Abstract
CXC chemokine ligand 12 (CXCL12; stromal cell–derived factor 1) is a unique homeostatic chemokine that signals through its cognate receptor, CXCR4. CXCL12/CXCR4 signaling is essential for the formation of blood vessels in the gastrointestinal tract during development, but its contribution to renal development remains unclear. Here, we found that CXCL12-secreting stromal cells surround CXCR4-positive epithelial components of early nephrons and blood vessels in the embryonic kidney. In glomeruli, we observed CXCL12-secreting podocytes in close proximity to CXCR4-positive endothelial cells. Both CXCL12- and CXCR4-deficient kidneys exhibited identical phenotypes; there were no apparent abnormalities in early nephrogenesis or in differentiation of podocytes and tubules, but there was defective formation of blood vessels, including ballooning of the developing glomerular tuft and disorganized patterning of the renal vasculature. To clarify the relative importance of different cellular defects resulting from ablation of CXCL12 and CXCR4, we established endothelial cell–specific CXCR4-deficient mice, which recapitulated the renal phenotypes of conventional CXCR4-deficient mice. We conclude that CXCL12 secreted from stromal cells or podocytes acts on endothelial cells to regulate vascular development in the kidney. These findings suggest new potential therapeutic targets for remodeling the injured kidney.
Nephrogenesis requires a coordinated process during development and has two distinct embryologic aspects. One is the development of epithelial components. They originate from interactions between the metanephric blastema, a group of mesenchymal cells in the genital ridge, and the ureteric bud (UB), an epithelial outgrowth of the nephric duct. When the tips of the UB invade the metanephric blastema, mutual inductive signals initiate a cascade of events, including UB branching and mesenchymal aggregation, which is followed by formation of nephrons. The other essential aspect is assembly of renal microcirculation, a multistep process including differentiation of endothelial progenitor cells, recruitment of endothelial cells into the glomerular vascular cleft, and maturation of functional vessels.1 Although great advances have been made in recent years, further effort is required to elucidate the molecular determinants regulating vessel formation in the kidney.
Chemokines are a family of structurally related chemoattractant cytokines. Among them, CXC chemokine ligand 12 (CXCL12; stromal cell-derived factor 1 [SDF-1]) is a unique homeostatic chemokine that signals through its cognate receptor CXCR4 and plays essential roles in hematopoiesis and organogenesis.2,3 Mice lacking CXCL12 and CXCR4 display identical and lethal phenotypes, indicating a monogamous relationship between these molecules.4 CXCL12 and CXCR4 are required for B cell development,5–8 colonization of bone marrow by hematopoietic stem cells,9,10 colonization of the gonads by primordial germ cells,11 and cardiac4,6,12 and cerebellar8,12 development. In addition, CXCL12 knockout (KO) or CXCR4 KO embryos display defects in vascularization of the gastrointestinal tract but not of the yolk sac, brain, or heart, demonstrating the essential organ-specific functions of CXCL12/CXCR4 in blood vessel formation4,13; however, we and another group8 observed that kidneys of CXCR4 KO mice have vascular congestion. Furthermore, it has been reported that CXCL12 is expressed in the developing glomeruli.14 These observations prompted us to investigate the roles of this chemokine in kidney development. Here we demonstrate the pivotal roles of the CXCL12/CXCR4 axis in kidney development with a focus on blood vessel formation, using conventional CXCL12 and CXCR4 KO mice and endothelial cell–specific CXCR4 KO mice.
Results
Expression of CXCL12 in the Developing Kidney
We first investigated the expression of CXCL12 in the developing kidney using CXCL12/GFP knock-in mice, in which the green fluorescence protein (GFP) gene has been inserted into the CXCL12 locus, resulting in recapitulation of the endogenous expression pattern of CXCL12 (Figure 1, Supplemental Figure 1).9 GFP was detected in the stromal cells surrounding developing nephrons (UBs, cap mesenchyme [CM], and pretubular aggregates; Figure 1B) and in the stromal cells surrounding blood vessels (Supplemental Figure 1, A and B) in the developing kidney and was expressed continuously during embryogenesis including at embryonic day 13.5 (E13.5; data not shown), E15.5 (Figure 1, Supplemental Figure 1), and E17.5 (data not shown). GFP-positive cells were negative for the pericyte marker PDGF receptor β (PDGFRβ; Supplemental Figure 1C). In the developing glomeruli, an adjacent part of the podocytes began to express CXCL12 (Figure 1C, arrows), and, thereafter, all podocytes expressed CXCL12 in the mature glomeruli (Figure 1D). CXCL12 was also expressed in some segments of arteries, including interlobular arteries (Supplemental Figure 1, A and D).
Figure 1.
Expression of CXCL12 in the developing kidney using CXCL12/GFP knock-in mice at E15.5. (A) Appearance of a whole section of the kidney at low magnification. (B) The section was immunostained red (Alexa555) for calbindin, a UB marker. GFP was expressed in the stromal cells surrounding developing nephrons (UB, CM [arrows], and pretubular aggregates [PA; arrowheads]). (C and D) Another section was immunostained with Alexa555-labeled anti-VEGFR2 antibody, a marker for endothelial cells. (C) CXCL12 was expressed in some developing podocytes (arrows) in the primitive glomeruli. (D) All podocytes express CXCL12 in the mature glomeruli. Magnifications: ×100 in A; ×400 in B; ×600 in C and D.
