Abstract
There are limited data regarding whether albuminuria and reduced estimated GFR (eGFR) are separate and independent risk factors for cardiovascular and renal events among individuals with type 2 diabetes. The Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study examined the effects of routine BP lowering on adverse outcomes in type 2 diabetes. We investigated the effects of urinary albumin-to-creatinine ratio (UACR) and eGFR on the risk for cardiovascular and renal events in 10,640 patients with available data. During an average 4.3-yr follow-up, 938 (8.8%) patients experienced a cardiovascular event and 107 (1.0%) experienced a renal event. The multivariable-adjusted hazard ratio for cardiovascular events was 2.48 (95% confidence interval 1.74 to 3.52) for every 10-fold increase in baseline UACR and 2.20 (95% confidence interval 1.09 to 4.43) for every halving of baseline eGFR, after adjustment for regression dilution. There was no evidence of interaction between the effects of higher UACR and lower eGFR. Patients with both UACR >300 mg/g and eGFR <60 ml/min per 1.73 m2 at baseline had a 3.2-fold higher risk for cardiovascular events and a 22.2-fold higher risk for renal events, compared with patients with neither of these risk factors. In conclusion, high albuminuria and low eGFR are independent risk factors for cardiovascular and renal events among patients with type 2 diabetes.
Diabetes is a major global health problem, currently affecting an estimated 246 million people worldwide, with a doubling of this prevalence expected in the next 30 yr.1 Compared with people without diabetes, affected individuals are at increased risk for both cardiovascular events and kidney disease.2,3 Increased urinary albumin excretion (albuminuria) and reduced GFR both have been demonstrated to be risk factors for progressive kidney failure and cardiovascular disease.4–9
Guidelines therefore recommend the annual assessment of albuminuria and GFR, and this has become accepted as common practice.10–13 Although both renal functional parameters are believed to be risk factors for cardiovascular events,4–9 there are limited data as to whether these two factors are associated with adverse outcomes independent not only of other known cardiovascular risk factors but also of each other in people with type 2 diabetes.14–19
The BP-lowering arm of the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study recently reported that the routine administration of a fixed combination of the angiotensin-converting enzyme inhibitor perindopril and the diuretic indapamide to a broad cross-section of patients with type 2 diabetes reduced the risk for cardiovascular and kidney outcomes, regardless of initial BP level.20 More recently, the glucose-lowering arm of ADVANCE reported that intensive glucose lowering based on gliclazide (modified release) reduced the risk for new or worsening nephropathy.21 Herein, we present the findings of observational analyses examining the association between albuminuria and GFR at baseline or during follow-up and the risk for cardiovascular events and renal events in type 2 diabetes.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the 10,640 patients for whom urinary albumin-to-creatinine ratio (UACR) and serum creatinine measurements were available at baseline. UACR levels at baseline were in the normoalbuminuric, microalbuminuric, and macroalbuminuric ranges in 69, 27, and 4% of patients, respectively. The proportions with estimated GFR (eGFR) of ≥90, 60 to 89, and <60 ml/min per 1.73 m2 were 25, 56, and 19%, respectively. Forty-three (0.4%) patients had eGFR of <30 ml/min per 1.73 m2. A total of 62% of patients with GFR <60 ml/min per 1.73 m2 had normoalbuminuria (Table 2).
Table 1.
Baseline characteristics of participants (N = 10,640)a
| Variable | Value |
|---|---|
| Age (yr; mean [SD]) | 66 (6) |
| Female (n [%]) | 4522 (43) |
| Duration of diabetes (yr; median [IQR]) | 7 (3 to 11) |
| Kidney factors | |
| UACR (mg/g; median [IQR]) | 15.0 (7.1 to 39.8) |
| <30 (n [%]) | 7377 (69) |
| 30 to 300 (n [%]) | 2862 (27) |
| >300 (n [%]) | 401 (4) |
| serum creatinine (μmol/L; median [IQR]) | 84.0 (70.7 to 97.2) |
| eGFR (ml/min per 1.73 m2; median [IQR]) | 75.9 (63.6 to 89.7) |
| eGFR ≥90 (n [%]) | 2611 (25) |
| eGFR 60 to 89 (n [%]) | 5996 (56) |
| eGFR <60 (n [%]) | 2033 (19) |
| BP (mmHg) | |
| SBP (mean [SD]) | 145 (21) |
| DBP (mean [SD]) | 81 (11) |
| history of currently treated hypertension (n [%]) | 7289 (69) |
| Previous vascular disease (n [%]) | |
| history of macrovascular disease | 3394 (32) |
| history of myocardial infarction | 1250 (12) |
| history of stroke | 971 (9) |
| Other major risk factors | |
| HbA1c (%; mean [SD]) | 7.5 (1.5) |
| serum total cholesterol (mmol/L; mean [SD]) | 5.2 (1.2) |
| serum LDL cholesterol (mmol/L; mean [SD]) | 3.1 (1) |
| serum HDL cholesterol (mmol/L; mean [SD]) | 1.3 (0.4) |
| serum triglycerides (mmol/L; median [IQR]) | 1.6 (1.2 to 2.3) |
| BMI (kg/m2; mean [SD]) | 28.3 (5.2) |
| electrocardiogram abnormalities (n [%])b | 1863 (18) |
| current smoker (n [%]) | 1594 (15) |
| current drinker (n [%]) | 3212 (30) |
| Randomized treatment | |
| perindopril-indapamide (n [%]) | 5313 (50) |
aDBP, diastolic BP; IQR, interquartile range.
