk of developing IFG and current smokers had 2.5 times greater likelihood of incident IFG compared to never smokers. The significant positive linear trend suggests a dose-dependent association between IFG and smoking even after adjustment for several putative risk factors for T2DM including visceral adiposity, family history, age, glucose, sex, and hypertension. Furthermore, in an analysis that combined dosage and duration (lifetime total pack-years) in conjunction with smoking status we noted that the OR of IFG was similar between former smokers whose total lifetime pack-years was > median and current smokers whose lifetime pack-years was < median, suggesting that the deleterious effects of past smoking did not dissipate over the six years of follow- up. We also showed an elevated risk among all smokers but highest among current heavy smokers.
Only two other studies have examined the association between smoking and IFG, both restricted to men. Among 1,266 Japanese male office workers (35–59 years) prospectively followed for five years, current smokers of ≥ 40.1 pack-years had an RR of IFG that was 2.01 (95% confidence interval 1.05,3.83) compared to never smokers(19). Contrastingly, a cross-sectional study of > 3,000 Japanese male self-defense workers (46–59 years, 98% between 50 and 54 years) found no association between cigarette smoking (past or current) and IFG, IGT, or T2DM (20) perhaps due to the study design, the narrow age range, or the select occupational cohort.
A number of mechanisms linking cigarette smoking to glucose metabolism have been proposed. Experimental studies have shown that smoking may impair insulin action mainly due to a lower peripheral glucose uptake leading to an insulin resistant state (29– 31). The mechanism(s) whereby smoking decreases insulin sensitivity remains a source of speculation. One explanation is that cigarette smoke may have a direct toxic effect on the endothelial lining of blood vessels which may lead to increased insulin resistance (23). Moreover, it is well known that smoking is associated with chronic inflammation which is shown to be predictive of Type 2 diabetes. (32–39)
Another hypothesis is that smoking may mediate disturbed glucose metabolism by promoting or inducing alteration in fat distribution. In cross-sectional and longitudinal studies smokers have been shown to be thinner than non-smokers (40, 41). Shimokata showed that the hip circumference was smaller in smokers than non-smokers, but the waist-to-hip ratio was greater in the smokers and increased progressively with the number of cigarettes smoked daily, suggesting that weight is differentially distributed in non-smokers and smokers and more centrally deposited in the smoking group (42). Evidence supporting this was demonstrated in our prospective data: there was a positive linear trend in waist circumference sagittal girth and waist-hip ratio by ascending pack-year category, but no difference in BMI (data not shown). The android pattern of fat deposition is a known risk factor for insulin resistance and diabetes (43–46).
Fifty-one percent (473/924) of subjects in the Western New York Health study smoked (former and current). Compared to non-smokers the crude RR (64/473 divided by 37/451) of IFG among former and current smokers combined was 1.65 (95% confidence interval: 1.29, 2.11). If the relationship between smoking and IFG is causal and if the effect of the exposure were irreversible our results suggest that about 25% (95 confidence interval: 19.2, 30.8) of incident IFG in the total population may be attributed to smoking and would have been preventable if smoking had never been initiated.
The strengths of this study include its population-based, prospective design, and the detailed exposure assessment. Also, we included men and women with a wide age range (35–79 years). One limitation to this study is that the vast majority of participants were White accordingly these results cannot be generalized to minority populations. Further studies in such populations are warranted because minority populations are at high risk for diabetes and some minority groups have a high prevalence of smoking behavior (47). Limitations also include the use of one fasting plasma glucose measure at the baseline and follow-up examinations to identify and categorize normoglycemic and IFG subjects. Reliance on a single measure could have resulted in misclassification: the directionality of the potential bias is unknown. Nevertheless the American Diabetes Association supports the use of one fasting measure (48) in epidemiologic studies partly because it is quicker, easier, and cheaper to obtain than an oral glucose tolerance test (OGTT), and also because it has less intra-individual variation than an OGTT (49–52). Our own data show that the correlation coefficient between baseline and follow-up glucose measures was r=0.60.
In summary this prospective, community based study, showed that the risk of incident IFG was associated with smoking status and intensity in a dose-dependent fashion. The mechanism(s) of action whereby cigarette smoking mediates disturbed glucose metabolism is likely multifactorial and requires additional study. Although IFG is not a clinical entity these results support the need for early identification of those at risk to develop diabetes, namely smokers. A recent NHIS report released by the CDC revealed that one-fourth of U.S. adults self-reported that they had pre-diabetes (IFG or IGT); when questioned about risk reduction behaviors i.e. weight loss, physical activity, or reduced fat or calories 42% engaged in one of these behaviors. (53). Notably absent was information on smoking which continues to be a significant public health concern contributing to a large proportion of chronic diseases.
