Abstract
Macrophages act as the first line of self defense by mounting an inflammatory response to antigen and as antigen presenting cells to initiate the adaptive immune response. Inhibition of macrophage activation is one of the possible approaches to modulate inflammation. Intravenous (i.v.) tolerance has proved to be an effective method for ameliorating experimental autoimmune diseases. Whether macrophages are involved in tolerance induction is still largely undefined. In the present study we found that i.v. tolerance induction resulted in lower B7.1, B7.2 and MHC class II molecules, and reduced phagocytosis by both peritoneal macrophages and adherent splenocytes. Macrophages from tolerized mice were associated with a significantly impaired response of MOG-sensitized T cells to MOG. Macrophages from tolerized mice produced low levels of pro-inflammatory molecules IL-12, TNF-α, IL-1β, RANTES and MCP-1 and high levels of IL-10 and TGF-β. Administration of anti-TGF-β led to a reduction of IL-10 in tolerized mice. Thus, i.v. tolerance inhibits macrophage classical activation and APC function, increases macrophage alternative activation and IL-10 and TGF-β production. These cytokines, in turn, induce enhanced production of IL-10 in macrophages in MOG i.v. mice.
Keywords: Macrophage, EAE/MS, intravenous, tolerance
1. Introduction
Macrophages are a major cell population of the innate immune system. They play an important role in mounting an inflammatory response, both in the absence and presence of antigen, by secreting a number of cytokines and chemokines (Allam and Anders, 2008). These cytokines and chemokines influence the maturation and differentiation of neighboring cells of both the innate and adaptive immune system, which further enhances inflammation. In addition to being the first line of defense, macrophages also act as important accessory cells in the adaptive immune response. Macrophages play a role in the activation of the adaptive immune system by functioning as antigen presenting cells (APCs), the most important outcome of macrophage activation and maturation (Fortier, 2001). Activated macrophages express MHC class II and costimulatory molecules such as CD80, CD86 and CD40 and induce an effective T cell response in the presence of an antigen-dependent inflammatory response. Successful T cell activation by macrophages requires MHC/T cell receptor interaction and expression of costimulatory molecules on T cells and macrophages, supplemented by macrophage-secreted cytokines and chemokines (Carlsen et al., 2006; Hara et al., 1997).
Macrophages are the major effector cells mediating immune responses in experimental autoimmune encephalomyelitis (EAE) (Bhasin et al., 2008; Sinha et al., 2008; Youssef et al., 2002) and they determine the sequential degradation of myelin (van der Goes et al., 2005). Macrophages infiltrate the blood-brain baeeier at an early stage of EAE and initiate an inflammatory response against myelin antigens; they also act as APCs, thereby activating an antigen-specific T cell response in the CNS (Kato et al., 2004). These primary macrophage infiltrating cells are recruited from the circulation in response to a danger signal provoked by the presence of autoantigen.
Recent studies have shown that macrophages are an extremely heterogeneous lineage displaying a combination of both pro-and anti-inflammatory functions (Fong et al., 2008; Gordon and Taylor, 2005). The two extremes in the spectrum of macrophage function are represented by the classically activated (or M1) and the alternative (or M2) phenotypes (Gordon, 2003; Li et al., 2008b). Classically activated macrophages, which are induced by a combination of IFN-γ and proinflammatory stimuli (such as LPS or TNF-α), have anti-microbial and cytotoxic properties, whereas alternatively activated macrophages are anti-inflammatory or reparative (Martinez et al., 2008; Schroder et al., 2006). It has also been reported that macrophages play a critical role in the suppression of immune response to apoptotic cell-associated antigens in the EAE model (Miyake et al., 2007).
Injection of soluble proteins often results in the induction of antigen-specific tolerance or deviation to helper, rather than inflammatory, T cell immunity (Hilliard et al., 2000). In animal models, it has been shown that i.v tolerance effectively ameliorates EAE (Li et al., 2008a; Zhang et al., 2005). Zhang et al. reported a reduction in the overall CNS inflammation specifically associated with a decrease in the number of CD11b+CD45+ infiltrating macrophage cells after MOG i.v., and selectively suppressive effects of MOG i.v. on TNF-α producing macrophages (Zhang et al., 2005). We have previously reported immunoregulatory effects of MOG i.v. on a myeloid-like dendritic cell subset (CD11b+CD11c+) differentiation and T cell proliferation (Li et al., 2008a). We have also shown that MOG i.v. increased production of Th2 cytokines, especially those with a strongly modulated macrophage function such as TGF-β, IL-4, IL-5, IL-13 and IL-10 (Li et al., 2008a; Zhang et al., 2005).
In the present study we investigated the effects of macrophages on immunoregulation after MOG i.v. We hypothesized that macrophage activation and function may be altered in MOG i.v. tolerized mice, and that MOG i.v. inhibits T cell proliferation partly by inhibiting macrophage maturation and APC function. Our results indicate that MOG i.v. suppresses phagocytosis and inhibits the antigen presenting function of macrophages by decreasing MHC class II molecule expression. Tolerization also decreases costimulatory molecule expression, the second signal essential to T cell activation, and inhibits production of proinflammatory cytokines and chemokines. Our results also show that production of IL-10 and TGF-β in macrophages of MOG-i.v. mice was significantly enhanced in vitro, and administration of anti-TGF-β antibody led to a reduction in IL-10 production in vivo after MOG i.v. However, the absence of IL-10 did not prevent an increase of TGF-β in tolerized IL-10 knockout (IL-10−/−) mice. These findings suggest that MOG i.v. inhibits macrophage classical activation and APC function while it increases alternative activation of macrophages.
2. Materials and Methods
2.1. Mice and reagents
Female C57BL/6, IL-10−/− mice, aged 8~10 weeks were purchased from The Jackson Laboratory. All mice were maintained in a special pathogen-free animal facility at Thomas Jefferson University. Neutralizing anti-human TGF-β1 Ab (anti-TGF-β Ab) was purchased from R&D Systems.
