Abstract
Background and objectives: Atypical hemolytic uremic syndrome (aHUS) is associated with mutations in genes encoding complement-regulatory proteins factor H, I and B and membrane cofactor protein. Recently, heterozygous gain-of-function mutations in the complement C3 gene have been found in patients with aHUS.
Design, setting, participants, & measurements: A large family with a C3 R570Q mutation is described. Clinical and laboratory findings of carriers of the mutation and unaffected family members are reported.
Results: The index patient suffered from recurrent aHUS at age 22 and developed end-stage renal failure. Of 24 family members, nine harbored the C3 R570Q mutation. Carriers showed reduced or borderline C3 levels. Arterial hypertension was found in six family members, microhematuria in five and chronic kidney disease stage 3 in two elderly carrier patients. Despite marked consumption of C3, serum terminal complement complex levels were not elevated in carriers compared with other family members.
Conclusions: The penetrance of the C3 R570Q mutation to induce aHUS is incomplete and lower compared with mutations in other genes predisposing to the disease. The mutation is possibly also associated with hypertension, hematuria and chronic kidney disease, all of which may represent consequences of long-term complement activation in the renal vasculature.
Hemolytic uremic syndrome (HUS) is a rare disease characterized by microangiopathic hemolytic anemia, thrombocytopenia and acute renal failure. In children, the most frequent form (90% of patients) is the so-called typical or postdiarrheal (D+) HUS, caused by infection with Shiga-toxin (Stx) producing Escherichia coli. Other cases are classified as atypical D-HUS or aHUS and can be either sporadic or familial. A clear link was demonstrated between the disease and genetic abnormalities in complement regulator genes. In about 50% of patients suffering from aHUS, predisposing mutations in genes of complement regulators are identified. These include loss-of-function mutations in soluble complement factors H (CFH) and I (CFI) and membrane-bound membrane co-factor protein (MCP, CD46) (1–9). In about ten percent of cases, loss-of-function is acquired and due to anti-factor H antibodies (10). In addition, gain-of-function mutations in complement factor B (BF) also predispose to aHUS (11). A recent report describes nine heterozygous mutations in the complement C3 gene that are associated with aHUS in 14 patients (12). Whereas two of these caused impaired C3 secretion, five mutated proteins showed reduced binding to MCP and resisted cleavage by CFI. Here we describe in detail a patient carrying one of these mutations (R570Q) and an investigation of the patient's family. We suggest that this mutation not only predisposes to aHUS, but is probably also associated with hypertension, minor urinary abnormalities and chronic kidney disease (CKD).
Patients and Methods
The methods for identifying the C3 mutation in the index case have been described in detail (12). Furthermore, mutation analysis of factor H, factor H-related proteins 1 and 3, membrane cofactor protein, factor I and factor B has been performed in the index case as described (3, 5,11, 13,14). In addition to the index patient, 24 other family members were available for investigation (in one seven-year-old boy only mutation analysis was available). This included medical history and BP measurement. Laboratory tests for blood count, schistocytes, lactic dehydrogenase, haptoglobin, serum creatinine, estimated GFR, hematuria, proteinuria, C3 and C4 were performed using routine methods. The concentration of terminal complement complex (TCC) in serum was quantitated using a specific ELISA (15). Complement C3 levels in affected and unaffected probands were compared by two-sided unpaired t test.