List of Abbreviations and Acronyms
- T2DM
Type 2 diabetes mellitus
- FPG
Fasting plasma glucose
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- OGTT
Oral glucose tolerance test
- NGT
Normoglycemia
- BMI
Body mass index
- HTN
Hypertension
- RR
Relative risk
- OR
Odds Ratio
- AR
Attributable risk
- CI
Confidence interval
- PA
Physical activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. [see comment] New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. [see comment] New England Journal of Medicine. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. 2008 ( www.diabetes.org/about-diabetes.jsp)
- 4.Sairenchi T, Iso H, Nishimura A, et al. Cigarette Smoking and Risk of Type 2 Diabetes Mellitus among Middle-aged and Elderly Japanese Men and Women. American Journal of Epidemiology. 2004;160:158–62. doi: 10.1093/aje/kwh183. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson S, Midthjell K, Grill V, et al. Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the Nord-Trondelag study. Diabetologia. 2004;47:1953–6. doi: 10.1007/s00125-004-1554-9. [DOI] [PubMed] [Google Scholar]
- 6.Rimm EB, Manson JE, Stampfer MJ, et al. Cigarette smoking and the risk of diabetes in women. American Journal of Public Health. 1993;83:211–4. doi: 10.2105/ajph.83.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rimm EB, Chan J, Stampfer MJ, et al. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. [see comment] BMJ. 1995;310:555–9. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Will JC, Galuska DA, Ford ES, et al. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. 2001:540–6. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 9.Feskens EJ, Kromhout D. Cardiovascular risk factors and the 25-year incidence of diabetes mellitus in middle-aged men. The Zutphen Study American Journal of Epidemiology. 1989;130:1101–8. doi: 10.1093/oxfordjournals.aje.a115437. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami N, Takatsuka N, Shimizu H, et al. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus. Replication and extension in a Japanese cohort of male employees. American Journal of Epidemiology. 1997;145:103–9. doi: 10.1093/oxfordjournals.aje.a009080. [DOI] [PubMed] [Google Scholar]
- 11.Uchimoto S, Tsumura K, Hayashi T, et al. Impact of cigarette smoking on the incidence of Type 2 diabetes mellitus in middle-aged Japanese men: the Osaka Health Survey. Diabet Med. 1999;16:951–5. doi: 10.1046/j.1464-5491.1999.00173.x. [DOI] [PubMed] [Google Scholar]
- 12.Persson PG, Carlsson S, Svanstrom L, et al. Cigarette smoking, oral moist snuff use and glucose intolerance. J Intern Med. 2000;248:103–10. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. [see comment] JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 14.Patja K, Jousilahti P, Hu G, et al. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J Intern Med. 2005;258:356–62. doi: 10.1111/j.1365-2796.2005.01545.x. [DOI] [PubMed] [Google Scholar]
- 15.Meisinger C, Doring A, Thorand B, et al. Association of cigarette smoking and tar and nicotine intake with development of type 2 diabetes mellitus in men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Diabetologia. 2006;49:1770–6. doi: 10.1007/s00125-006-0298-0. [DOI] [PubMed] [Google Scholar]
- 16.Waki K, Noda M, Sasaki S, et al. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabet Med. 2005;22:323–31. doi: 10.1111/j.1464-5491.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Ajani UA, Liu S, et al. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med. 2000;109:538–42. doi: 10.1016/s0002-9343(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 18.Foy CG, Bell RA, Farmer DF, et al. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2501–7. doi: 10.2337/diacare.28.10.2501. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi N, Nakamura K, Matsuo Y, et al. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Annals of Internal Medicine. 2000;133:183–91. doi: 10.7326/0003-4819-133-3-200008010-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sakai Y, Yamaji T, Tabata S, et al. Relation of alcohol use and smoking to glucose tolerance status in Japanese men. Diabetes Research & Clinical Practice. 2006;73:83–8. doi: 10.1016/j.diabres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Trevisan M, Dorn J, Falkner K, et al. Drinking pattern and risk of non-fatal myocardial infarction: a population-based case-control study. [see comment] Addiction. 2004;99:313–22. doi: 10.1111/j.1360-0443.2003.00630.x. [DOI] [PubMed] [Google Scholar]
- 22.Dorn JM, Hovey K, Muti P, et al. Alcohol drinking patterns differentially affect central adiposity as measured by abdominal height in women and men. Journal of Nutrition. 2003;133:2655–62. doi: 10.1093/jn/133.8.2655. [DOI] [PubMed] [Google Scholar]