2.2. Induction of EAE and i.v. tolerance
Mice were injected subcutaneously (s.c.) with 200 μg MOG35–55 in CFA containing 5 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) over 2 sites at the back. All mice received 200 ng pertussis toxins (Campbell, CA) i.v. injection on days 0 and 2 p.i. To induce i.v. tolerance, MOG35–55 (200 μg/mouse) was i.v. injected at days 3, 5 and 7 p.i.; mice that received the same volume (100 μl) of PBS i.v. in parallel served as controls. EAE was scored according to the following clinical scoring system (Li et al., 2008a): 1, limp tail or waddling gait with tail tonicity; 2, waddling gait with limp tail (ataxia); 2.5, ataxia with partial limb paralysis; 3, full paralysis of one limb; 3.5, full paralysis of one limb with partial paralysis of second limb; 4, full paralysis of 2 limbs; 4.5, moribund; and 5, death. Mice were examined daily in a blind fashion for signs of EAE. All work was performed in accordance with Thomas Jefferson University guidelines for animal use and care.
2.3. Isolation of macrophages and preparation of ascitic fluid samples
Peritoneal exudates macrophages were harvested by peritoneal lavage from EAE and MOG i.v.-tolerized C57BL/6 mice by i.p. injection with 1 ml of PBS for collection of cytokine samples or 5 ml of PBS for collection of peritoneal exudates cells (Wakabayashi et al., 2003). Cells were washed with PBS and resuspended in RPMI 1640 supplemented with 10% FBS. We have previously shown that this preparation procedure yielded > 95% pure CD11b+CD11c− macrophages with only 1.8% CD11c+ DCs (Guan et al., 2007).
The ascitic fluids were collected by peritoneal lavage with 10 ml of saline on days 12 and 23 p.i. Cell-free ascitic fluid was obtained by centrifugation (250 × g, 10 min) and filtered through a 0.22-pm filter (Millipore, Bedford, MA) to remove contaminating cells. As a control, ascitic fluid from EAE mice was used. The cell-free ascitic fluid was stored at −20°C until measurement of cytokine contents.
To prepare splenic macrophages, we followed a protocol described previously (Throsby et al., 1991; Throsby et al., 1993). Briefly, spleens were homogenized, and then passed through a plastic sieve to remove debris, and the cells were suspended for 3–5 min at 37°C in lysis buffer (1.66% ammonium chloride) to lyse the red blood cells. After two washing steps with RPMI 10% FBS medium, the cells were plated in a 37°C, 5% CO2 incubator. After 30 min of incubation, non-adherent cells were removed together with the culture medium and replated in new plates, while adherent cells were replenished with fresh culture medium.
2.4. Macrophage culture
Macrophages from EAE and MOG i.v.-tolerized mice were cultured in RPMI 1640 supplemented with 10% FBS in 96-well, flat-bottom plates at a density of 106 cells/ml. The cells were stimulated with LPS (100 ng/ml) for 24 h in a humidified 5% CO2 incubator. At the end of the incubation culture supernatants were collected and stored at −20°C for cytokine and chemokine assay.
2.5. In vitro phagocytosis
Mononuclear cells from spleen and peritoneal macrophages were isolated by centrifugation, using standard techniques (Gille et al., 2006). Cells were placed at 106 cells/ml in flat bottom cell culture plates in 10% heat inactivated fetal calf serum VLE-RPMI 1640. Recombinant IFN-γ (500 I.U./ml) was purchased from R&D Systems. Antibodies to CD14-PE (MUP9), CD16-PE (B73.1), and Ig-matched controls (IgG1, IgG2b) were purchased from BD Biosciences (PharMingen). Phenotypic analysis was performed on a FACSAria flow cytometer (BD Biosciences). Macrophages were identified by forward-, side-scatter, and CD14 expression as previously described (Gille et al., 2006).
E. coli DH5a, carrying the green fluorescent protein (GFP)-mut2 encoding plasmid pCD353 (E. coli-GFP), express a prokaryotic variant of GFP under control of a lactac promoter (Gille et al., 2006). Bacteria were freshly grown on agar plates supplemented with kanamycin (50 μg/ml; Sigma) and isopropyl-b-D-1-thiogalactopyranoside (1 mM; Sigma) for GFP induction. After 24 h, a single colony was grown in Lennox L broth-medium (Invitrogen, Karlsruhe, Germany) until early logarithmic growth phase (OD600=0.4–0.5). Bacteria were washed and resuspended in PBS, then used immediately. Bacterial viability was unaffected by the procedures. E. coli-GFP were added in 50:1 ratio to macrophages and incubated at 37°C for 45 min. After centrifugation of free bacteria, cells were fixed with 2% paraformaldehyde. Phagocytosis capacity was analyzed by mean GFP fluorescence intensity.
2.6. Purification of T cells and co-culture
T cells were purified from spleen of EAE mice with EAE by using anti-mouse CD4-coated magnetic beads (Miltenyi Biotec, Auburn, CA) (Hilliard et al., 2000). Briefly, splenic MNCs were incubated with anti-CD4 magnetic beads and then subjected to selection through MACS separation columns. Cells selected on the basis of CD4 expression routinely consisted of >98% viable cells. Enriched cells were washed and resuspended in RPMI 1640 supplemented with 10% FBS.
Macrophages from EAE and MOG-i.v. mice (1 × 106 cells/ml) were treated with LPS for 24 h in a humidified 5% CO2 incubator. Syngeneic T cells (106 cells/ml) purified from EAE splenocytes were added to the treated macrophages in the presence of MOG35–55 (25 μg/ml). Gamma irradiated splenocytes (1 × 105 cells/ml) from naïve mice were used as stimulator cells. After 3 days of incubation, T cell proliferation was measured based on 3H-thymidine incorporation. IL-2, and IFN-γ production in culture supernatants was assayed by ELISA (R&D Systems).
2.7. Flow cytometry analysis
Macrophages from EAE and MOG i.v.-tolerized mice (106 cells/ml) were treated with LPS for 24 h in a humidified 5% CO2 incubator, and were analyzed using B7.1-PE, B7.2-PE, CD40-PE, MHC II-PE mAb conjugates (eBiosciences) for costimulatory molecule and MHC II expression. Isotype controls were included as appropriate. Data were analyzed using FACSaria and Flowjo software (BD Biosciences, Mountain View, CA).
2.8. Measurement of cytokines and chemokines by ELISA
Culture supernatants from macrophages of EAE PBS i.v. or MOG i.v.-mice and macrophage-T cell cocultures were assayed for cytokines TNF-α, IL-12 and IL-1β, TGF-β, IL-10 and chemokines RANTES and MCP-1 by ELISA using assay kits from R&D Systems following the manufacturer’s instructions.
