Abstract
The fate of the three heterotrophic biofilm forming bacteria, Pseudomonas aeruginosa, Klebsiella pneumoniae and Flavobacterium sp. in pilot scale cooling towers was evaluated both by observing the persistence of each species in the recirculating water and the formation of biofilms on steel coupons placed in each cooling tower water reservoir. Two different cooling tower experiments were performed: a short-term study (6 days) to observe the initial bacterial colonization of the cooling tower, and a long-term study (3 months) to observe the ecological dynamics with repeated introduction of the test strains. An additional set of batch experiments (6 days) was carried out to evaluate the adhesion of each strain to steel surfaces under similar conditions to those found in the cooling tower experiments. Substantial differences were observed in the microbial communities that developed in the batch systems and cooling towers. P. aeruginosa showed a low degree of adherence to steel surfaces both in batch and in the cooling towers, but grew much faster than K. pneumoniae and Flavobacterium in mixed-species biofilms and ultimately became the dominant organism in the closed batch systems. However, the low degree of adherence caused P. aeruginosa to be rapidly washed out of the open cooling tower systems, and Flavobacterium became the dominant microorganism in the cooling towers in both the short-term and long-term experiments. These results indicate that adhesion, retention and growth on solid surfaces play important roles in the bacterial community that develops in cooling tower systems.
Keywords: cooling tower systems, pilot scale, microbial community, bacterial initial adhesion, bacterial growth rate, biofilm formation
Introduction
Cooling towers are heat rejection devices used in industrial settings to efficiently cool building water systems without the use of chemical refrigerants. Cooling towers function by transferring heat from recirculated water to the atmosphere, typically by means of dripping or spraying water over a fill material having high surface area. Cooling towers provide a unique environment for microbial growth and dissemination (Yamamoto et al. 1992; Breiman 1996). These devices generally have sizable water reservoirs, within which the temperature is typically maintained between 25°C and 35°C. Both microbes and substrata for microbial growth can either be present in the incoming water or introduced from the atmosphere. Many traditional water treatment chemicals, eg antiscalants and zinc-based corrosion inhibitors, provide nutrients that accelerate the growth of microbes in the towers (Kusnetsov et al. 1993). A wide variety of substrata can also be introduced by atmospheric deposition in open, roof-top cooling towers (Kusnetsov et al. 1993; Ceyhan and Ozdemir 2008). Dissolved and particulate matter that enters cooling towers is concentrated by the evaporation and recycling of water within these systems.
The presence of pathogenic microorganisms in cooling towers is thought to impose a significant public health risk. Cooling towers provide good environments for the multiplication of pathogenic microorganisms. The warm water present in cooling towers and building warm water loops particularly favor the growth of thermophilic organisms such as the species of Legionella, and cooling towers have been implicated in numerous outbreaks of Legionnaires’ disease and related illnesses (Kurtz et al. 1982; Orrison et al. 1983; Bhopal et al. 1991; Bentham and Broadbent 1993; Keller et al. 1996; WHO 2003; Berk et al. 2006; Sabria et al. 2006; Declerck et al. 2007). Cooling towers also produce and spread aerosols, which can result in air-borne transmission of disease over long distances (Addiss et al. 1989; Nguyen et al. 2006). Biofilms readily grow on the extensive and diverse surfaces present in cooling tower reservoirs and fill material. Biofilms both harbor and protect pathogens (Costerton et al. 1987, 1995; Wright et al. 1991; Murga et al. 2001; Hall-Stoodley et al. 2004), and microbial ecological interactions in biofilms are particularly important for Legionella species and other pathogens that infect amoebae (Berk et al. 1998; Newsome et al. 1998; Atlas 1999; Steinert et al. 2002; Donlan et al. 2005; Albert-Weissenberger et al. 2007; Declerck et al. 2007; Hilbi et al. 2007). Enhanced pathogen survival has previously been observed in defined, multi-species laboratory biofilms (Murga et al. 2001). In addition to the health concerns associated with the presence of pathogens in biofilms in cooling towers, biofilm formation also poses serious problems by causing equipment damage through corrosion and by decreasing energy efficiency by clogging hydraulic systems and increasing heat transfer resistance across fouled surfaces (Gaylarde and Morton 1999; Meesters et al. 2003).
While biofilm formation has been extensively studied and the importance of the microbial ecology of biofilms to pathogen virulence and survival has been well established (Costerton et al. 1987, 1995; Hall-Stoodley et al. 2004), little attention has been given to bacterial colonization of cooling tower surfaces. To date, only a few studies have been conducted to determine the role of biofilms in the establishment of microbial communities in simulated cooling tower systems (Turetgen 2004; Turetgen and Cotuk 2007). More frequently, stagnant and stirred batch systems and chemostats have been used to examine the growth of representative cooling tower biofilm communities under controlled conditions (Wright et al. 1991; Green 1993; Murga et al. 2001; Donlan et al. 2005), but the conditions in these types of systems are not directly representative of cooling tower environments, and certainly do not include the variety of habitat conditions that are normally found in cooling towers. Conversely, it is extremely difficult to conduct systematic studies of biofilms in industrial cooling towers, and studies to date have been limited to surveys designed to catalog organisms that are present ( Kurtz et al. 1982; Berk et al. 2006; Declerck et al. 2007). To the best of the authors’ knowledge, no controlled studies have been performed to date to investigate the growth of microbial consortia on cooling tower surfaces under realistic environmental conditions.
In the present study, experiments were performed in a set of pilot-scale cooling tower systems that were constructed following industrial standards and used the same materials as found in conventional, full-size cooling tower systems. These systems replicate all essential features found in industrial cooling towers, including a standing water reservoir, applied heat load, recirculation of water onto a high-surface area fill material to facilitate evaporation, and an open configuration with air flow over the fill material and both make-up and blow-down water flows (ASHRAE 2000). Therefore, these systems provide a realistic distribution of local environmental conditions for study of microbial growth in cooling towers while also providing the necessary degree of control for experimental investigations of microbial growth processes.
Bacterial adhesion to substratum surfaces is expected to be extremely important in the microbial ecology of cooling towers because of the high throughflow rates commonly found in these systems. Bacterial adhesion is regulated by multiple interaction forces, such as electrostatic double-layer forces, hydrophobic interactions, hydrogen bonding and steric interactions (Salerno et al. 2004; van Merode et al. 2006; Liu and Li 2008). Here, these interactions were evaluated through observation of bacterial cell surface hydrophobicity and cell surface charge, which have been demonstrated to correlate with bacterial adhesion to surfaces (Liu and Li 2008). Confocal laser scanning microscopy (CLSM), which provides direct non-invasive optical sectioning of microbial communities (Macedo et al. 2005; Stoodley et al. 2005), was also employed to observe the resulting morphology of the biofilms that grew within the cooling towers and the spatial distribution of cells within the biofilms. 16S ribosomal DNA (rDNA)-based molecular identification was used for the identification of unknown bacteria that colonized the biofilms during the long-term experiments. The advantages of using 16S ribosomal DNA sequencing include its universal distribution among bacteria and the presence of species-specific variable regions (Macedo et al. 2005; Simoes et al. 2007). Thus, 16S rDNA sequencing was used to provide rapid and objective discrimination of organisms within the cooling tower biofilm communities. The application of this suite of methods to realistic, pilot-scale systems provides new insight into the mechanisms that govern microbial colonization of cooling towers, and clarifies the processes that control the relative abundance of different bacteria in the planktonic and surface-attached communities in these systems.
