Abstract
Background and objectives: Oliguric, hypotensive patients who require large amounts of fluids may benefit from sustained low-efficiency dialysis performed continuously (C-SLED). C-SLED through higher clearance may improve survival, or through greater nutritional loss may worsen survival. No studies have assessed survival on C-SLED. The objective was to examine patient outcomes and survival predictors on C-SLED.
Design, setting, participants, & measurements: The data of 199 consecutive cancer patients treated with C-SLED were analyzed. The median duration of C-SLED was 50 h. With 48 h of C-SLED, the blood urea nitrogen (BUN) and serum creatinine levels had decreased by 80% and 73%, respectively. The mean arterial pressure (MAP) was maintained despite higher ultrafiltration and reduced vasopressor use. The 30-d mortality rate was 65%. Despite excellent dialysis, the sequential organ failure assessment (SOFA) score remained predictive of mortality. In the univariate model, higher SOFA scores and lower values for MAP, blood pH, and serum albumin and creatinine levels were associated with higher mortality. Administration of total parenteral nutrition (TPN) was, however, associated with lower mortality.
Results: In the multivariate model, the higher SOFA score and lower blood pH, MAP and C-SLED duration were associated with higher mortality. In a subset analysis of 129 patients who received C-SLED for at least 48 h, those with higher BUN levels, which were associated with higher TPN infusion, had a lower mortality risk.
Conclusion: This first detailed report on C-SLED indicates that C-SLED can be effective and suggests a link between nutrition and survival.
The incidence of acute renal failure (ARF) and the need for dialysis are increasing in intensive care units (ICUs), including cancer center ICUs (1,2). The University of Texas M. D. Anderson Cancer Center is a comprehensive cancer care center with its own nephrology service. Among the cancer patients admitted to our ICU, about 14% develop ARF (unpublished observation in 2006). Many are critically ill and hemodynamically unstable, requiring continuous infusions of large volumes of fluids.
Sustained (or slow) low-efficiency dialysis (SLED) benefits patients with ARF who are hemodynamically unstable (3,4). SLED is efficacious, cost effective, and easy to perform (5). Published reports indicate that SLED is performed either 8 h or 12 h per day usually for 5 or 6 d a week (5). At our institution, because of high rates of fluid administration in our critically ill patients, SLED once initiated is performed continuously (C-SLED), allowing the constant removal of fluid and solutes. Thus, C-SLED ends up providing two to three times more dialysis clearances than the 8 or 12 h SLED. Because of the higher clearances levels, C-SLED may potentially improve the patient outcomes; however, because of its higher associated electrolyte and nutritional losses, C-SLED may worsen the survival. To our knowledge, no studies have reported in detail on the clinical experience of C-SLED, and certainly none has examined on dialysis efficacy, patient outcomes or factors influencing the patient outcomes. We analyzed the data on 199 consecutive patients treated with C-SLED with the objective to provide detailed data on dialysis characteristics, survival and survival predictors.
Materials and Methods
Patients
After obtaining the Institutional Review Board approval, we generated and verified a list of 199 critically ill cancer patients who consecutively received C-SLED. The study period was from January 2006 through to June 2007 (18 mo). From our institution's electronic medical record system, we collected pertinent data on patient demographics, dialysis details, and patient outcomes. A sequential organ failure assessment (SOFA) score—which ranging from 0 to 24, with 24 being the highest severity score—on the day C-SLED began was calculated for each patient (6). The actual hours of C-SLED were calculated by subtracting any interruption time from the total time recorded. Data for patients who were withdrawn from life- sustaining support were also obtained. We gathered information from the patients' biochemical profiles for the first 48 h of C-SLED and obtained additional laboratory data for a group of patients who received C-SLED for more than a week.
C-SLED Procedures
Typically, C-SLED was initiated in hypotensive, oligoanuric patients with ARF who required pressors and large volumes of fluids. A standardized protocol based on a previously reported method for SLED was utilized to perform C-SLED, except that SLED was maintained continuously (4). C-SLED was initiated and discontinued by dialysis nurses, but was maintained by ICU nurses trained to oversee this procedure. C-SLED was performed with the use of Fresenius 2008H/K dialysis machines and Optiflux F16NR hemodialyzers (Fresenius Medical Care North America, Waltham, MA). The dialysate flow rate of 100 ml/min and blood flow rate of 200 ml/min were fixed; normal saline was infused continuously at 100 ml per hour in all patients from the start of C-SLED as prefilter, prepump infusion to minimize clotting of the extracorporeal circuit. The standard dialysate composition was 140 mEq/L of sodium, 4 mEq/L of potassium, and 30 mEq/L of bicarbonate. Their levels were adjusted as clinically warranted, especially when citrate anticoagulation was used. The dialysate temperature was maintained at 36.6 °C. Each dialysis machine had its own water purification system. Water entering the dialysis machine was purified by charcoal filtration, followed by deionization. Reverse osmosis was not used for water treatment. Samples of water from the deionizer and from the dialysis machine (mixed with dialysate) were tested monthly for bacterial counts and endotoxin levels. The results were reviewed by our Microbiology Department and the Director of Dialysis. The data are also reviewed at the quarterly Dialysis Quality Assurance meeting. The water quality was maintained consistently above the standards issued by the Association for the Advancement of Medical Instrumentation. Since the majority of patients were thrombocytopenic, dialyzer clotting was less frequent with prefilter saline infusion. Once clotted, patients were placed on citrate anticoagulation protocol and the concentrations of calcium, sodium and bicarbonate in the dialysate were adjusted. Since below-normal blood urea nitrogen (BUN) and serum creatinine levels were observed in a large percentage of patients receiving C-SLED, dialysis clearances were obtained on day two of C-SLED in four anuric patients. A dialysate flow rate of 100 ml/min and the mean of three blood and dialysate concentrations of BUN and creatinine obtained at 8-h intervals were used for the clearance calculation based on a standard formula, DV/P (where D is dialysate concentration of BUN and creatinine, P is the plasma concentration of BUN and creatinine, and V is the dialysate flow rate).