2.9. Statistical analysis
All experiments were done in triplicate. “n” represents the number of mice used for each experiment. Data are presented as the arithmetic mean ± standard deviation. Comparison between groups was analyzed using the Student’s t test. The accepted level of significance was p <0.05.
3. Results
3.1. MOG i.v.-treatment inhibits LPS-induced activation of macrophage
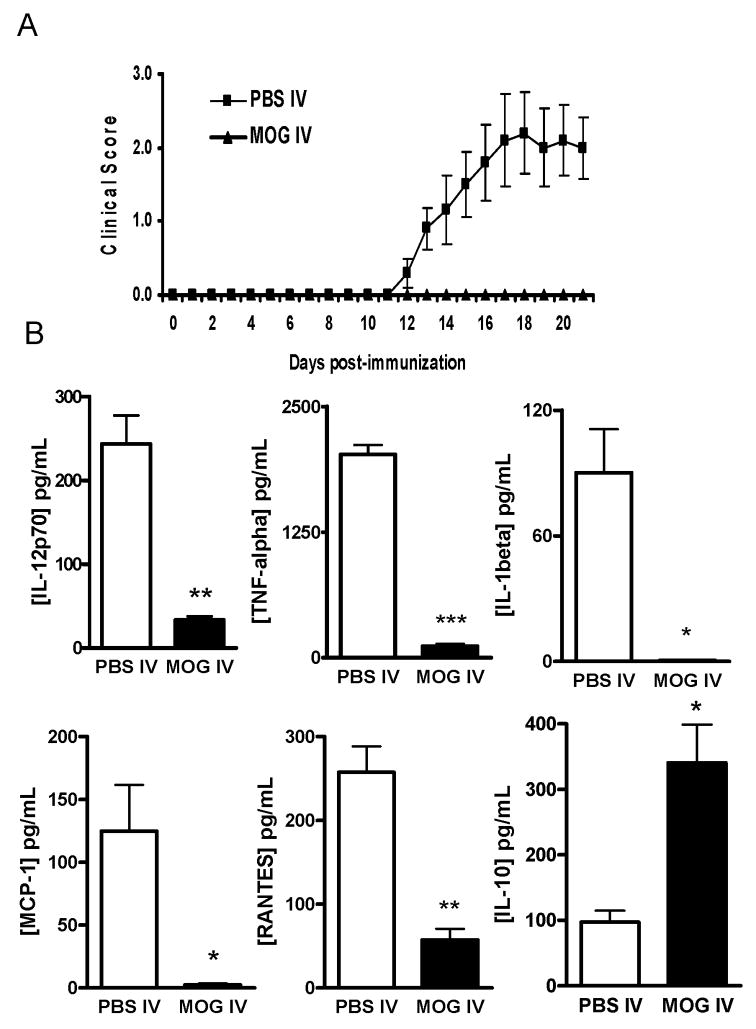
We i.v. administered MOG35–55 peptide to mice after induction of EAE. Mice that received i.v. PBS in parallel served as controls. All mice in the control group developed chronic-progressive EAE. In contrast, when MOG was administered i.v. on days 3, 5, and 7 p.i., the disease was markedly suppressed (Fig. 1A; p<0.01). Consistent with clinical signs, demyelination and extensive inflammatory infiltrates were mostly observed in white matter of spinal cords of PBS-treated EAE mice, while normal morphology was preserved in MOG i.v. treated mice (data not shown).
Fig. 1. Effect of MOG i.v. on cytokine and chemokine release by LPS-stimulated macrophages.
(A) I.v. injection of MOG35–55 prevents EAE. C57BL/6 mice were immunized with MOG35–55 + CFA. Pertussis toxin was injected on days 0 and 2 p.i. Two-hundred μg MOG35–55 was injected i.v. on days 3, 5, and 7 p.i. Mice that received i.v. PBS in parallel served as controls. Clinical EAE was scored according to a 0–5 scale (n = 5 in each group). One representative experiment of three is shown (total n = 15 in each group). (B) Peritoneal and splenic macrophages from PBS-i.v. or MOG-i.v. mice were isolated at the end of the experiment as described in Materials and Methods and stimulated with 100 ng/ml LPS for 24 h. The cell supernatants were collected and cytokine and chemokine production was determined by ELISA. Data represent mean ± S.D (n = 5). * p <0.05, ** p <0.01, and *** p <0.001 in comparison between macrophages from MOG-i.v. and PBS-i.v. mice.
To analyze the macrophage activation profile in immune tolerance, we harvested these cells from the peritoneal cavity and spleens of control EAE or tolerized mice. After stimulation with LPS in culture, supernatants were harvested and production of macrophage-related cytokines/chemokines was assayed by ELISA. LPS-induced activation resulted in increased production of TNF-α (2024.0 ± 93.5 pg/ml), IL-12 (343.7 ± 34.1 pg/ml) and IL-1β (90.2 ± 20.8 pg/ml) by murine macrophages from EAE mice. We observed a significant decrease in production of the above-mentioned cytokines in macrophages from MOG i.v.-treated mice (Fig. 1B). An almost 20-fold decrease in TNF-α production was observed in macrophages from MOG-i.v. mice (115.1 ± 23.4 pg/ml) in comparison to macrophages from EAE mice. LPS induced IL-1β, IL-12 production was significantly inhibited in macrophages from MOG i.v. mice. However, IL-10 production was after MOG i.v. treatment (97.0 ± 17.6 vs. 340.5 ± 58.4 pg/ml).
In addition, we observed a significant inhibition of LPS-induced chemokine production, namely RANTES and MCP-1, by MOG i.v. Production of RANTES was 57.4 ± 13.2 pg/ml in MOG-i.v. mice compared to 257.5 ± 30.9 pg/ml in EAE mice (p <0.01). A similar effect of MOG i.v. on MCP-1 production was also observed (Fig. 1B).
