Abstract
OBJECTIVE
Vascular remodeling is a physiological process that occurs in response to long-term changes in hemodynamic conditions, but may also contribute to the pathophysiology of intima-media thickening (IMT) and vascular disease. Shear stress detection by the endothelium is thought to be an important determinant of vascular remodeling. Previous work showed that Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a component of a mechanosensory complex that mediates endothelial cell (EC) responses to shear stress.
METHODS AND RESULTS
We tested the hypothesis that PECAM-1 contributes to vascular remodeling by analyzing the response to partial carotid artery ligation in PECAM-1 knockout mice and wild-type littermates. PECAM-1 deficiency resulted in impaired vascular remodeling and significantly reduced IMT in areas of low flow. Inward remodeling was associated with PECAM-1-dependent NFκB activation, surface adhesion molecule expression and leukocyte infiltration as well as Akt activation and vascular cell proliferation.
CONCLUSIONS
PECAM-1 plays a crucial role in the activation of the NFκB and Akt pathways and inflammatory cell accumulation during vascular remodeling and IMT. Elucidation of some of the signals that drive vascular remodeling represent pharmacologically tractable targets for the treatment of restenosis after balloon angioplasty or stent placement.
Keywords: hemodynamics, vascular remodeling, intima-media thickening, inflammation
Introduction
Vascular remodeling is the ability of the cells of the vessel wall to reorganize their cellular and extracellular components in response to a chronic stimulus 1. For instance, increases in blood flow due to an arteriovenous shunt will increase vessel diameter; conversely, reductions in blood flow initiate a signaling cascade that leads to a reduction in the vascular lumen 2. Vascular remodeling of the carotid artery, clinically defined as intima-media thickening (IMT), is an important predictive phenotype for human cardiovascular disease 3, 4.
An important stimulus for vascular remodeling is blood flow. As blood flows along a vessel, it creates shear stress on the vessel wall. Shear stress forces modulate endothelial structure and function. Due to normal laminar flow, shear stress is a critical factor in maintaining vascular homeostasis. On the other hand, disturbed shear stress is a determinant of localized atherosclerotic lesions at bifurcations and branch points. Many studies have described the correlations between local shear stress and plaque progression in human coronary arteries 5, 6. Even in healthy subjects, carotid IMT is inversely related to carotid shear stress. Shear stress and vascular remodeling are also responsible for restenosis after balloon angioplasty or stent implantation 7, 8.
Vascular ECs are ideally positioned to serve as transducers, to relay hemodynamic and biochemical changes into molecular events in the other layers of the vascular wall 9. EC surfaces are equipped with numerous mechanoreceptors capable of detecting and responding to shear stress, including caveolae, ion channels, integrins, receptor Tyr kinases, the apical glycocalyx, primary cilia, heterotrimeric G proteins, and intercellular junctions. In this context, we recently identified a mechanosensory complex comprised of PECAM-1, vascular endothelial cadherin (VE-cadherin), and vascular EC growth factor receptor-2 (VEGFR2) that mediates EC responses to shear stress 10. Based on the significant role of PECAM-1 in transducing shear stress in ECs in vitro, we hypothesized that PECAM-1 plays an important role in vascular remodeling and IMT associated with changes in flow in vivo.
Methods
Cell culture, shear stress assays
PECAM-1−/− cells and cells reconstituted with murine full-length PECAM-1 were prepared as described11. Levels of PECAM-1 in reconstituted cells are similar to wild-type levels, and the identity of the ECs was confirmed by expression of VE-cadherin and uptake of Ac-LDL (not shown). For oscillatory flow, ECs were sheared at ± 6.5 Dyne/cm2, 1 Hz (there was an average forward flow component of 0.4 dyne/cm2 to allow for nutrient delivery).
Animals
PECAM-1−/− C57BL/6 mice were kindly provided by Dr. P. Newman (Blood Research Institute, Blood Center of Wisconsin, Milwaukee), bred in house and used in accordance with the guideline of the National Institute of Health and for the care and use of laboratory animals (approved by the Institutional Animal Care and Use Committees of the University of North Carolina at Chapel Hill). Male PECAM-1−/− and age-matched littermates (PECAM-1+/+)(12–14 weeks) were used for all experiments. All analyses were conducted by observers blinded to animal phenotype.