Statistical Analyses
Mean arterial pressure (MAP) and biochemical measurements in the blood were analyzed as time-dependent variables. In addition to fitting serum creatinine and BUN as time-dependent continuous variables in the Cox regression models, we also performed Cox regression modeling for BUN and serum creatinine as categorical variables. BUN and creatinine were categorized by tertiles or dichotomized by the lower range of their respective normal values, i.e., dichotomized as equal to or lower than versus greater than the lower limit of the normal range, which was 8 mg/dl for BUN and 0.8 mg/dl for serum creatinine. Using t test or χ2 method, parameters of patients who deceased were compared with parameters of patients who survived. The probability of overall survival was estimated by the Kaplan-Meier method, and the Cox proportional hazards models, fitted with time-dependent covariates, were used to identify predictors of overall survival. All the covariates were assessed for correlation. To assess the effect of dialysis on survival predictors, we also analyzed a subset of 129 patients who received C-SLED for at least 48 h. For all multivariate survival analyses, clinically relevant variables with a P value of ≤0.1 in the univariate analysis were fitted and the backward selection procedure was used for model selection. Additionally, multivariate analysis was repeated by including all the clinically relevant covariates (regardless of their significance in the univariate analysis) in a single step. Variables with a two-tailed P value of <0.05 were considered statically significant. SAS 9.1 software (SAS institute Inc., Cary, NC) was used for data analysis.
Results
Patient and Dialysis Characteristics
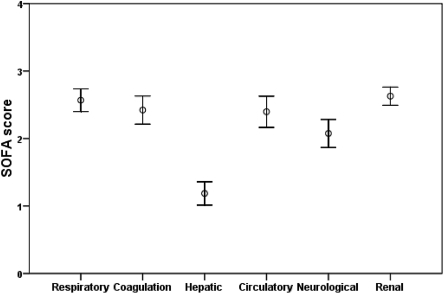
All the 199 consecutive patients, who had received C-SLED from January 2006 to June 2007, i.e.,18 mo, were included in the analysis. The demographic details along with cancer types and treatments are shown in Table 1. Hematologic malignancies were common (nearly 60%) in these patients, and most patients had received chemotherapy. Nearly one-fifth of the patients had received one or more stem cell transplants. The SOFA score for individual organs showed that multi-organ failures were common among these patients at the time of starting C-SLED (Figure 1). Sepsis was one of the clinical diagnoses in 54 patients (27%). Blood cultures had been drawn in 84% of patients while they were in ICU before C-SLED, and cultures were positive in 29% (Table 1). Many patients had been receiving potentially nephrotoxic drugs and many had multiple potential causes for AKI. Bladder catheterization had been performed as standard procedure and urinary obstruction had been routinely ruled out by ultrasonograhic examination. Clinical notes recorded by the consulting nephrologists showed that 63% of patients had ischemic AKI, including those with septic shock; 11% had nephrotoxic AKI; and 26% had multifactorial AKI, including patients with sepsis.
Table 1.
Details (% or mean ± SD) of 199 patients started on C-SLED
| Age (years) | 57 ± 16 |
| Sex (male %) | 68 |
| Race (%) | |
| White | 68 |
| Black | 11 |
| Hispanic | 11 |
| Asian | 9 |
| Weight (kg) | 87 ± 25 |
| Primary cancer (%) | |
| Leukemia | 38 |
| Lymphoma | 20 |
| Myeloma | 4 |
| Genitourinary | 14 |
| Gastrointestinal | 15 |
| Breast | 2.5 |
| Lung | 4 |
| Othera | 2.5 |
| Cancer therapyb (%) | |
| Chemotherapy | 86 |
| Stem cell transplantation | 18 |
| Surgery | 22 |
| Radiation | 20 |
| Other | 4 |
| Blood culturec (%) | |
| No growth | 71 |
| Gram positive | 14 |
| Gram negative | 10 |
| Gram positive and negative | 2 |
| Fungal | 3 |
| MAP (mmHg), pre–C-SLED | 75 ± 12 |
| SOFA score, pre–C-SLED | 13.0 ± 4.0 |
| Serum albumin (g/l), pre–C-SLED | 2.27 ± 0.72 |
| BUN (mg/dl), pre–C-SLED | 63 ± 30 |
| Serum creatinine (mg/dl), pre–C-SLED | 3.3 ± 1.7 |
BUN, blood urea nitrogen; C-SLED, continuous sustained low-efficiency dialysis; MAP, mean arterial pressure; SOFA, sequential organ failure assessment.