Expression of CXCR4 in the Developing Kidney
We then investigated the expression of CXCR4 by immunohistochemistry (Figure 2) and in situ hybridization (Supplemental Figure 2). The specificity of these methods in detecting CXCR4 was confirmed using CXCR4 null kidneys as negative controls (Figure 2C, right) and using sense probe (Supplemental Figure 2, B and D). CXCR4 was consistently expressed in the nephrogenic zone during embryogenesis, including at E13.5 (Figure 2A), E15.5 (data not shown), and E17.5 (Figure 2, B and C). At the early embryonic stage, CXCR4 was strongly expressed in UBs (Figure 2A, arrows) and pretubular aggregates (Figure 2A, arrowheads), whereas its expression at a later stage switched to CM (Figure 2C) instead of UBs. Along with nephrogenesis, CXCR4 was downregulated and completely disappeared from these epithelial components in S-shaped bodies (data not shown).
Figure 2.
Expression of CXCR4 in the developing kidney. CXCR4 was stained red (Alexa555) in the kidney sections of wild-type mice at E13.5 (A) and E17.5 (B through G). (A and B) Whole sections of the kidneys under low magnification revealed that CXCR4 is mainly expressed in the nephrogenic zone. (C) Co-immunostaining for cited1, a marker for CM, in green (Alexa488; left: CXCR4, middle: merged image) shows that CXCR4 is expressed in CM (arrows in inset) and PA (arrowheads in inset). Sections of CXCR4 null kidney are shown as negative control for CXCR4 staining (right). (D through F) Another section was co-immunostained for VEGFR2 in green (Alexa488; left: CXCR4, right: merged image). (D) CXCR4 was first detected only weakly in the endothelial cells in the cleft of the comma-shaped body (arrows) and more intensely in those at a basal position (arrowheads). (E) CXCR4 was detected in arterioles leading to the primitive glomeruli (arrows). Weak expression was detected in a stalk of glomerular endothelial cells (arrowheads). (F) In more mature glomeruli, CXCR4 was expressed in many glomerular endothelial cells. (G) Co-immunostaining for renin in green (Alexa488) shows that renin is expressed in pericytes surrounding CXCR4-positive endothelial cells, suggesting that CXCR4-positive vessels are afferent arterioles. Glm, glomerulus. Magnifications: ×100 in A and B; ×400 in C and E; ×600 in D, F, and G.
CXCR4 was also expressed in the vasculature. It was only weakly detected in the endothelial cells in the cleft of the comma-shaped body (Figure 2D, arrows), and was more intensely expressed in basal endothelial cells (Figure 2D, arrowheads). CXCR4 was intensely expressed in the arterioles connecting the capillary loop stage glomeruli (Figure 2E, arrows). Only glomerular endothelial cells in the stalk expressed CXCR4 at this stage (Figure 2E, arrowheads), but most of the glomerular endothelial cells expressed CXCR4 in mature glomeruli (Figure 2F). Co-immunostaining for renin revealed that renin-positive pericytes surrounded the CXCR4-positive arterioles, suggesting that CXCR4-positive arterioles were afferent arterioles (Figure 2G). The results from in situ hybridization of CXCR4 in embryonic kidney at E17.5 (Supplemental Figure 2) were largely consistent with those from the immunohistochemical analysis. CXCR4 mRNA was detected in early nephrons, including the UBs, CM, and pretubular aggregates (Supplemental Figure 2, A and C), and was also strongly detected in inner glomerular cells (Supplemental Figure 2E).
Spatial Relationship between CXCL12 and CXCR4 in Developing Kidney
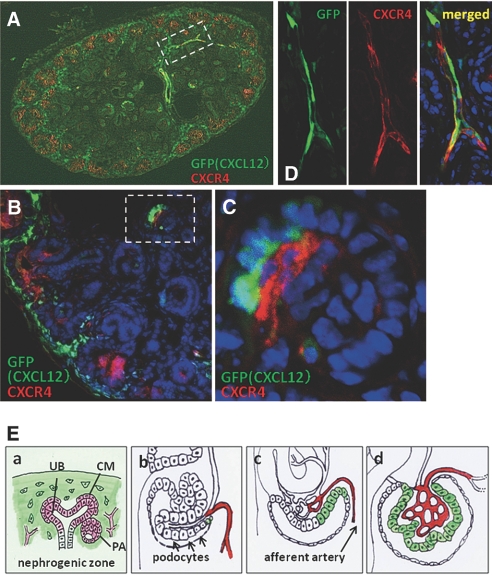
To clarify the spatial relationship between CXCL12- and CXCR4-positive cells, we performed co-immunostaining of CXCR4 in kidney sections from CXCL12/GFP knock-in mice at E17.5. In the nephrogenic zone, CXCL12-positive stromal cells surrounded CXCR4-positive developing early nephrons and blood vessels (Figure 3, A and B). In primitive glomeruli, some podocytes expressed CXCL12, and glomerular endothelial cells just adjacent to the CXCL12-positive podocytes expressed CXCR4 (Figure 3C). In contrast, all of the podocytes and endothelial cells in mature glomeruli expressed CXCL12 and CXCR4, respectively (data not shown). Of note, at least some of the interlobular arteries apparently expressed both CXCL12 and CXCR4 at the same time (Figure 3D), suggesting that CXCL12/CXCR4 signals may function in an autocrine manner to extend arteries.
