Abstract
Anticipating the sequencing of the human genome and description of the human proteome, the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik) was initiated in 2002. AGES-Reykjavik was designed to examine risk factors, including genetic susceptibility and gene/environment interaction, in relation to disease and disability in old age. The study is multidisciplinary, providing detailed phenotypes related to the cardiovascular, neurocognitive (including sensory), and musculoskeletal systems, and to body composition and metabolic regulation. Relevant quantitative traits, subclinical indicators of disease, and medical diagnoses are identified using biomarkers, imaging, and other physiologic indicators. The AGES-Reykjavik sample is drawn from an established population-based cohort, the Reykjavik Study. This cohort of men and women born between 1907 and 1935 has been followed in Iceland since 1967 by the Icelandic Heart Association. The AGES-Reykjavik cohort, with cardiovascular risk factor assessments earlier in life and detailed late life phenotypes of quantitative traits, will create a comprehensive study of aging nested in a relatively genetically homogeneous older population. This approach should facilitate identification of genetic factors that contribute to healthy aging as well as the chronic conditions common in old age.
Keywords: Aging, Population Genetics, Phenotype, Epidemiology, Cognition, Cardiovascular Disease, Osteoporosis, Body composition
Aging is a complex process that reflects a person’s social and biologic history. Aging may be accompanied by multiple pathologic conditions that increase disease, reduce cognitive and physical function, and impair quality of life. To understand better the determinants of aging, identify potential therapeutic interventions, and design effective prevention programs, a multidisciplinary approach to study well-defined older populations is needed. This approach also lends itself well to the study of genetics since the effects of genes often extend well beyond the single organ system to which a gene was thought to contribute. The rationale for establishing comprehensively evaluated phenotypes across organ systems was described by Freimer and Sabatti in what they term the “The Human Phenome Project.” (1). The Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik) was conceived and designed to provide an approach to study, among other risk factors, the genetic contribution to conditions of old age. This paper describes the rationale and design of AGES-Reykjavik, the measurements included in the study, and provides select descriptive data on the first 2,300 participants.
MATERIALS AND METHODS
Study rationale
AGES-Reykjavik is based on three general hypotheses: first, that genetic variation contributes to disease occurring in old age; second, that selected diseases common in old age share genetic, behavioral, and environmental risk factors; and third, that better classification of phenotypes based on multiple streams of data, including midlife history and subclinical disease, will further the exploration of how these risk factors are associated with complex traits and diseases manifest late in life.
AGES-Reykjavik is an epidemiologic study focusing on four biologic systems: vascular, neurocognitive (including sensory), musculoskeletal, and body composition/metabolism. These four systems were chosen because similar risk factors contribute to physiological changes and disease in these systems. For instance, inflammation is associated with atherosclerosis (2, 3) diabetes (4), obesity (5), smoking-related illnesses (6), dementia (7), osteoporosis (8), and macular degeneration (9).
AGES-Reykjavik stems from the Reykjavik Study, a cohort established in 1967 to prospectively study cardiovascular disease in Iceland. Combining midlife data from the Reykjavik Study and old age data from the AGES-Reykjavik allows a life course approach to better characterize phenotypes. This combination of data can be used to identify patterns of risk factors and evaluate whether these patterns have remained stable or changed with age. For instance, previous studies demonstrate convincingly that risk factors such as blood pressure, weight, and cholesterol measured in late life are influenced by prevalent old age morbidities and no longer reflect the exposures that initiated these pathologies (10, 11). Furthermore, the midlife data is unbiased with regard to health history and is far better than retrospective recall.
Apart from improved phenotypic description, the availability of the mid-life data allows for a complete assessment of nonresponse, particularly how death and refusals might contribute to bias. This assessment will be enhanced by additional information from hospital records, a national mortality index with authentication of all death certificates, a Minimum Data Set for Nursing Home (MDS-NH) and home-care patients (MDS-HC), and archival information from birth records all available for linkage with the cohort.
To define quantitative traits, subclinical and clinical disease, AGES-Reykjavik includes extensive state-of-the-art imaging techniques, biochemical measurements, and diagnostic evaluations. These measures should provide insights into preclinical disease states, identify patterns of concomitant traits, and increase our ability to understand prognostic indicators underlying pathophysiologic changes. Imaging techniques yield standardized information on morphometry of organs and tissues in vivo. Use of imaging in epidemiologic studies has been an effective way to understand subclinical disease particularly in the fields of osteoporosis (12), atherosclerosis (13), brain structure (14), and body composition (15). Since the imaging protocols used in AGES-Reykjavik are similar to protocols in other studies (16, 17), we can compare data directly with these studies. This multi-measurement strategy of phenotypic definition offers important advantages, and has been successfully employed elsewhere (18).
Some characteristics of Iceland and the Icelandic population should enhance the power to examine genetic and gene-environment interactions that modulate expression of genes in old age. The Icelandic population is relatively genetically homogeneous (19), which reduces the problem of population stratification. Thus, a greater proportion of people at the phenotypic extremes may share the same genetic susceptibility. Genealogic databases in Iceland allow identification of relationships in the cohort. The relative isolation and hardship due to deadly infectious epidemics, few major roads, and foreign rule, coupled with volcanic soil and cold climate, lead to restricted diet and increased physical activity, until recently. Nonetheless, Iceland has had high literacy rates and, across the last century, relatively low neonatal mortality. Lastly, Iceland is freer of air and water pollution than many other countries because most electrical energy is generated by a geothermal process (20), minimizing several environmental factors affecting health.
