Abstract
Background and objectives: Atypical hemolytic uremic syndrome (aHUS) is associated with a congenital or acquired dysregulation of the complement alternative pathway that leads to continuous complement activation on host cells causing inflammation and damage. Eculizumab, a humanized mAb against complement protein C5, inhibits activation of the terminal complement pathway.
Design, setting, participants, & measurements: We report an adolescent with relapsing unclassified aHUS. On admission, a high plasma creatinine level indicated a poor prognosis, and hemodialysis had to be started. Plasma exchanges were initially effective against the microangiopathic hemolytic activity and allowed a temporary improvement of renal function with termination of hemodialysis after 7 wk. Subsequently, plasma exchanges (three times per week) failed to prevent ongoing aHUS activity and progressive renal failure. After 12 wk, aHUS treatment was switched to eculizumab.
Results: Eculizumab was effective in terminating the microangiopathic hemolytic process in two aHUS relapses; however, after normalization of complement activity, aHUS recurred and ultimately led to anuric end-stage renal failure.
Conclusions: In this patient, complement inhibition by eculizumab temporarily terminated the microangiopathic hemolytic activity. Nevertheless, renal damage as a result of preceding and subsequent aHUS activity resulted in end-stage renal failure; therefore, therapeutic success may depend on early administration of eculizumab. The optimal duration of treatment may be variable and remains to be determined.
Hemolytic uremic syndrome (HUS) is a clinical triad of Coombs-negative microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure (1). In children, HUS is most commonly triggered by Shiga-like toxin (Stx)-producing bacteria (2). Approximately 10% of HUS cases are Stx negative (2). These atypical forms (aHUS) may occur sporadically or within families, are often recurrent, and generally have a poor outcome (2). After renal transplantation, there is a high risk for graft loss for aHUS recurrence or thrombosis (1).
aHUS is associated with an impairment of the complement alternative pathway regulation leading to deficient host cell protection and inappropriate complement activation on platelets and endothelial cells, particularly in the kidneys (3,4). Approximately 50% of patients with aHUS have mutations in one of the complement regulatory proteins: Factor H (CFH), factor I (CFI), or membrane co-factor protein (MCP) (3–5). More recently, mutations in factor B (CFB) and C3 have been associated with aHUS (6,7). The frequencies of homozygous deletions of CFH-related genes CFHR1/CFHR3 and of polymorphisms in CFH, MCP, and C4-binding protein are increased (8–10). Patients who have aHUS with combined mutations have been reported (4). Approximately 10% of children with aHUS have an acquired functional CFH deficiency caused by anti-CFH autoantibodies, frequently associated with absent CFHR1/CFHR3 (11–13). In mutation carriers, aHUS penetrance is approximately 50%, suggesting that other genetic or environmental modifiers are needed for disease expression (3). Identification of mutations or autoantibodies is important owing to differences in renal survival, outcome of renal transplantation, and mortality (14,15).
Current treatment of aHUS relies on plasma therapy with variable success (16). In anti-CFH autoantibody–positive patients, add-on immunosuppression may be reasonable (11). In CFH mutation carriers, liver-kidney transplantations have occasionally been performed (17). aHUS is characterized by an impaired complement regulation. Thus, treatment targeting at the common terminal pathway of complement activation seems to be reasonable. Eculizumab (Soliris; Alexion Pharmaceuticals, Cheshire, CT) is a humanized mAb against complement protein C5 that inhibits the generation of the proinflammatory peptide C5a and the formation of the membrane complement complex C5b-9 (18,19). Beneficial effects of eculizumab treatment have already been demonstrated in patients with paroxysmal nocturnal hemoglobinuria (20). Recently, eculizumab was reported to be effective also in aHUS (21,22). Remissions of aHUS were achieved in a case of congenital aHUS and in an adult with posttransplantation recurrence (21,22). In the adult patient, a single dose of eculizumab was effective to maintain a remission for 8 mo (22). We here describe the effect of eculizumab in the treatment of an adolescent with aHUS. In contrast to the patient reported by Nürnberger et al. (22), the effect of eculizumab was only transient and aHUS relapses occurred early after recovery of complement hemolytic activity.