Experimental Protocol
Blood flow reduction in the left common carotid artery (LCA) and blood flow measurements were performed as previously described 12–14. Briefly, C57Bl/6 mice were anesthetized with isoflurane (1.5%) and maintained at 37°C on heating pad. They received subsequent antibiotic (50 mg/kg cephazolin), and analgesic (10 mg/kg im Pentazocine) after wound closure. Sterile technique was used to isolate ~0.5-mm lengths of the left external carotid artery distal to the thyroid artery and the left internal carotid/occipital artery pair, followed by ligation with 7-0 suture. Partial blood flow (5–10% within 3 weeks after surgery) was maintained through the thyroid branch. The sham procedure consisted of vessel isolation and ligature placement without ligation. To measure blood flow, 0.7V series transonic flow probe was used according to the manufacturer’s instructions (http://www.transonic.com/workbook.shtml).
Morphometry
At 24-hr, 5-day or 3-week after the surgical procedure, the vasculature was fixed by transcardial perfusion at 100 mmHg with 25 ml sodium nitroprusside in 0.1mM PBS followed by 100 ml of 4% paraformaldehyde. The trachea, left and right carotid arteries, and surrounding tissue were removed en bloc, and fixed for 24 h in 4% paraformaldehyde. The central 5mm section was embedded in paraffin. Sections (5 µm) were cut every 300 µm through the central-most 2.5-mm length, and eight sections were mounted to a slide; additional slides were prepared in the same way by serially sectioning at these intervals along the vessel. For morphometric analyses, slides from the 3-week time point were stained with Masson's trichrome for morphometry or hematoxylin-eosin for cell density and morphology. Images were taken with a Zeiss inverted microscope. The circumferences of lumen (CL), internal and external elastic lamina of common carotid artery (IEL, EEL) were measured from the images using NIH Image J package. Calculations were performed as follows:
Immunohistochemistry
Immunohistochemistry for paraffin embedded cross sections was described previously 13. For en face preparations, the common carotid artery was perfusion fixed and dissected out under dissection microscope. The common carotid arteries were cut longitudinally and pinned flat with endothelium facing up onto a Surperfrost/Plus glass slide (Fisher Scientific, Pittsburgh, PA). We used antibodies to NFκB (1:100, BD Pharmingen, San Diego, CA), phosphorylated NFκB S536 (1:100, Cell Signaling), ICAM-1 (1:100, Santa Cruz, CA), VCAM-1 (1:100, Santa Cruz), phosphorylated Akt pS473 (1:100, Cell Signaling), CD45 (1:50, BD Pharmingen). Antigen retrieval was performed for cross sections with Retrogen (BD Pharmingen), except for NFκB and ICAM-1 antibodies. For Avidin—Biotin—peroxidase Complex (ABC) staining, primary antibodies were incubated at 4°C overnight, followed by 60 minutes for secondary antibody incubation at room temperature, and 30 minutes for ABC complex (Vector Elite). 3,3’ diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA) was used to visualize the peroxidase-binding sites, followed by Mayor modified hematoxylin staining. For en face, slides were permeabilized with 0.3% Triton-PBS for 40 minutes, and blocked with 10% fetal bovine serum for 60 minutes. After overnight incubation of primary antibodies at 4°C, the slides were incubated with FITC-labeled secondary antibodies for 60 minutes. En face preparations were evaluated with a Leica confocal microscope. For quantitation studies, 5 random fields and <100 cells were counted.
Luciferase activity assays
Luciferase activity assays were performed as described 15 using a vector (1.0µg) containing the PDGF-A-chain shear stress response element (PDGF-A/SSRE) regulating the expression of firefly luciferase, together with Renilla luciferase, under the control of a minimal promoter16. Transfections were performed using Lipofectamine LTX Reagent (Invitrogen).
Leukocyte adhesion assay
These assays were performed as previously described 17. To quantify adherent cells, five random fields were counted for each assay.
Statistical Analysis
Results are described as mean ± SEM. Statistical tests were performed with Microsoft Excel analysis package, using Student t test for 2 groups or one-way ANOVA followed by multiple comparisons with Honest Significance Difference (HSD) test. The level of P<0.05 was considered significant.
