Abstract
Nephrosis and a rapid decline in kidney function characterize HIV-associated nephropathy (HIVAN). Histologically, HIVAN is a collapsing focal segmental glomerulosclerosis with prominent tubular damage. We explored the expression of neutrophil gelatinase-associated lipocalin (NGAL), a marker of tubular injury, to determine whether this protein has the potential to aid in the noninvasive diagnosis of HIVAN. We found that expression of urinary NGAL was much higher in patients with biopsy-proven HIVAN than in HIV-positive and HIV-negative patients with other forms of chronic kidney disease. In the HIV-transgenic mouse model of HIVAN, NGAL mRNA was abundant in dilated, microcystic segments of the nephron. In contrast, urinary NGAL did not correlate with proteinuria in human or in mouse models. These data show that marked upregulation of NGAL accompanies HIVAN and support further study of uNGAL levels in large cohorts to aid in the noninvasive diagnosis of HIVAN and screen for HIVAN-related tubular damage.
In 2007 alone, there were 2.7 million new infections with HIV and 2 million HIV-related deaths worldwide.1 An important complication of HIV is a form of kidney disease called HIV-associated nephropathy (HIVAN), which occurs predominantly in patients of African descent.2–4 The prevalence of HIVAN may be as high as 15% of HIV patients (based upon autopsy data), and 4232 new cases of HIVAN reached ESRD between 2002 and 2006 in the United States.5
HIVAN is a rapidly progressive form of chronic kidney disease (CKD) characterized by nephrotic range proteinuria. Kidney biopsies demonstrate histologic abnormalities in both glomeruli and tubules, including collapsing FSGS, podocyte proliferation and dedifferentiation, tubular dilation, microcyst formation, and tubulointerstitial inflammation.6,7 The pathogenesis is believed to be due to dysregulation of podocytes and tubular epithelia by HIV-1 itself.8,9 Early identification of HIVAN is important because highly active antiretroviral therapy (HAART), corticosteroids, and inhibition of renin–angiotensin may delay disease progression6,10,11. Nonetheless, because HIV infection may be associated with other glomerular diseases, definitive diagnosis of HIVAN requires a kidney biopsy. In fact, half of all patients with presumed HIVAN demonstrated different types of lesions once biopsied.11,12
Neutrophil gelatinase-associated lipocalin (NGAL) is a 22-kD protein that is markedly upregulated in renal tubules and urine (uNGAL) in response to epithelial damage.13–15 Expression of NGAL peaks 12 h after acute injury14,15 but remains elevated if injury is severe.16 In our study of 650 patients presenting to an inner-city emergency department, we found that a single, spot uNGAL test could distinguish ongoing injury from physiologic changes in renal function found in prerenal azotemia and from periods of slow or limited progression found during the course of many types of CKD (“stable CKD”).13 However, in that study, we noted that one CKD patient later diagnosed with HIVAN (T. L. Nickolas, unpublished observations) had markedly elevated levels of uNGAL, suggesting that HIV-associated tubular disease regulated its expression.
To test the association of uNGAL and HIV, we examined a cohort of 13 patients with biopsy-proven HIVAN. Biopsied or autopsied kidneys showed global (41% of nephrons per section), collapsing (19%), and segmental (12%) glomerulosclerosis and tubular atrophy (48%) and microcysts (18%), consistent with HIVAN. This group was compared with 24 race-matched HIV-positive controls with normal kidney function, defined as an estimated GFR (eGFR) ≥60 ml/min and no evidence of proteinuria. Comparisons were also made to HIV-positive and HIV-negative cohorts with other forms of CKD.