Materials and Methods
Case Report
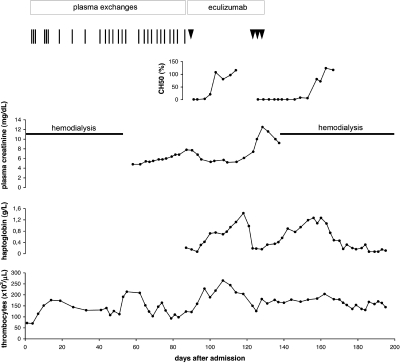
A 17.8-yr-old previously healthy boy presented with progressive weakness, calf pain, weight gain (5 kg), and generalized edema. On admission, he was alert and pale. There was no history of a preceding infection, diarrhea, or drug intake. BP was 205/120 mmHg. Blood chemistry showed Coombs-negative hemolytic anemia (hemoglobin level 5.5 g/dl) with red blood cell fragmentation and thrombocytopenia (72 × 103/μl). Serum haptoglobin was <0.07 g/L (normal 0.3 to 2.0 g/L). Plasma creatinine (PCr) was 22.4 mg/dl (normal 0.6 to 1.3 mg/dl). Diuresis was 220 ml/d, with microhematuria and proteinuria (4.3 g/L). Renal ultrasound showed enlarged kidneys with reduced perfusion. Hemodialysis was started immediately after admission. The diagnosis of sporadic, Stx-negative HUS was established. ADAMTS13 antigenic level and activity were 53 and 20%, respectively. No autoantibodies against ADAMTS13 were detected. Antinuclear factors and anticardiolipin antibodies were negative. Complement analyses indicated complement alternative pathway activation (see the Results section). Plasma exchanges (80 ml/kg per session) were started on day 3, with normalization of platelet counts (Figure 1). Urine output gradually increased; however, tapering of plasma exchanges repeatedly led to relapses (Figure 1). Hemodialysis could be terminated after 7 wk (steady-state PCr 4.8 mg/dl). Thereafter, despite continued plasma exchanges thrice weekly, microangiopathic hemolytic activity persisted and renal function deteriorated again (PCr 7.8 mg/dl). After signed informed consent and vaccination against Neisseria meningitidis (Mencevax [GlaxoSmithKline, Rixemstraat, Belgium] and NeisVac-C [Baxter Bioscience, Vienna, Austria]), a single dose of eculizumab (600 mg, using a 40-min intravenous infusion) was administered, resulting in normalization of platelet counts within 3 d and haptoglobin levels within 5 d. Subsequently, renal function improved again (PCr 5.2 mg/dl). Approximately 2 wk after recovery of complement hemolytic activity (CH50), aHUS relapsed and PCr increased to 12.5 mg/dl (Figure 1). Repetitive doses of eculizumab (3 × 600 mg within 6 d) again led to a normalization of haptoglobin levels within 6 d. Only minor renal recovery (PCr 9.2 mg/dl) ensued and severe hypervolemic hypertension required hemodialysis again. The platelet counts (normalization within 5 d) increased only moderately, probably related to superimposed hypertensive nephropathy. In view of end-stage renal failure, eculizumab was discontinued. A subsequent aHUS relapse led to anuria.
Figure 1.
Clinical course and laboratory findings. Vertical bar indicates plasma exchange, arrowhead indicates eculizumab infusion, and horizontal bar indicates hemodialysis.
