Abstract
Mutations of PKD1 and PKD2 account for 85 and 15% of cases of autosomal dominant polycystic kidney disease (ADPKD), respectively. Clinically, PKD1 is more severe than PKD2, with a median age at ESRD of 53.4 versus 72.7 yr. In this study, we explored whether a family history of renal disease severity predicts the mutated gene in ADPKD. We examined the renal function (estimated GFR and age at ESRD) of 484 affected members from 90 families who had ADPKD and whose underlying genotype was known. We found that the presence of at least one affected family member who developed ESRD at age ≤55 was highly predictive of a PKD1 mutation (positive predictive value 100%; sensitivity 72%). In contrast, the presence of at least one affected family member who continued to have sufficient renal function or developed ESRD at age >70 was highly predictive of a PKD2 mutation (positive predictive value 100%; sensitivity 74%). These data suggest that close attention to the family history of renal disease severity in ADPKD may provide a simple means of predicting the mutated gene, which has prognostic implications.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic renal disorder, with a prevalence of one in 500 to 1000 in the general population. It is the third most common single cause of ESRD in the United States, accounting for 5% of people with ESRD.1–5 ADPKD is genetically heterogeneous, with two disease genes (PKD1 on chromosome 16 and PKD2 on chromosome 4) accounting for most of the cases. Mutations of PKD1 and PKD2 are thought to account for 85 and 15% of cases, respectively, in linkage-characterized European populations.6,7 Although the clinical manifestations overlap completely between two gene types, there is a strong locus effect on renal disease severity. Patients with PKD1 have significantly more severe renal disease than patients with PKD2, with larger kidneys and earlier onset at ESRD (median age 53.4 versus 72.7 yr, respectively).8,9 By contrast, a weak allelic effect (based on the 5′ versus 3′ location of the germline mutations) on renal disease severity may exist for type 110 but not type 2 ADPKD.11 In addition, significant intrafamilial renal disease variability is evident, which is thought to be due to genetic and environmental modifiers.11,12
PKD1 is a large, complex gene containing 46 exons spanning 50 kb, with 33 of these exons at the 5′ end being duplicated elsewhere on chromosome 16. PKD2 is a single-copy gene, consisting of 15 exons spanning a 68-kb genomic region. There is marked allelic heterogeneity for both gene types, with 314 truncating mutations having been described in PKD1 and 91 truncating mutations in PKD2.1,2 PKD1 encodes polycystin 1 (PC1), a large receptor-like protein, and PKD2 encodes polycystin 2 (PC2), a nonselective cation channel that transports calcium. Both PC1 and PC2 physically interact to form a complex that regulates intracellular levels of calcium and are located in the primary cilia of renal tubular cells. Recent studies suggested that the polycystin complex in the primary cilia of renal tubular cells serves as a mechanosensor for urine flow and that dysfunction of this mechanosensor may lead to cellular proliferation and cystogenesis.1,2
Recent advances in our understanding of the molecular pathobiology of ADPKD have led to the discovery of a number of drugs (e.g., tolvaptan, somatostatin, mammalian target of rapamycin inhibitors) that may target cyst growth and delay renal disease progression.1,2 Several of these promising drugs are being or will be tested in clinical trials, and disease-modifying treatment may become a reality in the not-too-distant future.1 In this context, the knowledge of ADPKD gene type may allow for the optimization of the design of such clinical trials, and identification of those affected individuals who are most likely to benefit from these novel therapies should they become available; however, the gene type is seldom known for most families in the clinical setting. Although molecular genetic testing, either by linkage or direct mutation analysis, can elucidate the gene type, such testing has its limitations.13 Linkage studies require DNA samples from several affected family members and are of limited utility in small families or de novo cases. Mutation-based screening for ADPKD is expensive and yields a definitive pathogenic mutation in only 42 to 63% of cases because the large and complex structure of PKD1 results in many unclassified missense variants whose pathogenicity often cannot be predicted with complete certainty.14,15
In this study, we explored whether renal disease severity can be used as clinical predictors of underlying gene type in families with ADPKD. To predict PKD1, we explored various cutoffs of early age at ESRD as indicative of severe renal disease. To predict PKD2, we explored various cutoffs of late age with renal sufficiency or at ESRD as indicative of milder renal disease. We then evaluated the performance characteristics of these cutoffs to define the optimal criteria for clinical prediction of ADPKD gene type.