Genomic DNA was extracted from peripheral venous blood samples using standard procedures. The primer sequences, chosen to amplify the whole coding region and all splice sites of the C3 gene, are available from the authors on request. Oligonucleotide primers were designed from the NCBI annotations for C3 mRNA (NM_000064.2) and genomic reference (NC_000019.8) sequences (www.ncbi.nlm.nih.gov/). To amplify C3 exon, 14 harboring the c.1775G>A (R570Q mutation), a 289-bp fragment was PCR-amplified using oligonucleotide primers C3 14f, 5′- TCTTTCCACTCTAGCCCAGC, and C3 14r, 5′- CCTCCGCCTCTTCTCAGC. We amplified 15 to 25 ng of genomic DNA in a 25-μl reaction volume that included 1x GoTaq PCR buffer (Promega, Mannheim, Germany), 1.5 mM MgCl2, primers at 0.8 mM and dNTPs at 200 μM (final concentrations), and 0.5 U of GoTaq polymerase (Promega). The following PCR conditions were used for all sets of primers: initial denaturation at 95 °C for three minutes; 36 cycles of denaturation at 95 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 40 s; final extension at 72 °C for 8 min. PCR products were cleaned using ExoSap-IT Puffer und Enzyme (USB, Vienna, Austria), and subsequently sequenced on an ABI 3130 DNA sequencer, with BigDye terminator mix (Applera, Vienna, Austria). Patient and control chromatographs, and the reference sequence were aligned and analyzed using the SEQUENCHER computer program (GeneCodes Corporation, Ann Arbor, MI). DNA samples of family members were tested for the presence and zygosity of the R570Q mutation by means of an allele-specific PCR using reverse primer C3 592rt, 5′- acggccaccagtaccatct to amplify the mutant allele, and C3 592rc, 5′- acggccaccagtaccatcc to amplify the wild-type allele in distinct PCR reactions with a common forward primer, C3 592comf, 5′- cctttctgtctttccactctagc, each yielding 205-bp fragments. In these reactions, a primer pair, K1f, 5′- agagaatggtcagtagggacact, and K1r, 5′- ggacctcagatgtgctgtt, amplified a 531-bp fragment to control for PCR success.
Single nucleotide polymorphisms (SNP) in the MCP and CFH genes that determine risk haplotypes for aHUS were analyzed as described elsewhere (16,17).
A linkage analysis of the C3 R570Q mutation with hypertension was conducted assuming a disease allele frequency of 0.001, and four age-dependent phenocopy rates integrated as liability classes corresponding to prevalences of hypertension in the general Austrian population (<30 yr 9%, 30 to 40 yr 21%, 40 to 50 yr 29%, >50 yr 54%).
The whole study was approved by the institutional review board of Innsbruck Medical University. All patients or their parents gave informed consent to the investigations, in particular for molecular genetic analysis in the context of genetic counseling.
Results
The Index Patient
A 22-yr-old woman with an unremarkable medical history was admitted because of high fever and right flank pain. The diagnosis of acute pyelonephritis was confirmed by CT scan. She was treated with antibiotics. Two days later she complained of pain in the right upper abdomen. A sonogram showed acute cholecystitis, and she underwent a laparoscopic cholecystectomy. A blood culture grew E. coli. Two days later thrombocytopenia and elevated serum creatinine were noted, and she developed severe hemolytic anemia. Serum C3 levels were moderately reduced. Hemolytic uremic syndrome was diagnosed. Over the next two weeks, the patient underwent 10 plasma exchanges (50 ml/kg each) using fresh frozen plasma as substitution fluid. All laboratory values normalized. Over the next year several check-ups showed no abnormality except consistently lowered C3 levels to about 50% of normal. Fourteen months after the initial attack, the patient observed a brown discoloration of her urine. Laboratory controls performed two weeks later disclosed a relapse of the hemolytic uremic syndrome (Table 1). Within the next seven weeks, 24 plasma exchanges (50 ml/kg) were performed. Although hemolytic anemia and thrombocytopenia resolved, the patient did not regain sufficient renal function and remained on chronic hemodialysis treatment. She is currently on the waiting list for renal transplantation. Serotyping of E. coli grown in the blood culture was not performed. The patient did not develop IgM or IgG antibodies against O157 serotype, which makes infection with Shiga toxin-producing E. coli unlikely.
Table 1.
Laboratory values during attacks and remission of aHUS in the index patient
| Laboratory parameter | First Attack | Remission | Relapse |
|---|---|---|---|
| Hemoglobin (120–157 g/L) | 50 | 134 | 96 |
| Thrombocytes (150–380 G/L) | 7 | 255 | 38 |
| LDH (130–223 U/L) | 2280 | 166 | 1874 |
| Haptoglobin (42–176 mg/dl) | <20 | 112 | <7 |
| Creatinine (0.60–1.00 mg/dl) | 2.5 | 0.82 | 4.17 |
| C3 (90–180 mg/dl) | 70.0 | 51.5 | 58.7 |
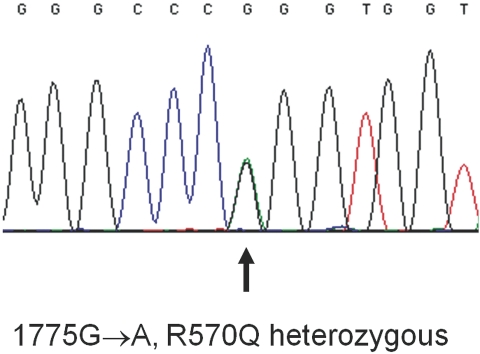
Mutation analysis revealed a heterozygous C3 R570Q mutation (Figure 1). The arginine-to-glutamine substitution at position 570 corresponds to the nucleotide substitution c.1775G→A. Mutations in other genes were not detected.