Complement Analyses
EDTA-plasma and serum samples were sequentially taken for complement analyses and stored at −70°C until assayed. Functional activity of classical (CH50) and alternative (APH50) complement pathway was measured by hemolytic titration according to the described procedures (23,24). Plasma C4 and C3 concentrations were determined by nephelometry. C3dg/C3d was measured by double-decker rocket immunoelectrophoresis (25), using rabbit anti-C3c antibodies in the lower gel and rabbit anti-C3d antibodies (Dako, Hamburg, Germany) in the upper gel. SC5b-9 ELISA was performed as described previously (26). Plasma concentrations of CFH and CFI were assessed by ELISA using polyclonal goat anti-human factor H IgG or monoclonal anti-human factor I IgG (Genzyme, Boston, MA), respectively, as capture antibodies. Bound regulator molecules were detected by rabbit anti-H IgG (ICN Biochemical, Eschwege, Germany) or goat anti-I IgG (ICN), respectively. The reactions were visualized by the appropriate peroxidase-conjugated third antibodies, using 2,2′-azino-bis-3-ethyl-benzothiazoline sulfonate as substrate. Optical density was measured at λ = 405 nm on an EAR 340 AT Microplate Reader (SLT; Calbiochem, Bad Soden, Germany). Purified CFH and CFI (Quidel, San Diego, CA) were taken as standards. Presence and mobility of CFH and CFHR1 were determined by Western blot analysis as described previously (8). Plasma was analyzed for anti-CFH autoantibodies by ELISA (12). Expression of MCP on peripheral blood mononuclear cells was assessed by incubation with a FITC-conjugated mouse anti-human mAb (FITC Mouse Anti-Human CD46; BD Biosciences Pharmingen, San Diego, CA) and analysis by FACSCalibur (BD Biosciences, Franklin Lakes, NJ).
Genetic Analyses
All exons of the complement regulatory genes were sequenced as described previously (7,27–29). The primer sequences for CFH, CFI, MCP, CFB, C3, and C4BP gene screening are available from the authors on request. We used the multiplex ligation-dependent probe amplification reaction for screening the CFHR1 gene using a specific CFHR1 probe located in exon 2 of this gene (MRC-Holland, Amsterdam, Netherlands). All 29 exons and flanking intron sequences of the ADAMTS13 gene were sequenced by standard procedures on an ABI Prism 3130 (AME Bioscience, Toroed, Norway). Sequence data were compared with genome database entries from GenBank (http://www.ncbi.nlm.nih.gov).
Results
Complement Analyses
On admission, plasma complement analyses showed normal C4 but decreased C3 (0.62 g/L; normal 0.89 to 1.87 g/L) and moderate increases of C3d (43 mU/L; normal <40 mU/L) and SC5b-9 (518 ng/ml; normal <320 ng/ml). CH50, APH50, plasma levels of CFH and CFI, and Western blot analyses of CFH and CFHR1 were normal. No anti-CFH autoantibodies were detected. MCP was normally expressed on peripheral blood mononuclear cells.
At distance from plasma exchanges, normal C3 levels were obtained as well. During aHUS relapses, C3 was variably decreased or low normal (data not shown).
Upon eculizumab administration, complement hemolytic activity (CH50 and APH50) became undetectable, indicating effective inhibition of the terminal complement sequence (Tables 1 and 2, Figure 1). After the first administration, C3 levels transiently normalized and SC5b-9 concentrations decreased (Table 1). After the second administration, C3 levels dropped, and SC5b-9 concentrations showed a delayed increase and remained high throughout the observation period (Table 2).
Table 1.
Results of complement analyses after eculizumab (1 × 600 mg)a
| Day | APH50 (%) | C3 (mg/ml) | C3d (mU/L) | SC5b-9 (ng/ml) |
|---|---|---|---|---|
| 1 | <5 | 0.70 | 48 | 634 |
| 3 | nt | 0.83 | 37 | 728 |
| 7 | 49 | 1.19 | 19 | 575 |
| 10 | nt | 1.00 | 58 | 392 |
| 13 | 134 | 1.47 | 56 | 360 |
| 17 | nt | 1.09 | 49 | 369 |
| 21 | 114 | 0.91 | 49 | 309 |
| 24 | nt | 0.76 | 56 | 256 |
nt, not tested.
Table 2.