Materials and methods
Pilot-scale cooling tower systems
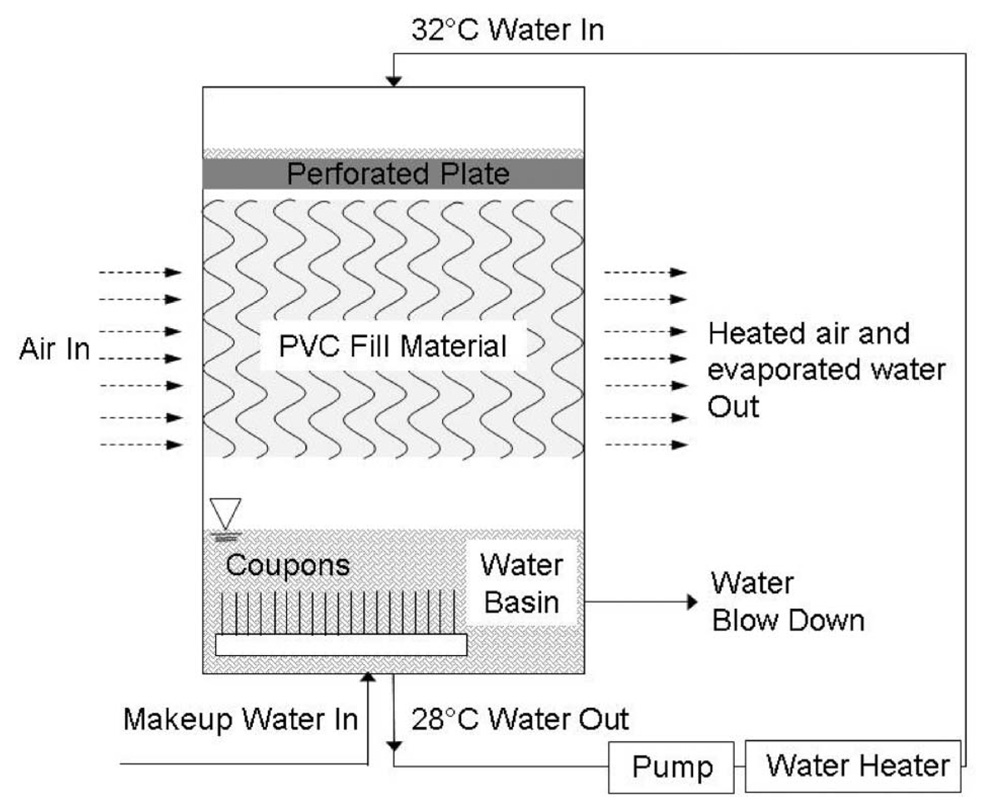
Experiments were performed in four pilot-scale cooling tower systems, each having the configuration depicted in Figure 1. Each cooling tower consisted of a chamber (56 l) containing a water reservoir (10.8 l) and three blocks of commercial cooling tower fill material composed of polyvinyl chloride (PVC) packed in an open, highly porous configuration (Brentwood Industries, PA). Each block of fill had overall dimensions of 0.2 × 0.3 × 0.5 m3. The water to be cooled was pumped from the water reservoir at the flow rate of 10,080 l day−1, through a heat exchanger, and then to the top of the tower, where it was distributed via a perforated plate over the top deck of the PVC fill. Air was drawn through the tower horizontally at a constant rate of 380 ± 18 m min−1 to cool the falling water. Air outflow was controlled by a large exhaust plenum by direct connection to the building laboratory ventilation system. The exhaust air was pulled through a dedicated HEPA filter unit (Comfill Farr, NJ) in order to ensure that live bacteria did not enter the building ventilation system.
Figure 1.
Schematic of pilot scale cooling tower system.
The cooling tower systems were operated continuously. High rates of evaporation occurred because of the low humidity of the laboratory air. During the course of the experiments, evaporative losses in each test cell averaged 57.6 ± 1.3 l day−1. As per standard industrial practice, each test cell was drained at a constant rate in order to maintain a steady concentration of dissolved solids in each cooling tower, and make-up water was provided to exactly match water losses from evaporation plus the blow-down drainage. Make-up water was from the laboratory tap water supply with chlorine removed by UV irradiation provided by two submerged BL-15 lamps (UVP LLC, CA). A commercial anti-scaling mixture (RP-1307 inhibitor, Southeastern Laboratories, Goldsboro, NC) was added to achieve a concentration of 3.65 × 10−6 mg l−1 in the makeup water and injected at a constant rate of 9 ml h−1 into the system. The blow-down rate for each cooling tower cell was set at 25.5 ± 1.1 l day−1 in order to obtain water conductivity of 1 ± 0.1 µS cm−1, which is roughly three times the make-up water conductivity of 0.3 ± 0.02 µS cm−1. Throughout the experiment, the temperature in the warm water loop of each cooling tower was kept constant at 32 ± 0.5°C, and the temperature in the cool water basin was 28 ± 0.38°C.
To observe the microbial colonization of cooling tower surfaces, numerous sterilized steel coupons (1 × 1 × 0.1 cm3) (cold rolled steel, Inland Steel Co, IL) were inserted vertically in a sample holding rack in each water basin before bacterial inoculation. The spacing between coupons was 0.5 cm, and each coupon was inserted in the sample holder to a depth of 0.2 cm. Coupons with accumulated biofilm were subsequently removed at different times during the experiment for analysis of biofilm growth. The steel had a regular matte surface finish, which provided a uniform dull surface, and is widely used for most applications of cold rolled steel sheet. The surface roughness of the steel coupons was measured and found to be 1.27 ± 0.18 µm. Before use, the coupons were disinfected by washing with 0.5% sodium hypochlorite solution, rinsed with distilled water, immersed in 80% ethanol for 10 min, and air dried.
Chemical and physical conditions
Water conductivity and temperature were measured daily by using a conductivity meter coupled with a thermister probe (ES-12, HORIBA, Ltd, Japan). Measurements of pH were performed twice a week with a pH triode (Orion, Thermo Fisher Scientific, Inc, MA) connected to a digital pH scale (Orion 720A, Thermo Fisher Scientific, Inc, MA). A colorimeter (DR/890, Hach Company, CO) was used to analyze water sample total chlorine, phosphonate and silica concentrations. Total chlorine measurements were performed twice a week, whereas phosphonate and silica concentrations were measured once a week. Alkalinity was tested once a week using a residential pool and spa kit (model K-1004, Taylor Advantage, IL). Airflow measurements were performed twice a week using a hand-held anemometer (Davis, model LCA30-VT). For each cooling tower, airflow readings were collected at the top, middle and bottom of the air inlet. The average of the three readings was then recorded as the overall cooling tower airflow rate. Copper, magnesium, silicon, phosphorus and iron concentrations were measured using inductively coupled plasma-atomic emission spectrometry (Varian, Inc, CA). Samples for all analyses were collected from the water reservoir of the cooling tower.
Bacterial strains and cell preparation
Three bacterial strains were used in these studies: Pseudomonas aeruginosa (ATCC 7700), Klebsiella pneumoniae (DMDS Lab. No. 92-08-28a) and Flavobacterium sp. (CDC-65). These microorganisms are all Gram-negative bacteria commonly found in potable-water environments, and this consortium has been used in prior studies of Legionella survival in multispecies biofilms (Murga et al. 2001). For each experiment, the stored strains were streaked onto R2A agar plates and incubated at 28°C for 16 h (K. pneumoniae and P. aeruginosa) or 36 h (Flavobacterium). A single colony was then transferred into 5 ml of growth medium containing 0.5 g l−1 yeast extract, proteose peptone no. 3, casamino acids and dextrose, 0.3 g l−1 sodium pyruvate and dibasic potassium phosphate and 0.05 g l−1 magnesium sulfate. The growth medium was prepared with reverse-osmosis purified water. The bacteria were grown in a shaker incubator at 200 rpm and 28°C until they reached the stationary phase (16 h for K. pneumoniae; 20 h for P. aeruginosa; 30 h for Flavobacterium).
Inoculation of cooling tower systems
In both short-term and long-term studies, bacterial cultures and growth medium were added to four identical cooling tower cells by pulse injection to reach an initial concentration of 108 CFU ml−1 for each of the three bacteria (K. pneumoniae, P. aeruginosa and Flavobacterium), and nutrient concentrations of 0.005 g l−1 yeast extract, proteose peptone no. 3, casamino acids and dextrose, 0.003 g l−1 sodium pyruvate and dibasic potassium phosphate and 0.0005 g l−1 magnesium sulfate.
The initial short-term experiment indicated that some of the strains were not able to colonize surfaces within the cooling tower, likely because of rapid washout. To ensure that the initial condition for the longterm experiment included colonization of surfaces by all the three test strains, a coupon rack loaded with 20 steel coupons (1 × 1 × 0.1 cm3) having pre-attached K. pneumoniae, P. aeruginosax and Flavobacterium of 104 CFU cm−2 was placed in each cooling tower water basin before injection of planktonic bacteria and nutrients.