bElectrocardiogram abnormalities were defined as presence of Q waves consistent with previous myocardial infarction, left ventricular hypertrophy and artrial fibrillation.
Table 2.
Number of patients according to UACR and GFR levels
| UACR Level at Baseline | eGFR Level at Baseline (ml/min per 1.73 m2; n [%]) |
||
|---|---|---|---|
| ≥90(n = 2611) | 60 to 89(n = 5996) | <60(n = 2033) | |
| Normoalbuminuria (UACR <30 mg/g) | 1800 (16.9) | 4325 (40.6) | 1252 (11.8) |
| Microalbuminuria (UACR 30 to 300 mg/g) | 729 (6.9) | 1492 (14.0) | 641 (6.0) |
| Macroalbuminuria (UACR >300 mg/g) | 82 (0.8) | 179 (1.7) | 140 (1.3) |
Risk for Cardiovascular Events, Cardiovascular Death, and Kidney Failure According to Baseline Albuminuria and eGFR Levels
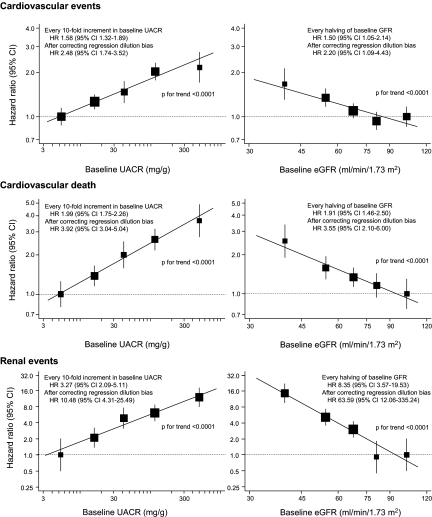
During an average follow-up of 4.3 yr, a total of 938 (8.8%) patients experienced a cardiovascular event, 432 (4.1%) of which were fatal, and 107 (1.0%) developed a renal event. Higher UACR levels at baseline were log-linearly associated with an increased risk for cardiovascular events, cardiovascular death, and renal events, after adjustment for age, gender, duration of diabetes, eGFR, systolic BP (SBP), history of currently treated hypertension, history of cardiovascular disease, hemoglobin A1c (HbA1c), serum LDL cholesterol, serum HDL cholesterol, serum triglycerides, body mass index, electrocardiogram abnormalities, current smoking, and current alcohol intake. These associations were observed even within the normoalbuminuric range (Figure 1). Similarly, the risk for each outcome increased log-linearly with lower eGFR levels. Every 10-fold increment in baseline UACR, which corresponds approximately to a change from one clinical stage of albuminuria to the next (i.e., from normo- to microalbuminuria or from micro- to macroalbuminuria), was associated with a 1.6-fold (95% confidence interval [CI] 1.3 to 1.9), two-fold (95% CI 1.8 to 2.3), and 3.3-fold (95% CI 2.1 to 5.1) higher, multivariable-adjusted risk of cardiovascular events, cardiovascular death, and renal events, respectively. For every halving of baseline eGFR, the risk for these outcomes increased 1.5-fold (95% CI 1.1 to 2.1), 1.9-fold (95% CI 1.5 to 2.5), and 8.4-fold (95% CI 3.6 to 19.5), respectively. After correction for regression dilution, these estimates were substantially increased. A 10-fold increment in UACR was associated with a 2.5-fold (95% CI 1.7 to 3.5), 3.9-fold (95% CI 3.0 to 5.0), and 10.5-fold (95% CI 4.3 to 25.5) higher, multivariable-adjusted risk for cardiovascular events, cardiovascular death, and renal events, respectively. For every halving of baseline eGFR, the risk for these outcomes increased 2.2-fold (95% CI 1.1 to 4.4), 3.6-fold (95% CI 2.1 to 6.0), and 63.6-fold (95% CI 12.1 to 335.2), respectively. These log-linear relationships were present in parallel between the randomized BP treatment groups, as well as the randomized glucose treatment groups, without any evidence of heterogeneity in the association (P > 0.16 for heterogeneity).
Figure 1.