Figure 1.
Sequence analysis showing the 1775G→A base exchange in the C3 gene. The genomic structure numbering begins with the start site ATG. The protein numbering starts with the first amino acid of mature C3 (exact nomenclature R570Q or p.Arg592Gln).
Other Carriers of the Mutation
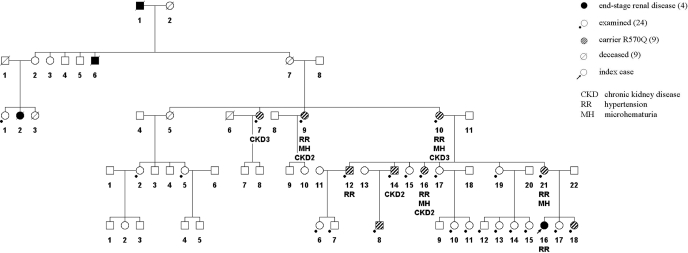
The family tree is shown in Figure 2. In addition to the index case, nine other family members were found to carry the C3 R570Q mutation. Clinical data on eight carriers (not including the seven-year-old boy) are summarized in Table 2. Six of the seven adult carriers suffered from hypertension, which required antihypertensive combination therapy in four of them. In addition, the index patient had also suffered from hypertension before development of aHUS. Five carriers had mild hematuria, and three had hyaline casts in the urinary sediment. Determination of renal function by eGFR showed CKD stage 3 in the two oldest affected family members and borderline renal function (CKD stage 2) in four additional carriers. Contrarily, only one of the 11 adult family members without the mutation had hypertension. Urinalysis and renal function were normal in all noncarriers.
Figure 2.
Pedigree of the family.
Table 2.
Demographic data and clinical characteristics of individuals carrying the C3 R570Q mutation
| Patient | Sex | Age | Serum Creatinine (mg/dl)a | eGFR (ml/min/1.73m2)b | Urine Abnormalities | Blood Pressure (mmHg) | Antihypertensives |
|---|---|---|---|---|---|---|---|
| III0.7 | F | 79 | 1.27 | 41 | Microhematuria, hyaline casts | 150/90 | ARB, CCB |
| III0.9 | F | 64 | 0.85 | 67 | Microhematuria | 180/90 | ARB |
| III0.10 | F | 77 | 1.57 | 32 | Microhematuria, hyaline casts | 130/80 | ARB, Carvedilol |
| IV0.12 | M | 55 | 0.89 | 89 | None | 160/110 | ARB, BB, Thiazide |
| IV0.14 | M | 41 | 1.17 | 70 | None | 110/75 | |
| IV0.16 | F | 52 | 0.78 | 78 | Microhematuria, hyaline casts | 145/90 | None |
| IV0.21 | F | 45 | 0.66 | 97 | Microhematuria | 160/110 | ARB |
| V0.8 | M | 7 | |||||
| V0.16 | F | 26 | ESRD | 160/110 | ACAI, BB, CCB | ||
| V0.18 | F | 10 | 0.54 | 153 | None | 110/80 | None |
Normal range 0.70–1.20 mg/dl for males and 0.70-1.10 mg/dl for females.
Normal range 90–120 ml/min/1.73 m2.
V.16 is the index case.
eGFR was calculated using the modified MDRD formula in adults and the Schwartz formula in V.18.
Clinical data of V.8 are not available.
ARB, angiotensin receptor blocker; CCB, calcium channel blocker; BB, beta blocker; ACEI, angiotensin converting enzyme inhibitor.