Results
During an 8-yr period, we identified from the Hereditary Kidney Disease Clinic at Toronto General Hospital 132 consecutive unrelated new patients who had ADPKD and consented to participate in our genotype-phenotype study. Approximately 60% of these patients were referred by family doctors and the remainder by community nephrologists. Most of them were younger than 45 yr, and none had ESRD at the time of their referral. Through these probands, we recruited 110 families into our study but not the remaining families because of a lack of interest from their family members. All study families were clinically ascertained and genetically characterized using a standardized protocol (see the Concise Methods sections). Of these 110 families, 13 were apparently of de novo onset with the probands being the only affected (both parents had negative ultrasound scans, but paternity was not confirmed), 75 were genetically characterized to be either PKD1 or PKD2, and 22 were indeterminate with respect to their gene type (all were small families uninformative for linkage, and eight probands from these families also underwent sequence-based screening of both PKD1/PKD2 by Athena Diagnostic but failed to identify any pathogenic mutations). We also included 14 families from Newfoundland who were previously and extensively characterized for this study,16 including the two branches of a bilineal family with heterozygous PKD1 and PKD2 germline mutations.17 The gene type data are shown in Supplemental Tables 1 and 2.
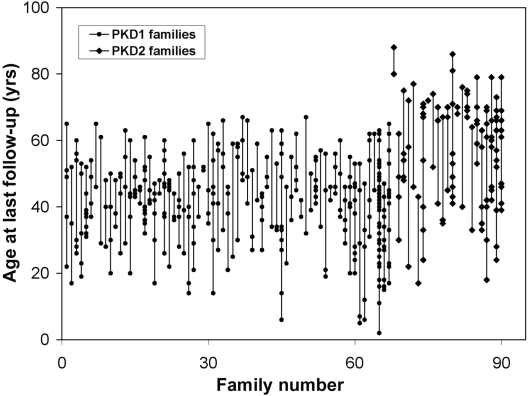
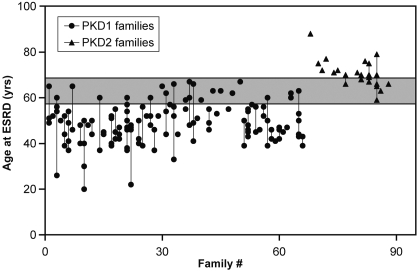
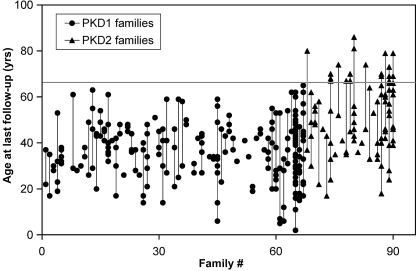
In total, we studied 484 affected individuals from 90 families with ADPKD: 367 of them belonged to 67 families with PKD1, and 117 belonged to 23 families with PKD2. In this study population, PKD1 and PKD2 accounted for 74 and 26% of the families, respectively. The mean age at last follow-up for patients with PKD1 was 41 yr (range 2 to 67 yr) and for patients with PKD2 was 56 yr (range 17 to 88 yr; Figure 1). A total of 140 (38%) study patients with PKD1 and 24 (21%) patients with PKD2 had ESRD, respectively. The mean age at ESRD for patients with PKD1 was 48.9 yr (95% confidence interval 47.4 to 50.4 yr) and for patients with PKD2 was 70.2 yr (95% confidence interval 68.0 to 72.8 yr). In families with two or more affected individuals with ESRD, there was significant variability in the age of onset at ESRD for both PKD1 and PKD2 (Figure 2). In addition, a significant number of patients with PKD2 but none with PKD1 remained renal sufficient despite an advanced age of ≥68 yr (Figure 3).
Figure 1.
Age at last follow-up for all of the affected study participants grouped by family. Each dot within a vertical line represents an affected member from the same family.
Figure 2.