Results of complement analyses after eculizumab (3 × 600 mg within 6 d)a
| Day | APH50 (%) | C3 (mg/ml) | C3d (mU/L) | SC5b-9 (ng/ml) |
|---|---|---|---|---|
| 3 | <5 | 1.04 | 40 | 1524 |
| 6 | nt | 0.93 | 35 | 1450 |
| 9 | <5 | 0.80 | 43 | 1348 |
| 12 | nt | 0.77 | 45 | 1093 |
| 14 | nt | 0.73 | 45 | 1774 |
| 16 | <5 | 0.87 | 37 | 2149 |
| 19 | nt | 0.78 | 17 | 1930 |
| 23 | 39 | 0.74 | 55 | 1676 |
| 26 | nt | 0.69 | 44 | 1894 |
| 30 | 47 | 0.70 | 46 | 2207 |
| 35 | nt | 0.87 | 44 | 1772 |
| 37 | 124 | 0.82 | 48 | 1809 |
| 40 | nt | 0.90 | 52 | 1896 |
| 44 | nt | 0.80 | 44 | 1064 |
| 47 | 134 | 0.69 | 38 | 1365 |
nt, not tested.
Genetic Analyses
After complete sequencing of all exons in CFH, CFI, MCP, CFB, C3, and ADAMTS13 genes, no genetic abnormality was detected and no association of any particular polymorphism was identified. Screening of CFHR1 deletion and CFH-CFHR1 hybrid gene with multiplex ligation-dependent probe amplification was negative.
Discussion
Despite remarkable progress in decoding the genetic basis of aHUS, a significant portion of aHUS cases—such as the patient presented here—remain unexplained. The two main mechanisms leading to Stx-negative HUS are a deficiency of the von Willebrand factor–cleaving metalloprotease ADAMTS13 and a dysregulation of the alternative C3 convertase (2). In this patient, ADAMTS13 activity was normal and no genetic abnormality in complement genes was detected. Nevertheless, on admission, the patient's plasma showed a decrease of C3, suggesting a complement activation.
In the unclassified aHUS group, the risk for end-stage renal failure within 1 yr after onset is approximately 30%, mainly already after the first episode (14,15). The high initial plasma creatinine level in this patient is considered to be a strong predictor of poor outcome (15). Relapse rates are similar to other aHUS groups, and disease recurrences in transplanted kidneys have been reported (14,15).
Empirical treatment guidelines for aHUS recommend plasma therapy as soon as possible (30). The presence of genetic abnormalities in circulating complement proteins in many patients with aHUS provides a rationale for plasma infusions to correct the complement regulatory dysfunction. Plasma exchanges may additionally remove autoantibodies or other potentially toxic substances from the patient's blood; however, several patients do not respond to plasma therapy or require long-term treatment in relapsing disease (16). In the patient described here, plasma exchanges were effective against the microangiopathic hemolytic process for approximately 8 wk and allowed a temporary improvement of renal function. Thereafter, plasma exchanges (three times per week) failed to prevent ongoing aHUS activity and progressive renal failure.
The dysregulated complement alternative pathway in aHUS results in increased formation of alternative C3 convertase on cell surfaces, providing exponential cleavage of C3b, generation of the anaphylatoxins C3a and C5a, and formation of the lytic membrane attack complex (4). In this setting, host cells become targets for repeated complement attacks, causing inflammation and cellular damage. Thus, blockage of C5 by eculizumab should be protective for host cells in aHUS. In our patient, eculizumab effectively blocked complement activity, as shown by suppressed CH50 and APH50, and went along with termination of the microangiopathic hemolytic process on two occasions; however, unlike the first treatment period with recovery of C3 levels and drop in concentrations of the activation marker SC5b-9, the second treatment with eculizumab was not followed by abolition of C3 consumption and SC5b-9 levels remained on a high level. It is not entirely clear whether and to what extent hemodialysis at this stage of disease contributed to the elevated levels of the complement activation product SC5b-9. In contrast to SC5b-9 concentrations, normal values of CH50 and APH50, as well as moderately elevated C3d levels, do not necessarily reflect local complement activation at aHUS recurrence.
Our observation again highlights the importance of complement activation in the pathogenesis of aHUS. Even despite the absence of an identified genetic deficiency in complement regulatory proteins, eculizumab was effective in terminating the microangiopathic hemolytic activity in a patient with aHUS. A possible hitherto unknown genetic susceptibility factor leading to insufficient tissue protection against complement deposition cannot be excluded. A high frequency of likely triggering infectious events has been reported (4); however, the pathogenetic mechanisms that induce and maintain the activation of the complement cascade are largely unknown. It is also conceivable that a very intense and strong trigger may induce a strong local complement activation that continues even in the absence of a detectable complement regulatory dysfunction. Because eculizumab has no effect on upstream complement activation, a persistence of the trigger might explain the recurrences of the microangiopathic hemolytic process.