Patients with HIVAN had significantly lower CD4 counts and higher viral loads, serum creatinine levels, and proteinuria compared with HIV-positive, race-matched controls (Table 1). In HIVAN, uNGAL was upregulated 11-fold in comparison with that of HIV-positive race-matched controls without kidney disease (mean values, 748 ± 1160 μg/g creatinine in HIVAN versus 68 ± 98 μg/g creatinine without HIVAN; P value 0.006). Furthermore, in comparison with HIV-positive patients with CKD of non-HIVAN etiology (CKD, HIV-positive), uNGAL was upregulated approximately fivefold regardless of race-matching (Table 2). Moreover, uNGAL was 34-fold higher in patients with HIVAN compared with HIV-negative patients with CKD secondary to membranous nephropathy (n = 16), non-HIVAN FSGS (n = 7), or diabetic and hypertensive kidney diseases (n = 12).13 In fact, uNGAL levels in patients with HIVAN were more typical of patients presenting to an inner-city emergency department with acute kidney injury (AKI) (n = 30, Table 2) than those in the same emergency department with stable CKD (CKD, HIV-negative, n = 106).
Table 1.
Mean values and cohort characteristics for HIV-positive patients with HIVAN or race-matched controlsa
| All Patients (n = 37) | HIVAN (n = 13) | HIV-Positive Race-Matched Controls (n = 24) | |
|---|---|---|---|
| Age (yrs) | 46 (10) | 42 (9.6) | 48 (10) |
| Male (%) | 68 | 62 | 71 |
| Hepatitis B (%) | 13 | 10 | 14 |
| Hepatitis C (%) | 33 | 10 | 45 |
| eGFR(ml/min) | 99 (57) | 42 (37) | 128 (42)b |
| Creatinine (mg/dl) | 2.3 (4.0) | 5.3 (6.1) | 0.8 (0.2)b |
| Proteinuria (mg/dl) | 0.5 (1.3) | 1.3 (1.9) | 0.0 (0.0) |
| CD4 (cells/mm3) | 277 (324) | 160 (258) | 340 (342)c |
| Viral load (IU/ml) | 121,755 (224,675) | 232,535 (279,496) | 73,591 (182,928)b |
| uNGAL (μg/g creatinine) | 307 (750) | 748 (1160) | 68 (98)b |
aContinuous data were log-transformed prior to statistical testing.
bP value <0.05 for comparison between HIVAN and HIV-positive race-matched controls.
cP value <0.01 for comparison between HIVAN and HIV-positive race-matched controls.
Table 2.
Median values of kidney injury biomarkers: HIV-positive patients with HIVAN and other cohortsa
| NGAL (μg/g creatinine) | Serum Creatinine (mg/dl) | GFR (ml/min) | Protein/Creatinine Ratio | |
|---|---|---|---|---|
| Control, HIV-positive, race-matched (n = 24) | 28 (4–481)c | 0.9 (0.4–1.2)c | 116 (83–251)c | 0.0 (0.0–0.0) |
| Control, HIV-positive, non-race-matched (n = 14) | 74 (5–361) | 0.8 (0.4–1.0)c | 114 (83–246)c | 0.0 (0.0–0.3)c |
| CKD, HIV-positive, HIVAN (n = 13) | 231 (18–4050) | 2.6 (0.9–18.5) | 30 (4–117) | 0.6 (0.0–9.6) |
| CKD, HIV-positive, race-matched (n = 6) | 88 (5–374) | 2.1 (1.1–3.4) | 37 (24–190) | 0.2 (0.0–8.2) |
| CKD, HIV-positive, non-race-matched (n = 10) | 51 (19–225) | 1.5 (0.3–2.2) | 57 (13–344) | 0.5 (0.0–9.2) |
| CKD, HIV-negative (n = 106) | 12 (1–344)c | 1.5 (1–12.6) | 43 (5–90) | N/A |
| CKD, HIV-negative, membranous (n = 16) | 8 (1–248)c | 1.3 (0.5–3.2)b | 56 (21–128) | 4.5 (1.0–15.3)b |
| CKD, HIV-negative, FSGS (n = 7) | 19 (4–67)b | 1.2 (1.1–3.7) | 73 (17–63) | 1.9 (1.0–2.4) |
| CKD, HIV-negative, diabetic and hypertensive kidney diseases (n = 12) | 32 (2–316)b | 2.7 (0.7–3.6) | 26 (18–98) | 2.3 (0.2–27.9) |
| AKI, all causes (n = 30) | 296 (11–1833) | 3.9 (0.7–28.6) | 16 (2–53) | N/A |
aAll data presented as median (range). N/A, not available.
bP value <0.05 in comparison with the HIVAN cohort.
cP value <0.01 in comparison with the HIVAN cohort.