Age plot for affected study participants who developed ESRD, grouped by family. Each dot within a vertical line represents the age at which an affected member of the same family developed ESRD. Significant intrafamilial variability is noted with the gray zone denoting the age range at which both patients with PKD1 and PKD2 overlap, presumably as a result of a modifier effect from genetic and environmental factors; however, having at least one family member who developed ESRD at or before the age of 58 is highly predictive of PKD1. Conversely, having at least one family member who developed ESRD at or after the age of 68 is highly predictive of PKD2,
Figure 3.
Age plot of affected study participants who remained renal sufficient at the last follow-up. A significant number of patients with PKD2 but none with PKD1 remained renal sufficient despite their old age (>68 yr as denoted by the gray line). Having at least one family member who maintained renal sufficiency at or after the age of 68 is highly predictive of PKD2.
We examined all of the affected study participants within each family to identify individuals at the extremes of the age cutoffs indicative of disease severity. We found the criterion of having at least one family member with early onset ESRD at or before 55 to 58 yr of age had a positive predictive value (PPV) of 100% and sensitivity of 72 to 75% for PKD1 (Table 1). Conversely, we found the criterion of having at least one family member who remained renal sufficient or developed ESRD at or after 68 to 70 yr of age had a PPV of 100% and a sensitivity of 74 to 78% for PKD2 (Table 2). These two criteria will be useful for clinical prediction of PKD1 and PKD2 and correctly predicted the gene type in approximately three quarters of the study families. Of the remaining study families, none of them had an affected individual who satisfied these criteria and were therefore indeterminate. Given that none of the test criteria provide a negative predictive value (NPV) of 100%, the absence of a family history for these test conditions cannot be used for exclusion of the specific gene type.
Table 1.
Performance characteristics for various cutoffs of early age at ESRD predictive of PKD1
| Age (≤ yr) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 55 | 72 | 100 | 100 | 55 |
| 56 | 72 | 100 | 100 | 55 |
| 57 | 75 | 100 | 100 | 58 |
| 58 | 75 | 100 | 100 | 58 |
| 59 | 76 | 96 | 98 | 58 |
| 60 | 78 | 96 | 98 | 60 |
| 61 | 78 | 96 | 98 | 60 |
| 62 | 79 | 96 | 98 | 61 |
| 63 | 82 | 91 | 97 | 64 |
| 64 | 82 | 91 | 97 | 64 |
| 65 | 84 | 91 | 97 | 66 |
| 66 | 84 | 87 | 95 | 65 |
| 67 | 85 | 78 | 92 | 64 |
| 68 | 85 | 74 | 91 | 63 |
| 69 | 85 | 74 | 91 | 63 |
| 70 | 85 | 74 | 91 | 63 |
Table 2.
Performance characteristics for various cutoffs of late age of renal sufficiency or ESRD predictive of PKD2
| Age (≥ yr) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 65 | 83 | 88 | 70 | 94 |
| 66 | 83 | 93 | 79 | 94 |
| 67 | 83 | 97 | 90 | 94 |
| 68 | 78 | 100 | 100 | 93 |
| 69 | 78 | 100 | 100 | 93 |
| 70 | 74 | 100 | 100 | 92 |
| 71 | 61 | 100 | 100 | 88 |
| 72 | 57 | 100 | 100 | 87 |
| 73 | 48 | 100 | 100 | 85 |
| 74 | 44 | 100 | 100 | 84 |
| 75 | 40 | 100 | 100 | 83 |
Only 21 study patients with PKD1 and 11 study patients with PKD2 developed stage 4 chronic kidney disease (CKD; data not shown). We did not find it useful to combine stage 4 CKD and stage 5 CKD (ESRD) for the prediction of ADPKD gene type (data not shown); however, the sample size of our patients with stage 4 CKD was small.
Discussion
The ADPKD gene type has important prognostic value, but this is seldom known in the clinical setting. The presence of a significant modifier effect makes it difficult for making such prediction, especially for an individual patient at an age when the disease has not yet fully manifested.11,12 Although molecular genetic testing is available for defining the ADPKD gene types, they have their limitations.13–15 Given the strong gene locus effect,9–11 clinicians often use the knowledge of renal disease severity of their patients or their affected relatives to guesstimate the underlying ADPKD gene type; however, this clinical intuition approach is qualitative and fuzzy and provides no specific risk estimates for a particular ADPKD gene type. This study examined for the first time the role of a comprehensive family history of renal disease severity in predicting the ADPKD gene type.