In the linkage analysis, maximal LOD score of 0.0018 was obtained with mutation C3 R570Q for hypertension, assuming a mutation penetrance of 0.8 (heterozygous or homozygous). Given the high population prevalence for hypertension, a LOD score of 0.0018 is close to the maximal LOD score that can be obtained with this single pedigree. Nevertheless, this LOD score between the C3 mutation and hypertension is inconclusive.
Three family members had died from renal failure many years ago. Patient I.1 had, according to his death certificate, died from renal failure in 1940, at age 67. Whether he suffered from acute or chronic renal failure is unclear. One of his sons, II.6, had died in 1910 from acute renal failure when he was six years old. Family member III.2 had died in 1965 at age 30 during pregnancy with a spontaneous abortion, followed by acute renal failure and severe anemia. Although impossible to prove, it can be assumed that at least the last two had suffered from aHUS. Individual II-7, who transmitted the mutation to the following generation, had died from heart failure.
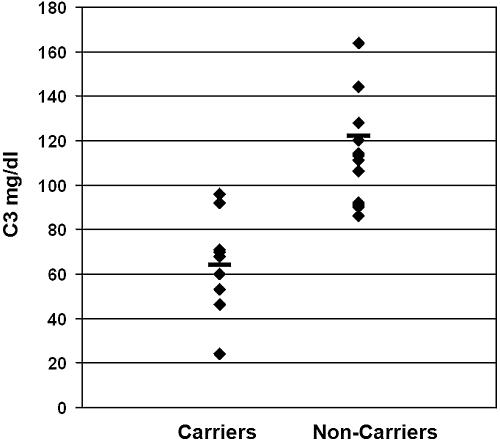
Complement C3 levels are shown in Figure 3. Whereas C3 was normal in 13 healthy individuals and borderline in one, it was reduced in seven carriers (including the index case) and at the lower limit of normal in two other ones (mean value 66 mg/dl in carriers versus 113 mg/dl in others, P < 0.001). Serum TCC levels were in the normal range in all family members (mean value 8.4 μg/ml in carriers, 15.7 μg/ml in others, normal range 2.5 to 53 μg/ml).
Figure 3.
Complement C3 serum levels of affected and unaffected family members.
Results of SNPs and risk haplotypes of the MCP and CFH genes are shown in Tables 3 and 4. The MCP ggaac haplotype has been identified as a susceptibility factor for aHUS (16). The index patient V.16 was homozygous for this haplotype. Carriers IV.12, IV.14, IV.16, IV.21 and V.18 were heterozygous. The CFH risk haplotype for aHUS is H3 (CFH gtgt) (17). The index patient is heterozygous for the H4 haplotype (CFH gtag) and for the H5 haplotype (CFH gcag) and does not carry the H3 risk haplotype. Carriers III.9 and III.10 are homozygous for the H3 risk haplotype.
Table 3.
Results of SNP and MCP risk haplotype analysis in carriers of the C3 R570Q mutation
| SNP | rs2796267 | rs2796268 | rs1962149 | rs859705 | rs7144 |
|---|---|---|---|---|---|
| Nucleotide | – 567 A>G | - 261 A>G | IVS9-78 G>A | IVS12 + 638 G>A | c.2232T>C |
| Patient | |||||
| III.7 | AA | AA | GG | GG | TT |
| III.9 | AA | AA | GG | GG | TT |
| III.10 | AA | AA | GG | GG | TT |
| IV. 12 | GA | GA | GA | nd | TC |
| IV.14 | GA | GA | GA | GA | TC |
| IV.16 | GA | GA | GA | GA | TC |
| IV.21 | GA | GA | GA | GA | TC |
| V.16a | GG | GG | AA | AA | CC |
| V.18 | GA | GA | GA | GA | TC |
V0.16 is the index patient. The MCPggaac haplotype is associated with increased susceptibility for aHUS.
Table 4.
Results of SNP and CFH risk haplotype analysis in carriers of the C3 R570Q mutation
| SNP | rs800292 | rs1061170 | rs3753396 | rs1065489 |
|---|---|---|---|---|
| Nucleotide | c.184G>A | c.1204T>C | c.2016A>G | c.2881G>T |
| Amino Acid | V26I | Y402H | Q672Q | D936E |
| SCR | SCR1 | SCR7 | SCR11 | SCR16 |
| Patient | ||||
| III.7 | GG | TC | GA | TG |
| III.9b | GG | TT | GG | TT |
| III.10b | GG | TT | GG | TT |
| IV.12 | GG | TT | GA | TG |
| IV.14 | GG | TT | GA | TG |
| IV.16 | GG | TT | GA | TG |
| IV.21 | GG | TT | GA | TG |
| V.16a | GG | TC | AA | GG |
| V.18 | GA | TT | AA | GG |
SCR, short consensus repeat.