Figure 3.
Spatial relationship between CXCL12 and CXCR4 in developing kidney. Kidney sections from CXCL12/GFP knock-in mice were stained red (Alexa555) for CXCR4. (A and B) Appearance of whole kidney section (A) and magnified image (B) demonstrates that CXCL12-positive stromal cells surround CXCR4-positive developing nephrons. (C) Higher magnification of the glomerulus in the dotted lines in B suggests that some podocytes express CXCL12 and that glomerular endothelial cells just adjacent to the podocytes express CXCR4. (D) Higher magnification of the region in the dotted lines in A of GFP (left) and CXCR4 staining (middle) in the interlobular arteries. Merged image (right) indicates that at least some interlobular arteries express both CXCL12 and CXCR4. (E) Summary of the expression of CXCL12 (green) and CXCR4 (red) in the developing early nephron (a) and glomeruli (b through d). See the Discussion section for more details. Magnifications: ×100 in A; ×400 in B; ×600 in C and D.
Analysis of CXCR4 and CXCL12 Null Kidneys
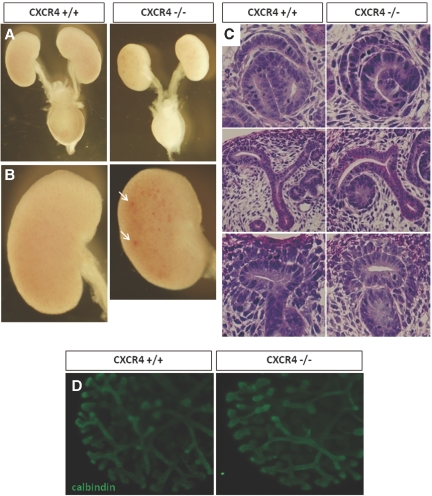
Next, we analyzed the kidneys of CXCR4 KO mice.4 Because approximately 50% of CXCR4 KO mice die before E18.5 and 100% die shortly after birth,4 we analyzed the embryonic kidney mainly at E17.5, when developing and mature nephrons could be observed in the same kidney. Macroscopically, the urinary tracts and bladders of the CXCR4 KO mice exhibited no abnormalities at E17.5 compared with wild-type mice (Figure 4A). CXCR4 null kidneys were smaller in weight by 10 to 15% than wild-type kidneys (Figure 4, A and B) and exhibited congestion of blood and/or petechial hemorrhage (Figure 4B, arrows).
Figure 4.
Analysis of CXCR4 null kidneys. (A and B) Stereoscopic appearances of wild-type (left) and CXCR4 null (right) urinary organs. Kidneys, urinary tracts, and bladders from mice at E17.5 are shown after removal of genital organs. (A) CXCR4 null kidneys are smaller, but the urinary tract and bladder show no macroscopic abnormalities. (B) Magnified images of CXCR4 null kidneys show petechial hemorrhages (arrows). (C) Assessment of early development of nephrons. Periodic acid-Schiff (PAS)-stained sections of wild-type (left) or CXCR4 null (right) kidneys at E13.5 are shown. Early nephrogenesis, including formation of comma- or S-shaped bodies (top), UB branching and formation of renal vesicles (middle), and aggregations of metanephric mesenchyme (lower), was not affected by ablation of CXCR4. (D) Branching morphogenesis of CXCR4 null kidneys. Kidneys were resected from wild-type or CXCR4 KO mice at E12.5 and cultured on Transwell for 48 h. They were then whole mount–immunostained for calbindin, revealing that UB branching was not affected by the ablation of CXCR4. Magnifications: ×10 in A and B; ×200 in C and D.