Results
Vascular Remodeling After Flow Alteration in PECAM-1−/− mice
In order to assess how changes in blood flow and attendant shear stress affect arterial remodeling in vivo, we used the partial carotid ligation model, and examined flow-dependent remodeling in the common carotid arteries of PECAM-1+/+ and PECAM-1−/− mice. This model limits thrombosis and EC denudation and is thus physiologically relevant when studying cardiovascular disease 12. Importantly, carotid flows were comparable in PECAM-1+/+ and PECAM-1−/− mice before and after ligation (Fig S1). While there were no differences in sham PECAM-1+/+ and PECAM-1−/− vessels, we did observe dramatic differences in the LCAs of ligated animals (Fig. 1). Wild-type ligated LCAs had reduced lumen diameter and a dramatic increase in intima-media thickness; in contrast, the LCA IMT of ligated PECAM-1−/− mice was dramatically thinner. Increased flow in the RCA caused a small compensatory increase in lumen area in the PECAM-1+/+ but not in the PECAM-1−/− vessels (Fig. 1). Thus, based on the fact that PECAM-1 deficiency resulted in a striking decrease in intima-media and adventitia thickening induced by ligation, we hypothesized that PECAM-1-dependent signaling might be important during vascular remodeling.
Figure 1. PECAM-1 is necessary for flow-induced remodeling in vivo.

(A). Cross-sections of carotid arteries 3 weeks after ligation. Sham LCAs shown as insets. Bracket shows IMT. Bar, 50µm. (B). Morphometric analysis of carotid artery lumen area and IMT. * P <0.05 vs sham.
Proliferation in PECAM-1−/− Mice
Several studies have shown that proliferation is a driving force for remodeling in response to low flow 12, 18, 19, while apoptosis is also thought to have a central role in IMT 19. Akt plays a particularly prominent part in signaling networks that modulate cellular proliferation, apoptosis and survival. To gain further insight into mechanisms by which PECAM-1 alters IMT, we evaluated phosphorylation of Akt, apoptosis and proliferation in the LCA. LCAs from PECAM-1+/+ and PECAM-1−/− mice were fixed, excised and examined en face. We observed very low levels of pAkt in the ECs of LCAs from shams (Fig. 2A; insets). pAkt levels were significantly upregulated in WT LCAs; however, there was a dramatic decrease in pAkt positive staining in the ECs of PECAM-1−/− (Fig. 2A). To assess proliferation, cross-sections of the carotid arteries were immunostained for PCNA with hematoxylin counterstain to obtain area and nuclear cell density of media and adventitia. In the LCA from sham operated animals from both genotypes we found no cell proliferation. As expected, PECAM-1+/+ animals showed increased proliferation in all three layers in the LCA; in contrast, proliferation was greatly reduced in the PECAM-1 −/− mice after ligation, especially in the intima layer (Fig. 2B). We also assayed apoptosis in both genotypes by staining for cleaved caspase-3. Despite the role for PECAM-1 in apoptosis 20, 21, we did not observe differences in apoptosis (not shown). We therefore conclude that PECAM-1 signaling is required for flow-induced Akt activation and proliferation observed in areas of low flow.