Our study cohort represents a typical sample of families with ADPKD seen in the clinic; however, we observed a higher proportion of our Canadian families affected with PKD2 compared with two studies of linkage-characterized European families from the late 1980s to early 1990s (26 versus 15%, respectively).6,7 Our finding is consistent with a recent population-based study from Olmsted County, MN, which reported a prevalence of PKD2 of 36%.18 Given that PKD2 is milder and may be underdetected before the era of widespread ultrasound screening, the two more recent studies suggest that its prevalence may be higher than previously estimated.
We also observed a large intrafamilial variability for the age at which ESRD occurred in our study population, a finding that has been described previously10–12; therefore, the age at which one family member develops ESRD cannot be used to predict whether and when other affected members will develop ESRD. Despite the large intrafamilial variability for the age at ESRD within families, however, we were able to formulate useful clinical criteria for predicting the ADPKD gene type. Given the marked gene locus effect for ADPKD, we examined early age at ESRD as an indicator for severe renal disease for predicting PKD1 and late age of renal sufficiency or ESRD as an indicator for mild renal disease for predicting PKD2 in our study families. Using these indicators, we found clear age cutoffs at which the effect of the gene type could be differentiated from a modifier effect (Figures 2 and 3). Specifically, we found that having at least one family member who developed ESRD at or before the age of 58 is highly predictive of PKD1, with a PPV of 100% and a sensitivity of 75%. Conversely, having at least one family member who maintained renal sufficiency or developed ESRD at or after the age of 68 is highly predictive of PKD2, with a PPV of 100% and a sensitivity of 78% (Table 1).
Given that we have studied only 90 families (albeit with 484 affected individuals), these positive predictive values may overestimate their true values. To minimize the risk for false-positive labeling, we therefore recommend a more stringent and conservative criterion of having at least one family member developed ESRD at or before age 55 yr for PKD1 prediction (PPV 100%; sensitivity 72%). Similarly, we also recommend the criterion of having at least one family member who maintained renal sufficiency or developed ESRD at or after age 70 yr for PKD2 prediction (PPV 100%; sensitivity 74%). These criteria will be useful for predicting the underlying ADPKD gene type especially in families with multiple older affected individuals; however, they may not be useful when a detailed family history is not available and in families with few affected individuals or de novo ADPKD. In the latter settings, molecular genetic testing remains the only means for determining the ADPKD gene type.
In conclusion, our clinical predictors based on a family history of renal disease severity can serve as a valuable adjunct to molecular testing in determining ADPKD gene type. Given the strong gene locus effect, age-adjusted total kidney volume may also be predictive of the ADPKD gene type,8 but its utility needs to be confirmed and defined in future studies. Distinguishing the gene type in individuals affected by ADPKD is useful for prognostication, for optimizing the design of novel therapeutic clinical trials, and possibly for selecting patients for disease-modifying drug treatment in the future. In addition, these clinical predictors can be integrated into a test strategy for gene type–specific ultrasound diagnosis of ADPKD.19
Concise Methods
Study Patients
A total of 168 consecutive unrelated new patients with ADPKD were assessed (by Y.P.) at the Hereditary Kidney Disease Clinic of the Toronto General Hospital between June 1, 2000, and May 30, 2008. All of them provided a detailed family history of ADPKD for at least three generations and were invited to participate in a genotype-phenotype epidemiologic study. A total of 132 patients (approximately 79%) consented and provided further contact information for their family members. We then invited and recruited all of their available key spouses and at-risk family members for our study. In addition, 18 families with ADPKD from Newfoundland were previously identified through a population-based screen and extensively studied.16 Fourteen of them with clearly defined PKD1/PKD2 gene type (including a bilineal family with heterozygous PKD1 and PKD2 mutations) were also included in this study.