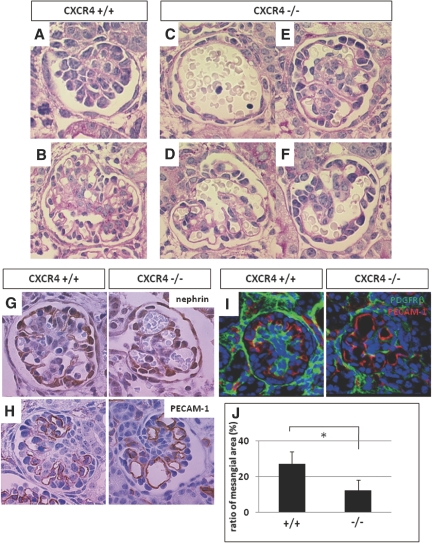
The observation that CXCL12-secreting stromal cells surround CXCR4-positive early nephrons prompted us to examine defects in nephrogenesis at early stages in the CXCR4 null kidney. Compared with wild-type kidney, periodic acid-Schiff–stained sections from CXCR4 null kidneys at E13.5 displayed no significant abnormalities in morphogenesis, showing normal formation of comma- or S-shaped bodies and aggregations of metanephric mesenchyme (Figure 4C). Platelet/endothelial cell adhesion molecule-1 (PECAM-1; CD31) staining to label endothelial cells revealed that the development of these cells in the cleft of early nephrons was not apparently disrupted in CXCR4 null kidneys (Supplemental Figure 3A), which is consistent with the observation that endothelial cells at this stage express CXCR4 weakly or not at all (Figure 2D). To investigate UB branching, we used a calbindin antibody to immunostain organ cultures of CXCR4 null or wild-type kidney at E12.5 that had been grown for 48 h (Figure 4D). No differences in calbindin staining between the two kidney types were observed; however, the CXCR4 null mice displayed severe abnormalities in glomerular morphogenesis from the capillary loop to mature stages (Figure 5, C through F) compared with wild-type mice (Figure 5, A and B). CXCR4 KO mice exhibited glomeruli with aneurismal dilation of the capillaries in which the complex tuft pattern was lost and a few distended or ballooned capillaries were observed. The severity of this abnormality differed between glomeruli: Some were composed of a single capillary tuft (Figure 5C), whereas others had a morphology similar to the wild-type (Figure 5E). To elucidate the cell lineages of abnormal glomeruli, sections were immunostained for nephrin (Figure 5G), synaptopodin, integrin α3, and Wilms' tumor 1 (data not shown) for podocytes, PECAM-1 (Figure 5H), and vascular endothelial growth factor receptor 2 (VEGFR2; data not shown) for endothelial cells and PDGFRβ (Figure 5I) for mesangial cells. A few distended capillary loops in CXCR4 null glomeruli were overlain with nephrin-positive podocytes and lined with PECAM-1–positive endothelial cells, both of which expressed the respective markers similar to wild-type glomeruli. In contrast, the number of PDGFRβ-positive mesangial cells in the total glomerular area was significantly lower in CXCR4 null glomeruli (Figure 5J). Electron microscopic analysis of mice at E17.5 demonstrated that foot processes were significantly effaced in CXCR4 null glomeruli, with endothelial cells and podocytes securely attached to the glomerular basement membrane (GBM; Figure 6). Furthermore, it was demonstrated that there were fewer fenestrations in CXCR4 null glomeruli than in normal glomeruli (Figure 6). Some of the glomeruli of CXCR4 KO mice exhibit phenotypes that resemble those of the PDGF, B polypeptide (PDGFB)15 and PDGFRβ16 KO mice; therefore, we investigated the expression of PDGFB on wild-type and CXCR4 null kidneys using in situ hybridization (Supplemental Figure 3B). We observed that glomerular endothelial cells continue to express PDGFB similar to the wild-type even after ablation of CXCR4, suggesting that the ballooning of glomerular tufts was not due to the reduction of PDGFB in endothelial cells.
Figure 5.
Analysis of glomerulogenesis in CXCR4 null kidneys. (A through F) Deficiencies in glomerulogenesis in CXCR4 null kidneys. PAS-stained images of premature to mature glomeruli of wild-type (A and B) or CXCR4 KO (C through F) mice at E17.5 are shown. Compared with wild-type kidney, CXCR4 null kidneys display abnormal glomerulogenesis: Some glomeruli consist of a single capillary tuft surrounded by a single array of cells (C), and other glomeruli show ballooning of capillary tufts to various extents (D through F). (G through I) Expression of markers for cell lineages in wild-type (left) and CXCR4 null (right) glomeruli at E17.5. Glomeruli were immunostained for nephrin for podocytes (G), PECAM-1 for endothelial cells (H), and PDGFRβ for mesangial cells (I). (J) Analysis of the ratio of the PDGFRβ-positive area (mesangial cells area) to the total glomerular area in wild-type and CXCR4 null kidneys. The ratio is expressed as the mean ± SD. *P < 0.05 versus wild-type glomeruli. Magnification, ×1000.
Figure 6.
Electron microscopic analysis of CXCR4 null glomeruli. Low-magnification (top) and high-magnification (middle) images show foot process effacement (arrows) after CXCR4 ablation, although endothelial cells and podocytes are securely attached to the GBM. (Bottom) There are fewer fenestrations in CXCR4 null glomeruli than in normal glomeruli (arrows). Magnifications: ×2000 in top; ×10,000 in middle and bottom.
Because dilated capillary structures and congestion of red blood cells were observed in the mutant metanephroi, we investigated formation of the vasculature in addition to the glomerular tuft. Whole-mount staining with anti–PECAM-1 antibody of the CXCR4 null kidneys at different stages demonstrated that the vascular patterning was grossly disorganized and that the vasculature diameters were irregular even at E13.5, which is before the maturation of glomeruli (Figure 7A). Similar abnormalities were also detected at later stages (E15.5 [Figure 7B]; E17.5 [Figure 7C]).