Figure 2. PECAM-1 is necessary for proliferation during vascular remodeling.
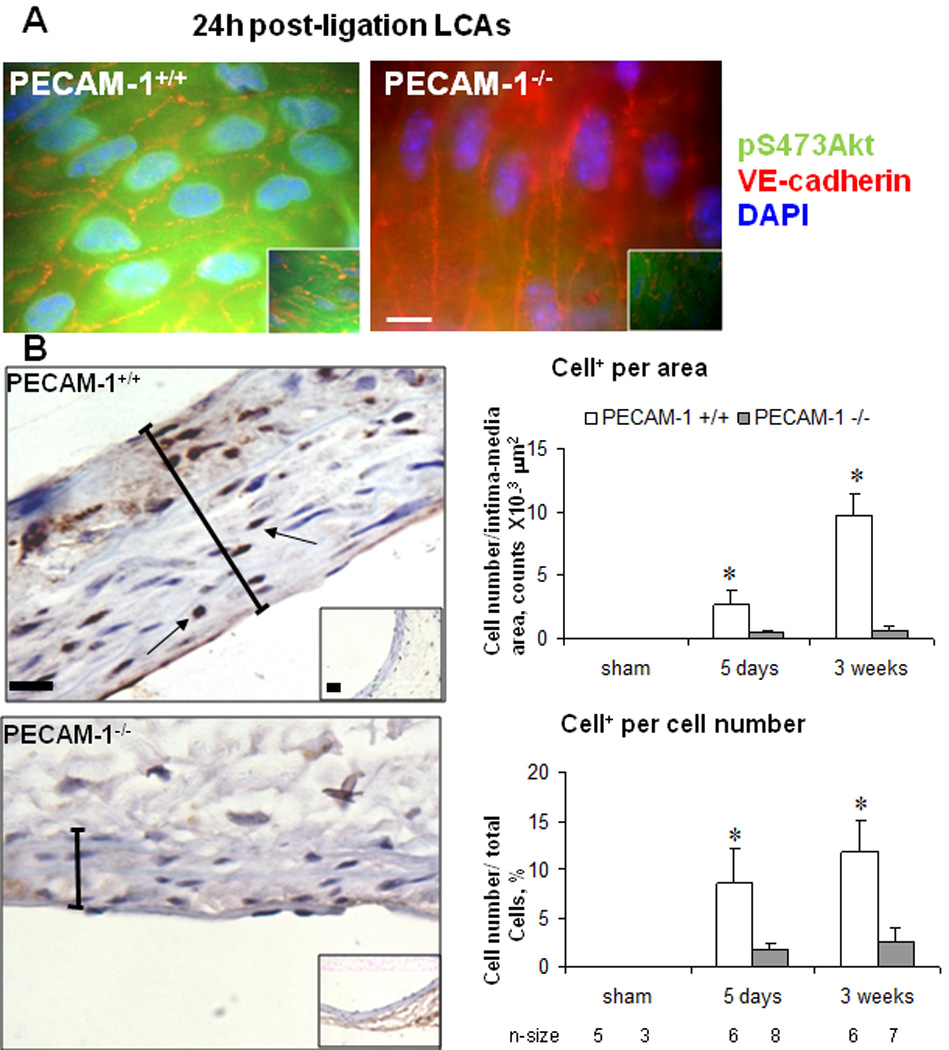
(A) En face LCAs stained for pS473Akt, VE-cadherin and DAPI. Bar, 10µm. (B) Cross-sections of LCAs stained for PCNA and counter stained with hematoxylin. PCNA positive cells were normalized per area, or per total cells. * P <0.05 vs. Bar=20µm
NFκB Pathway in Intima-Media in PECAM-1−/− Mice
Increased expression of a wide array of inflammatory genes, many of which contain NFκB consensus sites within their promoters, is a prominent feature of neointima formation 22. In addition, the NFκB signal transduction pathway is regulated by blood flow and is primed for activation in regions exposed to disturbed flow 23. The impaired IMT and vascular remodeling in PECAM-1−/− carotids raises the possibility that PECAM-1 participates in the regulation of the inflammatory response elicited by ligation. Because proximal to the site of stenosis (or ligation), disturbed shear stress prevails and adversely affects the biology of the arterial wall 24, we used an in vitro system to apply disturbed shear stress to ECs that express (PE-RC) or lack (PE-KO) PECAM-1. PE-RC and PE-KO ECs were exposed to oscillatory flow to allow sustained activation of NFκB, which was assessed by either staining with the phospho-p65 Ab (Fig.S1), or measuring transcriptional activation of NFκB using a luciferase reporter (Fig. 3A). Both assays showed that ECs expressing PECAM-1 are able to activate NFκB, in contrast to cells lacking PECAM-1.
Figure 3. PECAM-1 is necessary for flow-induced NFκB activation in response to flow and during vascular remodeling.

(A). PECAM-1 knockout (PE-KO) or reconstituted (PE-RC) ECs were exposed to oscillatory flow and assayed for NFκB transcriptional activity (* p < 0.05). (B) En face LCAs stained for p65, VE-cadherin and Dapi.. Bar, 10µm.
Because PECAM-1 is required for NFκB activation in response to oscillatory flow in vitro (Fig. 3A), we asked whether the NFκB pathway might explain differences in PECAM-1−/− and PECAM-1+/+ carotid remodeling. As shown in Fig. 3B, although ECs from PECAM-1+/+ LCAs showed nuclear accumulation for the p65 subunit of NFκB, ECs from PECAM-1−/− mice showed cytoplasmic localization for NFκB. Importantly, activation of NFκB in PECAM-1+/+ LCAs is an early event in response to ligation, it was observed as early as 2h and reached a maximum at 24h post ligation.