3.2. MOG i.v.-treatment inhibits LPS-induced phagocytic function of macrophages
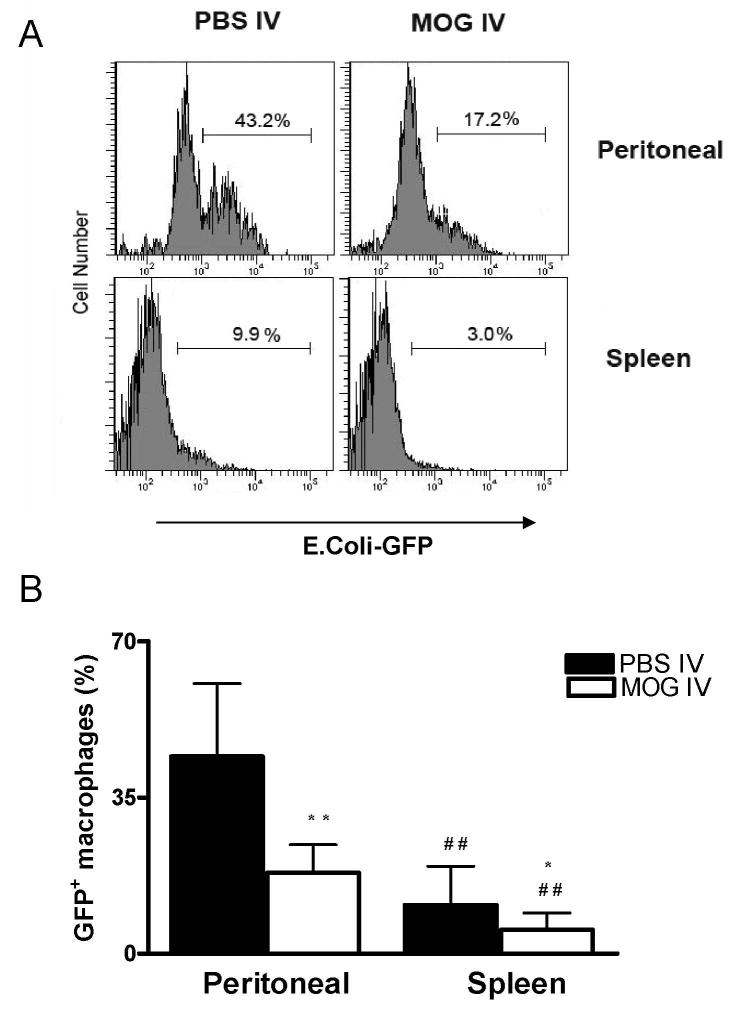
Phagocytosis assay using green fluorescent protein labeled E. coli is a new and useful method (Gille et al., 2006) for analysis of macrophage function. To evaluate the phagocytic effect of i.v. tolerance in EAE, we studied E. coli-mediated phagocytic function of peritoneal and spleen macrophages in MOG i.v. and EAE groups. A rapid down-regulation in the phagocytosis capacity for macrophages was found in MOG i.v. vs. EAE mice. This is true for both both peritoneal (44.2 ± 16.3% vs.18.1 ± 6.3%; P<0.01) and splenic macrophages (10.9 ± 8.7% vs. 5.4 ± 3.7%; P<0.05) (Fig. 2).
Fig. 2. Phagocytosis of GFP fluorescent transfected E. coli in vitro.
Freshly isolated peritoneal macrophages and spleen adherent cells from MOG-i.v. and PBS-i.v. mice were incubated with GFP E. coli for 1hr at 37°C. After washing the cells to remove extracellular E. coli, 1×106 macrophages were analyzed by flow cytometry. (A) GFP-E. coli within the phagocytic cells. Mean values ± S.D (n = 5) were presented in (B). * refers to comparison between PBS-i.v. and MOG-i.v. groups; # refers to comparison between peritoneal macrophages and splenic macrophages in the same groups. *, P<0.05, **, and ##, P<0.01.
There was an inverse relationship between cell number and fluorescence intensity (Groesdonk et al., 2006; Pinheiro da Silva et al., 2007). During the bacteria/cell incubation interval, the percentage of GFP+ changes due to bacterial ingestion increased shortly, and then decreased constantly (data not shown). Significantly smaller percentages of splenic macrophages ingested fluorescent E. coli compared to peritoneal macrophages in both EAE (44.2 ± 16.3% vs.10.9 ± 68.7%; P<0.01) and MOG-i.v. (18.1 ± 6.3% vs. 5.4 ± 3.7%; P<0.01) groups (Fig. 2), indicating that there were significantly fewer phagocytic cells taking up foreign material among splenic macrophages than in peritoneal macrophages.
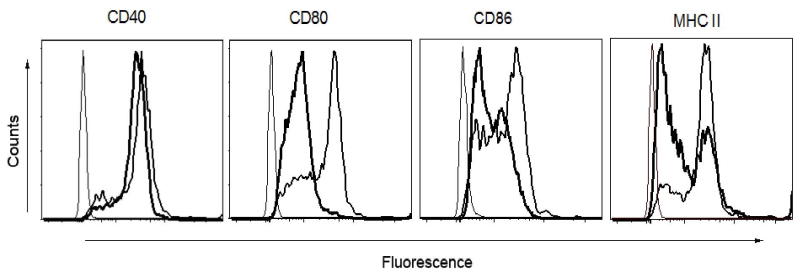
3.3. MOG i.v. suppresses costimulatory molecules and MHC II expression on LPS-stimulated macrophages
LPS stimulation results in macrophage activation and increased production of proinflammatory cytokines. LPS activity is further augmented by the key costimulatory pathway (interaction between CD28 and CD80-CD86) for T cell activation. Signaling through CD28 by CD80-CD86 is essential to maintain LPS activity, and it has been shown that LPS alone does not increase the expression of CD80-CD86 (Foss et al., 1999; Hoeve et al., 2006). We therefore further studied the effect of MOG i.v. treatment on the expression of CD80, CD86 and CD40 on macrophages stimulated by LPS stimulation. We observed a decrease in CD80 and CD86 expression on LPS-stimulated macrophages after MOG i.v. treatment compared with EAE mice. However, MOG i.v. had no such inhibitory effect on CD40 expression. Further, we observed that MOG i.v. decreases the expression of MHC class II molecules in LPS-stimulated macrophages (Fig. 3).
Fig. 3. Effect of MOG i.v. on costimulatory and MHC class II molecule expression in peritoneal macrophages.