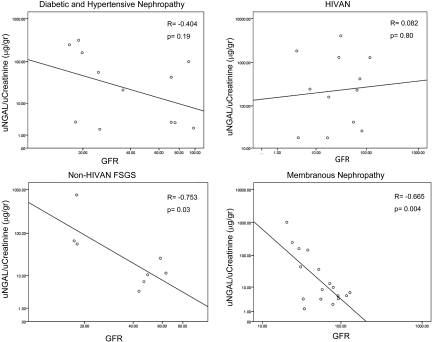
The enhanced expression of uNGAL in HIVAN was not due to higher levels of serum creatinine, lower eGFR, or proteinuria. uNGAL showed no correlation with eGFR in HIVAN (r = 0.082, P = 0.8), whereas it was inversely related to eGFR in two other forms of CKD (Figure 1), membranous nephropathy (r = − 0.665, P = 0.004), and FSGS (r = −0.753, P = 0.03). In fact, five HIVAN patients with relatively preserved kidney function (serum creatinine <2, mean eGFR 79.4, range 117.35 to 56.22) and limited proteinuria (0.68 g/L, range 0.0 to 3.0 mg/ml) demonstrated markedly elevated levels of uNGAL (mean 401 ng/ml, range 42 to 1285 ng/ml), suggesting that uNGAL can be expressed early in the course of progressive renal failure due to HIVAN. Consistently, there was no correlation between uNGAL and proteinuria (r = −0.28, P = 0.4). In contrast, uNGAL levels were significantly correlated with viral load (R = 0.469, P = 0.005), and there was a suggestion that uNGAL was suppressed by HAART (three of the patients with low levels of uNGAL were receiving HAART). In summary, rather than renal failure itself, characteristics of HIVAN appeared to accelerate uNGAL expression. The lack of association between uNGAL and glomerular functional markers (eGFR and proteinuria) implied that HIVAN stimulated uNGAL at a tubular site.
Figure 1.
uNGAL levels displayed as a function of eGFR in patients with CKD due to HIVAN, non-HIVAN FSGS, and diabetic and membranous Nephropathies. There was no correlation between the GFR and uNGAL in the HIVAN population (r = 0.082, P = 0.8) or the patients with diabetic nephropathy (r = −0.348, P = 0.4), but a significant correlation existed between GFR and uNGAL for both non-HIVAN FSGS (r = −0.753, P = 0.03) and membranous nephropathy (r = −0.665, P = 0.00). All data were log-transformed.
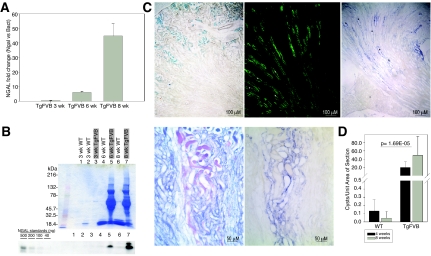
Because HIV-transgenic mice (TgFVB)17 display a syndrome identical to HIVAN18 and lack the confounds typically present in human cohorts, we measured NGAL expression in kidneys of TgFVB and wild-type (WT) littermates (Affymetrix, Mouse Genome 430 2.0 microarrays; Geo Accession Number Series GSE14221). NGAL was one of the most highly upregulated genes among 39,000 transcripts, demonstrating 62- and 109-fold increases at 6 and 8 wk, respectively (Figure 2A). After GC content-robust microarray averaging (GC-RMA) normalization of TgFVB and WT samples, NGAL was the most upregulated gene (TgFVB versus WT, P = 1.4 × 10−70). In fact, out of 23 proven AKI and 35 proven hypoxia-specific genes,19 (Figure 2, A and B) NGAL was the most differentially expressed gene, fivefold higher than the next best gene and 100-fold higher than most others. We confirmed the expression of NGAL in HIVAN kidneys using real-time-PCR (Figure 3A) and the presence of NGAL in the urine by immunoblot (Figure 3B). NGAL mRNA and uNGAL protein increased over time with the progression of kidney disease (Figure 3, A and B).