Study design: the Reykjavik Study and AGES-Reykjavik protocols
The Reykjavik Study (RS) originally was comprised of a random sample of 30,795 men and women born in 1907–1935 and living in Reykjavik in 1967 (21–30). The RS sample was divided into six groups (groups B, C, A, D, E, and F) by birth year and birth date within month (Table 1). Each group was invited to participate in specific stages. The B group was designated for longitudinal follow-up and was examined in all stages. The F group was designated a control group and not included in examinations until 1991. Men and women were examined in separate years for more efficient clinic operation. Table 1 shows the number from each group sampled at each stage, with the number examined in each stage in the last column labeled “Respondents”. Since a standard examination was performed in each stage (Tables 2 and 3 for measures), longitudinal and cross-sectional data could be used to study secular and individual changes over the 30-year follow-up period. The stage VI examination (1991–1996) focused on persons aged 70 and older from the F and B groups. It included the core exam components, plus measures of cognitive and physical function, social support, and other topics particularly relevant to aging. Surveillance for vital events and cardiovascular disease events has been continual in the cohort since 1967. Some of the major published research findings from the RS are summarized in Table 4.
Table 1.
Examinations for participants in the Reykjavik Study (1967–1996) and AGES-Reykjavik (2002–2004)*.
| Reykjavik Study Number of Participants |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subcohort | B | C | A | D | E | F | |||
| Total Sample | |||||||||
| Stage of Reykjavik Study | Dates of Examination | Men | 2,955 | 2,743 | 2,755 | 2,282 | 2,106 | 2,081 | |
| Women | 3,101 | 2,990 | 2,936 | 2,429 | 2,191 | 2,225 | Respondents | ||
| I | 1967–1968 | Men | 2,203 | 2,203 | |||||
| 1968–1969 | Women | 2,371 | 2,371 | ||||||
| II | 1970–1971 | Men | 2,072 | 1,985 | 4,057 | ||||
| 1971–1972 | Women | 2,049 | 2,134 | 4,183 | |||||
| III | 1974–1976 | Men | 1,916 | 1,785 | 1,859 | 5,560 | |||
| 1977–1979 | Women | 1,014 | 955 | 1,931 | 3,900 | ||||
| IV | 1979–1981 | Men | 1,801 | 1,443 | 3,244 | ||||
| 1981–1984 | Women | 1,968 | 1,619 | 3,587 | |||||
| V | 1985–1987 | Men | 1,477 | 1,115 | 2,592 | ||||
| 1987–1991 | Women | 1,765 | 1,266 | 3,028 | |||||
| VI | 1991–1994 | Men | 664 | 169 | 833 | ||||
| 1994–1996 | Women | 943 | 267 | 1,210 | |||||
|
AGES-Reykjavik Number of Participants |
|||||||||
| AGES-Reykjavik | 2002–2004 | Men | 344 | 320 | 305 | 2 | 5 | 0 | 976 |
| 2002–2004 | Women | 467 | 414 | 426 | 7 | 10 | 0 | 1,324 | |
This table shows the cohort recruitment and examination schedule for the Reykjavik Study (RS) and the Age, Gene/Environment Susceptibility- Reykjavik Study (AGES-Reykjavik) through February, 2004. The RS cohort was randomized into six groups or subcohorts (B, C, A, D, E, and F) based on birth dates. The RS examinations were done in six stages, listed on the left, during which different sub-cohorts groups were invited. The B group was designated for longitudinal follow-up and examined at each stage. Men and women were examined separately at each stage to optimize examination clinic logistics. At the bottom, the row labeled ‘AGES-Reykjavik’ represents the number of persons from each of the RS subcohorts who were recruited among the first 2,300 participants to enter the AGES-Reykjavik Study. When AGES-Reykjavik began, 4,800 men and 6.749 women from the RS were alive (as of March, 2002).
Table 2.
The Reykjavik Study and Ages-Reykjavik questionnaire components
| Questionnaire Components | Reykjavik Study 1967–1991 |
Reykjavik Study for age >70 1991–1996 |
AGES- Reykjavik 2002–2006 |
AGES- Reykjavik follow-up 2007–2011 |
|---|---|---|---|---|
| Proxy contact information | X | X | ||
| General health status and hospitalizations | X | X | X | X |
| Medical History | ||||
| Heart and arteries: general diagnosis, surgical procedures, chest pain history | X | X | X | X |
| Diabetes: general diagnosis, medications, diet | X | X | X | X |
| Lung disease | X | X | X | X |
| Hypertension: general diagnosis, medications | X | X | X | X |
| High cholesterol | X | X | ||
| Falls and broken bones | X | X | X | |
| Arthritis: type, location, related impairment | X | X | X | X |
| Migraines: symptoms | X | X | X | |
| Stroke or transient ischemic attack (TIA): general diagnosis, symptoms | X | X | X | |
| Parkinsonism symptoms | X | X | ||
| Restless leg syndrome symptoms | X | |||
| Other diseases | X | X | X | |
| Cancer | X | X | X | X |
| Hearing problems and ear diseases: occupational exposure, degree of impairment | X | X | ||
| Vision problems: cataracts, glaucoma, macular degeneration | X | X | ||
| Dentition: periodontal disease, dentures | X | |||
| Prostate disease (MEN) | X | X | ||
| Reproductive history (WOMEN): pregnancies, menopause, medications | X | X | ||
| Weight history | X | X | ||
| Sleeping habits | X | X | ||
| Urinary Incontinence | X | X | ||
| Anxiety | X | X | ||
| Geriatric Depression Scale | X | X | X | |
| Depression history and medications | X | X | X | |
| Subjective memory problems | X | X | X | |
| Social activity and contacts | X | X | X | |
| Coping and perceived stress | X | |||
| Cognitively stimulating leisure activities | X | X | ||
| Functional limitations: stairs, 500 m walk, activities of daily living (ADL), instrumental activities of daily living, use of assistive devices | X | X | X | |
| Family medical history | X | X | X | |
| Education and languages | X | X | X | |
| Occupational history | X | X | X | |
| Wealth indicators | X | X | X | |
| Residence location in youth and mid-life. | X | X | X | |
| Diet history: youth, mid-life, current (old-age) | X | X | ||
| Smoking and tobacco use history | X | X | X | |
| Alcohol | X | X | ||
| Physical activity: winter, summer, youth, mid-life | X | X | X | X |
Table 3.