Macrophages from PBS-i.v. or MOG-i.v. mice were isolated and stimulated with 100 ng/ml LPS for 24 h. The cells were harvested and stained with fluorophore conjugated mAbs against CD40, B7.1, B7.2 and MHC II and analyzed in FACSaria flow cytometer. Bold histogram line represents specific fluorescence for macrophages of MOG-i.v. mice; thinner histogram line represents that of PBS-i.v. EAE mice with isotype-matched control mAbs. One representative experiment of three is shown.
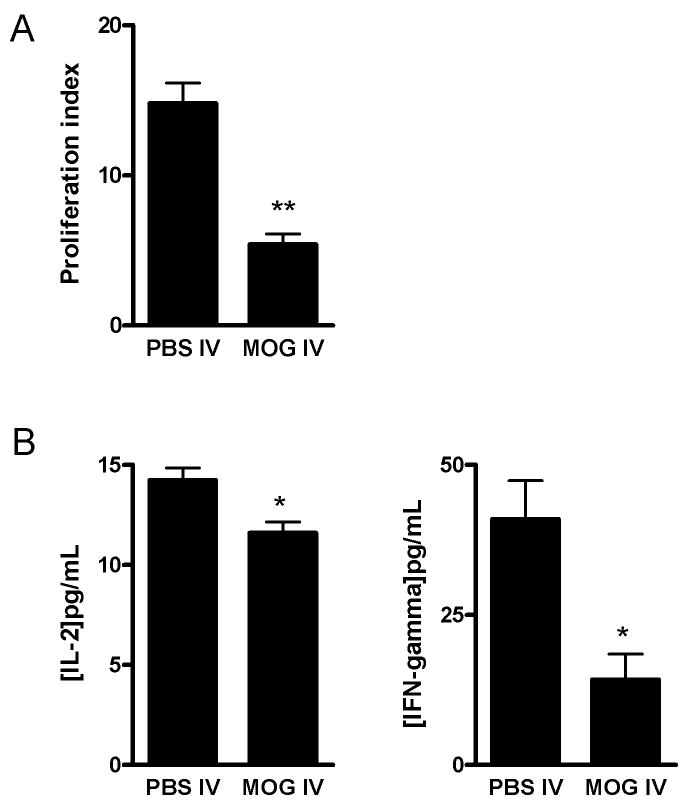
3.4. Macrophage from MOG i.v. treated mice restrict proliferation of autoantigen-induced T cells
A decreased expression of MHC class II and costimulatory molecules on macrophages after MOG i.v. treatment suggests that these macrophages are deficient in their role as APCs. To confirm the inhibitory effect of MOG i.v. on antigen presentation by macrophages, we set up a co-culture where MOG i.v.-treated macrophages were used as the only APC source. As shown in Fig. 4A, we observed that PBS i.v. macrophages as well as LPS-treated macrophages induced T cell proliferation in response to MOG35–55 stimulation. However, macrophages from MOG-i.v. mice were significantly less potent in stimulating T cell proliferation. We also detected significantly reduced production of IL-2 and IFN-γ in the co-culture supernatants using macrophages from MOG-i.v. mice the in presence of LPS stimulation (Fig. 4B).
Fig. 4. Effect of MOG i.v. on the antigen-presenting function of macrophages.
Macrophages from PBS-i.v. or MOG-i.v. mice were incubated with LPS for 24 h. These pre-treated macrophages were used as APCs along with T cells as responder cells in the presence of MOG35–55 (25 μg/ml). T cell proliferation was measured after 72 h incubation. Results are presented as proliferation index indicating the absorbance value (at 490 nm) of responder cells (A). IL-2 and IFN-γ production was measured in the culture supernatants after 72 h incubation period (B). Data represent mean ± S.D (n = 9). * p <0.05 and **p < 0.01 in comparison between two groups.
3.5. Effect of anti-TGF-β administration on production of TGF-β and IL-10 in vivo
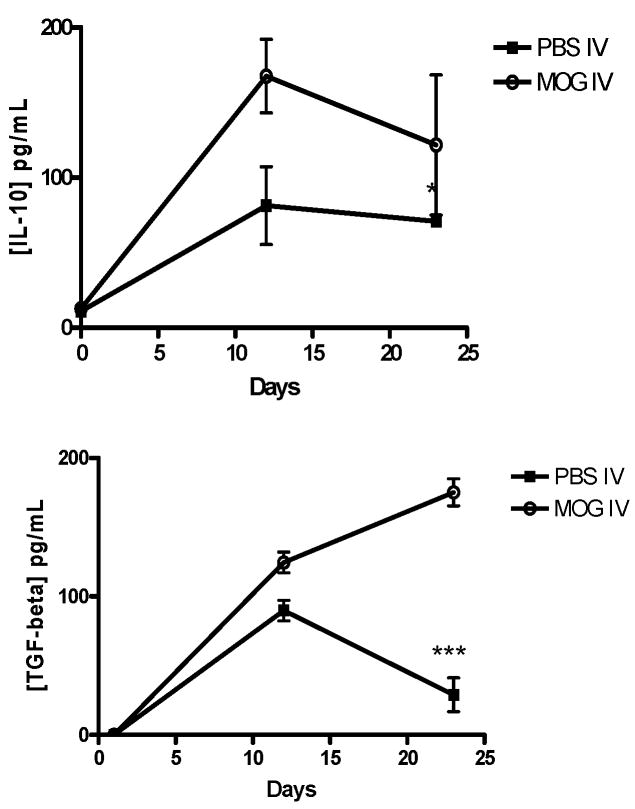
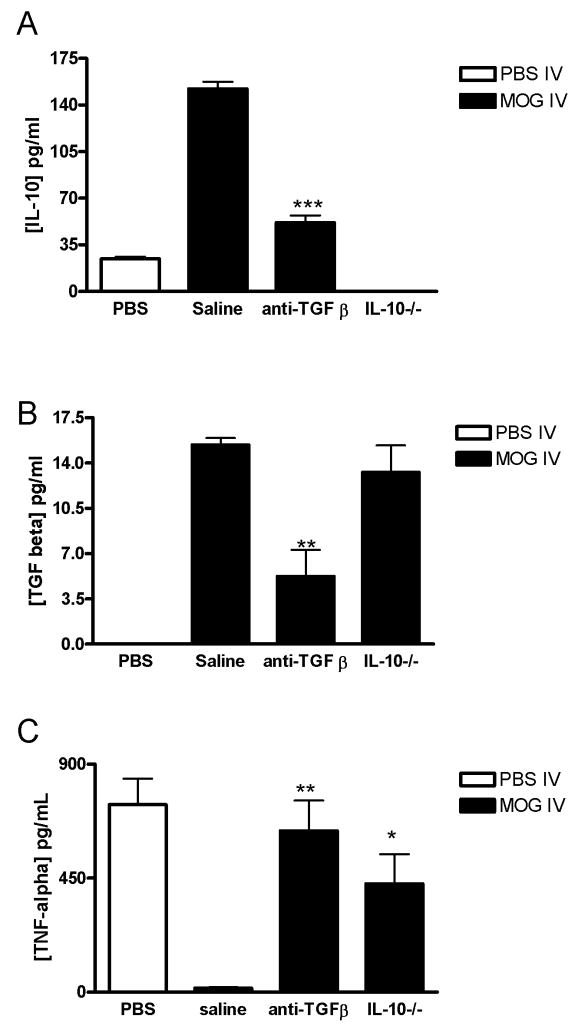
IL-10 production increased in parallel with elevation of the TGF-β level in ascitic fluid in MOG-i.v. mice (Fig. 5). Therefore, to clarify the relationship between IL-10 and TGF-β, we tried to prevent TGF-β activity by administering anti-TGF-β Ab to MOG i.v. mice. As shown in Fig. 6A, an increase in IL-10 contents was detected in the ascitic fluid from MOG i.v. controls. In contrast, a small amount of TGF-β was detected in the ascitic fluid from mice treated with anti-TGF-β Ab (Fig. 6B), indicating that the amounts of the Ab administered were enough to neutralize TGF-β activity in vivo. Furthermore, the enhanced level of IL-10 in MOG-i.v. mice was also significantly reduced by anti-TGF-β Ab administration (Fig. 6A). We also attempted to assess whether anti-IL-10 administration altered the TGF-β level in MOG i.v. mice. Although anti-IL-10 Ab administration to MOG-i.v. mice caused a reduction of the IL-10 level in ascitic fluid, no change in the TGF-β level was detected (data not shown). Asictes of MOG-i.v. IL-10−/− mice showed a similar TGF-β level as those from MOG-i.v. wildtype mice (Fig. 6B).