Others included gynecologic and neuroendocrine cancer and melanoma.
Therapies are often combined.
Based on blood cultures drawn in 84% patients while in intensive care units before C-SLED.
Figure 1.
Sequential organ failure assessment (SOFA) scores (mean ± 95% confidence interval) in 199 patients.
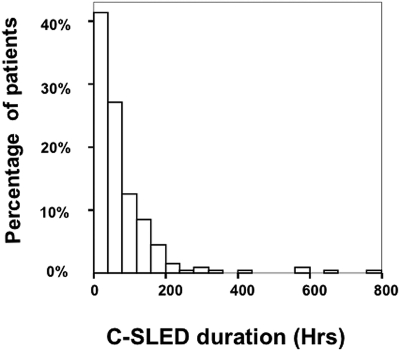
Figure 2 displays the frequency distribution of C-SLED duration corrected for C-SLED disruptions. The median duration of C-SLED was 50 h (range, 1 h to 32 d). Values for MAP, the vasopressor use and ultrafiltration rates are given in Table 2. Although study patients had had various types of cancers and had received various treatments (Table 1), all the patients had been extremely ill, and had had pressor-dependent hypotension, oligoanuria, and high obligatory fluid intake (Table 2). Our vasopressor-use was protocol-driven and vasopressors were always used as continuous infusions with a goal of maintaining the MAP above 65 to 70 mmHg. The vasopressors used in the order of their frequency were norepinephrine, vasopressin, phenylnephrine, dopamine, dobutamine and epinephrine.
Figure 2.
Frequency distribution of continuous sustained low-efficiency dialysis (C-SLED) duration in 199 patients.
Table 2.
MAP, vasopressor-use,a and UF rates in the first 48 h of C-SLED
| C-SLED Timing (h) | No. of Patients | MAP (mmHg)b | Pressors Used (%) |
UF Rate (ml/h)b |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Targeted | Achieved | |||
| 0 | 199 | 75 ± 12 | 25 | 34 | 23 | 13 | 5 | ||
| 0-12 | 188 | 78 ± 15 | 29 | 34 | 22 | 10 | 5 | 335 ± 140 | 360 ± 165e |
| 12-24 | 168 | 79 ± 13c | 36d | 32 | 20 | 7d | 5 | 424 ± 196 | 411 ± 169 |
| 24-48 | 137 | 78 ± 13 | 49d | 26d | 18d | 5d | 2d | 458 ± 143 | 432 ± 132e |
C-SLED, continuous sustained low-efficiency dialysis; MAP, mean arterial pressure; UF, ultrafiltration.
One or more vasopressors were used as continuous infusions.
Mean ± SD; MAP:
P < 0.05 versus 0 h; pressor:
P < 0.05 versus 0 h; UF:
P < 0.05 versus the respective targeted rate.
The mean values for MAP value before C-SLED were low, at 75 ± 12 mmHg (mean ± SD), with 75% of patients receiving at least one vasopressor. The MAP values were significantly higher after starting C-SLED, with fewer patients requiring vasopressors (Table 2). The range of average ultrafiltration obtained in the first 48 h of C-SLED was 360 to 432 ml/h (Table 2). C-SLED maintained fluid balance in most of the patients; for instance, the mean total fluid infused in the first 24 h of C-SLED was 10.97 ± 4.5 L (including saline infusion to reduce clotting), and the mean total fluid output, primarily through C-SLED, was 11.18 ± 4.52 L. The high obligatory fluid intake was to administer multiple pressors, antimicrobials, chemotherapeutic agents, multiple blood products, and nutritional supplements.
The mean platelet count in our patients at the time of starting C-SLED was low, at 36 ± 26 k/ul (normal 140 to 440 k/ul). All our patients received saline infusion prepump, but none received anticoagulation for dialysis at the start of C-SLED. In spite of saline infusion, 42% of patients developed at least one dialyzer-clotting in the first 48 h of C-SLED; these patients were started on citrate anticoagulation. Hypernatremia was noted in 17% of citrate-treated patients compared with 8% of noncitrate group (P < 0.05), and 25% of citrate-treated patients had hyperbicarbonatemia, compared with 6% of the noncitrate group (P < 0.05). These adverse effects, including variations in calcium levels, were correctable, and no patients had to discontinue citrate treatment.