The Reykjavik Study and Ages-Reykjavik examination components
| Measurements | Reykjavik Study 1967–1991 |
Reykjavik Study for age >70 1991–1996 |
AGES- Reykjavik 2002–2006 |
AGES- Reykjavik follow-up 2007–2007 |
|---|---|---|---|---|
| VASCULAR SYSTEM | ||||
| Pulse, blood pressure | X | X | X | X |
| Electrocardiogram: Heart rate, rhythm, ischemia, silent myocardial infarction (exercise test of subgroup in Reykjavik Study) | X | X | X | X |
| Heart Rate Variability (measured during cognitive and physical function assessment for stress response) | n=1023 | |||
| Ultrasonography of Carotid: Intimal/medial thickness, plaque count, carotid distensibility | X | |||
| Computerized tomography of vascular calcium: Coronary calcium, calcium volumes for aortic arch, and descending aorta | X | X | ||
| Digitized retinal photograph: Arterial damage, drusen, retinal exudates | X | |||
| Echocardiography: Left ventricular thickness, wall motion, valve structure/function | n=900 | |||
| Arterial tonometry: Pulse wave velocity | n=900 | X | ||
| Cardiac MRI with gadolinium enhancement: MRI defined MI, cardiac output, wall motion | n=1100 | |||
| Lipids (laboratory): Total, HDL, LDL cholesterol, triglycerides | X | X | X | X |
| Renal Function (laboratory): creatinine | X | X | X | X |
| NEUROCOGNITIVE | ||||
| Neuropsychological testing: Memory, speed of processing, working memory | X | X | X | |
| Mood: Depression symptoms, anxiety | X | X | X | |
| History of depression: depression diagnosis | X | X | ||
| MRI of the brain: Atrophy/ventricular size, infarct size and location, white matter lesion load and location, voxel-based morphometry | X | X | ||
| Dementia evaluation: Dementia diagnosis and subtype adjudication by clinical consensus | X | X | ||
| Visual acuity and functional vision | X | |||
| Audiometry evaluation | X | |||
| MUSCULOSKELETAL | ||||
| Computerized tomography of L1/L2 (1mm slices): Integral and trabecular bone quality, structural properties | X | X | ||
| Computerized tomography of hip (1 mm slices): Integral, cortical, and trabecular bone quality of total and regional femur, structural properties | X | X | ||
| Hand photographs for osteoarthritis assessment: phalangeal abnormalities | X | |||
| Goniometry of knee | X | |||
| OBESITY/SARCOPENIA AND METABOLISM | ||||
| Anthropometric measurements: Height, weight, waist circumference | X | X | X | X |
| Bioelectrical impedance: Total body fat and non-fat lean | X | X | ||
| Isometric dynamometry: Quadriceps strength, hand grip strength | X | X | ||
| Computerized tomography of L4/L5: Sagittal diameter, waist and thigh circumference, visceral, subcutaneous, intermuscular, intramuscular fat areas, total and selected muscle areas | X | X | ||
| Computerized tomography of thigh: Subcutaneous, intermuscular, intramuscular fat areas, total and selected muscle areas | X | X | ||
| INTEGRATIVE FUNCTION | ||||
| Health questionnaire: Behavioral risk factors, Social support/network, Medical history – see detailed questionnaire information in Table 2 | X | X | X | X |
| Motor and proprioceptive function: Balance platform, performance measures(TUG, 6meter walk) | X | X | Performance measures | |
| EuroQol EQ-5D questionnaire of health outcomes | X | X | ||
| Inflammation (laboratory): C-reactive protein, sedimentation rate | X | X | X | X |
| Stress Response (laboratory): Evening and morning salivary cortisol | X | |||
| Glucose Regulation (laboratory): fasting insulin, fasting glucose, hemoglobin A1C | X | X | X | X |
| Pulmonary Function: spirometry | X | X | 3000 | |
| Medications Inventory: prescriptions, over the counter | X | X | X | |
| IMAGE ARCHIVE: MRI, CT, Ultrasound, Retinal photographs | X | X | ||
| BIORESPOSITORY: serum, plasma, urine, and cells | X | X | X | X |
Abbreviations: MI= myocardial infarction, MRI=magnetic resonance imaging, CT=computerized tomography, HDL=high-density lipoprotein, LDL=low-density lipoprotein, TUG=Timed Up and Go Test,
Table 4.
Selected Findings from the Reykjavik Study
| Reference | Summary of finding |
|---|---|
| 21,22 |
Unrecognized Myocardial Infarction (MI) Risk factors and prognosis were similar for recognized and unrecognized MI. Risk of recurrent MI following an unrecognized MI was similar in men and women. Unrecognized MI is as common in women as in men |
| 22 |
Family history Family history of MI from questionnaire is an independent risk factor for MI that cannot be explained by the conventional risk factors |
| 24, 29, 30 |
Inflammation Erythrocyte sedimentation rate is an independent risk factor for MI. C-reactive protein is an independent risk factor for MI but does not add markedly to the conventional risk factors in prediction of MI. Mannose-binding lectin is predictive of MI in high-risk persons, such as diabetics or those with raised cholesterol. |
| 25, 26 |
Smoking and cancer Smoking was the most commonly associated risk factor for the development of neoplasms among the cardiovascular risk factors. Family history of lung cancer was shown to be an independent risk factor for lung cancer, even accounting for smoking. |
AGES-Reykjavik examinations began in 2002. At that time, there were 11,549 previously examined RS cohort members still alive. From these individuals, we randomly assigned recruitment order within the six RS groups. First we sampled from the A, B, and C groups, since these individuals had the largest amount of past examination data. We then sampled from the rest of the formerly examined participants (D and E groups). We did not sample within gender to preserve the fact that the RS had been initiated with a random sample of the population of Reykjavik in these birth cohorts. At the end of AGES-Reykjavik examinations in February, 2006, 5,764 survivors of the RS cohort had been examined (42 percent male). The AGES-Reykjavik examination is a single wave of examination, completed in three clinic visits, with a participant’s full examination completed within a four to six week time window.