Association of albuminuria level or eGFR at baseline with the risk for adverse outcomes. The centers of the square are placed at the point estimates, and vertical lines represent the corresponding 95% CIs. The area of each square is proportional to the inverse variance of each estimate. The estimates are adjusted for baseline covariates, including age, gender, duration of diabetes, log-transformed eGFR (or log-transformed UACR), SBP, history of currently treated hypertension, history of macrovascular disease, HbA1c, LDL cholesterol, HDL cholesterol, log-transformed triglycerides, body mass index (BMI), electrocardiogram abnormalities, current smoking, and current drinking. The hazard ratios (HRs) and 95% CIs for the regression lines were corrected with the regression dilution attenuation coefficient of log-transformed UACR (1.98) and log-transformed eGFR (1.96).
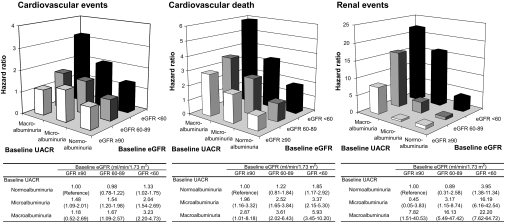
We also estimated the combined effects of baseline UACR and eGFR levels on the risk for cardiovascular events, cardiovascular death, and renal events (Figure 2). The effects of higher UACR and lower eGFR were independent of each other (all P > 0.20 for interaction). When compared with patients with normoalbuminuria and eGFR ≥90 ml/min per 1.73 m2, patients with both macroalbuminuria and eGFR <60 ml/min per 1.73 m2 were at 3.2-fold higher risk (95% CI 2.2 to 4.7) for cardiovascular events as well as 5.9-fold higher risk (95% CI 3.5 to 10.2) for cardiovascular death and 22.2-fold higher risk (95% CI 7.6 to 64.7) for renal events.
Figure 2.
Combined effects of albuminuria and eGFR levels at baseline on the risk for adverse outcomes. The estimates are adjusted for baseline covariates, including age, gender, duration of diabetes, SBP, history of currently treated hypertension, history of macrovascular disease, HbA1c, LDL cholesterol, HDL cholesterol, log-transformed triglycerides, BMI, electrocardiogram abnormalities, current smoking, and current drinking.
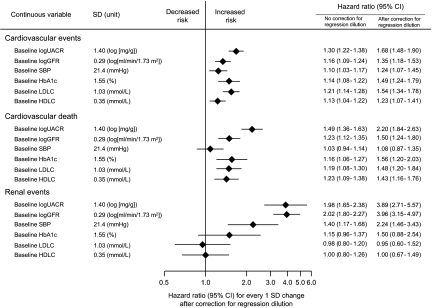
The effects of higher baseline UACR and lower baseline eGFR levels fitted as a continuous variable with cardiovascular events, cardiovascular death, and renal events were independent of each other and of other known risk factors, including SBP. On the basis of the magnitude of risk associated with 1-SD difference in the level of each factor, the effects of baseline UACR or baseline eGFR seemed to be of comparable magnitude to those of other established cardiovascular risk factors on the risk for cardiovascular events and cardiovascular death (Figure 3).
Figure 3.
Comparison of the impact of baseline factors on the risk for adverse outcomes. HRs and 95% CIs were estimated using a multivariable-adjusted model, including age, gender, baseline logUACR, baseline logGFR, baseline SBP, baseline HbA1c, baseline LDL cholesterol, baseline HDL cholesterol, and the following baseline covariates: Duration of diabetes, history of currently treated hypertension, history of macrovascular disease, triglycerides, BMI, electrocardiogram abnormalities, current smoker, and current drinker, and corrected with the attenuation coefficient of 1.98, 1.96, 2.37, 2.98, 2.29, and 1.74 for logUACR, log GFR, SBP, HbA1c, LDL cholesterol, and HDL cholesterol, respectively. The values are shown as the estimates per 1-SD increment (logUACR, SBP, HbA1c, and LDL cholesterol) or decrement (logGFR and HDL cholesterol) in each variable.
When the results were analyzed according to the clinical stage of chronic kidney disease (CKD) as defined by current guidelines, the risk for cardiovascular events and renal events generally increased according to CKD stage (Table 3). Furthermore, individuals with UACR of 30 mg/g and stage 3 CKD (eGFR 30 to 59 ml/min per 1.73 m2) were at greatest risk for all outcomes (Table 3). Similar findings were observed for all-cause mortality, major coronary events, and major cerebrovascular events (Supplemental Tables 1 through 3 and Supplemental Figures 1 and 2).
Table 3.