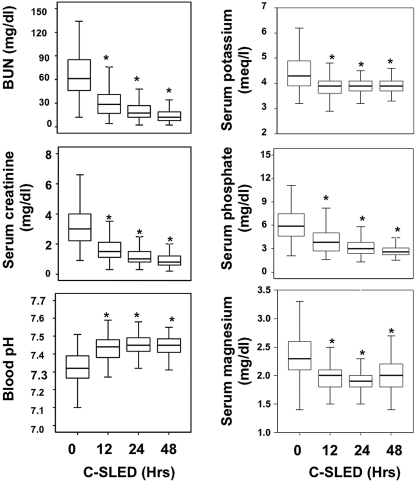
The mean values for various measurements taken before and during the first 48 h of C-SLED are displayed in Figure 3. BUN and serum creatinine values had decreased by 80% and 73%, respectively, at 48 h. In 23% of patients, BUN values were below the normal limit of normal of 8 mg/dl; in 46%, serum creatinine levels were below the normal limit of normal of 0.8 mg/dl. The mean dialysis clearances of BUN and creatinine measured in four anuric patients at 24 to 48 h after initiation of C-SLED were 76 ± 14 and 75 ± 12 ml/min, respectively. The mean blood pH in our study subjects increased steadily during C-SLED (Figure 3). Serum magnesium and serum phosphate levels decreased during C-SLED (Figure 3), and were infused daily at 3.2 ± 2 g and 25 ± 12 mmoles, respectively. We analyzed the serial measurements of serum BUN, creatinine, electrolytes, and divalent cations in 19 patients who continued receiving C-SLED for more than a week because of persistent ARF and hypotension; values shown in Figure 3 remained relatively stable throughout, with mean values within the normal range (data not shown).
Figure 3.
Box plots (median with interquartiles) for key biochemical measurements obtained during the first 48 h of continuous sustained low-efficiency dialysis (C-SLED). Error bars indicate confidence intervals. *P < 0.05 versus 0 h.
Survival Analyses for All Patients
By day 30 of C-SLED, 130 of the 199 patients died, yielding a 30-d mortality rate of 65%. Twenty-two patients (11%) had died within 24 h of starting C-SLED. Of the 130 patients who died within 30 d of starting C-SELD, 76 had been withdrawn eventually from life support because of the irreversible nature of their illnesses. The median survival time of all patients was 15 d (95% confidence interval [CI], 11 to 21 d) from the start of C-SLED. Among patients who survived 30 d, 65% had experienced renal recovery from ARF, defined as not requiring further dialysis support.
We compared pre–C-SLED characteristics of patients who were alive 30 d after starting C-SLED with pre–C-SLED characteristics of those who died during this period (Table 3). Sex, age, and body weight were not significantly different between the groups, whereas hematologic malignancies were more frequent in the patients who died within 30 d (64% versus 52%; P = 0.001). The mean SOFA score at the start of C-SLED was significantly higher in patients who died within 30 d (Table 3). Fewer patients who received total parenteral nutrition (TPN) died in 30 d compared with patients who did not receive TPN in the first 48-h (50% versus 68%, P < 0.05). Although pre–C-SLED MAPs were not significantly different between the groups, MAPs at 48 h were significantly higher in patients who survived 30 d (82 ± 14 versus 75 ± 14 mmHg; P = 0.008). Also, the levels of serum potassium, magnesium, and phosphate and the blood pH value before C-SLED and after 48 h of C-SLED were not significantly different between groups (data not provided).
Table 3.
Measurements (mean ± SD or percent) in patients who survived or died during the 30 days after starting C-SLED
| Alive (69) | Deceased (130) | P valuea | |
|---|---|---|---|
| Sex (male, %) | 65 | 69 | 0.43 |
| Age (years) | 58 ± 18 | 56 ± 15 | 0.20 |
| Weight (kg) | 88 ± 21 | 86 ± 22 | 0.47 |
| MAP (mmHg), pre–C-SLED | 76 ± 12 | 75 ± 13 | 0.19 |
| SOFA score, pre–C-SLED | 11.6 ± 4.1 | 14.2 ± 3.6 | 0.0001 |
| Serum albumin (g/l), pre–C-SLED | 2.47 ± 0.80 | 2.19 ± 0.70 | 0.043 |
| BUN (mg/dl), pre–C-SLED | 61 ± 30 | 64 ± 31 | 0.53 |
| Serum creatinine (mg/dl), pre–C-SLED | 3.9 ± 2.2 | 3.0 ± 1.3 | 0.006 |
BUN, blood urea nitrogen; C-SLED, continuous sustained low-efficiency dialysis; MAP, mean arterial pressure; SOFA, sequential organ failure assessment.
Comparison between alive and deceased groups.