Figure 2.
(A) Acute kidney injury (blue), hypoxia-associated genes (green), and NGAL (red) in kidneys of 8-wk-old HIVAN and age- and sex-matched WT littermate mice. (B) Table of AKI and hypoxia genes.
Figure 3.
Induction of uNGAL. (A) NGAL real-time PCR in TgFVB kidneys from 3-, 6-, and 8-wk-old mice. Data were normalized for NGAL expression in age- and sex-matched WT FVB/N littermate mice. (B) Coomasie blue stained gels and immunoblots of uNGAL in 3-, 6-, and 8-wk-old TgFVB and WT littermate mice. uNGAL increased between 6 and 8 wk in TgFVB mice, whereas proteinuria remained constant. (C) Sections of 8-wk-old TgFVB mouse kidneys. From left to right: Prussian blue staining demonstrates iron accumulation in cortical proximal tubules. Aquaporin-2 immunocytochemistry marks medullary collecting ducts while in situ hybridization in an adjacent section reveals NGAL expression (blue) in dilated collecting ducts (asterisk represents dilated tubules). Higher power demonstrates cast formation (periodic acid—Schiff stain, purple) and NGAL expression (blue) in two adjacent sections. (D) Increasing number of cysts per unit area in TgFVB kidneys with aging. TgFVB differed significantly from WT kidneys (n = 14, P = 0.00074 at 4 wk; n = 14, P = 0.001 at 8 wk).
TgFVB mice excrete as much as 10 g/L of high-molecular-mass proteins commonly seen in other glomerular diseases (Figure 3B).17 In addition, as with other types of nephrosis, the proximal tubule demonstrated Prussian-blue-positive iron accumulation, presumably the result of the capture of iron-bearing proteins from the filtrate (Figure 3C). Given that both proteinuria and iron filtration have been implicated as agents that damage the proximal tubule,20 we examined whether NGAL was expressed at this site. Surprisingly, in situ hybridization showed that NGAL mRNA was not expressed in the proximal tubule, where stainable iron was found, but rather in aquaporin-2-positive collecting ducts of TgFVB mice (Figure 3C). Although this may suggest that NGAL was activated downstream of the site of injury, NGAL was prominently expressed in dilated microcystic tubules rather than homogenously throughout a specific nephron segment, which would be typical of, for example, ischemia–reperfusion injury.16 NGAL mRNA was detected in 39% of microcysts (n = 2698 microcysts), in both medulla and cortex. In contrast, NGAL mRNA was not detected in noncystic tubules. This unexpected finding suggested that NGAL was likely induced by a local stimulus associated with cystogenesis rather than a global stimulus such as proteinuria, luminal debris, urinary iron, or ischemic injury. In this regard, it was notable that, although proteinuria was constant between 6 and 8 wk of life, NGAL expression increased over time, consistent with an increase in cyst number per area of section as these mice age (Figure 3D).
In the present study, we found that uNGAL is highly activated in HIVAN. Yet unlike our previous findings in CKD, uNGAL achieved levels more typical of AKI. The elevated levels of uNGAL may originate from the filtrate, but in our study, there was no correlation between uNGAL and proteinuria in either human or mouse HIVAN. Similarly, in a larger cohort of patients with biopsy-proven CKD of various etiologies (M. Sise, L. Allegri, and T. L. Nickolas, unpublished observations), there was no correlation between proteinuria and uNGAL (Pearson correlation, r = 0.085, P = 0.5, n = 88 patients), suggesting that sources of NGAL other than the glomerular filtrate contribute to uNGAL. In HIVAN, we identified this source as cystic tubular epithelia. This finding is consistent with the observation that uNGAL is expressed in patients with polycystic kidney disease,21 especially in those with rapid cyst enlargement.