Risk for adverse outcomes according to the clinical stage of CKD
| CKD Stage | No. of Patientsa | No. of Events | Annual Event Rate (%) | Age- and Gender-Adjusted |
Multivariable-Adjustedb |
||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Cardiovascular events | |||||||
| no CKD (eGFR ≥60 and UACR <30) | 6125 | 399 | 1.54 | 1.00 | Reference | 1.00 | Reference |
| stage 1 (eGFR ≥90 and UACR ≥30) | 811 | 74 | 2.19 | 1.60 | 1.25 to 2.06 | 1.46 | 1.14 to 1.89 |
| stage 2 (eGFR 60 to 89 and UACR ≥30) | 1671 | 196 | 2.87 | 1.82 | 1.53 to 2.15 | 1.57 | 1.32 to 1.88 |
| stage 3 (eGFR 30 to 59) | 1990 | 259 | 3.21 | 1.99 | 1.70 to 2.34 | 1.72 | 1.46 to 2.03 |
| UACR <30 in stage 3 | 1238 | 122 | 2.37 | 1.49 | 1.21 to 1.84 | 1.37 | 1.11 to 1.69 |
| UACR ≥30 in stage 3 | 752 | 137 | 4.67 | 2.79 | 2.30 to 3.40 | 2.24 | 1.83 to 2.75 |
| Cardiovascular death | |||||||
| no CKD (eGFR ≥60 and UACR <30) | 6125 | 144 | 0.54 | 1.00 | Reference | 1.00 | Reference |
| stage 1 (eGFR ≥90 and UACR ≥30) | 811 | 31 | 0.89 | 1.94 | 1.31 to 2.86 | 1.73 | 1.16 to 2.58 |
| stage 2 (eGFR 60 to 89 and UACR ≥30) | 1671 | 109 | 1.55 | 2.72 | 2.12 to 3.49 | 2.25 | 1.74 to 2.90 |
| stage 3 (eGFR 30 to 59) | 1990 | 143 | 1.71 | 2.79 | 2.20 to 3.54 | 2.30 | 1.80 to 2.93 |
| UACR <30 in stage 3 | 1238 | 58 | 1.09 | 1.80 | 1.32 to 2.46 | 1.60 | 1.17 to 2.20 |
| UACR ≥30 in stage 3 | 752 | 85 | 2.77 | 4.34 | 3.31 to 5.70 | 3.26 | 2.46 to 4.32 |
| Renal events | |||||||
| no CKD (eGFR ≥60 and UACR <30) | 6125 | 17 | 0.06 | 1.00 | Reference | 1.00 | Reference |
| stage 1 (eGFR ≥90 and UACR ≥30) | 811 | 3 | 0.09 | 1.39 | 0.41 to 4.75 | 1.24 | 0.36 to 4.27 |
| stage 2 (eGFR 60 to 89 and UACR ≥30) | 1671 | 27 | 0.38 | 5.96 | 3.25 to 10.95 | 4.89 | 2.61 to 9.16 |
| stage 3 (eGFR 30 to 59) | 1990 | 52 | 0.62 | 11.22 | 6.41 to 19.63 | 9.56 | 5.35 to 17.10 |
| UACR <30 in stage 3 | 1238 | 12 | 0.23 | 4.16 | 1.96 to 8.80 | 4.11 | 1.91 to 8.81 |
| UACR ≥30 in stage 3 | 752 | 40 | 1.32 | 21.75 | 12.22 to 38.70 | 16.66 | 9.08 to 30.56 |
aForty-three patients with eGFR <30 ml/min per 1.73m2 were excluded from this analysis.
bAdjusted for baseline covariates, including age, gender, duration of diabetes, SBP, history of currently treated hypertension, history of macrovascular disease, HbA1c, LDL cholesterol, HDL cholesterol, log-transformed triglycerides, BMI, electrocardiogram abnormalities, current smoker, and current drinker.
In sensitivity analyses, using the Cockcroft-Gault formula rather than the Modification of Diet in Renal Disease (MDRD) formula to estimate GFR, the results were similar. Albuminuria and reduced eGFR remained strong and continuous risk factors, independent of each other and of other known cardiovascular risk factors.
Risk for Cardiovascular Events and Death According to Albuminuria and GFR Levels during Follow-up
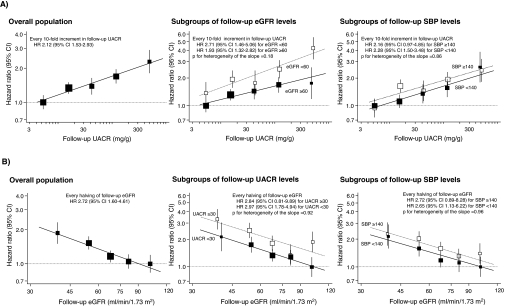
Because levels of albuminuria, kidney function, and other risk factors may change over time, potentially diluting their association with subsequent events, we additionally assessed the association of albuminuria and eGFR during follow-up with the risk for cardiovascular events after adjusting for follow-up levels of major cardiovascular risk factors. The median values of follow-up UACR and follow-up eGFR for each category were 5.3, 15.8, 42.0, 119.3, and 494.2 μg/mg and 39.5, 54.3, 67.7, 81.5, and 101.5 ml/min per 1.73 m2, respectively. The risk for cardiovascular events was associated log-linearly with the UACR and eGFR levels during follow-up in analyses adjusted for a wide range of potential confounding factors, including SBP (both P < 0.0001 for trend; Figure 4). There was no evidence of heterogeneity in the association between randomized treatment groups (both P > 0.47 for heterogeneity). These relationships were present for both factors, with similar associations for albuminuria detected in subgroups with follow-up eGFR above and below 60 ml/min per 1.73 m2, for eGFR in subgroups with follow-up UACR above and below the threshold for the definition of microalbuminuria, 30 mg/g, and for both parameters in subgroups with follow-up SBP above and below 140 mmHg (all P > 0.17 for heterogeneity). Similar findings were observed for cardiovascular death.