V0.16 is the index patient.
Carriers homozygous for the risk haplotype H3 CFHgtgt.
Discussion
In the present study, we report a large family with a gain-of-function mutation in the C3 gene and a large spectrum of renal disease from hypertension to atypical hemolytic uremic syndrome.
The functional consequences of the complement C3 R570Q mutation found in this family have been recently described (12). The mutated C3 protein shows markedly reduced binding to MCP (22% of normal binding), and therefore is cleaved and inactivated only to a minimal extent and slowly by factor I (reduction of cofactor activity >90%). In addition, the mutated molecule exhibits only 80% of wild-type binding to factor H. It can be assumed that mutated membrane-bound C3b forms the alternative pathway convertase C3bBb and causes uncontrolled activation of the amplification loop. Such a mechanism is in accordance with the reduced serum C3 levels observed in almost all carriers. The serum TCC levels did not differ between carriers and healthy family members. Another report describes a modest elevation of TCC levels in 17 patients suffering from recurrent HUS (18). Whether these results were obtained during active disease or remission is not described. TCC measurements during the acute disease are, to our knowledge, not available. In other glomerular diseases with complement activation, such as lupus nephritis, membranoproliferative glomerulonephritis type I or acute poststreptococcal nephritis, an increase in TCC in the blood is frequently found and correlates with disease activity and glomerular TCC deposition (19–22). However, as serum TCC levels may not reflect tissue TCC deposition, and due to the large normal range of our test, the biologic relevance of the results in that family is uncertain. Nonetheless, we propose that these individuals were able to somehow control further activation of the terminal complement pathway with its deleterious consequences. Hypothetically, once this control mechanism is overcome, possibly during further complement activation by an infection, full-blown complement activation including the terminal pathway and aHUS can occur. The urinary tract infection in the index case may have caused such a chain of events. In support of this hypothesis, two recent case reports describe effective treatment of aHUS with eculizumab, a monoclonal antibody against C5 and blocker of the terminal complement pathway (23,24).
The penetrance of aHUS in affected individuals is around 50% for mutation in the CFH, CFI, MCP and FB genes (5,11,14,25). In this family, one of 10 carriers (excluding the three individuals who died from renal failure years ago) developed aHUS, giving a penetrance of 10%. Whether such a low penetrance is also a feature of families affected by other C3 mutations is presently unknown. However, it is still possible that other carriers of that family may become affected in the future. For early detection of such an event, all carriers were advised to seek medical attention in case of infections or other symptoms of disease, and to treat all infections with antibiotics. Polymorphisms in the MCP and CFH genes predispose to development of aHUS and affect disease severity (13,16,26,27). The homozygosity for the MCP ggaac risk haplotype we identified in the index patient may have increased her susceptibility to aHUS. On the other hand, she did not carry a CFH risk haplotype. Two other carriers, however, 77 and 64 yr of age, were homozygous for the CFH H3 susceptibility haplotype. Therefore it could be that in carriers of the C3 R570Q mutation the MCP risk haplotype may be a stronger susceptibility factor compared with the CFH H3 risk haplotype. Transfection experiments of human embryonic kidney cells with genomic DNA from individuals homozygous for the MCP ggaac haplotype showed a 25% reduction of MCP transcription (16). In addition to the low binding capacity of the mutated C3 to MCP, the reduced transcription and surface expression of MCP may have put the index patient at extreme risk for uncontrolled complement activation and aHUS.
Little attention has been paid in the past to the carriers of mutations predisposing to aHUS without suffering from the disease. Our studies in such individuals harboring the C3 R570Q mutation suggest that, apart from aHUS, this mutation may possibly also be associated with other clinical symptoms. We observed a high frequency of arterial hypertension in these patients. Hypertension was quite severe with the need for multiple antihypertensives in some individuals. In addition, the majority also presented with microhematuria. Furthermore, GFR was markedly reduced in elderly affected family members. We hypothesize that low-grade complement activation in the renal vessels over many years may cause vascular damage, which in turn could explain the renal symptoms of these individuals.