In the patient with congenital aHUS, long-term eculizumab treatment led to persistent remission (21). In the adult patient with posttransplantation aHUS recurrence, a single dose of eculizumab induced long-term remission; however, an additional effect of tacrolimus withdrawal cannot be excluded (22). Both patients showed a complete renal recovery (21,22). In the patient reported here, eculizumab was administered as a rescue therapy after 12 wk. The high initial plasma creatinine level, requirement of hemodialysis for 7 wk, and progressive renal failure as a result of limited response to plasma therapy pointed to considerable preexisting renal damage. Established chronic renal failure and further damage by aHUS relapses after discontinuation of eculizumab treatments concurred toward anuric end-stage renal failure in this patient. Long-term treatment with eculizumab might have been beneficial in this patient, as reported for the patient with congenital aHUS (21). It is also conceivable that eculizumab is more effective in the early treatment of aHUS to protect the kidneys from ongoing complement-mediated damage and to allow recovery from reversible changes.
Complement-targeting therapy with eculizumab may be a valuable supplement or alternative to plasma therapy in aHUS, but efficacy seems to vary and the determinants of response to treatment are still unknown. Early administration of eculizumab in plasma-resistant cases may decrease the extent of irreversible renal damage; however, the optimal duration of eculizumab treatment remains to be determined with respect to differences in genetic background and triggering mechanisms.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Noris M, Remuzzi G: Hemolytic uremic syndrome. J Am Soc Nephrol 16: 1035–1050,2005 [DOI] [PubMed] [Google Scholar]
- 2.Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, Rizzoni G, Taylor CM, Van De Kar N, Zimmerhackl LBEuropean Paediatric Research Group for HUS: A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 70: 423–431,2006 [DOI] [PubMed] [Google Scholar]
- 3.Kavanagh D, Richards A, Atkinson J: Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med 59: 293–309,2008 [DOI] [PubMed] [Google Scholar]
- 4.Loirat C, Noris M, Frémeaux-Bacchi V: Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol 23: 1957–1972,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venables JP, Strain L, Routledge D, Bourn D, Powell HM, Warwicker P, Diaz-Torres ML, Sampson A, Mead P, Webb M, Pirson Y, Jackson MS, Hughes A, Wood KM, Goodship JA, Goodship TH: Atypical haemolytic uraemic syndrome associated with a hybrid complement gene. PLoS Med 3: e431 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goicoechea De Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, López-Trascasa M, Sánchez-Corral P, Morgan BP, Rodríguez de Córdoba S: Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A 104: 240–245,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frémeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault De Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zipfel PF, Edey M, Heinen S, Józsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship TH, Skerka C: Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet 3: e41 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frémeaux-Bacchi V, Kemp EJ, Goodship JA, Dragon-Durey MA, Strain L, Loirat C, Deng HW, Goodship TH: The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: Evidence from two independent cohorts. J Med Genet 42: 852–856,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blom AM, Bergström F, Edey M, Diaz-Torres M, Kavanagh D, Lampe A, Goodship JA, Stain L, Moghal N, McHugh M, Inward C, Tomson C, Frémeaux-Bacchi V, Villoutreix BO, Goodship TH: A novel non-synonymous polymorphism (p.Arg240His) in C4b-binding protein is associated with atypical hemolytic uremic syndrome and leads to impaired alternative pathway cofactor activity. J Immunol 180: 6385–6391,2008 [DOI] [PubMed] [Google Scholar]
- 11.Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Frémeaux-Bacchi V: Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 555–563,2005 [DOI] [PubMed] [Google Scholar]