Clinical Assessment
Upon obtaining informed consent, we reviewed the medical records of all of the available family members of the probands. We then screened all of the at-risk individuals for whom a diagnosis of ADPKD had not been made from each family with an abdominal ultrasound scan. We used the following criteria for the diagnosis of ADPKD: (1) Presence of at least two renal cysts (unilateral or bilateral) in an at-risk individual who was younger than 30 yr; (2) presence of at least two cysts in each kidney in an at-risk individual who was aged 30 to 59; or (3) presence of at least four cysts in each kidney in an at-risk individual who was aged ≥60 yr.20 All study participants provided a blood sample for serum creatinine and DNA genetic analysis. Estimated GFR was calculated from serum creatinine using a formula that adjusted for age, gender, and body weight.21 Stage 4 CKD was defined as estimated GFR of 15 to 30 ml/min per 1.73 m2. ESRD was defined as needing dialysis or renal transplantation. The institutional review boards of the University Health Network in Toronto and Memorial University in Newfoundland approved all of the protocols used for this study.
DNA Linkage and Haplotype Analysis
Genomic DNA was extracted from peripheral blood leukocytes using the FlexiDNA extraction kit (Qiagen, Mississauga, Ontario, Canada). We genotyped all of the available study participants with five simple-sequence repeat markers each at the PKD1 and PKD2 loci using an established protocol.22 The locations of these markers relative to the PKD1 locus are shown as follows (the number between markers denotes intermarker distance in cM): HBAP1-2.0-PKD1-0.1-CW4-0.1-SM6-0.6-D16S2618-2.0-D16S423. The locations of these markers relative to the PKD2 locus are shown as follows (the number between markers denotes intermarker distance in cM): D4S231-2.0-D5S1534-2.3-SPP1-0.2-PKD2-0.5-D4S1563-2.0-D4S423. Genotyping was performed by 32P α-dCTP labeling of the PCR products and analyzed by PAGE. All genotypes were performed and scored independently by K.R.W. without any knowledge of the clinical status of the study participants. Haplotypes were constructed by hand and using the program GENEHUNTER (v2.1_r5). Two-point linkage with “affected-only analysis” was performed using the M-LINK program of the FASTLINK 4.0 package (http://www.ncbi.nlm.nih.gov/CBBresearch/Schaffer/fastlink.html). Multipoint linkage with “affected-only analysis” was also performed in a number of larger families using GENEHUNTER (v2.1_r5). An autosomal dominant model with a disease allele frequency of 0.001 and a phenocopy rate of 0.001 was assumed. Marker allele frequencies were obtained from married-in participants and reconstruction of the genotypes of the founders.
Gene-Based Mutation Screening
In selected families with suspected PKD2, we amplified 18 PCR fragments from genomic DNA covering all 15 exons and splice junctions of PKD2, screened them by single-strand conformational polymorphism or dHPLC, and sequenced all fragments with sequence variants.11,22 In suspected families who had PKD2 and in which we failed to identify any pathogenic mutation and in selected families suspected to have PKD1, sequence analysis of both PKD1 and PKD2 was performed in a clinically affected subject using a commercial diagnostic service (Athena Diagnostics, Worcester, MA; http://www.athenadiagnostics.com/content/test-catalog/find-test/service). Briefly, genomic DNA was used for locus-specific long-range PCR amplification of eight segments encompassing the entire PKD1 duplicated region. The long-range PCR products served as templates for 43 nested PCRs, and the unique region of PKD1 and the entire PKD2 were amplified from genomic DNA in 28 additional PCRs. All 71 PCR products were bidirectionally sequenced, including the coding regions and exon-intron splice junctions of both genes.22
Prediction of ADPKD Gene Type
The underlying gene type (PKD1 versus PKD2) for each family was the primary outcome for the predictive testing. We examined all of the affected study participants within each family to identify individuals at the extremes of the age cutoffs indicative of disease severity. For PKD1 prediction, a family history of at least one affected member who developed ESRD at or before a specific age was used as the test criterion, and the sensitivity, specificity, PPV, and NPV were calculated for the different cutoffs of early age at ESRD indicative of severe disease. For PKD2 prediction, a family history of at least one affected member who remained renal sufficient or developed ESRD at or after a specific age was used as the test criterion, and the sensitivity, specificity, PPV, and NPV were calculated for the different cutoffs of late age of renal sufficiency or ESRD indicative of mild disease.23
Disclosures
None.