Figure 4.
Association of albuminuria and eGFR levels during follow-up with the risk for cardiovascular events. Closed and open squares represent HR in subgroups for eGFR of ≥60 and <60 ml/min per 1.73 m2, UACR of <30 and ≥30 mg/g, or SBP of <140 and ≥140 mmHg. The estimates are adjusted for age; gender; follow-up log-transformed eGFR (or follow-up log-transformed UACR); follow-up SBP; follow-up HbA1c; follow-up LDL cholesterol; follow-up HDL cholesterol; follow-up log-transformed triglycerides; follow-up BMI; randomized study treatment; and baseline covariates, including duration of diabetes, history of currently treated hypertension, history of macrovascular disease, electrocardiogram abnormalities, current smoking, and current drinking. In the subgroup analysis, the risk factor relevant to the subgroup was excluded from the multivariable model. The HRs and 95% CI for the regression lines were corrected with the regression dilution attenuation coefficient of log-transformed UACR (1.98) and log-transformed eGFR (1.96).
Discussion
These analyses demonstrate that both increased urinary albumin excretion and reduced eGFR are independently and continuously associated with the risk for both cardiovascular and kidney outcomes in patients with type 2 diabetes. There was no evidence of any interaction between these risk factors, so patients with both elevated albuminuria and reduced eGFR were at the highest risk, and the relationship was not mitigated by adjustment for other conventional risk factors. In fact, some of the cardiovascular and kidney outcomes were more strongly related to baseline and follow-up levels of albuminuria or eGFR than they were to other, very-well-established risk factors, such as SBP. These analyses therefore highlight the potential additional value of assessment of albuminuria and eGFR in risk assessment for individuals with type 2 diabetes.
This study has clearly shown that the associations with albuminuria and reduced eGFR are strong and independent across the range of observed values in a large population with type 2 diabetes. Comparable findings were also reported by several community-based studies.15–19 Data from 14,586 US community-based individuals demonstrated that individuals with both macroalbuminuria and eGFR <60 ml/min per 1.73 m2 were at four-fold higher risk for cardiovascular death and three-fold greater risk for all-cause death as compared individuals with normoalbuminuria and eGFR ≥90 ml/min per 1.73 m2.18 The Second Nord-Tr\ondelag Health (HUNT II) study also reported that the presence of microalbuminuria and reduced eGFR was associated with a higher risk for cardiovascular death in 9709 community-based participants, and the addition of UACR and eGFR to the traditional risk prediction model improved cardiovascular risk stratification.17 The HUNT II study was limited, however, by the fact that it could describe the relationship only for fatal events confirmed by death certificates, whereas this study demonstrates the relationship is present for fatal and nonfatal outcomes as adjudicated by an end point committee. These results suggest that assessment of both albuminuria and eGFR levels is needed to improve our ability to identify individuals with high risk for cardiovascular complications and to institute appropriate preventive measures.
The mechanism through which the relationship between albuminuria and GFR and cardiovascular and renal outcomes might be mediated is an area of great interest. Although albuminuria is considered a key aspect of the pathogenesis of progressive kidney dysfunction, progressive reduction in GFR was described in patients with type 1 diabetes and biopsy-proven diabetic nephropathy in the absence of proteinuria.22 Similar morphologic data are absent in type 2 diabetes, but this phenomenon of reduced GFR in the absence of significant albuminuria was previously described in several epidemiologic data of patients with type 2 diabetes,23–25 although the clinical sequelae in such patients has not been previously determined.
It has been suggested that albuminuria and reduced GFR may simply represent the renal manifestations of systemic endothelial dysfunction26,27 and systemic atherosclerosis,28,29 respectively. Indeed, it is likely that albuminuria and reduced GFR may be markers of different pathologic processes. The pathophysiology of the independently increased risk associated with both risk factors requires further exploration.
This study showed that patients with stages 1 and 2 CKD, defined as eGFR ≥60 ml/min per 1.73 m2 and UACR ≥30 mg/g,11 have a substantially increased risk for cardiovascular disease and kidney failure compared with those without CKD manifestations. Although the risk in people with stage 3 CKD was higher overall than for stages 1 and 2, important differences were observed within this group. Specifically, the risk for cardiovascular and renal events was lower in patients with normoalbuminuria and stage 3 CKD than in those with stage 2 CKD, manifested “only” by albuminuria. A similar finding was reported from a community-based cohort study.30 These findings suggest that additional stratification of stage 3 CKD into subgroups with differing risks for cardiovascular and kidney disease is possible by considering the presence or absence of albuminuria.