Batch experiments for bacterial adhesion and growth rate
Short-term studies also were performed in batch systems to evaluate the impact of bacterial growth rate on the adhesion and survival on coupons. In each test, bacterial cells were inoculated in test tubes with pre-inserted sterilized steel sample coupons. The initial cell and nutrient concentrations in the test tubes were identical to those in the cooling tower systems, and the same water used for the cooling tower makeup supply and for the solution medium for the batch studies. Four tests were carried out in triplicate: (1) Flavobacterium culture in cooling tower makeup water, (2) K. pneumoniae culture in cooling tower makeup water, (3) P. aeruginosa culture in cooling tower makeup water, (4) a Flavobacterium, K. pneumoniae and P. aeruginosa mixed culture in cooling tower makeup water.
Bacterial enumeration procedures
Bacterial growth was monitored daily both in the cooling tower basin (planktonic cells) and on the emplaced steel coupons (biofilm growth). To disaggregate surface-attached cells for enumeration and to disassociate bacteria and extracellular polymeric substance (EPS) bound to the coupon surfaces, sample coupons were placed in 15 ml centrifuge tubes containing 2 ml PBS buffer and then subjected to 10-min ultrasonication (FS 20H, Fisher Scientific Inc, IL), followed by vortexing (Genie 2, Fisher Scientific Inc, IL) at the maximum speed for 30 s. This method has previously been shown to be most effective in detaching biofilms from surfaces for bulk analysis (Liu et al. 2007, 2008).
Viable bacterial cell counts were obtained using the drop plate method (Liu et al. 2007). A series of 10-fold dilutions was performed, and 10 µl of each dilution was plated on R2A agar plates in triplicate. Plates were incubated at 28°C for 36 h before counting. The three types of bacteria were distinguished by the appearance of the colonies (white colonies: K. pneumoniae; yellow colonies: Flavobacterium; light green colonies: P. aeruginosa). The lower limit of this detection method is ~1 CFU 10 µl−1, or 102 CFU ml−1.
Physico-chemical characterization of bacterial cells
Cells were harvested by centrifugation (3000 g, 4°C, 10 min). The growth medium was decanted and the pellets were resuspended in cooling tower makeup water. The centrifugation–resuspension process was repeated three times to remove traces of the growth medium.
A ZetaPALS Analyzer (Brookhaven Instruments Corp, NY) was used to measure bacterial cell electrophoretic mobility and zeta potential in cooling tower makeup water at pHs of 5.8, 7.0 and 9.0 (the pH was adjusted to 5.8 and 7.0 with 0.1N HCl). The measurements were replicated 10 times with 30 cycles for each assay.
The hydrophobicities of the bacterial cell surfaces were inferred from the plateau contact angles, θ, of liquid drops placed on lawns of bacteria. Lawns of bacteria were prepared by filtering 10–20 ml of cell suspension (108 cells ml−1 in early stationary phase) onto an aluminum oxide filter (0.4 pore size, 25 mm diameter; Whatman Corp, UK) under vacuum for 20–40 min. Lawns were dried in a desiccator for 30 min and measured within 2 h. Previous studies have shown that, after a drying time of 30 min, measurements on lawns are stable for 3 h (Vanloosdrecht et al. 1987; Razatos et al. 1998). Contact angles were measured within 2 s for 2 µl droplets (triplicate samples) of cooling tower makeup water at various pHs (5.8, 7.0 and 9.0) by a drop shape analysis equipment (Newport Optical Inc, RI). The shape of the sessile drop was fitted by mathematical expressions and used to calculate the contact angle.
CLSM for the characterization of biofilms
The structures of the attached bacterial cells and biofilms in the cooling tower water reservoirs were evaluated by analyzing coupons using an upright Leica confocal laser scanning microscope (CLSM) model DM RXE-7 (Leica Microsystems, Germany). Concanavalin A conjugated with Texas Red was used to stain biofilm EPS, and SYTO 9 was used to stain bacterial cells (Invitrogen Corporation, CA). Fluorescence stained coupons were placed in PBS buffer, and biofilm surface structures were visualized under a 40× water immersion objective. All CLSM images were collected within 3 h after sample coupons were taken from cooling tower test cells.
16S ribosomal DNA sequencing analysis
After 20 days cooling tower operation, colonies of non-introduced bacteria were detected in samples from the cooling tower system. 16S rDNA sequencing analysis was used to identify these unknown species, as well as to confirm the identification of colonies of inoculated strains. Single colonies were selected from R2A agar plates based on their distinctive morphology, transferred to different R2A plates, and incubated under 28°C. Cells were then grown in 5 ml growth medium at 28°C until they reached stationary phase. Genomic DNA was extracted using the phenol/chloroform method (Zoetendal et al. 2006). Fifty microliter of buffer EB (10 mM Tris · Cl, pH 8.5) was used to elute DNA. The concentration of resulting genomic DNA was determined using NanoDrop ND-1000 spectrophotometer (NanoDrop products, DE).
Partial 16S rDNA fragments were amplified from the genomic DNA by PCR using primer 63f (forward) (5′-CAG GCC TAA CAC ATG CAA GTC-3′, positions 43–63 in 16S rDNA of E. coli) and primer 1387r (reverse) (5′-GGG CGG WGT GTA CAA GGC-3′, positions 1387–1404 in 16S rDNA of E. coli) (Marchesi et al. 1998). Each 50 µl PCR mixture contained 1 × PCR buffer with 1.5 mM MgCl2, 0.25 mM deoxynucleoside triphosphate solution, 2 µM primers and 1.25 U DNA polymerase. The total amount of genomic DNA added to PCR mixtures was ~50 ng for pure cultures. Thermocycling, which was conducted in a Primus 96 instrument (MWG Biotech Ltd, Ireland), started with an initial denaturation for 3 min at 95°C. A total of 36 cycles, each including 30 s at 95 C, 45 s at 56°C, and 90 s at 72°C, was followed by a final primer extension step of 10 min at 72°C. The PCR products were Agarose gel purified before sequencing using QIAquick Spin Columns (QIAGEN Sciences, MD), following the manufacturer’s instructions.
The sequences of purified PCR products were determined by Eton Bioscience, Inc (CA). Selected bacterial 16S rDNA sequences were compared with the sequence libraries of GenBank (Benson et al. 2004).
Results
Electrokinetic potential and contact angle of bacterial cells
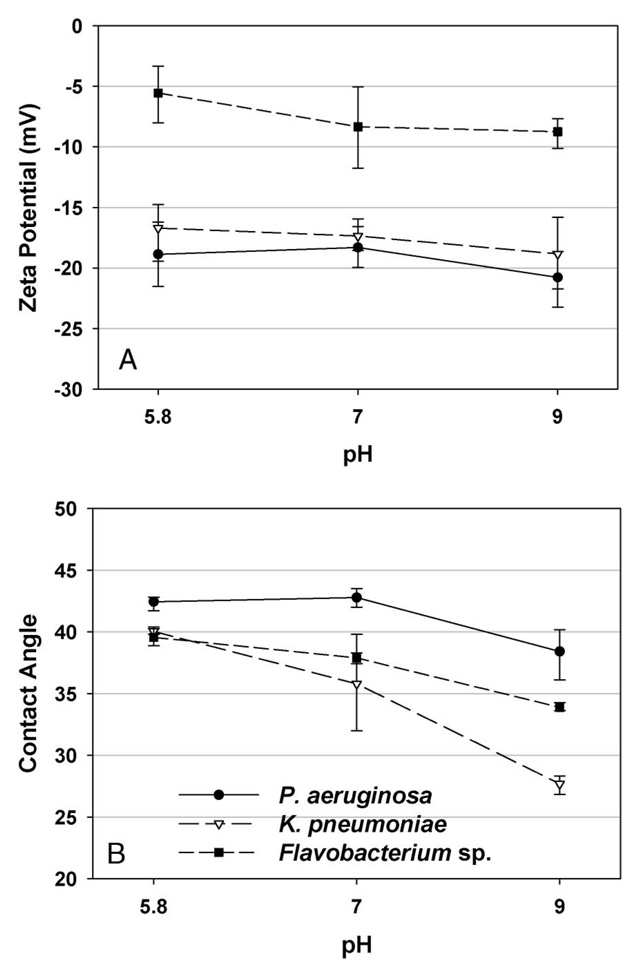
Figure 2A presents the influence of solution pH on the zeta potential of cells of Flavobacterium, K. pneumoniae and P. aeruginosa. Under the conditions investigated, all the three bacterial strains exhibited negative zeta potentials and their surface charges became less negative with decreasing pH. Flavobacterium had the lowest zeta potential among the three bacterial cells, indicating a less negatively charged cell surface. K. pneumoniae and P. aeruginosa had almost identical electrokinetic potential (P = 0.01 by paired-sample t test).