Acknowledgments
This study was supported by grants from the Kidney Foundation of Canada and Canadian Institute of Health Research (MOP77806) to Y.P., a Canada Research Chair in Genetics of Complex Diseases to A.D.P., and the Canadian Institute of Health Research Distinguished Scientist Award to P.P.
We are indebted to all of the participants of the study. We also thank Dr. R.M.A. Richardson for critique of this article.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Harris P, Torres V: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong A, Harris PC: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Iglesias C, Torres V, Offord K, Holley K, Beard C, Kurland L: Epidemiology of adult polycystic kidney disease. Am J Kidney Dis 2: 630–639, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Davies F, Coles G, Harper P, Williams AJ, Evans C, Cochlin D: Polycystic kidney disease re-evaluated: A population-based study. Q J Med 79: 477–485, 1991 [PubMed] [Google Scholar]
- 5.US Renal Data System: Excerpts from the USRDS 2004 Annual Data Report. Am J Kidney Dis 45 [Suppl]: S1–S280, 2005 [Google Scholar]
- 6.Peters D, Sandkuijl L: Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol 97: 128–139, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Dobin A, Kimberling W, Pettinger W, Bailey-Wilson JE, Shugart Y, Gabow P: Segregation analysis of autosomal dominant polycystic kidney disease. Genet Epidemiol 10: 189–200, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang Q, Thompson PA, Zhu F, Miller JPCRISP Consortium: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hateboer N, van Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Rossetti S, Burton S, Strmecki L, Pond GR, San Millan JL, Klaus Z, Barratt TM, Ozen S, Torres VE, Bergstralh EJ, Winearls CG, Harris P: The position of the polycystic kidney disease 1 gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 13: 1230–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Magistroni R, He N, Wang KR, Andrew R, Johnson A, Gabow P, Dicks E, Parfrey P, Torra R, San-Millan JL, Coto E, Van Dijk M, Breuning M, Peters D, Bogdanova N, Ligabue G, Albertazzi A, Hateboer N, Demetriou K, Pierides A, Deltas C, St. George-Hyslop P, Ravine D, Pei Y: Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 1164–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Paterson AD, Magistroni R, He N, Wang K, Johnson A, Fain PR, Dicks E, Parfrey P, St. George-Hyslop P, Pei Y: Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 16: 755–762, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Pei Y: Diagnostic approach in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 1: 1108–1114, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Rossetti S, Consugar M, Chapman A, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang Q, Thompson PA, Miller JP, Harris PCCRISP Consortium: Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Gonzalez MA, Jones JG, Allen SK, Palatucci CM, Batish SD, Seltzer WK, Lan Z, Allen E, Qian F, Lens XM, Pei Y, Germino GG, Watnick TJ: Evaluating the clinical utility of a molecular genetic test for polycystic kidney disease. Mol Genet Metab 92: 160–167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dicks E, Ravani P, Langman D, Davidson WS, Pei Y, Parfey PS: Incident renal events and risk factors in autosomal dominant polycystic kidney disease: A population and family-based cohort followed for 22 years. Clin J Am Soc Nephrol 1: 710–717, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Pei Y, Paterson AD, Wang KR, He N, Hefferton D, Watnick T, Germino GG, Parfrey P, Somlo S, St. George-Hyslop P: Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet 68: 355–363, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossetti S, Adeva M, Kubly V, Consugar MB, Torres VE, Harris PC: An Olmsted Country population-based study indicates that PKD2 is more common than previously described [Abstract]. J Am Soc Nephrol SA-PO093, 2007 [Google Scholar]
- 19.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasound diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Diseases Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Paterson AD, Zahirieh A, He N, Wan K, Pei Y: Molecular diagnostics in autosomal dominant polycystic kidney disease: Utility and limitations. Clin J Am Soc Nephrol 3: 146–152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB: Diagnosis and Screening. In: Evidence-Based Medicine: How to Practice and Teach EBM, 2nd Ed., edited by Parkinson M.Edinburgh, Churchill Livingstone, 2000, pp 73–108 [Google Scholar]