Fig. 5. Modulated production of TGF-β and IL-10 in ascitic fluids from MOG i.v. mice.
The ascitic fluids were collected by peritoneal lavage with 10 ml of saline on indicated days after MOG immunization. Cell-free ascitic fluid was obtained by centrifugation and filtered through a 0.22-pm filter to remove contaminating cells. The cell-free ascitic fluid was stored at −20°C until measurement of cytokine contents. Quantities of TCF-β and IL-10 were measured by ELlSA kits. The results are expressed as the mean with SD of cytokine levels in five individual ascites. Significant at * p < 0.05 or *** p < 0.001 compared with the result between these two groups.
Fig. 6. Cytokine production from PBS-i.v. and MOG i.v. mice treated with anti-TGF-β.
MOG-i.v. C57BL/6 mice were given i.p. anti-TGF-β Ab (50 μg/mouse) from day 12 for 4 consecutive days. Mice that received saline (saline) i.p. in parallel served as controls. MOG-i.v. IL-10−/− mice were injected i.p. with saline. PBS-i.v. mice served as a control. Ascitic fluids were collected as described in Fig. 5 at day 1 after the final anti-TGF-β Ab administration. Production of IL-10 (A) and TGF-β (B) was measured by ELISA. The results are expressed as the mean with SD of cytokine levels in five individual ascites. (C) Peritoneal macrophages were cultured (1 ×106/ml) in the presence or absence of LPS for 24 h in vitro as described in Fig. 1B. Macrophages obtained from PBS-i.v. mice were used as a control. TNF-α concentrations in the supernatant were measured by ELISA. Significant at *p < 0.05, **p < 0.01, ***p <0.001 compared with saline injected MOG-i.v. control mice.
3.6. Effect of lL-10 and TGF-β on macrophage production of TNF-α
To investigate the effect of IL-10 and TGF-β on macrophage activity of MOG i.v. mice, we also measured TNF-α production by peritoneal macrophages from MOG-i.v. in IL-10−/− mice or in wild type mice treated with anti-TGF-β Ab. As shown in Fig. 6C, TNF-α production by macrophages from untreated MOG-i.v. mice was markedly suppressed, compared with that of non-tolerized EAE mice. In contrast, IL-10 deficiency caused significant enhancement of TNF-α production by macrophages from the MOG i.v. mice. A similar result was observed in macrophages when MOG-i.v. mice were treated with anti-TGF-β Ab. These results demonstrate that, in addition to TGF-β, overproduced IL-10 also suppresses classical macrophage activity in MOG i.v. mice.
4. Discussion
Intravenous tolerance has been proved effective in suppressing EAE; however, the mechanism underlying this phenomenon has not been fully elucidated. Our previous studies have shown that MOG i.v. modulates CD11b+CD11c+ DC function and T cell activation (Li et al., 2008a; Zhang et al., 2005). Our present study shows for the first time the mechanism by which MOG i.v. also exerts its modulatory effects on CD11b+CD11c− peritoneal macrophages (Guan et al., 2007) and splenic macrophages (Throsby et al., 1991; Throsby et al., 1993). MOG i.v. can inhibit proinflammatory cytokine production by classical macrophage activation, and can decrease macrophage production of IFN-γ, the key proinflammatory mediator of T cells.
