Abstract
Both innate and adaptive mechanisms participate in the pathogenesis of kidney ischemia-reperfusion injury (IRI), but the role of regulatory immune mechanisms is unknown. We hypothesized that the anti-inflammatory effects of CD4+CD25+FoxP3+ regulatory T cells (Tregs) protect against renal IRI. Partial depletion of Tregs with an anti-CD25 mAb potentiated kidney damage induced by IRI. Reducing the number of Tregs resulted in more neutrophils, macrophages, and innate cytokine transcription in the kidney after IRI but did not affect CD4+ T cells or B cells. We performed adoptive transfer of lymph node cells from wild-type mice or FoxP3-deficient Scurfy mice into T cell– and B cell–deficient RAG-1 knockout mice to generate mice with and without FoxP3+ Tregs, respectively. FoxP3+ Treg–deficient mice accumulated a greater number of inflammatory leukocytes after renal IRI than mice containing Tregs. To confirm that a lack of Tregs potentiated renal injury, we co-transferred isolated Tregs and Scurfy lymph node cells; Treg repletion significantly attenuated IRI-induced renal injury and leukocyte accumulation. Furthermore, although adoptive transfer of wild-type Tregs into RAG-1 knockout mice was sufficient to prevent kidney IRI, transfer of IL-10–deficient Tregs was not. Taken together, these results demonstrate that Tregs modulate injury after kidney IRI through IL-10–mediated suppression of the innate immune system.
Accumulation and activation of immune system cells in the kidney during ischemia-reperfusion (IR) is a major mediator of kidney injury. Numerous studies have demonstrated the involvement of the immune system and inflammation in the pathogenesis of IR-induced acute kidney injury (AKI).1–6 Experiments using animal models have revealed that both the innate and adaptive immune systems mediate IR injury (IRI) in the kidney. Accumulation of neutrophils and macrophages is a consistent early finding in the kidney during reperfusion, and depletion of either leukocyte subset protects mice from IR-induced AKI.4,5 Conversely, mice lacking T cells and/or B cells sustain less kidney damage after IR,1–3 demonstrating that the adaptive immune system also contributes to renal IRI.
Regulatory T cells (Tregs) are lymphocytes with immunosuppressive properties. Tregs are commonly identified by their expression of CD4 and CD25 on the cell surface and upregulation of the transcription factor FoxP3.7 The actions of Tregs can be mediated by the production of anti-inflammatory cytokines such as IL-10 or TGF-β, by direct cell–cell contact or CTLA-4–mediated inhibition, or by production of extracellular adenosine.8–11 Furthermore, Tregs have the ability to traffic to areas of inflammation to mitigate immune reactions.12,13
In addition to Tregs' widely known ability to prevent effector T cell proliferation and function, Tregs can suppress the innate immune response.14–20 In vivo, CD4+CD25+FoxP3+ Tregs inhibit Adriamycin-induced macrophage accumulation and kidney injury and, in vitro, suppress the macrophage inflammatory phenotype.18 Neutrophil reactive oxygen species generation and cytokine production are inhibited in the presence of Tregs, and co-culture with Tregs markedly increases the rate of neutrophil cell death.19 CD4+CD25+FoxP3+ Tregs were recently identified in normal mouse kidneys by flow cytometry.21 In summary, Tregs are intrinsic anti-inflammatory leukocytes that reside in the kidney under normal conditions, but their involvement in renal IRI has not been investigated.