We first identified NGAL as a tubulogenic factor,22 a property supported by studies in cell lines.23 Likewise, in adult kidney,14 thyroid cells,24 and gastric epithelia,25 NGAL acts as a growth factor, whereas in other cells, such as polycystic-kidney-disease-related renal cysts, NGAL may serve as a proapoptotic factor.26 A common growth mechanism may underlie these phenomena, perhaps with iron loaded NGAL,22,24 but exact mechanisms are unclear. Nonetheless, it is striking that proliferation and apoptosis have been noted in HIVAN cysts,27 and the intensive expression of NGAL at these sites implies that one or both of these activities might be modulated by NGAL. Further analysis must be based on mouse models that place the NGAL knockout allele on the HIVAN background.
In conclusion, uNGAL was markedly elevated in patients with biopsy-proven HIVAN and in a mouse model of HIVAN. NGAL was produced by tubular cysts and secreted into the urine. These data suggest the possibility that uNGAL may be useful to monitor the formation of renal tubular cysts and consequently distinguish HIVAN from common forms of CKD, such as diabetes or hypertension, or other glomerulopathies presenting in the HIV patient. In this light, the very high levels of uNGAL associated with HIVAN may provide a rationale for biopsy and aggressive HAART therapy to prevent the progression of HIVAN to ESRD.28 However, this proposal requires further testing in a large cohort where we can determine the temporal relationships between NGAL expression and disease onset and between NGAL expression and HAART.
Concise Methods
Patients
This protocol was approved by the Institutional Review Board of Columbia University. Deidentified urine samples from patients with biopsy-proven HIVAN were obtained from Mount Sinai School of Medicine (n = 4) and the Manhattan HIV Brain Bank (n = 9),29. Urine samples were collected at the time of kidney biopsy (n = 2), between 20 and 36 mo after kidney biopsy (n = 2), or between 0.3 and 37 mo before autopsy (n = 9) and then frozen at −80°C. HIV-positive controls and HIV-positive patients with other biopsy-proven CKD were derived from the same cohorts as HIVAN patients. HIV-positive controls divided into race and nonrace groups are presented separately in Table 2. HIV-positive controls had normal kidney function (defined by an estimated Modification of Diet in Renal Disease GFR of ≥60 ml/min),30 lacked proteinuria, and had no clinical evidence of HIVAN. Investigators responsible for the laboratory and statistical analysis (C.S.F. and T.L.N.) were blinded to the clinical diagnosis of both patients and controls.
For comparison of uNGAL expression in HIVAN with other types of kidney disease, we used cohorts of HIV-negative patients with AKI or CKD. Patients with AKI and nonbiopsied, stable CKD were derived from our previously described investigation of the ability of uNGAL to detect AKI in patients presenting to an emergency department.13 Patients with biopsy-proven glomerular or tubulointerstitial etiologies of CKD were obtained from the Kidney Biopsy Registry, University of Parma, Italy. These patients were >18-yr-old and underwent kidney biopsy as part of routine care (January 1, 2005, through April 1, 2008). Urine was collected at the time of biopsy. Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin/eosin, silver methenamine, and periodic acid–Schiff stain. Patients with AKI or CKD were stratified by type of kidney disease.
Statistical Analysis
SAS 9.1 (Cary, NC) was used for statistical analyses. All continuous data were log-transformed before analyses and are presented as non-log-transformed values. Pearson's correlation was used to determine relationships among uNGAL and other continuous variables, and a t test for unequal variances was used for comparisons. Fisher's exact test was used to compare categorical data among patients with and without HIVAN.