NFκB dimers bind to a shear stress responsive element found in the promoter of several atherogenic genes, including ICAM-1 and VCAM-1, which regulate monocyte recruitment 25. To test if PECAM-1-dependent NFκB activation translates to altered gene expression, we assayed expression of ICAM-1 and VCAM-1 in response to oscillatory flow. Flow cytometric analysis and immunofluorescence surface staining showed that cells expressing PECAM-1 upregulated both ICAM-1 and VCAM-1. In contrast, PECAM-1 null ECs were unresponsive to flow (Fig. S3). Thus, PECAM-1 expression contributes to the pro-inflammatory phenotype of the endothelium induced by disturbed shear stress.
We then assayed upregulation of these NFκB target genes during flow-induced IMT. We did not observe any differences in VCAM-1 or ICAM-1 staining between sham-operated PECAM-1+/+ and PECAM-1−/− mice. However, expression of VCAM-1 and ICAM-1 in ligated LCAs was much lower in PECAM-1−/− compared to PECAM-1+/+ animals (Fig. 4). We also analyzed expression of ICAM-1 and VCAM-1 in ECs using cross-sections of the carotid (Fig.S2). Interestingly, the NFκB activation described above was temporally followed by CAM upregulation, which was seen as early as 24–48h and lasted for up to 2 weeks after ligation in PECAM-1+/+ LCAs (not shown). Taken together, these results demonstrate that lack of PECAM-1 signaling leads to a disruption of the NFκB pathway, thereby regulating vascular remodeling and IMT.
Figure 4. PECAM-1 is necessary for inflammatory gene expression during vascular remodeling.

En face LCAs stained for ICAM-1 and VE-cadherin. # P <0.05 vs sham. Bar, 60µm
Role of PECAM-1 in flow-induced Inflammation
Upregulation of CAMs is a fundamental prerequisite for the attraction and adhesion of monocytes. This inflammatory response is also important during vascular remodeling 12. Because disturbed flow induces monocyte adhesion both in vivo and in vitro by upregulating CAM expression, we hypothesized that disruption of the NFκB pathway in PECAM-1−/− mice affects monocyte adhesion in response to disturbed flow and during vascular remodeling. Exposure of PECAM-1-expressing ECs to disturbed flow significantly increased monocyte adhesion (Fig. 5A). In contrast, PECAM-1 null ECs could not support monocyte adhesion in response to disturbed flow. Importantly, treatment with TNF-α increased monocyte adhesion independently of PECAM-1 expression, suggesting that the inflammation defect is due to disrupted mechanosignaling rather than due to a direct role for PECAM-1 in transmigration in this system (Fig. 5A). To test whether loss of PECAM-1 signaling affected flow-induced inflammation during vascular remodeling, we used immunohistochemistry for CD45. We found much greater CD45 positive cell accumulation in LCAs of wild-type mice compared to matched areas of PECAM-1−/− mice (Fig. 5B). As was previously reported for C57Bl/6 mice, inflammatory cells were not detected in LCA from sham operated animals in both genotypes. These results therefore suggest that PECAM-1 regulates CAM expression and monocyte adhesion in response changes in flow in vitro and in vivo.
Figure 5. PECAM-1 promotes leukocyte accumulation during vascular remodeling.

(A) PE-RC or PE-KO cells were exposed to oscillatory flow or TNF-α and monocyte cell adhesion was assayed. Bar, 50µm. Quantitation of monocyte binding assays in also shown. (* p < 0.05). (B) Cross-sections of LCAs were stained with CD45 antibody and counter stained with hematoxylin. * P <0.05 vs sham. Bar, 20µm.