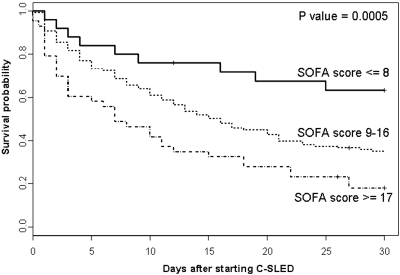
As displayed in Figure 4, higher SOFA score tertiles were associated with a stepwise increase in overall mortality. Also, a higher SOFA score in the Cox regression model was associated with a significantly higher mortality risk (Table 4). In contrast, higher levels of serum creatinine, serum albumin, blood pH, and MAP were associated with significantly lower mortality risk (Table 4). The use of TPN at any time within the first 48 h of C-SLED was marginally associated with lower mortality risk (hazard ratio [HR] = 0.62; P = 0.07).
Figure 4.
Probability of 30-d survival in 199 patients treated with continuous sustained low-efficiency dialysis (C-SLED) as a function of sequential organ failure assessment (SOFA) score tertiles.
Table 4.
Cox proportional hazards models in 199 patients who received C-SLED
| Variable | No. of Patients | HR (95% CI) | P Value |
|---|---|---|---|
| Univariate | |||
| Age | 199 | 0.999 (0.988–1.009) | 0.83 |
| Sex (female vs. male) | 199 | 0.84 (0.58–1.22) | 0.37 |
| Primary cancer (solid vs. hematologic) | 199 | 0.83 (0.58–1.18) | 0.29 |
| BMI | 198 | 0.98 (0.96–1.007) | 0.18 |
| C-SLED duration | 199 | 0.998 (0.996–1) | 0.07 |
| SOFA score | 199 | 1.12 (1.07–1.18) | 0.0001 |
| BUNa | 199 | 0.997 (0.98–1.01) | 0.65 |
| Serum creatininea | 199 | 0.8 (0.66–0.98) | 0.03 |
| Serum albumina | 199 | 0.68 (0.51–0.92) | 0.01 |
| Serum magnesiuma | 199 | 1.31 (0.76–2.24) | 0.33 |
| Serum phosphatea | 199 | 1.20 (1.08–1.33) | 0.0005 |
| Serum potassiuma | 199 | 2.05 (1.48–2.84) | 0.0001 |
| Serum ionized calciuma | 199 | 1 (0.81–1.24) | 1.00 |
| Blood pHa | 199 | 0.001 (0–0.007) | 0.0001 |
| MAPa | 199 | 0.97 (0.96–0.98) | 0.0001 |
| BUN at start of C-SLED | 196 | 1.002 (0.996–1.007) | 0.53 |
| Serum creatinine at start of C-SLED | 197 | 0.83 (0.73–0.94) | 0.004 |
| Serum albumin at start of C-SLED | 167 | 0.75 (0.58–0.98) | 0.035 |
| TPN anytime during the first 48 h (yes vs. no) | 199 | 0.62 (0.36–1.06) | 0.07 |
| Multivariate | |||
| Blood pHa | 199 | 0.005 (0.001–0.04) | <0.0001 |
| MAPa | 199 | 0.98 (0.96–0.99) | 0.0002 |
| SOFA Score | 199 | 1.12 (1.07–1.19) | <0.0001 |
| Duration of SLED | 199 | 0.997 (0.995–0.999) | 0.004 |
BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; C-SLED, continuous sustained low-efficiency dialysis; HR, hazard ratio; MAP, mean arterial pressure; SOFA, sequential organ failure assessment; TPN, total parenteral nutrition.
Indicates fitted as time-dependent variables.
In the multivariate analysis, higher SOFA scores and lower duration of SLED, lower blood pH, and lower MAP were significant predictors of higher overall mortality (Table 4).
The multivariate analysis in Table 4 repeated with including all the physiologic relevant variables (age, gender, BMI, and cancer type), regardless of statistical significance in the univariate analysis, still provided similar results as in Table 4, except age had turned out to be a significant predictor of survival (HR 0.99; 95% CI: 0.97 to 0.99; P < 0.026). Again, although we had included serum phosphorous and potassium in the model, repeat analysis excluding them from the model had no effect on the model prediction (data not shown).
Survival Analyses for the Subset of Patients Who Received C-SLED for At Least 48 h
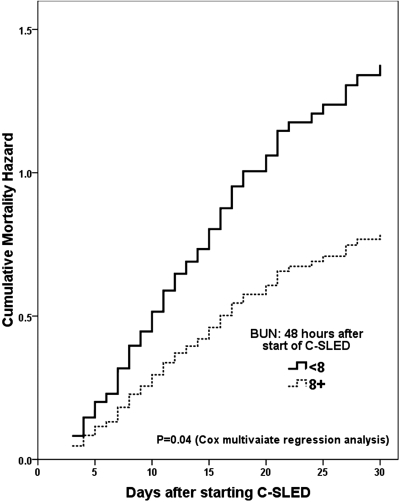
We also analyzed the survival rates of a subset of 129 patients who received C-SLED for at least 48 h to determine the effect of dialysis on survival factors. In the univariate Cox regression model, despite the fact that patients received excellent dialysis therapy, the higher SOFA scores remained closely correlated with higher mortality risk (Table 5). Lower 48-h MAP and blood pH levels were also associated with higher mortality risk. In contrast, patients with higher BUN levels had a significantly lower mortality risk than did patients with very low BUN levels (HR: 0.57; 95% CI, 0.34 to 0.94; P = 0.03). In the multivariate analysis of patients who received at least 48 h of C-SLED, lower BUN levels, along with the higher SOFA scores and lower 48-h MAP values, were a significant and independent predictor of higher mortality (displayed graphically in Figure 5). The multivariate analysis in Table 5 repeated with including all the physiologic relevant variables (age, gender, BMI, and cancer type), regardless of significance, provided similar results as in Table 5 (data not shown).