The independent continuous relation between albuminuria observed during follow-up and cardiovascular events suggests that measuring albuminuria as well as BP may be useful in monitoring BP-lowering treatment effects.31,32 In the main analyses of the ADVANCE BP20,33 and glucose-lowering21 arms, both interventions were shown to reduce the likelihood of progression in albuminuric state, as well as suggesting positive effects for macrovascular events. Long-term follow-up of the United Kingdom Prospective Diabetes Study (UKPDS)34 suggested that the benefits of these interventions on cardiovascular events may continue to grow, and it is tempting to speculate that reduction in albuminuria may play a role in this benefit.
In this study, every halving of UACR during follow-up was associated with a 20% lower risk for cardiovascular events. Interestingly, this association was similar in patients with higher and lower SBP during follow-up and was directly comparable to the 18% reduction in the risk for cardiovascular disease for every halving of albuminuria rates reported from the Reduction in Endpoints in Noninsulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) study.35 In that study, discordant responses between albuminuria and BP were observed in a significant proportion of individuals, adding more support for albuminuria as a therapeutic target in addition to BP in patients with type 2 diabetes; however, both the analyses from RENAAL and this study are observational and should therefore be regarded as primarily exploratory.
The strengths of this study include the large sample size that allowed for precise estimations of the independent effects of albuminuria and GFR level on cardiovascular risk. In addition, the effects of regression dilution bias of risk factor levels on the risk estimates were measured and corrected in this analysis, providing a more accurate measure of the magnitude of the associations.36 The limitations of this study should also be noted. First, it is widely recognized that GFR estimated using the MDRD equation leads to a certain degree of misclassification of eGFR levels; however, this limitation is unlikely to change our conclusions, because the sensitivity analysis using the Cockcroft-Gault equation to estimate GFR made little differences to the findings. Second, relatively few participants had macroalbuminuria and/or a eGFR <30 ml/min per 1.73 m2, thereby limiting the ability to assess the impact of these factors in more advanced nephropathy. Third, the generalizability of our findings may be limited, because the population studied was patients who had type 2 diabetes and were willing to participate in a trial; however, we believe that this study population would be representative of those seen in the community36 because of the inclusion of a broad spectrum of individuals with type 2 diabetes. Finally, measurements of serum creatinine and UACR were based on a single blood or urine sample at each visit. Assays were conducted locally rather than at a central laboratory and without calibration among laboratories, introducing a source of variability that may have reduced the precision of the results.
In conclusion, albuminuria and reduced eGFR are continuous risk factors for cardiovascular and kidney outcomes in patients with type 2 diabetes that are independent of each other and of other known risk factors. Routine measurement of both albuminuria and eGFR may therefore improve current tools for risk assessment in patients with type 2 diabetes.
Concise Methods
Study Design and Participants
ADVANCE is a factorial, randomized, controlled trial evaluating the effects of BP lowering and intensive blood glucose control on vascular outcomes. A detailed description of the design has been published previously.37 In brief, 11,140 individuals who had type 2 diabetes, were aged ≥55 yr, and had at least one additional risk factor for cardiovascular disease were enrolled from 215 centers in 20 countries. No participant inclusion or exclusion criteria were based on levels of BP or GFR, but the presence of albuminuria was one of a number of potential eligibility criteria for inclusion. Eligible participants were randomly assigned to either a fixed combination of perindopril and indapamide (4 mg/1.25 mg) or matching placebo and to either a gliclazide (modified release)–based intensive glucose control regimen or standard glucose control based on local guidelines of participating countries, after a 6-wk active run-in period. For this study, a total of 10,640 patients were studied after the exclusion of 500 patients for whom levels of UACR or serum creatinine at baseline were unavailable. Approval for the trial was obtained from each center's institutional review board, and all participants provided written informed consent.
Follow-up and Assessments
Participants were seen at 3, 4, and 6 mo after randomization and subsequently every 6 mo. Measurement of UACR was performed on spot urine samples at baseline, 24 mo, and 48 mo after randomization and at the end of follow-up. Serum creatinine was measured at baseline, at the conclusion of the run-in period, 4 and 12 mo after randomization, at subsequent yearly intervals, and at the end of follow-up. Both UACR and serum creatinine were measured at local laboratories. eGFR was calculated by the four-variable MDRD equation.10 At each study visit, BP was recorded as the mean of two measurements made in the seated position using an automated sphygmomanometer (Omron HEM-705 CP; Omron, Tokyo, Japan).