LPS leads to an effective classical macrophage activation (Hoeve et al., 2006). Classically activated macrophages are highly proinflammatory and they secrete IL-12. In the presence of IL-12 a Th1 response predominates. Activated Th1 cells produce IFN-γ, which initiates a positive feedback loop by priming and activating more macrophages and by up-regulating their MHC class II expression. IFN-γ also acts synergistically with TNF-α in macrophage activation initiating three different signaling arms via: 1) FADD dependent binding and activation of caspase-8; 2) activation of JNK pathway; and 3) activation of NFκB. IL-1β also plays a role in macrophage activation (Suzuki et al., 2003; Zhang and Mosser, 2008). Binding of IL-1β to IL-1R1 activates both MAPK-AP1 and I-κB kinase-NFκB pathways. MOG i.v. may exert an inhibitory effect on the production of IL-12, thereby indirectly suppressing the production of IFN-γ and its positive feedback loop of macrophage activation. Inhibition of macrophage activation is further enhanced by a drastic decrease in TNF-α production along with inhibition of IL-1β production. In addition to inhibition of macrophage activation, MOG i.v. also negatively affects monocyte and leukocyte migration as evidenced by inhibition of RANTES and MCP-1 production. Increased RANTES production is associated with a wide range of inflammatory disorders. It acts by promoting leukocyte infiltration to the site of inflammation. At high concentrations, RANTES induces activation of T cells in a manner not dissimilar to mitogens (Krensky and Ahn, 2007; Wong et al., 2001). The activation of T cells by RANTES is followed by increased production of IL-2 and IFN-γ. Inhibition of RANTES production by MOG i.v. not only suppresses inflammation, but also restricts T cell activation, as observed in decreased IFN-γ production. MCP-1 is a pro-inflammatory chemokine that is responsible for the infiltration of monocytes into inflammatory sites (Gu et al., 2000; Mahad and Ransohoff, 2003; Mishra et al., 2008). MOG i.v. inhibits the production of MCP-1, indicating an inhibition of macrophage activation. Classically activated macrophages play an antigen-presenting role in Th1 cellular response by upregulating surface molecules like MHC class II and B7. MOG i.v. down regulates expression of both MHC class II and B7 molecules and, in turn, inhibits the activation of CD4+ T lymphocytes, as evidenced by decreased IL-2 and IFN-γ production as well as inhibition of CD4+ T lymphocyte proliferation in response to autoantigen.
The present study demonstrates a novel inhibitory property of MOG i.v. on classical macrophage activation. I.v-tolerance-mediated downregulation of MHC class II molecules and mature markers CD80 and CD86 on macrophages (Gerlini et al., 2008; Smit et al., 2008) are accompanied by a reduction in their phagocytosis, suggesting that MOG i.v. may have an inhibitory effect on macrophage maturation and activation as well as on their APC function. Antigen presentation by both MHC class II molecules and costimulatory signals is necessary to initiate effective T cell activation. A decrease in the expression of either or both fails to bring about T cell activation followed by proliferation. We show a negative effect of MOG i.v. macrophages on T cell proliferation, which is an indirect effect mediated by inhibition of macrophage activation.
It has been suggested that TGF-β enhances macrophage ability to produce IL-10 in normal mice (Gordon, 2003; Maeda et al., 1995). A similar phenomenon has also been found in MOG-induced tolerance in EAE mice in the present study, in which we also found that IL-10 and TGF-β increased in MOG-i.v. mice in parallel with tolerance progression, and anti-TGF-β Ab administration resulted in reduction of IL-10 accumulation in the ascitic fluid. These findings indicate that TGF-β enhances IL-10-producing ability in macrophages from MOG-i.v. mice. Joetham et al. reported that anti-TGF-β administration reduced regulatory T cell activity dependent on production of IL-10 in the mouse model of lung allergic responses (Joetham et al., 2007), supporting our findings in another immunological condition. However, MOG-i.v. IL-10−/− mice showed no effects on TGF-β production in the ascitic fluid, suggesting that a feedback regulation between TGF-β and IL-10 production may not be occurring in macrophages of MOG i.v. mice. Given that only TGF-β itself could not induce the production of IL-10 in macrophages (Anderson et al., 2002; Gerber and Mosser, 2001; Gordon, 2003), increased IL-10 production by TGF-β in i.v. tolerized mice may reflect an indirect effect of TGF-β, in synergy with other immunoregulatory molecules, on macrophages.
In conclusion, we found that MOG i.v., an effective treatment strategy for MS patients, has a significant suppressive effect on both macrophage activation and T cell proliferation. The down-regulation of macrophage activation observed in MOG-i.v. mice might occur in the following way: First, TGF-β is produced in systemic circulation after MOG i.v. Second, TGF-β enhances macrophage production of IL-10. The production of large quantities of TGF-β and IL-10 may lead to pronounced downregulation of host-immune effector cells. Our observations have revealed a novel feature of effector-target interaction in MOG i.v.-induced immunosuppression. Future studies will focus on elucidating the signal transduction pathways in macrophages inhibited by MOG i.v. tolerance.
Acknowledgments
This work was funded by the NIH and the NMSS. We thank Katherine Regan for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interest.