Table 5.
Cox proportional hazards models in a subset of 129 patients who received C-SLED for at least 48 h
| Variable | No. of Patients | HR (95% CI) | P Value |
|---|---|---|---|
| Univariate | |||
| Age | 129 | 0.99 (0.99–1.01) | 0.91 |
| Gender (female vs. male) | 129 | 0.86 (0.54–1.39) | 0.15 |
| Primary cancer (solid vs. hematological) | 129 | 0.82 (0.56–1.22) | 0.54 |
| SOFA score | 129 | 1.10 (1.03–1.17) | 0.003 |
| BMI | 129 | 0.97 (0.94–1.01) | 0.09 |
| TPN (yes vs. no) | 129 | 0.61 (0.31–1.23) | 0.17 |
| BUN at 48 h | 129 | 0.99 (0.99–1.02) | 0.57 |
| BUN at 48 h (≥8 vs. <8 mg/dl) | 129 | 0.57 (0.34–0.94) | 0.03 |
| BUN at 48 h (<9 mg/dl) | 28 | 1.00 (Ref) | |
| BUN at 48 h (≥ 9 & <16 mg/dl) | 45 | 0.55 (0.31, 0.99) | 0.045 |
| BUN at 48 (≥16 mg/dl) | 46 | 0.78 (0.46, 1.34) | 0.37 |
| Serum creatinine at 48 h | 129 | 0.73 (0.49–1.09) | 0.13 |
| Serum albumin at 48 h | 129 | 0.70 (0.17–2.86) | 0.62 |
| Serum magnesium at 48 h | 126 | 0.86 (0.40–1.86) | 0.78 |
| Serum phosphate at 48 h | 126 | 1.03 (0.83–1.29) | 0.78 |
| Serum potassium at 48 h | 129 | 0.85 (0.42–1.74) | 0.65 |
| Serum ionized calcium at 48 h | 125 | 1.28 (0.99–1.64) | 0.06 |
| Blood pH at 48 h | 126 | 0.004 (0–0.17) | 0.004 |
| MAP at 48 h | 129 | 0.97 (0.95–0.99) | 0.0005 |
| C-SLED duration | 129 | 0.99 (0.99–1.001) | 0.43 |
| Multivariate | |||
| SOFA score | 129 | 1.10 (1.03–1.18) | 0.003 |
| MAP at 48 h | 129 | 0.98 (0.96–0.997) | 0.02 |
| BUN at 48 (≥8 vs. <8 mg/dl)a | 129 | 0.57 (0.33–0.98) | 0.04 |
BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; C-SLED, continuous sustained low-efficiency dialysis; HR, hazard ratio; MAP, mean arterial pressure; SOFA, sequential organ failure assessment; TPN, total parenteral nutrition.
See Figure 4.
Figure 5.
Cumulative mortality risk in patients with 48-h blood urea nitrogen (BUN) levels of ≥8 mg/dl or <8 mg/dl. The lower limit of normal value for BUN is 8 mg/dl. This graph is based on the multivariate Cox regression model of a subset of 129 patients who received continuous sustained low-efficiency dialysis (C-SLED) for at least 48 h (Table 5).
Characteristics of Patients According to 48-h BUN Levels
We grouped patients according to 48-h BUN levels (lower BUN, <8 mg/dl; higher BUN, ≥8 mg/dl; the lower limit of normal for BUN is 8 mg/dl) and compared their survival-related measurements (Table 6). Body weight was significantly lower in the lower-BUN group, whereas initial serum albumin and serum creatinine levels were not significantly different between the two groups. However, TPN infusion rates both before and during C-SLED were significantly higher in the higher-BUN group (Table 6).
Table 6.