Figure 2.
Surface zeta potential and contact angle of three bacterial strains, P. aeruginosa, K. pneumoniae and Flavobacterium sp., as a function of solution pH. Error bars represent standard deviations (SDs) of 10 replicate measurements.
The experimental results from bacterial contact angle testing are shown in Figure 2B. All three bacterial strains were hydrophilic with water contact angles ranging from 28° to 42°. P. aeruginosa had a higher water/solid contact angle than K. pneumoniae and Flavobacterium over the range of pHs tested in this study, indicating the lowest degree of hydrophilicity (P < 0.005).
Water chemistry in cooling towers
The chemical conditions in each experiment are reported in Table 1. The chemical composition remained essentially constant throughout the experiments. The average pH was 9.0 in the water basin due to the addition of the scale and corrosion inhibitor.
Table 1.
Cooling tower water chemistry and physical properties.
| Chemical and physical parameters | Results |
|---|---|
| Alkalinity | 465 ± 28 ppm |
| Conductivity | 0.94 ± 0.04 mS |
| Total chlorine | 0.17 ± 0.05 ppm |
| Phosphonate | 8.20 ± 0.73 ppm |
| pH | 9.0 ± 0.03 |
| Airflow | 118.5 ± 1.9 m min−1 |
| Ca | 111.5 ± 14.9 mg l−1 |
| Mg | 39.2 ± 6.5 mg l−1 |
| P | 2.68 ± 0.14 mg l−1 |
| Si | 3.65 ± 0.34 mg l−1 |
| Cu | <0.9 × 10−3 ppm |
| Fe | <0.3 × 10−3 ppm |
Cu and Fe concentrations were less than the detection limit.
Bacterial community in batch experiments
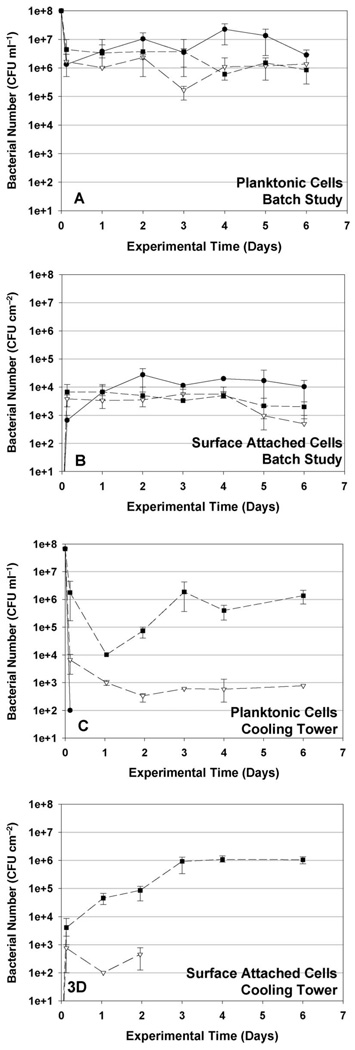
The bacterial cell numbers observed in suspension and on steel coupons from the test tube experiments are shown in Figure 3A and B. When the three species were co-cultured in the cooling tower makeup solution, all the three bacteria survived for 6 days in the low nutrient solution. The concentrations of viable planktonic P. aeruginosa, K. pneumoniaex and Flavobacterium all decreased by one to two orders of magnitude within hours after the initial inoculation, presumably due to a combination of deposition on surfaces within the batch systems and cell lysis due to the sudden dilution of the growth medium and water osmosis inside the cells (Halverson et al. 2000). The planktonic P. aeruginosa concentrations subsequently reached 5 × 106 CFU ml−1. The planktonic concentrations of K. pneumoniae and Flavobacterium decreased to a greater extent than P. aeruginosa, reaching an apparently steady concentration of 1 × 106CFU m−1 for both by the end of the experiment. Conversely, K. pneumoniae and Flavobacterium more readily associated with the coupon surfaces, rapidly reaching concentrations of almost 104 CFU cm−2, whereas P. aeruginosa required 24 h to reach this concentration on the coupons. However, P. aeruginosa subsequently out-competed K. pneumoniae and Flavobacterium in the biofilm, ultimately leading to surface concentrations of P. aeruginosa > 104 CFU cm−2 while concentrations of K. pneumoniae and Flavobacterium decreased to 5 × 102 CFU cm−2 and 2 × 103 CFU cm−2, respectively, by the end of the experiment. Further, comparing with the bacterial survival observed in pure cultures, shown in Figure 5, the survival of P. aeruginosa in the mixed culture was not affected by the presence of the other two bacteria, whereas the survival of K. pneumoniae and Flavobacterium in the mixed culture biofilm decreased following the initial deposition onto the surface.
Figure 3.
Concentrations of three introduced bacterial strains in liquid phase (A and C) and on coupon surfaces (B and D) observed in batch studies (A and B) and cooling tower experiments (C and D). Error bars represent SDs of replicate experiments. (–––●––– P. aeruginosa, – –∇– – K. pneumoniae, – – ∎ – – Flavobacterium sp.).
Figure 5.
Bacteria concentration in liquid phase (A) and on coupon surfaces (B) observed in mono-species batch experiments. Error bars represent SDs of triplicate experiments. (–––●––– P. aeruginosa, – – ∇ – – K. pneumoniae, – – ∎ – – Flavobacterium sp.).
Bacterial community in short-term cooling tower experiments
Three bacterial cultures (P. aeruginosa, K. pneumoniae and Flavobacterium) were mixed and inoculated into cooling towers each at an initial cell concentration of 108 CFU ml−1. Flavobacterium quickly became the dominant species in the cooling tower basins, and remained the most prolific throughout the experiment. As shown in Figure 3C, 3 h after initial bacterial injection, the Flavobacterium and K. pneumoniae concentrations had decreased two and four orders of magnitude, respectively, whereas P. aeruginosa declined more rapidly; below the sensitivity limit of the analytical method (102 cells ml−1) within 3 h. The loss of bacterial cells is primarily attributed to the high rate of water outflow required to maintain the steady-state salt concentrations in the cooling towers (blow-down), and can also result from cell lysis. In the water basin, planktonic K. pneumoniae reached a constant concentration of 2 × 102 CFU ml−1 2 days after initial injection. Flavobacterium decreased by four orders of magnitude in 1 day, but then increased rapidly over the next 2 days, presumably because of fast bacterial growth after initial colonization of solid surfaces. The Flavobacterium concentration remained constant after Day 4. The constant Flavobacterium and K. pneumoniae concentrations observed towards the end of the experiment suggest that the cooling tower communities reached steady state, ie where the rate of bacterial loss by death and wash-out equaled the rate of bacterial growth.
The concentrations of bacterial cells recovered from the steel coupons during the short-term experiment are reported in Figure 3D. As found for the planktonic community, P. aeruginosa cells were not detected on steel coupon surfaces 3 h after initial injection. In contrast, Flavobacterium reached 4 × 103 CFU cm−2 on coupons 3 h after the initial injection, and K. pneumoniae reached 8 × 102 CFU cm−2 in this period. The density of Flavobacterium increased for 3 days until a constant concentration of 106 CFU cm−2 was achieved, whereas K. pneumoniae density fell to below the detection limit 2 days after inoculation. These results show that Flavobacterium became the dominant species in the surface-attached microbial consortium, and strongly suggest that the ongoing presence of planktonic Flavobacterium in the cooling towers was directly related to the establishment of a substantial surface-attached community of this organism.