Discussion
Shear stress is a biomechanical force generated by fluid flow on the surface of the endothelium and plays a major role in vascular physiology and disease. However, the mechanisms by which ECs sense this mechanical stimulus and transform it into intracellular biochemical signals that change vessel structure and function have not been fully characterized in vivo. PECAM-1 is thought to be involved in flow mechanosensing or transduction, based on changes in its phosphorylation with altered pressure and flow 26–28 and in vitro experiments showing PECAM-1-dependent activation of flow-mediated intracellular signaling pathways 26, 29–32. More recently, researchers used ex vivo approaches: isolated skeletal muscle arterioles of PECAM-1−/− mice to show reduced NO-mediated dilation in response to high temporal gradients of wall shear stress 33, as well as a requirement for PECAM-1 for flow-mediated dilation in the mouse coronary circulation 34. During preparation of this manuscript, two studies reported a role for PECAM-1 in regulating atherosclerosis35, 36. Our findings support and extend these studies by showing that PECAM-1 is also important for vascular remodeling. Although it is important to understand the contribution of PECAM-1 in atherosclerosis, atherosclerosis is a multi-factorial disease influenced by both systemic and mechanical factors. Our findings provide the first demonstration that PECAM-1 is required for flow-mediated remodeling and IMT. We show that activation of NFκB; downstream upregulation of ICAM-1; and inflammatory cell accumulation in the low flow areas do not occur in the PECAM-1−/− mice. Additionally, PECAM-1−/− LCAs had significantly impaired Akt activation and proliferation. As a consequence, PECAM-1 deficient vessels are protected from hypertrophic remodeling seen in the low flow areas. Interestingly, the dramatically reduced IMT in PECAM-1−/− LCAs is similar to that seen in mice deficient in p105, the precursor of p50, one of the components of NFκB 22. These data support the importance of the PECAM-1/ NFκB axis in vascular remodeling Importantly, although PECAM-1 is important in transendothelial migration of monocytes and neutrophils37, 38, its role in transendothelial migration depends on the mouse strain, as C57BL/6 mice showed no discernible effects in two models of inflammation39. Because we investigated flow-mediated remodeling in mice on a C57BL/6 background, it is unlikely that PECAM-1 absence significantly curbed remodeling by inhibiting transmigration. However, we cannot rule out the contribution of the inflammatory role of PECAM-1 to the observed phenotype. PECAM-1 may also regulate vascular remodeling by mechanisms not investigated here. PECAM-1−/− mice have increased bleeding times40 which might affect vascular remodeling and IMT. PECAM-1 has also been shown to regulate eNOS activity32–34, which in turn affects remodeling41.
Flow-dependent vascular remodeling involves multiple genes, cell types and processes 42; indeed, recent studies using inbred mouse or rat strains emphasized the role of genetic factors in remodeling and IMT 18, 43. Amongst candidate mediators that have been extensively studied in mechanotransduction in vitro and remodeling in vivo are caveolae44, 45. Interestingly, flow-dependent remodeling in Caveolin-1 null carotids phenocopies the abnormal remodeling in eNOS-deficient mice41, thus underscoring the importance of both in vascular remodeling. Components of integrin signaling, such as β1 integrin, focal adhesion kinase and vimentin are also important for vascular remodeling 46–48. In addition, generation of reactive oxygen species (ROS) plays a central role in mechanotransduction and vascular remodeling 49, 50. Finally, the role of inflammation and white blood cells in vascular remodeling has become increasingly apparent 19, 51.
Shear stress detection by the endothelium has been proposed to be the primary mediator of flow-induced remodeling 2. The elucidation of a previously unrecognized role for PECAM-1 signaling in regulating vascular remodeling and the elucidation of some of the signals that drive remodeling considerably expand our understanding of the repertoire of molecular regulators controlling this process. Therefore, further characterization of PECAM-1 signaling and its pharmacological modulation might lead to novel therapeutic strategies for the treatment of restenosis after balloon angioplasty or stent placement.
Acknowledgements
A) Sources of Funding: This work was supported in part by an NIH grant (HL088632) to ET and AHA Postdoctoral Fellowship to ZC. ET is an Ellison Medical Foundation New Scholar. B) Acknowledgements: We thank Peter Newman (Blood Center of Wisconsin) for generously providing us with the PECAM-1−/− mice. We also thank James Faber and Hua Zhang for assistance with surgeries; Kirk McNaughton for expert histology assistance; Mauricio Rojas for the flow measurements; and the UNC Flow Cytometry Core Facility. We also thank Slava Korshunov, Debra Newman, Mark Majesky and John Catravas for helpful advice, discussions and critical reading of the manuscript.
Footnotes
C) The authors disclose no conflict of interest.