Figure 7.
Assessment of vascular patterning of whole kidneys from CXCR4 KO mice. (A through C) Whole-mount immunostaining for PECAM-1 was performed using kidneys from wild-type (left) and CXCR4 KO (right) mice at E13.5 (A), E15.5 (B), and E17.5 (C). Positive staining was visualized by the horseradish peroxidase–DAB method. Magnified images are also presented in the insets. In the CXCR4 null kidney, regular vascular pattering is disrupted, with narrowing (arrows) or dilation (arrowheads) observed at all stages. Magnification, ×10.
Next, we explored the kidneys of CXCL12 KO mice,6 which have been reported to exhibit identical phenotypes as CXCR4 KO mice.4 CXCL12- and CXCR4 null kidneys exhibited similar renal phenotypes, including capillary ballooning in glomeruli (Supplemental Figure 4A), disorganized vascular patterning (Supplemental Figure 4B), and reduction in mesangial cell numbers (data not shown).
Analysis of Kidneys of Endothelial Cell–Specific CXCR4 KO Mice
Nonendothelial cells also contribute to proper glomerular capillary development.1 Because we demonstrated that CXCR4 was temporally expressed in nonendothelial cells, it was unclear which CXCR4-expressing component is responsible for the defect in glomerular capillary development. To determine whether endothelial expression of CXCR4 predominantly affects glomerular capillary development, we established endothelial cell–specific CXCR4 KO mice. We used mice with a CXCR4 allele flanked by loxP sites17 and crossed them with transgenic mice that express Cre under the endothelial-specific Tie2 promoter.18 We found that most Tie2-Cre CXCR4 flox/flox mice died in utero just before birth and that E17.5 embryos developed defects in glomeruli and blood vessels similar to conventional CXCR4 KO mice (Supplemental Figure 4C). Kidneys from Tie2-Cre CXCR4 flox/flox mice surviving for 3 d (n = 4) exhibited dilated proximal tubules with vacuolation (Figure 8, A through D) in addition to ballooning of glomerular tufts (Figure 8, E and F). Notably, compared with conventional CXCR4 KO mice at E17.5, substantial numbers of glomerular endothelial cells were detached from the GBM in the endothelial cell–specific CXCR4 KO mice on day 3 (Figure 8, E through G).
Figure 8.
Analysis of the kidneys from endothelial cell–specific CXCR4 KO mice. (A through F) PAS-stained kidney sections of CXCR4 flox/+ (as a control; A) and Tie2-Cre CXCR4 flox/flox mice (B through F) 3 d after birth. (A and B) Low-magnification images. Arrows indicate abnormally dilated tubules. (C and D) Magnified view of B (right). Proximal tubules are dilated and flattened in shape with vacuolations (arrows). (E and F) Glomeruli of Tie2-Cre CXCR4 flox/flox mice exhibit ballooning of the capillary tuft, identical to conventional CXCR4 null glomeruli. Some endothelial cells are detached from the GBM (arrows). (G) Electron microscopic images of glomeruli from Tie2-Cre CXCR4 flox/flox mice. Endothelial cells detached from the GBM are shown magnified (middle and right). Magnifications: ×100 in A and B; ×200 in C; ×1000 in D through F; ×2000 in G, left; ×10,000 in G, middle and right.
Discussion
In this study, we elucidated the divergent expression pattern of CXCL12 and CXCR4 in the developing kidney. Using conventional and conditional CXCR4 KO mice, we also demonstrated that the CXCL12/CXCR4 axis is essential for the development of vasculature in the kidney.
Although expression of CXCL12/CXCR4 has already been described in the embryonic kidney of mice14 and humans19 and in crescentic glomerulonephritis of mice and human kidney,14 our data showed the spatial and temporal relationship between CXCL12- and CXCR4-positive cells in more detail. These data are summarized in Figure 3E. The features are as follows: (1) CXCL12-secreting cells are always in close contact with CXCR4-positive cells in the nephrogenic zone and in glomeruli, suggesting the presence of paracrine signaling from stromal cells and podocytes to the developing nephrons and endothelial cells, respectively; (2) both CXCL12 and CXCR4 are expressed in various cell types and show increased and decreased expression in various lineages; and (3) some vascular segments, including the interlobular arteries, express both CXCL12 and CXCR4 at the same time, suggesting that the CXCL12/CXCR4 axis promotes the process of angiogenic sprouting using an autocrine mechanism (Figure 3D).
On the basis of expression analysis, we speculated that CXCL12 would support nephrogenesis and vascular development of the kidney. To investigate this hypothesis, we first explored the development of the epithelial components of the nephron, including UB branching, aggregation of mesenchymal cells, formation of comma- and S-shaped bodies, and differentiation of podocytes and tubules of CXCR4 null kidneys; however, we found no differences in these features compared with wild-type kidneys, although it is possible that there is a subtle branching defect of UB that we cannot detect with whole-mount immunostaining on the explants. Because embryonic lethality precludes analysis of CXCL12/CXCR4 signaling for longer periods, additional analysis is required to conclude that this signaling is not essential for the development of early nephrons or differentiation of epithelial components; however, the redundancy of the chemokine system is thought to provide a high degree of flexibility in vivo, such that other chemokines may compensate for the loss of CXCR4.