Animals
TgFVB mice17 were bred with FVB/N mouse strain to produce heterozygotes. The HIV transgene correlated with the presence of cataracts.
Assays
The area of each kidney section was determined with Adobe Photoshop from 1× images, and total cysts per section were divided by this area to yield the cysts per unit area of each section.
NGAL was quantified by Western blots, using nonreducing 4 to 15% Tris-HCl gels (Bio-Rad Laboratories, Inc., Hercules, CA) and monoclonal (1:1000; AntibodyShop, Gentofte, Denmark) or rabbit polyclonal antibodies (RDSystems, Minneapolis, MN) together with standards (0.2 to 10 ng) of human or mouse recombinant NGAL protein. NGAL was reproducibly detected to 0.4 ng per lane. NGAL expression was quantified using ImageJ software (National Institute of Mental Health). NGAL mRNA was detected using digoxigenin-labeled antisense riboprobes generated from cDNAs encoding Ngal (exons 1 to 7, 566 bp) by linearization with XhoI followed by T7 RNA polymerase. Prussian blue staining in frozen sections used freshly prepared 2.5% potassium ferrocyanide and 2.5% hydrochloric acid for 20 min at room temperature.
Microarrays and real-time PCR utilized RNA isolated with the mirVANA RNA extraction kit (Ambion) and quantified by NanoDrop and gel electrophoresis. For real-time PCR analysis, samples were processed according to Bio-Rad SYBR GREEN, iCyclerMyiQ protocols. Target genes utilized respectively: Ngal 116 forward primer 5′-ctcagaacttgatccctgcc-3′ and NGALa 593 reverse 5′-tccttgaggcccagacactt-3′; β-actin 415 forward primer 5′-ctaaggccaaccgtgaaaag-3′ and β-actin 696 reverse primer 5′-tctcagctgtggtggtgaag-3′. The ΔΔCT method was used to calculated fold amplification of transcripts. For microarray analysis, double-stranded cDNA was synthesized from total RNA (7 μg) extracted from whole kidneys, and in vitro transcription biotin-labeled cRNA was generated for GeneChip hybridization (GeneChip One-Cycle Target Labeling Kit, Affymetrix). Fragmented biotin-labeled cRNA was hybridized to Affymetrix Mouse Genome 430 2.0 GeneChips. The results of the Affymetrix chip experiments were normalized using GC-RMA (Bioinformatics Toolbox, Matlab R2008a, The Mathworks, Inc.). After standardization using WT information, the data were averaged. Given the distribution of expression profiles, the Bonferroni-corrected significance threshold was calculated to 3.17 × 10−6.
Disclosures
Columbia University and Cincinnati Children's Hospital Medical Center have received licensing fees from Biosite and Abbott Diagnostics.
Acknowledgments
This work was supported by grants from the Emerald Foundation, the March of Dimes, the National Institute of Diabetes and Digestive and Kidney Diseases (DK-55388 and DK-58872), and the Glomerular Center of Columbia University to J.B. The collection of patient specimens was supported by National Institutes of Health Grants R24MH59724 and U01MH083501 (The Manhattan HIV Brain Bank, to S.M.) and P01DK56492 (to P.K.) and by the Clinical Research Center of the Mount Sinai School of Medicine (M01-RR-00071).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Development of Urinary Biomarkers for Kidney Disease Is the Search for Our Renal Troponin,” on pages 1656–1657.