Bacterial community in long-term cooling tower experiments
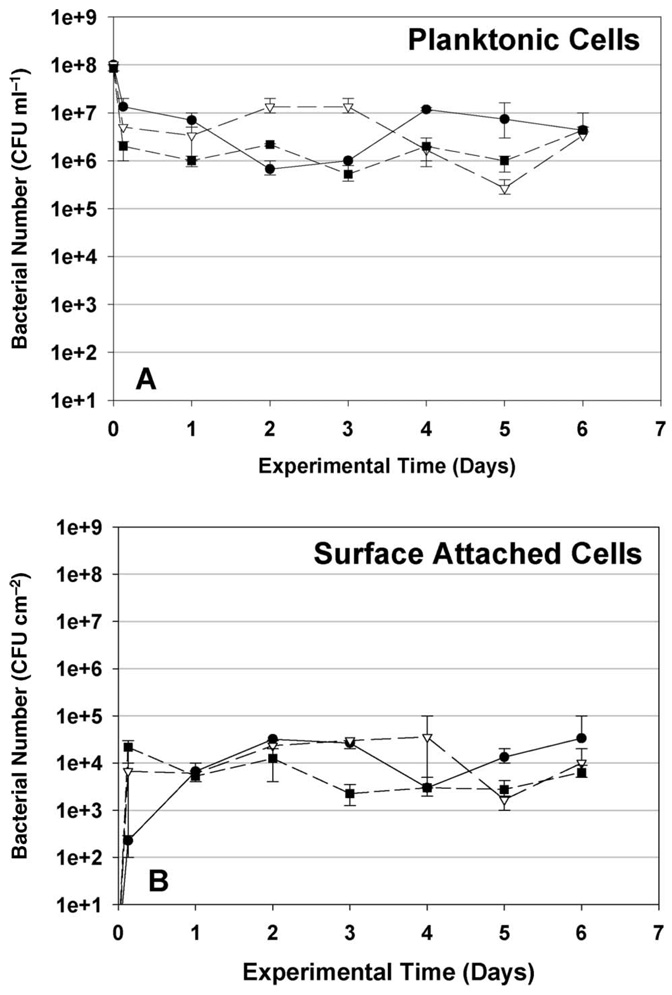
The persistence of P. aeruginosa, K. pneumoniae and Flavobacterium sp., was also observed in the bulk and biofilm phases in a separate long-term experiment. As shown in Figure 4A, planktonic K. pneumoniae and P. aeruginosa reached the concentrations of 104 CFU ml−1 and 103 CFU ml−1, respectively, in the water basin 1 day after the initial inoculation. Five days after the bacterial injection, the concentrations of both of these bacteria decreased to below the detection limit. Flavobacterium decreased by three orders-of-magnitude in 5 days, and then remained at a constant concentration of 105 CFU ml−1 throughout the rest of the experiment.
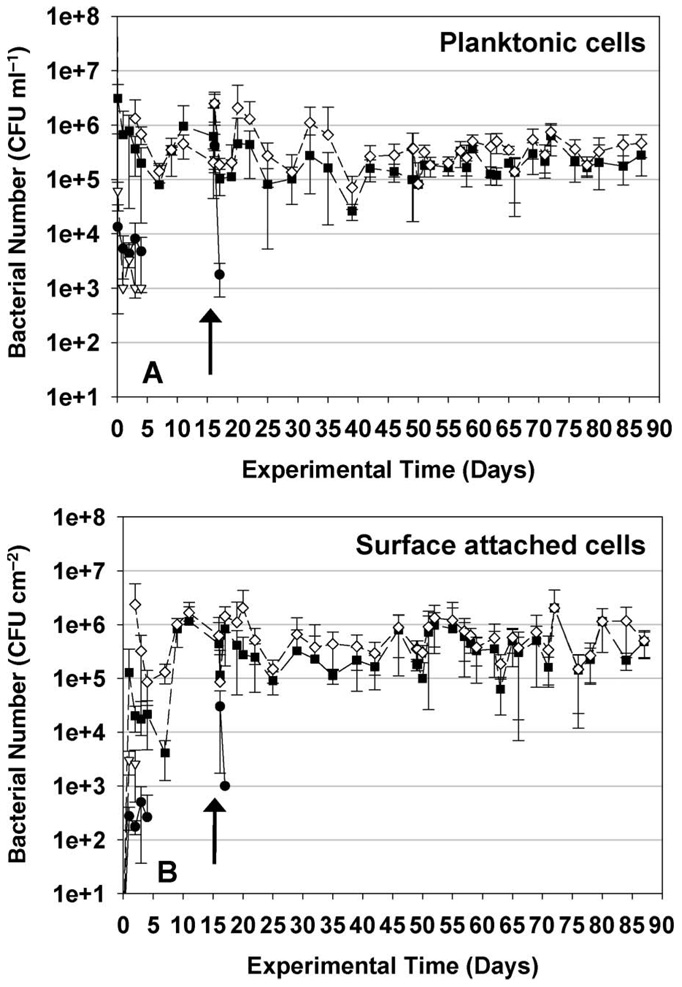
Figure 4.
Concentrations of three introduced bacterial strains in liquid phase (A) and on coupon surfaces (B) in cooling tower long-term study. Error bars represent SDs of triplicate experiments. Arrows represent the date when bacteria culture was reinoculated. (–––●––– K. pneumoniae, – – ∇ – – P. aeruginosa, – – ∎ – – Flavobacterium sp., – – ◊ – – total bacteria).
Figure 4B presents results on the colonization of sample coupons in the cooling towers over time. P. aeruginosa and K. pneumoniae both showed a low degree of retention on coupon surfaces, and were washed out of the cooling tower systems after 4 days. Flavobacterium sp. rapidly colonized and formed biofilms on the surfaces of the sampling coupons, and became the dominant microorganism in the cooling towers within a few days. By Day 10, Flavobacterium reached a constant growth rate, and the concentration of this bacterium in the cooling tower water basin and on the steel coupons remained stable for the rest of the study. K. pneumoniae and P. aeruginosa were observed to persist for a longer period of time in the long-term study than in the short-term experiments. This is attributed to the slow release of bacterial cells from the pre-inoculated coupons that were placed in the cooling towers at the beginning of the long-term experiment (compared with the introduction of only planktonic bacteria in the short-term experiment).
The three bacterial cultures were reinoculated into each cooling tower at Day 16 in order to observe whether colonization of a pre-established biofilm would yield different results than those from initial colonization of the system. Reinoculation times are indicated by arrows in Figure 4. P. aeruginosa disappeared from the system within 1 day after re-inoculation, whereas K. pneumoniae cells were only retained in the cooling tower system for 2 days following re-inoculation. These results confirm that the behavior observed in the original short-term experiment continues after the cooling tower surfaces are fully colonized by a biofilm. In particular, the ability of each strain to colonize available surfaces in the cooling tower and to grow in biofilms on these surfaces is critical to long-term persistence in the cooling tower community. These results are in agreement with the previous studies performed by Liu et al. (2008), who showed that high biofilm surface hydrophobicity and polymeric interaction between biofilm and newly injected bacterial cells may discourage adhesion to the biofilm surfaces. The new results presented here show that this behavior is important to both initial colonization of cooling towers and to the long-term microbial ecology of these systems; eg following the later introduction of immigrant bacteria from building water loops, make-up water supplies, or from the atmosphere.
Such immigrant strains were detected in the long-term experiments after 20 days of continuous cooling tower operation. Sequencing results showed that Methylobacterium, Sphingomonas and Rhodococcus spp. successfully colonized the cooling towers. These bacteria are common microbes in soils and aqueous systems, and are known to be able to survive under low nutrient conditions. Methylobacterium metabolizes methanol and methylamine. Methylbacterium is thought to be a part of the natural human foot flora, and some strains have been found living inside the human mouth (Lidstrom and Chistoserdova 2002). Sphingomonas is distributed widely in the environment due to its ability to utilize a wide range of organic compounds (Fredrickson et al. 1999). Rhodococcus sp. is known as one of the most important industrial organisms, as it grows under both mesophilic (Lichtinger et al. 2000) and psychrophilic (Patrauchan et al. 2005) conditions. The study reported here also confirmed the long-term persistence of the introduced Flavobacterium strain using 16S rDNA sequencing. Although the species composition of the biofilms was observed to change over time, Flavobacterium was the dominant community member in all of the investigated biofilms.