Phenotypic data in AGES-Reykjavik are collected using standardized protocols (Table 3). The first clinic visit includes a blood draw, blood pressure, electrocardiography, anthropometry, and measures of different domains of physical and cognitive function. The questionnaire, based on the original RS questions, includes health history, life-style practices, a medication survey, and a food history including early life diet and social aspects of daily life (Table 2). Serum, plasma, salivary swabs, and urine are obtained for metabolic, hormonal, and inflammatory markers. White blood cells are obtained, processed, and stored. Chemical measurements are carried out in the laboratory of the Icelandic Heart Association with independent external standards. Cells have been saved for transformation for more than half the cohort.
The second exam day includes imaging protocols using magnetic resonance imaging (MRI), computerized tomography (CT), and ultrasound instrumentation (Table 3). The third exam includes vision screening, assessment of intraocular pressure, digital retinal photographs through dilated pupils, a hearing test, a dementia assessment, if indicated, and the exit interview with a physician or nurse. The clinic, laboratory, and imaging suite are all housed in the same building. For those unable or unwilling to come to the clinic, a home examination has been available but was used sparingly.
Dementia case ascertainment is done in a 3-step process. The Mini-Mental State Examination (31) and the Digit Symbol Substitution Test (32) are administered to all participants. Individuals screen-positive based on a combination of these tests are administered a second, more diagnostic test battery, and a subset of these are selected for a neurologic exam. Proxies for this latter group are interviewed about medical history, and social, cognitive and daily functioning relevant to the diagnosis. A consensus diagnosis based on international guidelines is made by a panel that includes a geriatrician, neurologist, neuropsychologist, and neuroradiologist. We also screen for depression at visit one with follow-up testing for screen-positives with the M.I.N.I., which gives more detailed diagnostic information about psychiatric morbidity (33).
The image acquisition and reading protocols were designed in conjunction with expert consultants. Image acquisition is performed by a team of radiographers who have been trained and certified in each of the protocols. This group, augmented by trained lay readers, also analyzes all images except the retinal photographs, which are read by an independent reading center. Scans are first reviewed by a radiologist for major clinical abnormalities. Image analysis is generally semi-automated. All information, including images are de-identified prior to transfer into the permanent study database.
Phenotypic data will be combined with supplemental data on clinical outcomes. Sources of supplemental data include registries of vital status, cardiovascular disease and procedures, fractures; hospital records with International Classification of Diseases (ICD) codes; the MDS-NH (34), and the MDS-HC (35, 36). Registries are based on medical record data using predetermined algorithmic criteria.
Standardized quality control protocols have been established for the clinical and laboratory measures, the image acquisition, and image analysis. For all image modalities, a five to 10 percent random sample is re-read by consulting experts. In addition, a standard set of scans for each core measure is re-read over the year by the image analysis team to monitor drift in the readings. For the laboratory, all analyses are controlled with a set of daily internal quality control samples and quality assurance samples are measured monthly in accordance with the Scandinavian External Quality Assessment (EQA) organizers. Imaging machines are also monitored with daily, weekly, and monthly measures.
Genotyping will be carried out both at the Icelandic Heart Association and at other laboratories. With high throughput genotyping becoming more available, collaborations with other studies with similar phenotypic data are planned, for initial gene discovery and for replication.
AGES-Reykjavik was approved by the National Bioethics Committee in Iceland that acts as the Institutional Review Board for the Icelandic Heart Association (approval number: VSN-00-063), and by the National Institute on Aging Intramural Institutional Review Board. A multistage consent is obtained in AGES-Reykjavik to cover participation, use of specimens and DNA, and access to administrative records. All requests to merge AGES-Reykjavik data with administrative, genealogic, hospital, or nationally maintained databases are reviewed by the Icelandic Data Protection Committee. Release of data for analysis is governed by rules created by these bodies to protect the privacy of Icelandic participants.
Starting in 2007, all surviving AGES-Reykjavik participants will be recruited to a second examination. This examination is restricted to components that are central to testing hypotheses related to the four study areas and will show change over time. The planned measurements are shown in Tables 2 and 3.
Statistical methods
Selected cardiovascular risk factors are compared in all RS participants eligible for AGES-Reykjavik, in the first 1,310 men and 1,933 women invited to AGES-Reykjavik, and in the first 976 men and 1,324 women enrolled. Not described are the additional 3,464 participants enrolled in AGES-Reykjavik. Eligible are compared to invited and non-responding invited are compared to enrolled. Comparisons are made for the following: total cholesterol, triglycerides (log-transformed and then back transformed), fasting glucose, systolic blood pressure, and body mass index (weight in kilogram divided by height in meters squared) (22). In AGES-Reykjavik, lipids and glucose were analyzed using a Hitachi 912 (Roche Diagnostics, Switzerland, 1999) with comparable quality assessment standards as used in the RS.
Using SAS Proc Genmod (37), all age-adjusted regression models were created separately for men and women (Tables 5 and 6). Midlife data was adjusted to age=50 and AGES-Reykjavik data to age=76. Age-adjusted linear regression was used to compare groups on continuously distributed data; logistic regression models were used for smoking.