Measurements (mean ± SD or percent) in patients with 48-h BUN levels <8 mg/dl or ≥8 mg/dl
| BUN mg/dl |
P Value | ||
|---|---|---|---|
| <8 mg/dl (30) | ≥8 mg/dl (99) | ||
| Age (years) | 55 ± 16 (30) | 57 ± 16 (99) | 0.60 |
| Weight (kg) | 76 ± 21 (30) | 90 ± 22 (99) | 0.001 |
| Serum albumin, pre–C-SLED | 2.40 ± 0.80 (28) | 2.24 ± 0.70 (81) | 0.10 |
| SOFA score | 12.6 ± 4.1 (30) | 13.2 ± 3.7 (99) | 0.44 |
| Mortality (%) | 70 (30) | 55 (99) | 0.028 |
| BUN (mg/dl), pre–C-SLED | 46 ± 18 (30) | 73 ± 33 (99) | 0.001 |
| BUN (mg/dl), 24-h C-SLED | 9 ± 5 (28) | 23 ± 12 (97) | 0.002 |
| BUN (mg/dl), 48-h C-SLED | 4.5 ± 1.8 (30) | 17.0 ± 9.8 (99) | 0.0001 |
| TPN (%), pre–C-SLED | 3 (30) | 9 (99) | 0.001 |
| TPN (%), 24-h C-SLED | 4 (30) | 14 (99) | 0.001 |
| TPN (%), 48-h C-SLED | 7 (30) | 19 (99) | 0.0001 |
| Serum creatinine (mg/dl), pre–C-SLED | 3.25 ± 1.24 (30) | 3.32 ± 1.80 (99) | 0.86 |
| Serum creatinine (mg/dl), 48-h C-SLED | 0.78 ± 0.36 (30) | 1.06 ± 0.83 (98) | 0.09 |
BUN, blood urea nitrogen; C-SLED, continuous sustained low-efficiency dialysis; SOFA, sequential organ failure assessment; TPN, total parenteral nutrition. The lower limit of normal value for BUN is 8 mg/dl.
Discussion
With use of SLED in the continuous mode, we were able to provide continuous solute and fluid clearances in critically ill patients using SLED. Our analyses also yielded the unexpected and interesting finding that nutrition-related parameters may be important to survival in critically ill patients receiving continuous dialysis.
Like other Continuous Renal Replacement Therapies (CRRTs), C-SLED provides continuous dialysis but with the added advantages of SLED, such as ease of use, significant cost savings, and better clearances (5,7,8). Moreover, C-SLED, by providing dialysis continuously, provides twice the clearance of SLED (9), which we confirmed in this study by clearance measurements. Consistent with high clearance were the marked decreases in BUN and serum creatinine levels in our patients receiving C-SLED.
Our study provided the most comprehensive clinical experience with SLED therapy and the largest number of patients in a SLED study to date (5,7,8). Our patients had had various types of cancer and had received various cancer therapies. However, from the perspectives of dialysis and critical care, the population was homogenous in terms of ATN, oligoanuria, vasopressor-dependent hypotension, multi-organ failure, and high obligatory fluid intake.
Although ARF and dialysis are becoming increasingly common among cancer patients admitted to ICUs, few studies have described the types or outcomes of dialysis administered in this population. A few studies have indicated that ARF, especially when dialysis is required, is associated with very high mortality, but these were limited to cancer patients undergoing stem cell transplantation (10,11). Our study, therefore, provides some of the information that was lacking on the demographics of critically ill cancer patients who develop ARF. Our data also show that sepsis is frequent in immunocompromised patients, as would be expected, and that ARF in these patients is often multifactorial.
Our finding that severity of illness factors such as multi-organ failure, hypotension or acidosis was a strong and independent predictor of survival on CRRT is not new or unexpected (12). Our finding that extremely low BUN level in patients receiving C-SLED may be a predictor of higher mortality risk is, however, new and of interest. The higher-BUN group in our study had consistently received higher rates of TPN infusions before and during the first 48 h of C-SLED (Table 6), which could have contributed to the higher BUN values. The lower-BUN group had significantly lower body weights. A higher dialysis clearance in the low-BUN group merely due to lower body weight could not have accounted for the very low BUN level because the mean 48-h serum creatinine levels were not significantly different between the two groups (Table 6). Moreover, although the percentage reduction in BUN was greater in the lower-BUN group, such a calculation does not take into account the possible increase in the generation of BUN in the higher-BUN group receiving a higher rate of TPN. Thus, the very low BUN levels after C-SLED initiation could have been due to higher C-SLED urea clearance along with inadequate protein intake. Inadequate protein intake in these critically ill patients receiving dialysis can readily lead to a negative nitrogen balance due to hypercatabolism of protein and excessive loss of amino acids during dialysis. The possibility of poor nutrition contributing to a higher risk of mortality in our patients is suggested by our findings that lower levels of other nutritional markers, such as serum albumin and creatinine, were also associated with a higher risk of mortality and that administration of TPN appeared to be associated with better survival rates.
Protein hypercatabolism driven by stress and inflammation is a hallmark finding in critically ill patients (13). A similar finding of deranged protein metabolism is also well recognized in patients with ARF, especially in those receiving dialysis therapy (14). Among the various types of dialysis therapy, CRRT engenders a greater loss of amino acids (14–16). Free amino acids have very low molecular weights, averaging 140 Daltons, and as a result, they have a sieving coefficient of 1, allowing them to flow freely through the dialysis membranes. The estimated loss of amino acids during dialysis varies widely, ranging from 5% to 21% of daily amino acid intake or 5 to 15 g/d in the dialysate (14). Since C-SLED provides two to three times the clearance of other CRRTs, the daily loss of amino acids during C-SLED can be substantially higher. Little is known about amino acid loss in either Intermittent-SLED (I-SLED) or C-SLED.