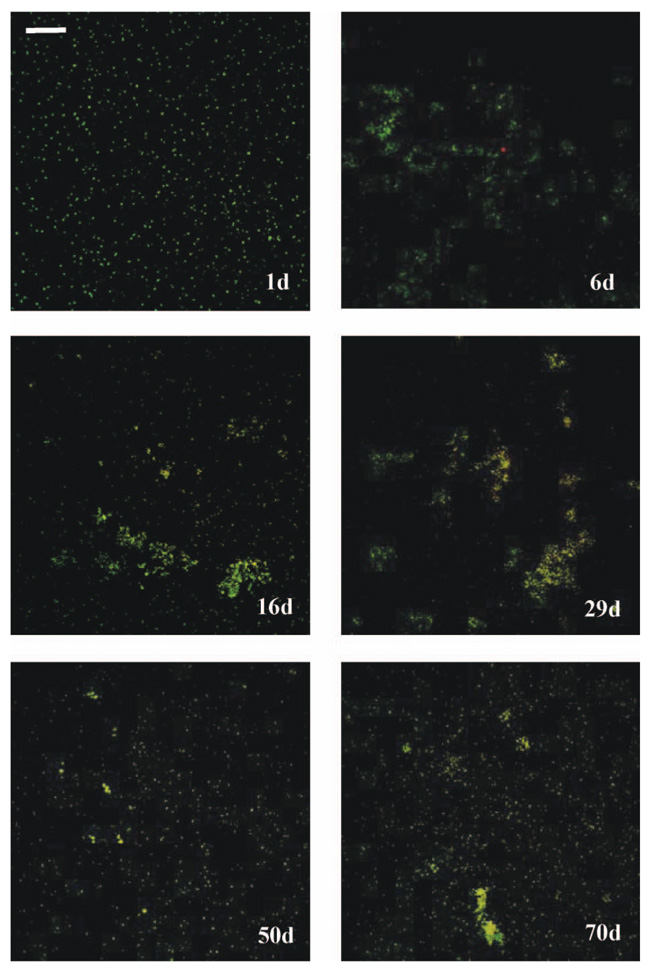
CLSM images provided direct evidence of surface colonization in the cooling tower water reservoir (Figure 6). On Day 1, the attached cells were widely spread over the coupon surfaces with an average thickness of 7 µm, whereas bacterial clusters having thicknesses up to 60 µm were observed at Day 6. The structures of the biofilms remained stable for the remainder of the experiment, indicating that the spatial organization of biofilms did not change after the period of initial colonization.
Figure 6.
Confocal images for the surface attached cells on coupons in cooling tower systems. Bar = 50 µm.
Discussion of bacterial adhesion to surfaces and persistence in cooling towers
Effect of bacterial adhesion on the development of cooling tower microbial communities
Significant differences in the bacterial community composition were observed between open cooling towers and closed batch systems. In batch systems, P. aeruginosa adhered to steel surfaces less effectively than Flavobacterium and K. pneumoniae, resulting in fewer surface-attached P. aeruginosa cells 3 h after the initial inoculation. However, P. aeruginosa grew much faster after adhesion and dominated the mixed culture by Day 2. Very different results were observed in the cooling towers. The low rate of adherence of P. aeruginosa to the steel coupon surfaces led to it being rapidly eliminated from the cooling tower system. These results strongly suggest that the adherence of individual strains plays a very important role in the microbial ecology of cooling tower microbial communities because of the high degree of wash-out associated with the drainage (blow-down) of these systems.
Bacterial adhesion is mediated by electrostatic forces as described by classical Derjaguin–Landau–Verwey–Overbeek (DLVO) theory (Bhattacharjee et al. 1998; Tufenkji and Elimelech 2004). According to the DLVO theory, more negatively charged bacterial surfaces pose higher repulsion to the negatively charged steel coupon surfaces (Ryu et al. 2004). In the study reported here, it was observed that K. pneumoniae and P. aeruginosa had similar zeta potentials in the cooling tower makeup water, and both were more negatively charged than Flavobacterium. These observations can explain the greater degree of Flavobacterium adhesion on the cooling tower surfaces. However, the deposition of K. pneumoniae and P. aeruginosa observed in both systems strongly implies bacterial deposition on steel also occurs by non-DLVO mechanisms. Hydrophobic moieties on cell surfaces have been shown to increase the adhesion of many bacteria by removing water adsorbed to surfaces. In the study reported here, it was observed that P. aeruginosa had the greatest hydrophobicity, but the least adhesion to steel coupons in batch systems and also the least persistence in the cooling tower systems, indicating that hydrophobicity was not the main mechanism controlling bacterial retention on cooling tower surfaces. These results are in agreement with Vanhaecke et al. (1990), who found a weak correlation between hydrophobicity and propensity of P. aeruginosa strains to adhere to stainless steel surfaces.
Bacterial motility is also known to affect adhesion. When motile cells are brought near surfaces (eg by hydrodynamic transport), they can swim along the surface at different orientations, either parallel to the surface, perpendicular to the surface, or tracing out circles along the surface (Frymier et al. 1995; Vigeant and Ford 1997). Cell motility can either enhance or deter cell adhesion (Jenneman et al. 1985; Korber et al. 1994). P. aeruginosa and Flavobacterium are both motile (Stewart et al. 1997; Hunnicutt et al. 2002). P. aeruginosa has twitching and swarming motility, which requires the extension and retraction of pili and flagella (Stewart et al. 1997; O’Toole and Kolter 1998; Reimmann et al. 2002). In this study, motility was confirmed by observing the fluorescent-stained bacterial cells under an epifluorescent microscope. Flavobacterium does not possess the conventional motility structures that influence deposition onto surfaces, namely, flagella or typical type IV pili (Hunnicutt and McBride 2000; Hunnicutt et al. 2002). The fact that K. pneumoniae is non-motile (Sturman et al. 1994) can explain the observation in this study that K. pneumoniae cells adhered better to cooling tower surfaces than P. aeruginosa.
Bacterial survival and biofilm formation
After initial cell attachment, the bacterial cells survived in both the closed-batch systems and cooling tower systems for an extended period of time. In monospecies batch experiments, K. pneumoniae grew faster than P. aeruginosa, and both bacteria multiplied more than twice as quickly as Flavobacterium. However, K. pneumoniae was out-competed by P. aeruginosa and Flavobacterium in both multi-species batch experiments and in the cooling tower systems. In batch experiments, P. aeruginosa became the dominant species in biofilms despite its low initial adherence to steel surfaces. In the cooling towers, low P. aeruginosa colonization of the surfaces led to wash-out of this organism, allowing Flavobacterium to dominate both the biofilm and planktonic communities. Additional experiments were carried out to compare the survival rates of these three bacteria subject to antiscaling chemicals, pH and sudden dilution under the condition tested in this study. No significant difference was observed in the killing of the three species. These results strongly imply that growth rate was not the main mechanism that governed the development of the bacterial community in the cooling towers, and instead initial adhesion to solid surfaces and subsequent biofilm formation ultimately controlled the persistence of different organisms in cooling towers.
Conclusions and implications
Bacterial adhesion and survival were studied in both pilot scale cooling towers and closed batch systems. The results demonstrate that adhesion, retention and growth on solid surfaces play important roles in the microbial ecology of cooling towers.
Short-term (6 days) experiments indicated that the microbial community in cooling tower systems can be greatly affected by the initial adhesion of bacterial cells to surfaces within the cooling towers. The community structure in this system was quite different from the one that developed in a closed batch system, where bacterial growth rates played a more important role in controlling the relative concentrations of each strain in the consortium. The surface charge and motility of the individual strains appeared to be the most important factors influencing the initial adhesion of the bacteria to surfaces under the experimental conditions tested.