Table 5.
Midlife values (adjusted to age 50) of selected disease risk factors in eligible, invited and the first 2300 AGES-Reykjavik Study participants: Men.
| MEN | Eligible from Reykjavik Study cohort members N=4,800 | Invited for AGES-Reykjavik N=1,310 | Non-responders to AGES-Reykjavik N=334 | AGES-Reykjavik enrollees N=976 | ||||
|---|---|---|---|---|---|---|---|---|
| Selected Risk Factors | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| TC (mmo1/L) | 6.32 | 6.29,6.35 | 6.39 * | 6.33,6.45 | 6.34 | 6.22,6.46 | 6.4 | 6.34,6.47 |
| TG (mmo1/L) | 1.15 | 1.13,1.17 | 1.11 * | 1.08,1.13 | 1.16 | 1.11,1.23 | 1.08 † | 1.05,1.11 |
| Fasting Glucose (mmol/L) | 4.48 | 4.46,4.50 | 4.47 | 4.44,4.50 | 4.52 | 4.46,4.58 | 4.45 † | 4.41,4.48 |
| SBP (mmHg) | 136.4 | 135.8,137.0 | 137.6 * | 136.6,138.6 | 142.5 | 140.2,144.9 | 135.6 ‡ | 134.5,136.7 |
| BMI (kg/m2) | 25.7 | 25.6,25.8 | 25.5 * | 25.3,25.7 | 25.7 | 25.3,26.0 | 25.4 | 25.2,25.6 |
| Smokers (%) | 50.2 | 48.7,51.7 | 52.1 | 49.3,54.8 | 55.1 | 49.6,60.6 | 51 | 47.8,54.2 |
Abbreviations: CI=Confidence Interval, TC=Total Cholesterol, TG=Triglycerides, SBP=Systolic Blood Pressure, BMI=Body Mass Index
p<0.05 between midlife data of invited and eligible Reykjavik Study cohort members
p<0.05 between midlife data of non-responders and AGES-Reykjavik Study enrollees
p<0.01 between midlife data of non-responders and AGES-Reykjavik Study enrollees
Table 6.
Midlife values (adjusted to age 50) of selected disease risk factors in eligible, invited and the first 2300 AGES-Reykjavik Study participants: Women
| WOMEN | Eligible from Reykjavik Study cohort members N=6,749 | Invited for AGES-Reykjavik N=1,933 | Non-responders to AGES-Reykjavik N=609 | AGES-Reykjavik enrollees N=1,324 | ||||
|---|---|---|---|---|---|---|---|---|
| Selected Risk Factors | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| TC (mmo1/L) | 6.32 | 6.28,6.35 | 6.28 | 6.23,6.33 | 6.36 | 6.25,6.46 | 6.26 | 6.20,6.32 |
| TG (mmo1/L) | 0.91 | 0.90,0.93 | 0.88 * | 0.87,0.90 | 0.89 | 0.86,0.93 | 0.88 | 0.86,0.90 |
| Fasting Glucose (mmol/L) | 4.29 | 4.27,4.31 | 4.25 * | 4.23,4.28 | 4.29 | 4.22,4.36 | 4.23 ‡ | 4.21,4.27 |
| SBP (mmHg) | 128.1 | 127.6,128.7 | 128.5 | 127.6,129.4 | 133.1 | 131.2,135.0 | 126.7 § | 125.7,127.8 |
| BMI (kg/m2) | 24.9 | 24.8,25.0 | 24.7 † | 24.5,24.8 | 24.7 | 24.3,25.1 | 24.6 | 24.4,24.9 |
| Smokers (%) | 36.3 | 35.0,37.7 | 32.3 * | 30.1,34.5 | 36.3 | 32.1, 40.8 | 30.8 ‡ | 28.4,33.5 |
Abbreviations: CI=Confidence Interval, TC=Total Cholesterol, TG=Triglycerides, SBP=Systolic Blood Pressure, BMI=Body Mass Index
p<0.01 between midlife data of eligible Reykjavik Study cohort members and those invited
p<0.05 between midlife data of eligible Reykjavik Study cohort members and those invited
p<0.05 between midlife data of non-responders and AGES-Reykjavik Study enrollees
p<0.0001 between midlife data of non-responders and AGES-Reykjavik Study enrollees
Among the first 2300 enrolled participants, we compared measures of cardiovascular risk factors from midlife with their current measurements (Table 7). Repeated measures generalized estimation models were used, with age at entry and time between visits as covariates.
Table 7.
Comparison of midlife Reykjavik Study and late life AGES-Reykjavik measurements of selected cardiovascular risk factors
| Gender | Variable | Reykjavik Study age-adjusted values | AGES-Reykjavik age-adjusted values | Pearson Correlation between values | 10-year change | 26-year change | p-value for correlation |
|---|---|---|---|---|---|---|---|
| MEN N=976 | Total cholesterol (mmol/L) | 6.40 | 5.27 | 0.26 | −0.41 | −1.06 | <0.01 |
| Triglyceride (mmol/L)* | 1.08 | 1.07 | 0.44 | −0.01 | −0.02 | 0.24 | |
| Serum glucose (mmol/L)† | 5.52 | 5.97 | 0.24 | 0.17 | 0.43 | <0.01 | |
| Systolic blood pressure (mmHg) | 135.6 | 141.9 | 0.20 | 2.4 | 6.2 | <0.01 | |
| Body mass index (kg/m2) | 25.4 | 26.7 | 0.66 | 0.4 | 1.16 | <0.01 | |
| WOMEN N=1,324 | Total cholesterol (mmol/L) | 6.26 | 6.11 | 0.27 | −0.08 | −0.21 | <0.01 |
| Triglyceride (mmol/L)* | 0.88 | 1.15 | 0.46 | 0.10 | 0.26 | <0.01 | |
| Serum glucose (mmol/L)† | 5.32 | 5.70 | 0.30 | 0.17 | 0.43 | <0.01 | |
| Systolic blood pressure (mmHg) | 126.7 | 141.4 | 0.31 | 5.5 | 14.4 | <0.01 | |
| Body mass index (kg/m2) | 24.6 | 27.1 | 0.69 | 0.9 | 2.4 | <0.01 |
Analysis on log transformed values. 10 year change back transformed
The Reykjavik Study (RS) value is blood sugar. Conversion to serum glucose was 1.47+0.91x(RS blood sugar).