A renal histology from one of the carriers with microhematuria or low eGFR would be very helpful to support our hypothesis. We considered a renal biopsy for scientific interest only unjustified. Whether such clinical abnormalities can also be found in carriers of other mutations associated with aHUS, is presently unknown. We strongly recommend, however, that a careful clinical investigation including BP measurement, urinalysis and determination of renal function be performed in such individuals.
Are aHUS patients with C3 mutations candidates for plasma exchange? Removal of the dysfunctional C3 molecule may have a beneficial effect. However, membrane-bound abnormal C3, which is responsible for alternative pathway activation, cannot be eliminated by plasma exchange, and continued supplementation of C3 may enhance further complement activation. Our patient made a complete recovery after the first attack of aHUS while undergoing intensive plasma exchange, whereas the second attack caused irreversible renal failure despite such therapy. The major reason for the different outcomes probably was the time interval between beginning of aHUS and start of therapy. This period was only a few days at the first episode, but two weeks at the second one, a time long enough to have caused irreversible renal damage. Before plasma exchange, serum creatinine was 2.5 mg/dl at the first and 4.1 mg/dl at the second attack. Further studies are needed to determine whether plasma exchange has a positive effect on that condition.
In the initial report, eight out of 14 patients with C3 mutations and aHUS developed end-stage renal disease. In these patients, 12 renal transplants were performed; recurrence of disease was observed in five transplants. It seems that the outcome of renal transplantation with regard to aHUS and C3 mutations is certainly better than for CFH and FI mutations, but not as good as in patients with MCP mutations (14,28–30). Recently, promising results of combined kidney and liver transplantation have been reported in patients with factor H mutations, when performed in combination with preoperative plasma exchange (31). In contrast to factor H, which is synthesized exclusively by the liver, about ten percent of C3 are produced by other cells (32). A liver transplantation would therefore reduce the proportion of mutated C3 from 50 to 5%. Whether this reduction is sufficient to protect against recurrence of disease is unknown.
In summary, we report on a large family with 10 individuals harboring the C3 R570Q complement C3 mutation. One of them developed aHUS and end-stage renal disease. The mutation probably also predisposes to arterial hypertension, hematuria and chronic kidney disease. We suggest that all carriers of mutations associated with aHUS be screened for these symptoms.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA: Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 53: 836–844, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Caprioli J, Bettinaglio P, Zipfel PF, Amadei B, Daina E, Gamba S, Skerka C, Marziliano N, Remuzzi G, Noris M: The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol 12: 297–307, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Neumann HP, Salzmann M, Bohnert-Iwan B, Mannuelian T, Skerka C, Lenk D, Bender BU, Cybulla M, Riegler P, Konigsrainer A, Neyer U, Bock A, Widmer U, Male DA, Franke G, Zipfel PF: Haemolytic uraemic syndrome and mutations of the factor H gene: A registry-based study of German speaking countries. J Med Genet 40: 676–681, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Herman Fridman W, Weiss L: Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: Report and genetic analysis of 16 cases. J Am Soc Nephrol 15: 787–795, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, Vigneau C, Kuypers D, Boudailliez B, Loirat C, Rondeau E, Fridman WH: Complement factor I: A susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet 41: e84–795, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavanagh D, Kemp EJ, Mayland E, Winney RJ, Duffield JS, Warwick G, Richards A, Ward R, Goodship JA, Goodship TH: Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 2150–2155, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Noris M, Brioschi S, Caprioli J, Todeschini M, Bresin E, Porrati F, Gamba S, Remuzzi G: Familial haemolytic uraemic syndrome and an MCP mutation. Lancet 362: 1542–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Richards A, Kemp EJ, Liszewski MK, Goodship JA, Lampe AK, Decorte R, Muslumanoglu MH, Kavukcu S, Filler G, Pirson Y, Wen LS, Atkinson JP, Goodship TH: Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A 100: 12966–12971, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodship TH, Liszewski MK, Kemp EJ, Richards A, Atkinson JP: Mutations in CD46, a complement regulatory protein, predispose to atypical HUS. Trends Mol Med 10: 