Of note is that significantly fewer patients who received TPN died in 30 d compared with patients who did not receive TPN in the first 48 h. On the basis of this finding, we hypothesize that to realize the survival potential of increased dialysis during CRRT, nutritional supplementation should be increased in tandem with the initiation of C-SLED. A recent prospective dialysis trial in patients with ATN (ATN trial) has challenged the earlier finding that higher CRRT clearance may be associated with better survival (17,18). The highest dialysis clearance studied in the ATN trial was 35 ml/kg/h. Whether C-SLED, which can nearly double the highest clearance studied in the ATN trial, combined with adequate nutrition, can improve the survival of critically ill patients with ARF is an open question. A study to compare I-SLED (12 h) with C-SLED (24 h) after adjusting for nutrition loss and ultrafiltration would be of interest to address whether solute clearance per se is critical for survival improvement.
There were some limitations to our study. Because this was a retrospective observational study, unintended bias in patient selection cannot be ruled out. However, to minimize bias, we included all the patients for the specified period. Moreover, our sample size was large relative to other SLED studies; the study period was recent and limited to 18 mo. Moreover, C-SLED in this study was performed according to a standardized protocol. Another limitation was that because the study was intended to assess C-SLED feasibility and patient outcome, the nutritional data that we could gather were limited.
In summary, our study showed that C-SLED can provide CRRT with excellent metabolic and fluid clearances in patients who are hemodynamically unstable and have high obligatory fluid intake. Our data suggest, perhaps for the first time, that very low BUN levels in patients receiving C-SLED may be a surrogate marker for negative nitrogen balance and unmet protein needs. Moreover, considering that TPN was associated with higher BUN levels that were significantly linked to lower mortality in our population, more aggressive and early nutritional support may be important to survival in patients receiving continuous dialysis. Future studies are therefore warranted to assess the loss of amino acids, vitamins, and other essential elements during continuous dialysis and the effects of nutritional supplementation on patient survival rates.
Disclosures
None.
Acknowledgments
We acknowledge the excellent support of the dialysis and intensive care unit nurses at The University of Texas M. D. Anderson Cancer Center and Ms. Barbara Liddle in preparing this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Hoste EA, Schurgers M: Epidemiology of acute kidney injury: How big is the problem? Crit Care Med 36: S146–S151, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Soares M, Salluh JI, Carvalho MS, Darmon M, Rocco JR, Spector N: Prognosis of critically ill patients with cancer and acute renal dysfunction. J Clin Oncol 24: 4003–4010, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Schlaeper C, Amerling R, Manns M, Levin NW: High clearance continuous renal replacement therapy with a modified dialysis machine. Kidney Int Suppl 72: S20–S23, 1999 [PubMed] [Google Scholar]
- 4.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK: Sustained low-efficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int 60: 777–785, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Tolwani AJ, Wheeler TS, Wille KM: Sustained low-efficiency dialysis. Contrib Nephrol 156: 320–324, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, De Mendonca A, Cantraine F: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26: 1793–1800, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Berbece AN, Richardson RM: Sustained low-efficiency dialysis in th ICU: Cost, anticoagulation, and solute removal. Kidney Int 70: 963–968, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Marshall MR, Ma T, Galler D, Rankin AP, Williams AB: Sustained low-efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: Towards an adequate therapy. Nephrol Dial Transplant 19: 877–884, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK: Urea kinetics during sustained low-efficiency dialysis in critically ill patients requiring renal replacement therapy. Am J Kidney Dis 39: 556–570, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Zager RA, O'Quigley J, Zager BK: Acute renal failure following bone marrow transplantation: A retrospective study of 272 patients. Am J Kidney Dis 13: 210–216, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Parikh CR, McSweeney P, Schrier RW: Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int 67: 1999–2005, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Wendon J, Smithies M, Sheppard M, Bullen K, Tinker J, Bihari D: Continuous high volume venous-venous haemofiltration in acute renal failure. Intensive Care Med 15: 358–363, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Powell-Tuck J: Nutritional interventions in critical illness. Proc Nutr Soc 66: 16–24, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Btaiche IF, Mohammad RA, Alaniz C, Mueller BA: Amino acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy 28: 600–613, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Ikizler TA, Flakoll PJ, Parker RA, Hakim RM: Amino acid and albumin losses during hemodialysis. Kidney Int 46: 830–837, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Martin H, Parkin G, Love J, Kearley Y, Boyce N: Continuous arteriovenous haemodiafiltration in the critically ill: Influence on major nutrient balances. Intensive Care Med 17: 399–402, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Palevsky PM, Zhang JH, O'Connor TZ: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronco C, Bellomo R, Homel P: Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: A prospective randomised trial. Lancet 356: 26–30, 2000 [DOI] [PubMed] [Google Scholar]