To illustrate the power of obtaining detailed measures on several biologic systems, we identified a key measurement from each of the four focus areas of the study and examined their joint prevalence in the first 2,300 of the total 5,764 persons enrolled in the cohort. We examined trabecular bone mass, performance on two cognitive tests, fasting insulin, and arterial calcification (Table 8). Trabecular bone mass was measured from the quantitative CT scans of the femoral neck and spine (38). For insulin, cognition, and trabecular bone density, scores below gender-specific medians were considered low scores (Table 8). Higher arterial calcification, imaged with helical CT and calculated as an Agatston score (39), was defined as having calcification in four of the five sites examined, including the ascending and descending aorta, the combined coronary arteries, and in the thoracic and abdominal aorta. For individuals missing data on one site, if all other sites analyzed had calcium present, they were considered at high risk. For this illustrative example, we selected cut-points that would provide overlap between traits; if other cut-points had been defined, the overlap proportions would have changed.
Table 8.
Cut-points used to examine overlap in the four focus areas for AGES-Reykjavik participants
| Men (N=976) | Women (N=1,324) | |||
|---|---|---|---|---|
| Median or % | 25th and 75th percentiles | Median or % | 25th and 75th percentiles | |
| Trabecular bone mineral density mg/cm3 | ||||
| Lumbar spine | 0.09 | 0.07,0.11 | 0.07 | 0.05,0.09 |
| Femoral neck | 0.03 | 0.01,0.06 | 0.01 | −0.01,0.04 |
| Glucose metabolism: | ||||
| Serum Insulin mU/L | 8.52 | 5.67,12.72 | 7.85 | 5.31,11.20 |
| Cognition: | ||||
| Mini-Mental State Exam | 27 | 25, 29 | 28 | 26, 29 |
| Digit Symbol Substitution Test | 28 | 21, 36 | 29 | 21, 36 |
| Calcification of arteries (% with any calcification)* | ||||
| Coronary arteries | 96.40% | 81.60% | ||
| Ascending aorta | 98.70% | 98.60% | ||
| Descending aorta | 84.50% | 84.80% | ||
| Abdominal aorta L1/L2 | 96.30% | 96.50% | ||
| Abdominal aorta L4/L5 | 91.80% | 89.50% | ||
| In 4 of 5 aortic areas and coronary arteries (%) | 91.10% | 85.10% | ||
Agatston scores and calcification measurements in the abdominal aorta at vertebral levels L1/L2 and L4/L5 with values greater than zero indicate that some degree of calcification is present. The percentages in this table reflect the percent of the cohort with any calcification present at the noted location.
RESULTS
Total eligible RS cohort versus randomly selected AGES-Reykjavik invitees
There were 11,549 participants from the RS alive as of March 2002, including 4,800 men (41.6 percent of those alive). From this group, a random sample of 1,310 men was invited to the AGES-Reykjavik clinic through February 2004. We first compared mean midlife values of cardiovascular risk factors for the 4,800 living, eligible men to the 1,310 invited to the AGES-Reykjavik examination (Table 5). Those invited had higher total cholesterol, lower triglycerides, higher systolic blood pressure, and lower BMI in midlife than the average midlife values for the pool of men alive. A similar analysis for women also showed differences between women who participated in the Reykjavik Study and those invited to participate in AGES-Reykjavik, but the factors that differed were not the same as in men. Of the 6,749 living, eligible women, a random sample of 1,933 women was invited to attend the AGES-Reykjavik exam. Compared with all the living RS women, the 1,933 invited had significantly lower triglycerides, fasting blood glucose, lower BMI, and included a smaller percentage of smokers (Table 6).
Responders versus non-responders through February 2004
Among the 1,310 men invited, 976 (response rate of 75 percent) agreed to participate in the study. Compared to those who refused, participants had significantly lower midlife triglycerides, fasting blood glucose, and systolic blood pressure (Table 5). The percent of men who smoked in midlife was similar in the two groups as was midlife total cholesterol and body mass index (BMI). Of the 1,933 women invited, 1,324 women participated in the examination (response rate of 68 percent). Women who participated in AGES-Reykjavik had significantly lower midlife glucose and systolic blood pressure, and were less likely to have been a smoker than non-responders (Table 6). BMI, total cholesterol, and triglycerides did not differ between these groups. In both men and women, nonresponse was greater among persons with a previously poor cardiovascular risk profile, particularly for systolic blood pressure and blood glucose.
Midlife versus late-life characteristics of first 2,300 participants recruited to the AGES-Reykjavik Study
Among the first 2,300 participants, all measures differed significantly between the mid-life and late-life measures with the exception of triglyceride levels in men (Table 7). Interestingly, other than BMI, midlife and older age measurements were only moderately correlated, with the lowest correlations for systolic blood pressure and fasting glucose. BMI, glucose, and systolic blood pressure all increased into old age, as did triglyceride levels in women; only total cholesterol decreased.