References
- Allam R, Anders HJ. The role of innate immunity in autoimmune tissue injury. Curr Opin Rheumatol. 2008;20:538–544. doi: 10.1097/BOR.0b013e3283025ed4. [DOI] [PubMed] [Google Scholar]
- Anderson CF, Gerber JS, Mosser DM. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 2002;8:477–481. doi: 10.1179/096805102125001118. [DOI] [PubMed] [Google Scholar]
- Bhasin JM, Chakrabarti E, Peng DQ, Kulkarni A, Chen X, Smith JD. Sex specific gene regulation and expression QTLs in mouse macrophages from a strain intercross. PLoS ONE. 2008;3:e1435. doi: 10.1371/journal.pone.0001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen HS, Yamanaka T, Scott H, Rugtveit J, Brandtzaeg P. The proportion of CD40+ mucosal macrophages is increased in inflammatory bowel disease whereas CD40 ligand (CD154)+ T cells are relatively decreased, suggesting differential modulation of these costimulatory molecules in human gut lamina propria. Inflamm Bowel Dis. 2006;12:1013–1024. doi: 10.1097/01.mib.0000234135.43336.72. [DOI] [PubMed] [Google Scholar]
- Fong CH, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, Karin M, Lawrence T. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med. 2008;205:1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier AH. Activation of murine macrophages. Curr Protoc Immunol Chapter 14, Unit 14 14. 2001 doi: 10.1002/0471142735.im1404s11. [DOI] [PubMed] [Google Scholar]
- Foss DL, Zilliox MJ, Murtaugh MP. Differential regulation of macrophage interleukin-1 (IL-1), IL-12, and CD80-CD86 by two bacterial toxins. Infect Immun. 1999;67:5275–5281. doi: 10.1128/iai.67.10.5275-5281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber JS, Mosser DM. Stimulatory and inhibitory signals originating from the macrophage Fcgamma receptors. Microbes Infect. 2001;3:131–139. doi: 10.1016/s1286-4579(00)01360-5. [DOI] [PubMed] [Google Scholar]
- Gerlini G, Mariotti G, Chiarugi A, Di Gennaro P, Caporale R, Parenti A, Cavone L, Tun-Kyi A, Prignano F, Saccardi R, Borgognoni L, Pimpinelli N. Induction of CD83+CD14+ nondendritic antigen-presenting cells by exposure of monocytes to IFN-alpha. J Immunol. 2008;181:2999–3008. doi: 10.4049/jimmunol.181.5.2999. [DOI] [PubMed] [Google Scholar]
- Gille C, Spring B, Tewes L, Poets CF, Orlikowsky T. A new method to quantify phagocytosis and intracellular degradation using green fluorescent protein-labeled Escherichia coli: comparison of cord blood macrophages and peripheral blood macrophages of healthy adults. Cytometry A. 2006;69:152–154. doi: 10.1002/cyto.a.20222. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Groesdonk HV, Schlottmann S, Richter F, Georgieff M, Senftleben U. Escherichia coli prevents phagocytosis-induced death of macrophages via classical NF-kappaB signaling, a link to T-cell activation. Infect Immun. 2006;74:5989–6000. doi: 10.1128/IAI.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yu S, Zhao Z, Ciric B, Zhang GX, Rostami A. Antigen presenting cells treated in vitro by macrophage colony-stimulating factor and autoantigen protect mice from autoimmunity. J Neuroimmunol. 2007;192:68–78. doi: 10.1016/j.jneuroim.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Ohtani H, Matsumoto T, Nakamura S, Kitano A, Arakawa T, Nagura H, Kobayashi K. Expression of costimulatory molecules B7-1 and B7-2 in macrophages and granulomas of Crohn’s disease: demonstration of cell-to-cell contact with T lymphocytes. Lab Invest. 1997;77:175–184. [PubMed] [Google Scholar]
- Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- Hoeve MA, Savage ND, de Boer T, Langenberg DM, de Waal Malefyt R, Ottenhoff TH, Verreck FA. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol. 2006;36:661–670. doi: 10.1002/eji.200535239. [DOI] [PubMed] [Google Scholar]
- Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, Ueda R, Suzumura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- Krensky AM, Ahn YT. Mechanisms of disease: regulation of RANTES (CCL5) in renal disease. Nat Clin Pract Nephrol. 2007;3:164–170. doi: 10.1038/ncpneph0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, Rostami A. CD11c+ CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008a;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu S, Zhang T, Pan W, Yang X, Cao X. Splenic Stromal Microenvironment Negatively Regulates Virus-Activated Plasmacytoid Dendritic Cells through TGF-{beta} J Immunol. 2008b;180:2951–2956. doi: 10.4049/jimmunol.180.5.2951. [DOI] [PubMed] [Google Scholar]
- Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Mishra RS, Carnevale KA, Cathcart MK. iPLA2beta: front and center in human monocyte chemotaxis to MCP-1. J Exp Med. 2008;205:347–359. doi: 10.1084/jem.20071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro da Silva F, Aloulou M, Skurnik D, Benhamou M, Andremont A, Velasco IT, Chiamolera M, Verbeek JS, Launay P, Monteiro RC. CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Sinha S, Kaler LJ, Proctor TM, Teuscher C, Vandenbark AA, Offner H. IL-13-mediated gender difference in susceptibility to autoimmune encephalomyelitis. J Immunol. 2008;180:2679–2685. doi: 10.4049/jimmunol.180.4.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E, Pretorius E, Anderson R, Oommen J, Potjo M. Differentiation of human monocytes in vitro following exposure to Canova in the absence of cytokines. Ultrastruct Pathol. 2008;32:147–152. doi: 10.1080/01913120802062729. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Eriksson U, Hara H, Mirtosis C, Chen NJ, Wada T, Bouchard D, Hwang I, Takeda K, Fujita T, Der S, Penninger JM, Akira S, Saito T, Yeh WC. IL-1R-associated kinase 4 is required for lipopolysaccharide-induced activation of APC. J Immunol. 2003;171:6065–6071. doi: 10.4049/jimmunol.171.11.6065. [DOI] [PubMed] [Google Scholar]
- Throsby M, Lee D, Huang WQ, Yang ZY, Copolov DL, Lim AT. Evidence for atrial natriuretic peptide-(5–28) production by macrophages of the rat spleen: an immunochemical and nonradioactive in situ hybridization approach. Endocrinology. 1991;129:991–1000. doi: 10.1210/endo-129-2-991. [DOI] [PubMed] [Google Scholar]
- Throsby M, Yang Z, Lee D, Huang W, Copolov DL, Lim AT. Coexpression of atrial natriuretic factor and beta-endorphin in a subpopulation of rat splenic macrophages: age-related differences. Endocrinology. 1993;133:2889–2896. doi: 10.1210/endo.133.6.8243316. [DOI] [PubMed] [Google Scholar]
- van der Goes A, Boorsma W, Hoekstra K, Montagne L, de Groot CJ, Dijkstra CD. Determination of the sequential degradation of myelin proteins by macrophages. J Neuroimmunol. 2005;161:12–20. doi: 10.1016/j.jneuroim.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Wakabayashi H, Fukushima H, Yamada T, Kawase M, Shirataki Y, Satoh K, Tobe T, Hashimoto K, Kurihara T, Motohashi N, Sakagami H. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by Barbados cherry, a fruit of Malpighia emarginata DC. Anticancer Res. 2003;23:3237–3241. [PubMed] [Google Scholar]
- Wong M, Uddin S, Majchrzak B, Huynh T, Proudfoot AE, Platanias LC, Fish EN. Rantes activates Jak2 and Jak3 to regulate engagement of multiple signaling pathways in T cells. J Biol Chem. 2001;276:11427–11431. doi: 10.1074/jbc.M010750200. [DOI] [PubMed] [Google Scholar]
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Yu S, Li Y, Ventura ES, Gran B, Rostami A. A paradoxical role of APCs in the induction of intravenous tolerance in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;161:101–112. doi: 10.1016/j.jneuroim.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]