Next, we explored the development of renal vasculature. The observation that some podocytes at the vascular pole began to secrete CXCL12 and that the “stalk” of glomerular endothelial cells, which are just adjacent to these podocytes, express CXCR4 suggests that a gradient of CXCL12 may function as a guide for glomerular capillary tuft growth. In support of this idea, we observed abnormal glomerular capillary development in the CXCR4 null kidneys. CXCR4 null glomeruli at the capillary loop to mature stages exhibited aneurismal dilation of the capillaries, which is variable in extent. A few distended capillary loops in mutant glomeruli were overlain with podocytes and lined with endothelial cells, both of which expressed the respective markers similar to wild-type glomeruli. In contrast, the number of mesangial cells was significantly lower in CXCR4 null glomeruli. Considering that PDGFB was expressed similarly in glomerular endothelial cells of the CXCR4 KO mice, it is speculated that another factor, which is secreted from endothelial cells by signaling through CXCR4, is responsible for the recruitment and/or proliferation of mesangial cells. We would like to seek to understand how the CXCL12/CXCR4 axis is involved in mesangial cell development in the future. Electron microscopic analysis of mice at E17.5 demonstrated that there were fewer fenestrations in endothelial cells of CXCR4 null glomeruli than in those of normal glomeruli (Figure 6), indicating that the CXCL12/CXCR4 axis may modulate signaling through VEGFR, which is a key inducer of fenestrations.20
In addition, we demonstrated that embryonic kidneys from CXCL12 KO mice have an identical phenotype as kidneys from CXCR4 null mice. Recently, CXCR7 (RDC-1) was reported to be a second CXCL12 receptor.21 Conventional and endothelial cell–specific CXCR7 KO mice die at birth with ventricular septal defects and semilunar heart valve malformation.22 Thus, we investigated the expression of CXCR7 in the embryonic kidney and showed that CXCR7 is expressed mainly in renal tubules (Supplemental Figure 5). Further investigation of the phenotypes of CXCR7 null kidneys will be required, but CXCR7 may not be involved in the vascular abnormalities seen in CXCL12 and CXCR4 mutant kidneys.
It has been demonstrated that tightly coordinated cross-talk between glomerular cells is required for the formation of a functional glomerular filtration barrier.1 For example, mice in which expression of PDGFRβ in mesangial cells16 or integrin α3 in podocytes23 is disrupted exhibit a similar phenotype in the glomeruli, including ballooning of the glomerular tuft. Because CXCR4 is temporally expressed in pretubular aggregates containing presumptive podocytes, we hypothesized that deletion of CXCR4 in endothelial cells would recapitulate the defects in blood vessel formation observed in CXCR4 KO mice. To address this question, we examined the kidneys of mice with endothelial cell–specific deletion of CXCR4 and found defects identical to those observed in conventional CXCR4 KO mice. This supports the notion that CXCL12 secreted from stromal cells and podocytes acts on endothelial cells to regulate vascularization of the kidney, including glomeruli; however, we cannot exclude the possibility that the defects in blood vessel formation in the mutants may be due to dysfunction of Tie2-expressing hematopoietic cells, because evidence is emerging that cells derived from bone marrow may also contribute to embryonic and postnatal angiogenesis.24 Further study is required to discriminate accurately the relative contribution of the two cell lineages (hematopoietic stem cells and endothelial cells) to blood vessel formation in the kidney.
Our expression analysis suggests that CXCL12 secreted from podocytes may act on the stalk of glomerular endothelial cells and on afferent arterioles that strongly express CXCR4 to attract them during tuft formation. Alternatively, CXCL12 secreted from podocytes may upregulate the expression of CXCR4 on glomerular endothelial cells, allowing them to induce glomerular tuft formation. The molecular mechanism underlying the CXCL12/CXCR4 signaling that regulates blood vessel formation in the kidney remains unclear. One possibility is that CXCL12/CXCR4 signaling acts on endothelial cells to promote survival signals and/or cell proliferation. In support of this idea, the p44/42 mitogen-activated protein kinase and Akt pathways both are activated by treatment of human umbilical vein endothelial cells with recombinant CXCL12 (data not shown). Although we did not find evidence that CXCR4 null endothelial cells undergo apoptosis or proliferate poorly, our observation that glomerular endothelial cells tend to detach from the GBM in Tie2-Cre CXCR4 flox/flox mice that survived 3 d after birth is consistent with this possibility.