Joint prevalence of health measures
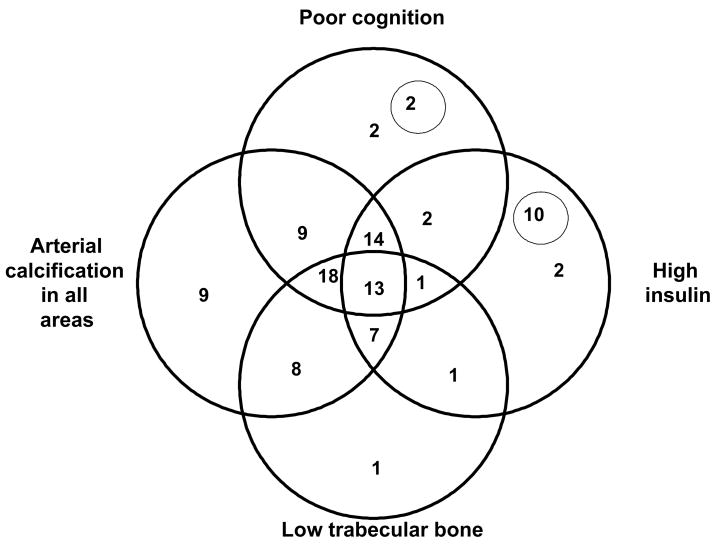
In this older population, overlap between measures representing the four focus areas of the study (trabecular bone mass, cognitive test performance, fasting insulin, and arterial calcification) was more common than the occurrence of a single characteristic (Figure 1) -- each alone was less than three percent, except for arterial calcification which as nine percent. Forty percent of the participants had three of the four defined characteristics, with the most common combination being lower trabecular bone, more arterial calcification, and lower cognitive score (18 percent), while the least common combination involved lower trabecular bone, poorer cognition, and higher insulin (one percent). Variation among these characteristics can be used to study successful aging, with few diseases, or to study the extreme of frailty, often accompanied by multiple health conditions.
Figure 1. Independence and overlap of prevalent phenotypes in the AGES-Reykjavik Study, 2002–2004.
Phenotypes from the Age, Gene/Environment Susceptibility Study (AGES-Reykjavik) are represented by the overlapping circles in this figure, representing the traits of poor cognition, arterial calcification in all areas, high insulin, and low trabecular bone. The phenotypes are further defined in Methods section. Numbers within the circles represent the percent of the cohort with each of these traits. Numbers in areas of overlap indicate the percent of the cohort that has more than one trait. Two percent of the cohort had none of the phenotypes and only 13 percent share all the traits. The number inside the small circle within the ‘poor cognition’ phenotype represents the percent of the cohort which had both poor cognition and low trabecular bone. Similarly, the number inside the small circle within the ‘high insulin’ phenotype represents the portion of the cohort that has both high insulin and arterial calcification in all areas.
DISCUSSION
A major goal of AGES-Reykjavik is intensive quantitative trait identification, within and across biologic systems, for studying the genetic contribution to diseases of old age. Because of the in-depth characterization within and between multiple physiologic systems, this study should also create a valuable resource for a comprehensive study of aging.
Many system-specific studies of the contribution of genetics to complex disorders have been undertaken. To our knowledge, this is one of the few studies designed a priori to comprehensively phenotype a cohort for multiple diseases, where the target conditions were selected based on the potential of genetic factors that contribute either to the discrete disease state or to quantitative traits that might underlie these conditions. This should allow for broader exploration of contributing genes and should be particularly valuable for analysis with whole genome SNP markers. The range of phenotypic characterization of the cohort, from clinically recognized conditions defined by criteria-based diagnoses to novel intermediate endophenotypes based on non-invasive technologies integrated with genetic, biochemical, physiologic, and performance-based measures of health and function, should provide a rich basis for newly-proposed analytic approaches, such as reverse phenotyping (40).
As the world’s population ages, a major challenge is to unravel the pathways to disease and disability in older persons. Iceland shares the same major chronic diseases as in other industrialized countries with similar rates of cognitive and physical impairment. Focusing on this population will allow innovative approaches to the study of how people reach old age and what factors allow older persons to enjoy a healthy old age. Practically, studies such as this, which require extensive long-term data, can only be achieved by leveraging longitudinal studies onto existing cohorts that have already accrued data, thereby facilitating a life course approach to understanding the trajectories of disease and disability. Studies like this complement the “organ-specific” studies of health in old age and provide an opportunity for extension of the findings in a context that can identify homologies between and among conditions that may better show factors that impact on multiple conditions. From this perspective, measurements in the study were selected based on well-designed population studies contemporary with AGES-Reykjavik and collaborations with investigators outside of the study will continue to be sought to augment these measurements.
Studies like AGES-Reykjavik that take advantage of existing data resources, can also address methodologic problems. The question of selective survival or selective participation often arises in studies of older populations, although it has been argued that the relationships of risk factors within the survivors is unaffected by the bias. Because data from earlier life exists in from the original study, it will be possible to model the effect that both survival and nonparticipation might have on the direction and strength of associations observed between risk factors and outcomes. This might be particularly important for estimating risks in older women, who tend to live longer but to be frailer and therefore have lower participation rates in studies. Selective participation of healthier older persons in this cohort is reflected in at least two ways. The response rate for older women is lower than for older men as older women are frailer and more likely to be institutionalized. Second, the midlife profile of the non-responders shows higher blood pressure and higher glucose, both major contributors to health in old age. Again, nesting the study within the Reykjavik Study, these potential biases are known (unlike most studies of aging where sampling of older persons is carried out de novo) and we hope to use the earlier data to model sensitivity of our results to these factors.
The design of the AGES-Reykjavik Study represents an integrative approach to methodologic problems that may affect studies of genetics and studies of aging. As with many of the ongoing major cohort studies, it is hoped that this study will serve as the basis for ancillary studies that utilize the biologic specimens and the image database for studies consistent with the original consent obtained from the participants.
Acknowledgments
This study has been funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Components of the study were also supported by the NEI, NIDCD, and NHLBI.