226–231, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Fremeaux-Bacchi V: Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 555–563, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Goicoechea De Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, Lopez-Trascasa M, Sanchez-Corral P, Morgan BP, Rodriguez De Cordoba S: Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A 104: 240–245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault De Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJ, Goodship TH, Atkinson JP: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipfel PF, Edey M, Heinen S, Jozsi M, Richter H, Misselwitz J, Hoppe B, Routledge D, Strain L, Hughes AE, Goodship JA, Licht C, Goodship TH, Skerka C: Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet 3: e41–4952, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wurzner R, Schulze M, Happe L, Franzke A, Bieber FA, Oppermann M, Gotze O: Inhibition of terminal complement complex formation and cell lysis by monoclonal antibodies. Complement Inflamm 8: 328–340, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Esparza-Gordillo J, Goicoechea De Jorge E, Buil A, Carreras Berges L, Lopez-Trascasa M, Sanchez-Corral P, Rodriguez De Cordoba S: Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet 14: 703–712, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Pickering MC, De Jorge EG, Martinez-Barricarte R, Recalde S, Garcia-Layana A, Rose KL, Moss J, Walport MJ, Cook HT, De Cordoba SR, Botto M: Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med 204: 1249–1256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prufer F, Scheiring J, Sautter S, Jensen DB, Treichl R, Wurzner R, Zimmerhackl LB: Terminal complement complex (C5b-9) in children with recurrent hemolytic uremic syndrome. Semin Thromb Haemost 32: 121–127, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Horigome I, Seino J, Sudo K, Kinoshita Y, Saito T, Yoshinaga K: Terminal complement complex in plasma from patients with systemic lupus erythematosus and other glomerular diseases. Clin Exp Immunol 70: 417–424, 1987 [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu YY, Nisihara RM, Wurzner R, Kirschfink M, de Messias-Reason IJ: SC5b-9 is the most sensitive marker in assessing disease activity in Brazilian SLE patients. J Investig Allergol Clin Immunol 8: 239–244, 1998 [PubMed] [Google Scholar]
- 21.Kobayashi Y, Hasegawa O, Honda M: Terminal complement complexes in childhood type I membranoproliferative glomerulonephritis. J Nephrol 19: 746–750, 2006 [PubMed] [Google Scholar]
- 22.Matsell DG, Wyatt RJ, Gaber LW: Terminal complement complexes in acute poststreptococcal glomerulonephritis. Pediatr Nephrol 8: 671–676, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Gruppo RA, Rother RP: Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med 360: 544–546, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Nurnberger J, Witzke O, Saez AO, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M: Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 360: 542–544, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Kavanagh D, Richards A, Atkinson J: Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med 59: 293–309, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Caprioli J, Castelletti F, Bucchioni S, Bettinaglio P, Bresin E, Pianetti G, Gamba S, Brioschi S, Daina E, Remuzzi G, Noris M: Complement factor H mutations and gene polymorphisms in haemolytic uraemic syndrome: The C-257T, the A2089G and the G2881T polymorphisms are strongly associated with the disease. Hum Mol Genet 12: 3385–3395, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Fremeaux-Bacchi V, Kemp EJ, Goodship JA, Dragon-Durey MA, Strain L, Loirat C, Deng HW, Goodship TH: The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: Evidence from two independent cohorts. J Med Genet 42: 852–856, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavanagh D, Goodship TH: Membrane cofactor protein and factor I: Mutations and transplantation. Semin Thromb Haemost 32: 155–159, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C: Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 18: 2392–2400, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Zimmerhackl LB, Scheiring J, Prufer F, Taylor CM, Loirat C: Renal transplantation in HUS patients with disorders of complement regulation. Pediatr Nephrol 22: 10–16, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Saland JM, Emre SH, Shneider BL, Benchimol C, Ames S, Bromberg JS, Remuzzi G, Strain L, Goodship TH: Favorable long-term outcome after liver-kidney transplant for recurrent hemolytic uremic syndrome associated with a factor H mutation. Am J Transplant 6: 1948–1952, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Barnum SR, Fey G, Tack BF: Biosynthesis and genetics of C3. Curr Top Microbiol Immunol 153: 23–43, 1990 [DOI] [PubMed] [Google Scholar]