The observation that there are organ-restricted (gastrointestinal4 and renal [this article]) vascular phenotypes in CXCR4 KO mice is intriguing. The precise mechanism is unclear, because endothelial cells in other organs also express CXCR4 (data not shown). One possibility is that blood vessel formation develops in an organ-specific manner and the role of the CXCL12/CXCR4 axis differs in each organ. In the developing small intestine, for example, many short, interconnecting vessels form between larger superior mesenteric arteries and the neighboring primary capillary plexus surrounding the primitive gut, which elongate and become the arteries supplying the small intestine.13 CXCL12 or CXCR4 KO mice lack filopodial extension and intussusceptions from endothelial cells of superior mesenteric arteries; thus, these interconnecting vessels fail to form, leading to gastrointestinal bleeding. In addition, because the CXCL12/CXCR4 axis may play an essential role in later stages of blood vessel formation (e.g., maturation), CXCR4 mutant embryos may die before a vascular phenotype fully appears, especially in organs in which blood vessel formation develops after birth (e.g., retina).
Although we confined our analysis to the roles of the CXCL12/CXCR4 axis in development, our findings may help determine the critical steps in microvascular repair after renal injury. Additional investigation will be necessary to provide a more complete understanding of microvessel formation in general.
Concise Methods
Mice
CXCL12 KO mice,6 CXCR4 KO mice,4 CXCL12/GFP knock-in mice,9 CXCR4 flox/+ mice,13 and Tie2-Cre transgenic mice18 have been described previously. They were backcrossed more than seven times with C57BL/6 mice. All animal experiments were conducted in accordance with institutional guidelines.
Antibodies
We used the following antibodies: Antibodies for CXCR4, clone 2B11 (BD Pharmingen, San Diego, CA); VEGFR2, clone 55B11 (Cell Signaling Technology, Danvers, MA); PDGFRβ, clone 28E1 (Cell Signaling Technology); calbindin, KD-15 (Sigma, St. Louis, MO); renin, provided by Prof. T. Inagami (Vanderbilt University School of Medicine, Nashville, TN); cited1, RB9201 (Lab Vision, Fremont, CA); PECAM-1, clone MEC13.3 (BD Pharmingen); nephrin, GP-N2 (Progen, Heidelberg, Germany); CXCR7 (RDC1), ab38089 (Abcam, Cambridge, UK); horseradish peroxidase–conjugated anti-rat antibody (DAKO, Glostrup, Denmark); biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA); and Alexa555- and Alexa488-conjugated secondary antibodies (Molecular Probes, Carlsbad, CA).
Immunohistochemistry
Except when detecting co-localization of GFP, we adopted the atypical “zinc-fixed frozen method” for immunostaining of CXCR4, because this method preserves antigenicity to a greater extent than the conventional formalin-fixed frozen method. Excised samples were immersed in ice-cold zinc fixative (BD Pharmingen) for approximately 12 h with gentle shaking. After the samples were rinsed with distilled water several times, they were immersed in graded concentrations of sucrose solution in PBS. Sections in 30% sucrose in PBS were embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan) and quickly chilled in liquid nitrogen. Sections 5 μm thick were blocked in 1% BSA (Sigma) in Tris-buffered saline–Tween 20 (20 mM Tris-HCl [pH 7.5], 137 mM NaCl, and 0.1% Tween 20 [vol/vol]) for 1 h at room temperature and incubated in primary antibody solution overnight at 4°C. For unconjugated primary antibody, appropriate secondary antibodies were used. Slides were analyzed using confocal microscopy (Radiance 2100; Carl Zeiss, Jena, Germany). For immunostaining of CXCR4 on the sections from CXCL12/GFP knock-in mice or for other proteins, we used 4% paraformaldehyde (PFA) in PBS for fixation. Sections were fixed for approximately 4 to 6 h on ice and processed as already described.
Immunohistochemical staining of nephrin and PECAM-1 on paraffin-embedded sections was performed using a streptavidin-biotin staining method (Vector ABC kit; Vector Laboratories). Sections were autoclaved in 0.01 M citrate buffer (pH 6.0) for 10 min at 120°C to retrieve the antigen.
Mesangial area was evaluated as glomerular expression of PDGFRβ by computerized image analysis using the Mac SCOPE program. In brief, 60 glomeruli of wild-type and CXCR4 null kidney were photographed, and positive areas were highlighted on the captured images. The area of positive staining relative to each total glomerular area was automatically calculated as a percentage with determined threshold settings. All data are expressed as means ± SD. Statistical significance (defined as P < 0.05) was evaluated using t test.