- 12.Józsi M, Strobel S, Dahse HM, Liu WS, Hoyer PF, Oppermann M, Skerka C, Zipfel PF: Anti-factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood 110: 1516–1518,2007 [DOI] [PubMed] [Google Scholar]
- 13.Józsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, Zipfel PF, Skerka C: Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 111: 1512–1514,2008 [DOI] [PubMed] [Google Scholar]
- 14.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi GInternational Registry of Recurrent and Familial HUS/TTP: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellier-Leclerc AL, Frémeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C.French Society of Pediatric Nephrology: Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 18: 2392–2400 2007 [DOI] [PubMed] [Google Scholar]
- 16.Noris M, Remuzzi G: Translational mini-review series on complement factor H: Therapies of renal diseases associated with complement factor H abnormalities—Atypical haemolytic uraemic syndrome and membranoproliferative glomerulonephritis. Clin Exp Immunol 151: 199–209,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalanko H, Peltonen S, Koskinen A, Puntila J, Isoniemi H, Holmberg C, Pinomäki A, Armstrong E, Koivusalo A, Tukiainen E, Mäkisalo H, Saland J, Remuzzi G, De Cordoba S, Lassila R, Meri S, Jokiranta TS: Successful liver-kidney transplantation in two children with aHUS caused by a mutation in complement factor H. Am J Transplant 8: 216–221,2008 [DOI] [PubMed] [Google Scholar]
- 18.Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP, Evans MJ: Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol 33: 1389–1401,1996 [DOI] [PubMed] [Google Scholar]
- 19.Mollnes TE, Kirschfink M: Strategies of therapeutic complement inhibition. Mol Immunol 43: 107–121,2006 [DOI] [PubMed] [Google Scholar]
- 20.Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, De Castro C, Fu CL, Maciejewski JP, Bessler M, Kroon HA, Rother RP, Hillmen P: Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 111: 1840–1847,2008 [DOI] [PubMed] [Google Scholar]
- 21.Gruppo RA, Rother RP: Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med 360: 544–546,2009 [DOI] [PubMed] [Google Scholar]
- 22.Nürnberger J, Witzke O, Saez AO, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M: Eculizumab for atypical hemolytic uremic syndrome. N Engl J Med 360: 542–544,2009 [DOI] [PubMed] [Google Scholar]
- 23.Mayer MM: Complement and complement fixation. In: Experimental Immunochemistry, edited by Kabat E, Mayer MM, Springfield, Charles C Thomas, 1961, pp 133–240 [Google Scholar]
- 24.Joiner KA, Hawinger A, Gelfand JA: A study of optimal reaction conditions for an assay of the human alternative complement pathway. Am J Clin Pathol 79: 65–72,1983 [DOI] [PubMed] [Google Scholar]
- 25.Brandslund I, Siersted HC, Svehag SE, Teisner B: Double-decker rocket immunoelectrophoresis for direct quantitation of complement C3 split products with C3d specificities in plasma. J Immunol Methods 44: 63–71,1981 [DOI] [PubMed] [Google Scholar]
- 26.Kotnik V, Luznik-Bufon T, Schneider PM, Kirschfink M: Molecular, genetic, and functional analysis of homozygous C8 beta-chain deficiency in two siblings. Immunopharmacology 38: 215–221,1997 [DOI] [PubMed] [Google Scholar]
- 27.Dragon-Durey MA, Frémeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Fridman WH, Weiss L: Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: Report and genetic analysis of 16 cases. J Am Soc Nephrol 15: 787–795,2004 [DOI] [PubMed] [Google Scholar]
- 28.Frémeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH: Complement factor I: A susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet 41: e84–795,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frémeaux-Bacchi V, Moulton EA, Kavanagh D, Dragon-Durey MA, Blouin J, Caudy A, Arzouk N, Cleper R, Francois M, Guest G, Pourrat J, Seligman R, Fridman WH, Loirat C, Atkinson JP: Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol 17: 2017–2025,2006 [DOI] [PubMed] [Google Scholar]
- 30.Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, Loirat C, Pecoraro C, Taylor CM, Van De Kar N, Vandewalle J, Zimmerhackl LBEuropean Paediatric Study Group for HUS: Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol 24: 687–696,2009 [DOI] [PubMed] [Google Scholar]