References
- 1.Freimer N, Sabatti C. The human phenome project. Nat Genet. 2003;34:15–21. doi: 10.1038/ng0503-15. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 4.Festa A, D’Agostino R, Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–7. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 5.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–66. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–21. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 7.McGeer PL, McGeer EG. Inflammatory pathogenesis in Alzheimer’s disease: biological mechanisms and cognitive sequeli. Neurobiol Aging. 2001;22:799–809. [Google Scholar]
- 8.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 9.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Launer LJ, Masaki K, Petrovitch H, et al. The association between midlife blood pressure levels and late-life cognitive function: The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 11.Harris TB, Ballard-Barbasch R, Madans J, et al. Overweight, weight loss, and risk of coronary heart disease in older women. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1993;137:1318–27. doi: 10.1093/oxfordjournals.aje.a116641. [DOI] [PubMed] [Google Scholar]
- 12.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 13.Wagenknecht LE, Langefeld CD, Carr JJ, et al. Race-specific relationships between coronary and carotid artery calcification and carotid intimal medial thickness. Stroke. 2004;35:e97–9. doi: 10.1161/01.STR.0000127081.99767.1d. [DOI] [PubMed] [Google Scholar]
- 14.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–98. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Breteler MM, van den Ouweland FA, Grobbee DE, et al. A community-based study of dementia: the Rotterdam Elderly Study. Neuroepidemiology. 1992;11:23–8. doi: 10.1159/000110957. [DOI] [PubMed] [Google Scholar]
- 18.Dick DM, Jones K, Saccone N, et al. Endophenotypes Successfully Lead to Gene Identification: Results from the Collaborative Study on the Genetics of Alcoholism. Behav Genet. 2005:1–15. doi: 10.1007/s10519-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 19.Helgason A, Nicholson G, Stefansson K, et al. A reassessment of genetic diversity in Icelanders: strong evidence from multiple loci for relative homogeneity caused by genetic drift. Ann Hum Genet. 2003;67:281–97. doi: 10.1046/j.1469-1809.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson PV. Letters from Reykjavik. Annals of Intern Med. 1998;128:941–945. doi: 10.7326/0003-4819-128-11-199806010-00014. [DOI] [PubMed] [Google Scholar]
- 21.Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Jónsdóttir LS, Sigfusson N, Sigvaldason H, et al. Incidence and prevalence of recognized and unrecognized myocardial infarction in women. The Reykjavik Study. Eur Heart J. 1998;19:1011–1018. doi: 10.1053/euhj.1998.0980. [DOI] [PubMed] [Google Scholar]
- 23.Andresdottir MB, Sigurdsson G, Sigvaldason H, et al. Fifteen percent of myocardial infarctions and coronary revascularizations explained by family history unrelated to conventional risk factors. The Reykjavik Cohort Study. Eur Heart J. 2002;23:1655–63. doi: 10.1053/euhj.2002.3235. [DOI] [PubMed] [Google Scholar]
- 24.Saevarsdottir S, Oskarsson OO, Aspelund T, et al. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med. 2005;201:117–25. doi: 10.1084/jem.20041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tulinius H, Sigfússon N, Sigvaldason H, et al. Risk factors for malignant diseases: A cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–873. [PubMed] [Google Scholar]
- 26.Jonsson S, Thorsteinsdottir U, Gudbjartsson DF, et al. Familial risk of lung carcinoma in the Icelandic population. JAMA. 2004;292:3026–9. doi: 10.1001/jama.292.24.2977. [DOI] [PubMed] [Google Scholar]
- 27.Vilbergsson S, Sigurdsson G, Sigvaldason H, et al. Prevalence and incidence of NIDDM in Iceland: evidence for stable incidence among males and females 1967–1991--the Reykjavik Study. Diabet Med. 1997;14:491–8. doi: 10.1002/(SICI)1096-9136(199706)14:6<491::AID-DIA365>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Gunnarsdottir I, Birgisdottir BE, Thorsdottir I, et al. Size at birth and coronary artery disease in a population with high birth weight. Am J Clin Nutr. 2002;76:1290–4. doi: 10.1093/ajcn/76.6.1290. [DOI] [PubMed] [Google Scholar]
- 29.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 30.Andresdottir MB, Sigfusson N, Sigvaldason H, et al. Erythrocyte sedimentation rate, an independent predictor of coronary heart disease in men and women: The Reykjavik Study. Am J Epidemiol. 2003;158:844–51. doi: 10.1093/aje/kwg222. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. Mini-Mental Status, a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale – Revised. New York: The psychological Corporation; 1981. [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 34.Johannesdottir GB, Jonsson PV. Nursing Home Pre-admission Assessment in Reykjavík 1992. Arctic Med Res. 1994;53:512–514. [Google Scholar]
- 35.Morris JN, Hawes C, Fries BE, et al. Designing the national resident assessment instrument for nursing homes. Gerontologist. 1990;30:293–307. doi: 10.1093/geront/30.3.293. [DOI] [PubMed] [Google Scholar]
- 36.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. J Am Geriatr Soc. 1997;45:1017–24. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 37.SAS Institute Inc. SAS/STAT 9.1 User’s Guide. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 38.Siggurdsson G, Aspelund T, Chang MR, Jonsdottir B, Sigurdson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing gender difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik) Bone. doi: 10.1016/j.bone.2006.03.020. (in press) [DOI] [PubMed] [Google Scholar]
- 39.Budoff MJ, Georgiou D, Brody A, et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter study. Circulation. 1996;93:898–904. doi: 10.1161/01.cir.93.5.898. [DOI] [PubMed] [Google Scholar]
- 40.Schultze TG, McMahon FJ. Defining the phenotype in human genetic studies: Forward genetics and reverse phenotyping. Hum Hered. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]