Whole-Mount Immunostaining
For whole-mount immunostaining for PECAM-1, embryonic kidneys at respective stages were immersed immediately after excision in 4% PFA in PBS for 12 h at 4°C and washed in ice-cold PBS several times. Thereafter, kidneys were dehydrated with graded concentrations of ice-cold methanol (up to 100%). For blocking of internal peroxidase activity, they were incubated in 0.3% (1:100) H2O2 in methanol for 30 min on ice. They were hydrated in graded concentrations of ice-cold methanol and then blocked in 2% skim milk (commercial), 1% Triton X-100, and 0.2% BSA in PBS (PBSMT) for 24 h at 4°C. The kidneys were then incubated in primary antibody solution (1 μg/ml) for 24 h at 4°C. Kidneys were washed in PBSMT on ice five times for 60 min each and then incubated in horseradish peroxidase–conjugated anti-rat antibody (0.5 μg/ml) for 24 h at 4°C. Next the kidneys were washed in PBSMT on ice five times for 60 min each time, incubated in 0.1% Triton X-100 in PBS (PBST) twice for 10 min each and incubated in 3,3′-diaminobenzidene (DAB; Wako, Osaka, Japan) in PBST (250 μg/ml) without H2O2 for 1 h. They were then incubated in DAB in PBST with H2O2 (1:10,000; 0.003%) on ice for the appropriate time while observing them under a stereoscopic microscope. The kidneys were then rinsed in PBST three times and the reaction completely quenched with 4% PFA in PBS. After refixation, the kidneys were dehydrated in graded methanol (up to 100%) and cleared in benzyl benzoate:benzyl alcohol (Sigma) 2:1 solution at room temperature. Cleared kidneys were examined by stereoscopy.
In Situ Hybridization
Mouse cDNA was obtained by reverse transcription using mRNA extracted from mouse embryonic kidney. DNA fragments corresponding to positions 699 through 1149 of CXCR4 mRNA (accession no. NM_009911) and to positions 1410 through 1933 of PDGFB mRNA (accession no. NM_011057) were amplified by PCR and subcloned into pSTBlue-1 vector (Stratagene, La Jolla, CA). Digoxigenin-labeled RNA probe was prepared using a Digoxigenin RNA labeling kit (Roche Diagnostics, Manheim, Germany) according to the manufacturer's protocol. Kidneys were dissected from mouse embryos at E17.5 and fixed in tissue fixative (Genostaff, Tokyo, Japan), embedded in paraffin, and sectioned at 6-μm thickness. Tissue sections were processed as described previously.25
Organ Culture
Kidneys were removed from CXCR4 KO or wild-type mice at E12.5 and cultured on Transwell (Corning, NY) in DMEM (Sigma) containing 10% FCS (Life Technologies, Grand Island, NY) and antibiotics. After culturing for 48 h at 37°C and in 5% CO2, pedicles were washed in PBS three times and fixed in ice-cold methanol for 10 min. After rinsing in PBS, they were blocked in 5% goat serum in PBS for 1 h and incubated in anti-calbindin antibody overnight. After intensive washing, they were incubated in Alexa488-conjugated anti-rabbit antibody for 1 h. After washing, the pedicles were mounted on slides and examined using fluorescence microscopy.
Transmission Electron Microscopy
Kidneys were dissected and fixed in 4% glutaraldehyde (Wako) for 6 h, postfixed in 1% osmium tetroxide, dehydrated in graded acetones, and embedded in Epon-Araldite. Ultrathin sections, cut into 0.08-μm thickness and stained with uranyl acetate and lead citrate, were examined with Hitachi H-7100 (Hitachi, Tokyo, Japan).
Disclosures
None.
Supplementary Material
Acknowledgments
Y.T. was supported by grant from Osaka Kidney Foundation (OKF 060001).
We thank Prof. T. Inagami (Vanderbilt University School of Medicine, Nashville, TN) for kindly providing the anti-renin antibody and N. Horimoto for technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The SDF-1/CXCR4 Axis Is a Novel Driver of Vascular Development of the Glomerulus,” on pages 1659–1661.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Vaughan MR, Quaggin SE: How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa T: A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol 72: 408–411, 2000 [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J: The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 20: 1915–1924, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T: The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393: 591–594, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa T, Kikutani H, Kishimoto T: Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A 91: 2305–2309, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T: Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382: 635–638, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa T: Microenvironmental niches in the bone marrow for B-cell development. Nat Rev Immunol 6: 107–116, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA: Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 95: 9448–9453, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T: Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19: 257–267, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Kohli L, Kim CH, Lee Y, Mantel C, Cooper S, Hangoc G, Shaheen M, Li X, Clapp DW: Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G alpha i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol 73: 630–638, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T: Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci U S A 100: 5319–5323, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR: Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393: 595–599, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T: The role of CXCL12 in the organ-specific process of artery formation. Blood 105: 3155–3161, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8: 1875–1887, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Soriano P: Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8: 1888–1896, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T: Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20: 707–718, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA: Conditional vascular cell adhesion molecule 1 deletion in mice: Impaired lymphocyte migration to bone marrow. J Exp Med 193: 741–754, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gröne HJ, Cohen CD, Gröne E, Schmidt C, Kretzler M, Schlöndorff D, Nelson PJ: Spatial and temporally restricted expression of chemokines and chemokine receptors in the developing human kidney. J Am Soc Nephrol 13: 957–967, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280: 35760–35766, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Sierro F, Biben C, Martínez-Muñoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martínez-A C, Mackay CR, Mackay F: Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A 104: 14759–14764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R: α3 β1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T: A role for hematopoietic stem cells in promoting angiogenesis. Cell 102: 199–209, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Ito T, Imai E, Yamato M, Iwatani H, Kawachi H, Hori M: Retinoids regulate the repairing process of the podocytes in puromycin aminonucleoside-induced nephrotic rats. J Am Soc Nephrol 14: 981–991, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.