Long-term (3 months) studies in cooling tower systems showed that the microbial community observed in the short-term studies is robust and persists for an extended period of time. Repeated reintroduction of the test strains did not change the microbial community structure, indicating that similar processes regulate the ability of the organisms to form biofilms and to colonize pre-existing biofilms on cooling tower surfaces. Thus, the fate and persistence of bacteria in cooling towers is likely highly dependent on the basic cell surface properties that govern their interactions with materials found in cooling towers and associated building piping systems. Use of materials that generally hinder bacterial adhesion can potentially be helpful in reducing colonization of cooling towers. The growth rate of bacteria was found to be less important than the adherence and colonization of surfaces in controlling biofilm development in the cooling tower systems.
A better understanding of microbial community diversity within cooling towers is needed in order to reduce the risks associated with the growth of pathogenic species such as L. pneumophila. The results presented here suggest that it is important to consider local habitat conditions, eg temperature, material composition, flow rates and water chemistry, when investigating microbial growth in cooling towers and other similar building systems. Conventional monitoring often relies only on samples obtained from water reservoirs or pipe loops, which reflect only the planktonic community. The results presented here show that colonization of surfaces plays an important role in the development and persistence of microbial communities in cooling towers. Therefore, standard evaluations of cooling towers should involve sampling of multiple possible pathogen reservoirs, including biofilms growing on all of the different types of surfaces found within the system.
Acknowledgements
This publication was made possible by Grant Number 5K25AI062977 to AIP. from the National Institute of Allergy and Infectious Disease (NIAID) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIAID. Construction of the cooling tower experimental system was supported by a contract from the American Society of Heating, Refrigeration and Air-conditioning Engineers (ASHRAE) awarded to NPC and AIP. The authors thank Barry Fields for providing the bacterial strains used in the experiments.
References
- Addiss DG, Davis JP, Laventure M, Wand PJ, Hutchinson MA, McKinney RM. Community-acquired Legionnaires’-disease associated with a cooling-tower-evidence for longer-distance transport of Legionella pneumophila. Am J Epidemiol. 1989;130:557–568. doi: 10.1093/oxfordjournals.aje.a115370. [DOI] [PubMed] [Google Scholar]
- Albert-Weissenberger C, Cazalet C, Buchrieser C. Legionella pneumophila - a human pathogen that coevolved with fresh water protozoa. Cell Mol Life Sci. 2007;64:432–448. doi: 10.1007/s00018-006-6391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHRAE. ASHRAE HVAC Systems & Equipment Handbook 2000. ASHRAE; Atlanta (GA): 2000. [Google Scholar]
- Atlas RM. Legionella: from environmental habitats to disease pathology, detection and control. Environ Microbiol. 1999;1:283–293. doi: 10.1046/j.1462-2920.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank: update. Nucleic Acids Res. 2004;32:D23–D26. doi: 10.1093/nar/gkh045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham RH, Broadbent CR. A model for autumn outbreaks of Legionnaires’ disease associated with cooling-towers, linked to system operation and size. Epidemiol Infect. 1993;111:287–295. doi: 10.1017/s0950268800056995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk SG, Ting RS, Turner GW, Ashburn RJ. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64:279–286. doi: 10.1128/aem.64.1.279-286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk SG, Gunderson JH, Newsome AL, Farone AL, Hayes BJ, Redding KS, Uddin N, Williams EL, Johnson RA, Farsian M, et al. Occurrence of infected amoebae in cooling towers compared with natural aquatic environments: implications for emerging pathogens. Environ Sci Technol. 2006;40:7440–7444. doi: 10.1021/es0604257. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Elimelech M, Borkovec M. DLVO interaction between colloidal particles: beyond Derjaguin’s approximation. Croat Chem Acta. 1998;71:883–903. [Google Scholar]
- Bhopal RS, Fallon RJ, Buist EC, Black RJ, Urquhart JD. Proximity of the home to a cooling-tower and risk of non-outbreak Legionnaires’ disease. Br Med J. 1991;302:378–383. doi: 10.1136/bmj.302.6773.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman RF. Impact of technology on the emergence of infectious diseases. Epidemiol Rev. 1996;18:4–9. doi: 10.1093/oxfordjournals.epirev.a017915. [DOI] [PubMed] [Google Scholar]
- Ceyhan N, Ozdemir G. Extracellular polysaccharides produced by cooling water tower biofilm bacteria and their possible degradation. Biofouling. 2008;24:129–135. doi: 10.1080/08927010801911316. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Cheng K-J, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Declerck P, Behets J, van Hoef V, Ollevier F. Detection of Legionella spp. and some of their amoeba hosts in floating biofilms from anthropogenic and natural aquatic environments. Water Res. 2007;41:3159–3167. doi: 10.1016/j.watres.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Forster T, Murga R, Brown E, Lucas C, Carpenter J, Fields B. Legionella pneumophila associated with the protozoan Hartmannella vermiformis in a model multi-species biofilm has reduced susceptibility to disinfectants. Biofouling. 2005;21:1–7. doi: 10.1080/08927010500044286. [DOI] [PubMed] [Google Scholar]
- Fredrickson JK, Balkwill DL, Romine MF, Shi T. Ecology, physiology, and phylogeny of deep subsurface Sphingomonas sp. J Ind Microbiol Biotechnol. 1999;23:273–283. doi: 10.1038/sj.jim.2900741. [DOI] [PubMed] [Google Scholar]
- Frymier PD, Ford RM, Berg HC, Cummings PT. 3-dimensional tracking of motile bacteria near a solid planar surface. Proc Natl Acad Sci USA. 1995;92:6195–6199. doi: 10.1073/pnas.92.13.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylarde CC, Morton LHG. Deteriogenic biofilms on buildings and their control: a review. Biofouling. 1999;14:59–74. [Google Scholar]
- Green PN. Efficacy of biocides on laboratory-generated Legionella biofilms. Lett Appl Microbiol. 1993;17:158–161. doi: 10.1111/j.1472-765x.1993.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Halverson LJ, Jones TM, Firestone MK. Release of intracellular solutes by four soil bacteria exposed to dilution stress. Soil Sci Soc Am J. 2000;64:1630–1637. [Google Scholar]
- Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. Environmental predators as models for bacterial pathogenesis. Environ Microbiol. 2007;9:563–575. doi: 10.1111/j.1462-2920.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- Hunnicutt DW, McBride MJ. Cloning and characterization of the Flavobacterium johnsoniae gliding-motility genes gldB and gldC. J Bacteriol. 2000;182:911–918. doi: 10.1128/jb.182.4.911-918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt DW, Kempf MJ, McBride MJ. Mutations in Flavobacterium johnsoniae gldF and gldG disrupt gliding motility and interfere with membrane localization of GldA. J Bacteriol. 2002;184:2370–2378. doi: 10.1128/JB.184.9.2370-2378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenneman GE, McInerney MJ, Knapp RM. Microbial penetration through nutrient-saturated Berea sandstone. Appl Environ Microbiol. 1985;50:383–391. doi: 10.1128/aem.50.2.383-391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DW, Hajjeh R, DeMaria A, Fields BS, Pruckler JM, Benson RS, Kludt PE, Lett SM, Mermel LA, Giorgio C, et al. Community outbreak of Legionnaires’ disease: an investigation confirming the potential for cooling towers to transmit Legionella species. Clin Infect Dis. 1996;22:257–261. doi: 10.1093/clinids/22.2.257. [DOI] [PubMed] [Google Scholar]