References
- 1.UNAIDS: Report on the Global AIDS Epidemic, 2008. Available at http://www.unaids.org/en/knowledgecentre/hivdata/globalreport/2008/default.asp Accessed May 2009
- 2.Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, Friedman EA: Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 310: 669–673, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Winston JA, Klotman ME, Klotman PE: HIV-associated nephropathy is a late, not early, manifestation of HIV-1 infection. Kidney Int 55: 1036–1040, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD: Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: A 12-year cohort study. AIDS 18: 541–546, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Shahinian V, Rajaraman S, Borucki M, Grady J, Hollander WM, Ahuja TS: Prevalence of HIV-associated nephropathy in autopsies of HIV-infected patients. Am J Kidney Dis 35: 884–888, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Atta MG, Lucas GM, Fine DM: HIV-associated nephropathy: Epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther 6: 365–371, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Wyatt CM, Klotman PE, D'Agati VD: HIV-associated nephropathy: Clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin Nephrol 28: 513–522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman ES, Klotman PE: HIV-associated nephropathy: Epidemiology, pathogenesis, and treatment. Semin Nephrol 23: 200–208, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE: Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest 100: 84–92, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Post FA, Campbell LJ, Hamzah L, Collins L, Jones R, Siwani R, Johnson L, Fisher M, Holt SG, Bhagani S, Frankel AH, Wilkins E, Ainsworth JG, Larbalestier N, Macallan DC, Banerjee D, Baily G, Thuraisingham RC, Donohoe P, Hendry BM, Hilton RM, Edwards SG, Hangartner R, Howie AJ, Connolly JO, Easterbrook PJ: Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis 46: 1282–1289, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Gupta SK, Eustace JA, Winston JA, Boydstun II, Ahuja TS, Rodriguez RA, Tashima KT, Roland M, Franceschini N, Palella FJ, Lennox JL, Klotman PE, Nachman SA, Hall SD, Szczech LA: Guidelines for the management of chronic kidney disease in HIV-infected patients: Recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 40: 1559–1585, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA: The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 66: 1145–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J: Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE: Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA 89: 1577–1581, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann R, Laroche D, Buchner K, Hucho F, Rudd C, Lindschau C, Ludwig P, Hoer A, Oberdisse E, Kopp J, Korner IJ, Repke H: The HIV-1 surface protein gp120 has no effect on transmembrane signal transduction in T cells. J Acquir Immune Defic Syndr 5: 760–770, 1992 [PubMed] [Google Scholar]
- 19.Vaidya VS, Ferguson MA, Bonventre JV: Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48: 463–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nankivell BJ, Boadle RA, Harris DC: Iron accumulation in human chronic renal disease. Am J Kidney Dis 20: 580–584, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J: An iron delivery pathway mediated by a lipocalin. Mol Cell 10: 1045–1056, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Gwira JA, Wei F, Ishibe S, Ueland JM, Barasch J, Cantley LG: Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J Biol Chem 280: 7875–7882, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Iannetti A, Pacifico F, Acquaviva R, Lavorgna A, Crescenzi E, Vascotto C, Tell G, Salzano AM, Scaloni A, Vuttariello E, Chiappetta G, Formisano S, Leonardi A: The neutrophil gelatinase-associated lipocalin (NGAL), a NF-κB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci USA 105: 14058–14063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Playford RJ, Belo A, Poulsom R, Fitzgerald AJ, Harris K, Pawluczyk I, Ryon J, Darby T, Nilsen-Hamilton M, Ghosh S, Marchbank T: Effects of mouse and human lipocalin homologues 24p3/lcn2 and neutrophil gelatinase-associated lipocalin on gastrointestinal mucosal integrity and repair. Gastroenterology 131: 809–817, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Wei F, Karihaloo A, Yu Z, Marlier A, Seth P, Shibazaki S, Wang T, Sukhatme VP, Somlo S, Cantley LG: Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int 74: 1310–1318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray PE, Liu XH, Robinson LR, Reid W, Xu L, Owens JW, Jones OD, Denaro F, Davis HG, Bryant JL: A novel HIV-1 transgenic rat model of childhood HIV-1-associated nephropathy. Kidney Int 63: 2242–2253, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Wyatt CM, Klotman PE: HIV-1 and HIV-associated nephropathy 25 years later. Clin J Am Soc Nephrol 2: S20–S24, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wyatt CM, Morgello S, Katz-Malamed R, Wei C, Klotman ME, Klotman PE, D'Agati VD: The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int 75: 428–434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]