- 23.Donahue RP, Rejman K, Rafalson LB, et al. Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care. 2007;30:354–9. doi: 10.2337/dc06-1772. [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report.[see comment][erratum appears in JAMA. 2003 Jul 9;290(2):197] JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 25.Stranges S, Trevisan M, Dorn JM, et al. Body fat distribution, liver enzymes, and risk of hypertension: evidence from the Western New York Study. Hypertension. 2005;46:1186–93. doi: 10.1161/01.HYP.0000185688.81320.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. American Journal of Epidemiology. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 27.Levin M. The occurrence of lung cancer in man. Acta Un intern Cancer. 1953;9:531–41. [PubMed] [Google Scholar]
- 28.Szklo M, Nieto EJ. Epidemiology Beyond the Basics. Gaithersburg: Aspen Publishers Inc; 2000. p. 104. [Google Scholar]
- 29.Frati AC, Iniestra F, Ariza CR. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes Care. 1996;19:112–8. doi: 10.2337/diacare.19.2.112. [DOI] [PubMed] [Google Scholar]
- 30.Attvall S, Fowelin J, Lager I, et al. Smoking induces insulin resistance--a potential link with the insulin resistance syndrome. J Intern Med. 1993;233:327–32. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 31.Facchini FS, Hollenbeck CB, Jeppesen J, et al. Insulin resistance and cigarette smoking. [see comment][erratum appears in Lancet 1992 Jun 13;339(8807):1492] Lancet. 1992;339:1128–30. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 32.Thorand B, Lowel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003;163:93–9. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Hu FB, Meigs JB, Li TY, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 34.Han TS, Sattar N, Williams K, et al. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. [see comment] Diabetes Care. 2002;25:2016–21. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 35.Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 36.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 37.Doi Y, Kiyohara Y, Kubo M, et al. Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: the Hisayama Study. Diabetes Care. 2005;28:2497–500. doi: 10.2337/diacare.28.10.2497. [DOI] [PubMed] [Google Scholar]
- 38.Barzilay JI, Abraham L, Heckbert SR, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–9. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 39.Stranges S, Rafalson LB, Dmochowski J, et al. Additional Contribution of Emerging Risk Factors to the Prediction of the Risk of Type 2 Diabetes: Evidence From the Western New York Study. Obesity. 2008;16:1370–6. doi: 10.1038/oby.2008.59. [DOI] [PubMed] [Google Scholar]
- 40.Canoy D, Wareham N, Luben R, et al. Cigarette smoking and fat distribution in 21,828 British men and women: a population-based study. Obesity Research. 2005;13:1466–75. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Khaw K-T. Cigarette Smoking and Increased Central Adiposity. Annals of Internal Medicine. 1989;111:783. doi: 10.7326/0003-4819-111-10-783. [DOI] [PubMed] [Google Scholar]
- 42.Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. [see comment] JAMA. 1989;261:1169–73. [PubMed] [Google Scholar]
- 43.Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145:614–9. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 44.Kaye SA, Folsom AR, Sprafka JM, et al. Increased incidence of diabetes mellitus in relation to abdominal adiposity in older women. J Clin Epidemiol. 1991;44:329–34. doi: 10.1016/0895-4356(91)90044-a. [DOI] [PubMed] [Google Scholar]
- 45.Lundgren H, Bengtsson C, Blohme G, et al. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes. 1989;13:413–23. [PubMed] [Google Scholar]
- 46.Wahrenberg H, Hertel K, Leijonhufvud BM, et al. Use of waist circumference to predict insulin resistance: retrospective study. Bmj. 2005;330:1363–4. doi: 10.1136/bmj.38429.473310.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CDC. Cigarette Smoking Among Adults- United States, 2007. MMWR Weekly. 2008 November 14;57:1221–6. [PubMed] [Google Scholar]
- 48.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 49.Davies MJ, Raymond NT, Day JL, et al. Impaired glucose tolerance and fasting hyperglycaemia have different characteristics. Diabet Med. 2000;17:433–40. doi: 10.1046/j.1464-5491.2000.00246.x. [DOI] [PubMed] [Google Scholar]
- 50.Olefsky JM, Reaven GM. Insulin and glucose responses to identical oral glucose tolerance tests performed forty-eight hours apart. Diabetes. 1974;23:449–53. doi: 10.2337/diab.23.5.449. [DOI] [PubMed] [Google Scholar]
- 51.Feskens EJ, Bowles CH, Kromhout D. Intra- and interindividual variability of glucose tolerance in an elderly population. Journal of Clinical Epidemiology. 1991;44:947–53. doi: 10.1016/0895-4356(91)90058-h. [DOI] [PubMed] [Google Scholar]
- 52.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 53.Self-reported prediabetes and risk-reduction activities--United States, 2006. Mmwr. 2008;57:1203–5. [PubMed] [Google Scholar]