- Korber DR, Lawrence JR, Caldwell DE. Effect of motility on surface colonization and reproductive success of Pseudomonas fluorescens in dual-dilution continuous-culture and batch culture systems. Appl Environ Microbiol. 1994;60:421–1429. doi: 10.1128/aem.60.5.1421-1429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz JB, Bartlett CLR, Newton UA, White RA, Jones NL. Legionella pneumophila in cooling water-systems -report of a survey of cooling-towers in London and a pilot trial of selected biocides. J Hyg. 1982;88:369–381. doi: 10.1017/s0022172400070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnetsov JM, Martikainen PJ, Jousimiessomer HR, Vaisanen ML, Tulkki AI, Ahonen HE, Nevalainen AI. Physical, chemical and microbiological water characteristics associated with the occurrence of Legionella in cooling-tower systems. Water Res. 1993;27:85–90. [Google Scholar]
- Lichtinger T, Reiss G, Benz R. Biochemical identification and biophysical characterization of a channel-forming protein from Rhodococcus erythropolis. J Bacteriol. 2000;182:764–770. doi: 10.1128/jb.182.3.764-770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidstrom ME, Chistoserdova L. Plants in the pink: cytokinin production by Methylobacterium. J Bacteriol. 2002;184:1818. doi: 10.1128/JB.184.7.1818.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li J. Role of Pseudomonas aeruginosa biofilmin the initial adhesion, growth and detachment of Escherichia coli in porous media. Environ Sci Technol. 2008;42:443–449. doi: 10.1021/es071861b. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang CH, Li J. Influence of extracellular polymeric substances on Pseudomonas aeruginosa transport and deposition profiles in porous media. Environ Sci Technol. 2007;41:198–205. doi: 10.1021/es061731n. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang CH, Li J. Adhesion and retention of a bacterial phytopathogen Erwinia chrysanthemi in biofilm-coated porous media. Environ Sci Technol. 2008;42:159–165. doi: 10.1021/es071698k. [DOI] [PubMed] [Google Scholar]
- Macedo AJ, Kuhlicke U, Neu TR, Timmis KN, Abraham WR. Three stages of a biofilm community developing at the liquid-liquid interface between polychlorinated biphenyls and water. Appl Environ Microbiol. 2005;71:7301–7309. doi: 10.1128/AEM.71.11.7301-7309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meesters KPH, Van Groenestijn JW, Gerritse J. Biofouling reduction in recirculating cooling systems through biofiltration of process water. Water Res. 2003;37:525–532. doi: 10.1016/s0043-1354(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology. 2001;147:3121–3126. doi: 10.1099/00221287-147-11-3121. [DOI] [PubMed] [Google Scholar]
- Newsome AL, Scott TM, Benson RF, Fields BS. Isolation of an amoeba naturally harboring a distinctive Legionella species. Appl Environ Microbiol. 1998;64:1688–1693. doi: 10.1128/aem.64.5.1688-1693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TMN, Ilef D, Jarraud S, Rouil L, Campese C, Che D, Haeghebaert S, Ganiayre FO, Marcel F, Etienne J, et al. A community-wide outbreak of Legionnaires’ disease linked to industrial cooling towers - how far can contaminated aerosols spread? J Infect Dis. 2006;193:102–111. doi: 10.1086/498575. [DOI] [PubMed] [Google Scholar]
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Orrison LH, Cherry WB, Tyndall RL, Fliermans CB, Gough SB, Lambert MA, McDougal LK, Bibb WF, Brenner DJ. Legionella oakridgensis - unusual new species isolated from cooling-tower water. Appl Environ Microbiol. 1983;45:536–545. doi: 10.1128/aem.45.2.536-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrauchan MA, Florizone C, Dosanjh M, Molm WW, Davies J, Eltis LD. Catabolism of benzoate and phthalate in Rhodococcus sp. strain RHA1: redundancies and convergence. J Bacteriol. 2005;187:4050–4063. doi: 10.1128/JB.187.12.4050-4063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razatos A, Ong YL, Sharma MM, Georgiou G. Molecular determinants of bacterial adhesion monitored by atomic force microscopy. Proc Natl Acad Sci USA. 1998;95:11059–11064. doi: 10.1073/pnas.95.19.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimmann C, Ginet N, Michel L, Keel C, Michaux P, Krishnapillai V, Zala M, Heurlier K, Triandafillu K, Harms H, et al. Genetically programmed auto-inducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology. 2002;148:923–932. doi: 10.1099/00221287-148-4-923. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Kim H, Frank JF, Beuchat LR. Attachment and biofilm formation on stainless steel by Escherichia coli O157: H7 as affected by curli production. Lett Appl Microbiol. 2004;39:359–362. doi: 10.1111/j.1472-765X.2004.01591.x. [DOI] [PubMed] [Google Scholar]
- Sabria M, Alvarez J, Dominguez A, Pedrol A, Sauca G, Salleras L, Lopez A, Garcia-Nunez MA, Parron I, Barrufet MP. A community outbreak of Legion-naires’ disease: evidence of a cooling tower as the source. Clin Microbiol Infect. 2006;12:642–647. doi: 10.1111/j.1469-0691.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- Salerno MB, Logan BE, Velegol D. Importance of molecular details in predicting bacterial adhesion to hydrophobic surfaces. Langmuir. 2004;20:10625–10629. doi: 10.1021/la048372z. [DOI] [PubMed] [Google Scholar]
- Simoes LC, Simoes M, Oliveira R, Vieira MJ. Potential of the adhesion of bacteria isolated from drinking water to materials. J Basic Microbiol. 2007;47:174–183. doi: 10.1002/jobm.200610224. [DOI] [PubMed] [Google Scholar]
- Steinert M, Hentschel U, Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol Rev. 2002;26:149–162. doi: 10.1111/j.1574-6976.2002.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Camper AK, Handran SD, Huang CT, Warnecke M. Spatial distribution and coexistence of Klebsiella pneumoniae and Pseudomonas aeruginosa in biofilms. Microb Ecol. 1997;33:2–10. doi: 10.1007/s002489900002. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Kathju S, Hu FZ, Erdos G, Levenson JE, Mehta N, Dice B, Johnson S, Hall-Stoodley L, Nistico L, et al. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res. 2005;437:31–40. doi: 10.1097/01.blo.0000175129.83084.d5. [DOI] [PubMed] [Google Scholar]
- Sturman PJ, Jones WL, Characklis WG. Interspecies competition in colonized porous pellets. Water Res. 1994;28:831–839. [Google Scholar]
- Tufenkji N, Elimelech M. Deviation from the classical colloid filtration theory in the presence of repulsive DLVO interactions. Langmuir. 2004;20:10818–10828. doi: 10.1021/la0486638. [DOI] [PubMed] [Google Scholar]
- Turetgen I. Comparison of the efficacy of free residual chlorine and monochloramine against biofilms in model and full scale cooling towers. Biofouling. 2004;20:81–85. doi: 10.1080/08927010410001710027. [DOI] [PubMed] [Google Scholar]
- Turetgen I, Cotuk A. Monitoring of biofilm-associated Legionella pneumophila on different substrata in model cooling tower system. Environ Monit Assess. 2007;125:271–279. doi: 10.1007/s10661-006-9519-8. [DOI] [PubMed] [Google Scholar]
- Vanhaecke E, Remon JP, Moors M, Raes F, Derudder D, Vanpeteghem A. Kinetics of Pseudomonas aeruginosa adhesion to 304 and 316-L stainless steel - role of cell surface hydrophobicity. Appl Environ Microbiol. 1990;56:788–795. doi: 10.1128/aem.56.3.788-795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanloosdrecht MCM, Lyklema J, Norde W, Schraa G, Zehnder AJB. The role of bacterial-cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Merode AEJ, van der Mei HC, Busscher HJ, Krom BP. Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis. J Bacteriol. 2006;188:2421–2426. doi: 10.1128/JB.188.7.2421-2426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeant MAS, Ford RM. Interactions between motile Escherichia coli and glass in media with various ionic strengths, as observed with a three-dimensional-tracking microscope. Appl Environ Microbiol. 1997;63:3474–3479. doi: 10.1128/aem.63.9.3474-3479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Health Organization; Emerging issues in water and infectious disease. 2003
- Wright JB, Ruseska I, Costerton JW. Decreased biocide susceptibility of adherent Legionella pneumophila. J Appl Bacteriol. 1991;71:531–538. doi: 10.1111/j.1365-2672.1991.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sugiura M, Kusunoki S, Ezaki T, Ikedo M, Yabuuchi E. Factors stimulating propagation of legionellae in cooling-tower water. Appl Environ Microbiol. 1992;58:1394–1397. doi: 10.1128/aem.58.4.1394-1397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, Heilig H, Klaassens ES, Booijink C, Kleerebezem M, Smidt H, de Vos WM. Isolation of DNA from bacterial samples of the human gastrointestinal tract. Nat Protoc. 2006;1:870–873. doi: 10.1038/nprot.2006.142. [DOI] [PubMed] [Google Scholar]