Albuminuria and GFR Categories
Microalbuminuria was defined as a UACR of 30 to 300 mg/g using ordinal cutoff points and macroalbuminuria as a UACR of >300 mg/g.10 Albuminuria levels in the normoalbuminuric and microalbuminuric range were further divided at the median level into the following categories: Low-normal (<9.1 mg/g), high-normal (9.1 to 29.9 mg/g), low microalbuminuria (30.0 to 65.5 mg/g), and high microalbuminuria (65.6 to 300.0 mg/g). Baseline eGFR levels were divided at 15-ml/min per 1.73 m2 intervals into five categories8,9: ≥90, 75 to 89, 60 to 74, 45 to 59, and <45 ml/min per 1.73 m2. The clinical stages of CKD were classified according to the recommendations of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines11: No CKD (eGFR ≥60 ml/min per 1.73 m2 and UACR <30 mg/g), stage 1 (eGFR ≥90 ml/min per 1.73 m2 and UACR ≥30 mg/g), stage 2 (eGFR 60 to 89 ml/min per 1.73 m2 and UACR ≥30 mg/g), and stage 3 (eGFR 30 to 59 ml/min per 1.73 m2). In addition, stage 3 was subclassified into two categories according to the status of albuminuria: UACR <30 mg/g and UACR ≥30 mg/g.
Outcomes
The main outcomes for this study were cardiovascular events (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke), cardiovascular death, and renal events (death as a result of kidney disease, requirement for dialysis or transplantation, or doubling of serum creatinine to >200 μmol/L). The secondary outcomes were all-cause death, major coronary events (death as a result of coronary heart disease [including sudden death] or nonfatal myocardial infarction), and major cerebrovascular events (death as a result of cerebrovascular disease or nonfatal stroke). Only the first event of the relevant outcome type was included in each analysis. All of these events were reviewed and validated by an independent end point adjudication committee.
Statistical Analysis
UACR and eGFR were transformed into natural logarithms because of their skewed distribution. The risk estimates for each outcome associated with UACR or eGFR at baseline were estimated using a Poisson log-linear regression model after adjustment for potentially confounding baseline covariates. The selection of variables was based on identifying all measured clinical variables of known or suspected prognostic importance for the outcomes of interest. Forty-three missing values of eGFR at baseline were imputed by a value recorded during the run-in period.
Associations between follow-up levels of UACR, eGFR, and other known risk factors with the risk for each outcome were assessed using the pooling of repeated observations method.38,39 Briefly, each participant's follow-up period was divided into a series of intervals defined by the 2-yr visits. The presence or absence of the relevant outcome was documented during each interval and coupled to the UACR levels recorded at the beginning of the interval and to the time-weighted average of eGFR levels and other risk factors recorded during the interval, before the development of the relevant event. Missing values for a variable at any one visit were imputed by using the value recorded during the previous visit. The proportions of missing values over time were <9.6% for all of the relevant variables in this analysis.40 The participants generated 30,900 intervals. These intervals were divided into five ordinal categories of follow-up UACR and eGFR using the same definition of categories described already for the baseline analyses.
Trends in relationships between categories of the relevant factor and the risk for outcomes were tested by adding the median value of each factor for each category to the relevant Poisson model. The variances of each risk estimate were calculated by using the floating absolute risk method.41,42 The regression line for the risk estimates according to UACR and eGFR levels at baseline or during follow-up were fitted using regression analysis with inverse variance weighting.42 The heterogeneity in the relationship between subgroups was tested by adding interaction terms between median values of each variable and subgroups to the model. The repeated measures at baseline and during the follow-up period in the placebo group were used to estimate a regression dilution attenuation coefficient for the relevant variable by using a linear mixed model36 to correct for regression dilution bias in the continuous association between each factor and each outcome.
The SAS 9.1 for Windows (SAS Institute, Cary, NC) was used to perform statistical analyses. All P values were calculated from two-tailed tests of statistical significance with a type I error rate of 5%.
Disclosures
The ADVANCE study was funded by grants from Servier (the major financial sponsor) and the National Health and Medical Research Council of Australia (211086 and 358395). S.M. and J.C. hold research grants from Servier as principal investigators for ADVANCE. V.P., B.E.d.G., S.Z., A.Pa., A.C., B.N., N.P., C.-E.M., M.C., M.M., B.W., P.H., G.M., M.W., S.M., and J.C. have received lecturing fees from Servier. T.N. holds fellowships of Banyu Life Science Foundation Fellowship and International Society of Hypertension Visiting Postdoctoral Award from the Foundation for High Blood Pressure Research in Australia. S.Z. holds a National Health and Medical Research Council of Australia Health Professional Research Fellowship. A.C. holds a Senior Research Fellowship from the Australian National Health and medical Research Council.
Supplementary Material
Acknowledgments
All members of the ADVANCE Collaborating Group have been listed in full previously.20 We thank the patients and all of the investigators at the participating centers.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This trial has been registered at http://www.clinicaltrials.gov (identifier NCT00145925).