References
- 1.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 2.Langille BL, Reidy MA, Kline RL. Injury and repair of endothelium at sites of flow disturbances near abdominal aortic coarctations in rabbits. Arteriosclerosis. 1986;6:146–154. doi: 10.1161/01.atv.6.2.146. [DOI] [PubMed] [Google Scholar]
- 3.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ Res. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 4.Cheng KS, Tiwari A, Baker CR, Morris R, Hamilton G, Seifalian AM. Impaired carotid and femoral viscoelastic properties and elevated intima-media thickness in peripheral vascular disease. Atherosclerosis. 2002;164:113–120. doi: 10.1016/s0021-9150(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 5.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18:527–533. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 6.Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, Gerrity RG, Daley W, Rogers C, Edelman ER, Feldman CL, Stone PH. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254. [DOI] [PubMed] [Google Scholar]
- 7.Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89:2816–2821. doi: 10.1161/01.cir.89.6.2816. [DOI] [PubMed] [Google Scholar]
- 8.Wentzel JJ, Gijsen FJ, Stergiopulos N, Serruys PW, Slager CJ, Krams R. Shear stress, vascular remodeling and neointimal formation. J Biomech. 2003;36:681–688. doi: 10.1016/s0021-9290(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 11.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1- deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 13.Erami C, Zhang H, Tanoue A, Tsujimoto G, Thomas SA, Faber JE. Adrenergic catecholamine trophic activity contributes to flow-mediated arterial remodeling. Am J Physiol Heart Circ Physiol. 2005;289:H744–H753. doi: 10.1152/ajpheart.00129.2005. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:1100–1105. doi: 10.1161/01.atv.0000023230.17493.e3. [DOI] [PubMed] [Google Scholar]
- 15.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. Embo J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khachigian LM, Anderson KR, Halnon NJ, Gimbrone MA, Jr, Resnick N, Collins T. Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A- chain promoter. Arterioscler Thromb Vasc Biol. 1997;17:2280–2286. doi: 10.1161/01.atv.17.10.2280. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Sweet D, Irani-Tehrani M, Maeda N, Tzima E. Shc coordinates signals from intercellular junctions and integrins to regulate flow-induced inflammation. J. Cell Biol. 2008 doi: 10.1083/jcb.200709176. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the "Glagov phenomenon" is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 19.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- 20.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–179. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- 21.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squadrito F, Deodato B, Bova A, Marini H, Saporito F, Calo M, Giacca M, Minutoli L, Venuti FS, Caputi AP, Altavilla D. Crucial role of nuclear factor-kappaB in neointimal hyperplasia of the mouse carotid artery after interruption of blood flow. Atherosclerosis. 2003;166:233–242. doi: 10.1016/s0021-9150(02)00336-2. [DOI] [PubMed] [Google Scholar]
- 23.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinlay S, Grewal J, Manuelin D, Fang JC, Selwyn AP, Bittl JA, Ganz P. Coronary flow velocity and disturbed flow predict adverse clinical outcome after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2002;22:1334–1340. doi: 10.1161/01.atv.0000024569.80106.b4. [DOI] [PubMed] [Google Scholar]
- 25.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara K, Masuda M, Osawa M, Kano Y, Katoh K. Is PECAM-1 a mechanoresponsive molecule? Cell Struct Funct. 2001;26:11–17. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- 27.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? Journal of Cell Biology. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osawa M, Masuda M, Harada N, Lopes RB, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule- 1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- 30.Fujiwara K, Masuda M, Osawa M, Katoh K, Kano Y, Harada N, Lopes RB. Response of vascular endothelial cells to fluid flow. Biol Bull. 1998;194:384–385. doi: 10.2307/1543120. discussion 385–386. [DOI] [PubMed] [Google Scholar]
- 31.Osawa M, Masuda M, Kusano KI, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;12:12. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 33.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R57–R65. doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- 35.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-Specific Effects of PECAM-1 on Atherosclerosis in LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial Cell PECAM-1 Promotes Atherosclerotic Lesions in Areas of Disturbed Flow in ApoE-Deficient Mice. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 39.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 40.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, Madri JA. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim J, Miyashiro JK, Berk BC. Shear stress is differentially regulated among inbred rat strains. Circ Res. 2003;92:1001–1009. doi: 10.1161/01.RES.0000069687.54486.B1. [DOI] [PubMed] [Google Scholar]
- 44.Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004;43:8312–8321. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei L, Liu D, Huang Y, Jovin I, Shai SY, Kyriakides T, Ross RS, Giordano FJ. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol Cell Biol. 2008;28:794–802. doi: 10.1128/MCB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koshida R, Rocic P, Saito S, Kiyooka T, Zhang C, Chilian WM. Role of focal adhesion kinase in flow-induced dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2005;25:2548–2553. doi: 10.1161/01.ATV.0000188511.84138.9b. [DOI] [PubMed] [Google Scholar]
- 48.Schiffers PM, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi GE, Levy BI, De Mey JG. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- 49.Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid Redox Signal. 2006;8:1121–1129. doi: 10.1089/ars.2006.8.1121. [DOI] [PubMed] [Google Scholar]
- 50.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phoxdependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 51.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]