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.International Diabetes Federation: Diabetes Atlas 2006, 3rd Ed., Brussels, International Diabetes Federation, 2006 [Google Scholar]
- 2.Asia Pacific Cohort Studies Collaboration: The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. The Asia Pacific Cohort Studies Collaboration. Diabetes Care 26: 360–366, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Barzi F, Woodward M: Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies. BMJ 332: 73–78, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf SHOPE Study Investigators: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Valmadrid CT, Klein R, Moss SE, Klein BE: The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 160: 1093–1100, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, Jardine M, Gallagher M, Turnbull F, Chalmers J, Craig J, Huxley R: The relationship between proteinuria and coronary risk: A systematic review and meta-analysis. PLoS Med 5: e207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ninomiya T, Perkovic V, Verdon C, Barzi F, Cass A, Gallagher M, Jardine M, Anderson C, Chalmers J, Craig JC, Huxley R: Proteinuria and stroke: A meta-analysis of cohort studies. Am J Kidney Dis 53: 417–425, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 51: 1285–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW.American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 11.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39 [Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 12.European Society of Hypertension-European Society of Cardiology Guidelines Committee: 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 21: 1011–1053, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Canadian Diabetes Association: 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 32 [Suppl 1]: S126–S133, 2008. Available at: http://www.diabetes.ca/files/cpg2008/cpg-2008.pdf [Google Scholar]
- 14.Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M: Cholesterol and Recurrent Events (CARE) Trial Investigators: Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: Analysis of a previously conducted randomised trial. BMJ 332: 1426–1432, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, Kanashiki M, Saito Y, Ota H, Nose T: The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 69: 1264–1271, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Foster MC, Hwang SJ, Larson MG, Parikh NI, Meigs JB, Vasan RS, Wang TJ, Levy D, Fox CS: Cross-classification of microalbuminuria and reduced glomerular filtration rate: Associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med 167: 1386–1392, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J: Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 167: 2490–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 167: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Cirillo M, Lanti MP, Menotti A, Laurenzi M, Mancini M, Zanchetti A, De Santo NG: Definition of kidney dysfunction as a cardiovascular risk factor: Use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med 168: 617–624, 2008 [DOI] [PubMed] [Google Scholar]
- 20.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B: Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 370: 829–840, 2007 [DOI] [PubMed] [Google Scholar]
- 21.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Caramori ML, Fioretto P, Mauer M: Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes 52: 1036–1040, 2003 [DOI] [PubMed] [Google Scholar]
- 23.MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, Matthews PG, Thomas MC, Power DA, Jerums G: Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care 29: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Tsalamandris C, Allen TJ, Gilbert RE, Sinha A, Panagiotopoulos S, Cooper ME, Jerums G: Progressive decline in renal function in diabetic patients with and without albuminuria. Diabetes 43: 649–655, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM: Albuminuria and renal insufficiency prevalence guides population screening: Results from the NHANES III. Kidney Int 61: 2165–2175, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Stehouwer CD, Smulders YM: Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J Am Soc Nephrol 17: 2106–2111, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Jefferson JA, Shankland SJ, Pichler RH: Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int 74: 22–36, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM: Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens 9: 827–833, 1995 [PubMed] [Google Scholar]
- 29.Diamond JR: Analogous pathobiologic mechanisms in glomerulosclerosis and atherosclerosis. Kidney Int 31 [Suppl]: S29–S34, 1991 [PubMed] [Google Scholar]
- 30.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RTPREVEND Study Group: Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: The importance of urinary albumin excretion. Nephrol Dial Transplant 23: 3851–3858, 2008 [DOI] [PubMed] [Google Scholar]
- 31.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlöf B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y: Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan intervention for endpoint reduction in hypertension study. Hypertension 45: 198–202, 2005 [DOI] [PubMed] [Google Scholar]
- 33.de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen C, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers JADVANCE Collaborative Group: Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20: 883–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Thomas MC, Atkins R: Assessment and management of hypertension in patients with type 2 diabetes. Intern Med J 39: 143–149, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Asia Pacific Cohort Studies Collaboration. Kengne AP, Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Gu DF, Suh I, Woodward M: Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region. J Hypertens 25: 1205–1213, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Rationale and design of the ADVANCE study: A randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. J Hypertens Suppl 19: S21–S28, 2001 [PubMed] [Google Scholar]
- 38.Cupples LA, D'Agostino RB, Anderson K, Kannel WB: Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med 7: 205–222, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, Macmahon S, Neal BPROGRESS Collaborative Group: Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens 24: 1201–1208, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Barzi F, Woodward M: Imputations of missing values in practice: Results from imputations of serum cholesterol in 28 cohort studies. Am J Epidemiol 160: 34–45, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Easton DF, Peto J, Babiker AG: Floating absolute risk: An alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med 10: 1025–1035, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Woodward M: Epidemiology: Study Design and Data Analysis, 2nd Ed., Boca Raton, Chapman and Hall/CRC Press, 2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.