Results
Antibody-Mediated Treg Depletion Potentiates Kidney IRI
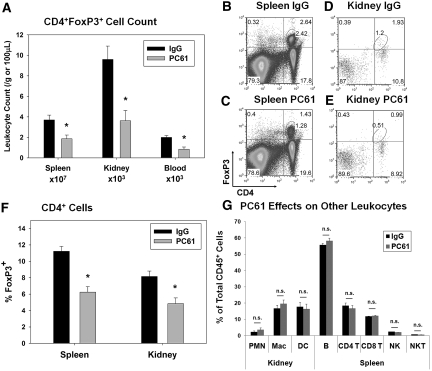
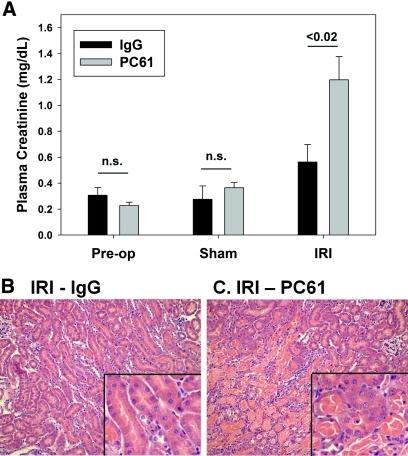
To investigate the role of Tregs in kidney IRI in normal C57Bl/6 mice, we used an anti-mouse CD25 mAb (PC61).22–24 Administration of PC61 (300 μg intravenously) significantly decreased (approximately 50%) spleen, kidney, and blood Tregs 5 d after injection compared with mice treated with control IgG (300 μg intravenously; Figure 1, A through E). This dosing strategy was chosen on the basis of the kinetic analysis of single-dose PC61 on FoxP3+ Tregs in C57Bl/6 mice.22 In addition to decreasing the total number of CD4+FoxP3+ cells, PC61 treatment significantly decreased the percentage of CD4+ cells expressing FoxP3 (Figure 1F). No significant effect of PC61 administration was observed on kidney neutrophils, macrophages or dendritic cells, spleen B cells, CD4+ T or CD8+ T cells, or natural killer (NK) or NK T cells measured at 5 d after injection (Figure 1G). Five days after PC61 or IgG injection, mice underwent 24 min of bilateral renal ischemia or sham surgery, and kidneys were allowed to reperfuse for 24 h. Whereas there was no difference in plasma creatinine (PCr) levels before surgery or after sham surgery, mice with decreased Tregs (PC61) developed more severe IRI compared with IgG-treated mice as measured by PCr levels (Figure 2A) and outer medulla acute tubular necrosis (ATN; Figure 2, B and C; ATN scores: IgG − IRI 1.2 ± 0.3 and PC61 − IRI 2.5 ± 0.3, P < 0.05).
Figure 1.
CD25 mAb (PC61) administration reduces Treg numbers in mouse spleen, kidney, and blood. (A) Five days after intravenous injection of 300 μg of PC61 or control IgG in C57Bl/6 mice, spleen, kidney, and blood cells were analyzed by flow cytometry to determine the number of Tregs (CD4+FoxP3+) per gram of tissue or 100 μl of blood as described in the Concise Methods section. (B through E) Representative flow cytometry results from mouse spleen or kidney 5 d after IgG (B and D) and PC61 (C and E) injection; total spleen or kidney cells were gated on the CD45+ and Fixable Green Live/Dead Stain–negative populations before the analysis shown. (F) The percentage of total CD4+ cells staining positive for intracellular FoxP3 in spleen and kidney was determined by flow cytometry. (G) The effect of PC61 administration versus IgG was determined by comparing in each group the percentage of CD45+ cells that were kidney neutrophils (PMN, GR-1+/CD11b+), macrophages (F4/80low/CD11b+), or dendritic cells (F4/80high/CD11b+) or spleen B cells (B220+/CD19+), CD4+ (CD3+/CD4+) or CD8+ (CD3+/CD8+) T cells, or NK (CD3-/NK1.1+) or NK T (CD3+/NK1.1+) cells. Data are means ± SEM (A, F, and G); n = 7 for spleen and kidney and n = 3 for blood. *P < 0.05 versus IgG for same organ.
Figure 2.
Partial Treg depletion potentiates kidney IRI. Five days after intravenous injection of 300 μg of PC61 or control IgG, C57Bl/6 mice underwent sham surgery or bilateral renal ischemia for 24 min. (A) After 24 h of reperfusion, renal function was assessed by measurement of PCr levels. Data are means ± SEM; n = 6 to 11 per group. n.s., P > 0.05. The extent of outer medulla tubular necrosis was determined by hematoxylin and eosin (H&E) staining. (B, C) Images are representative of six to eight animals per group. Magnifications: ×200 in B and C; ×600 in insets.
PC61-Induced Reduction in Kidney Treg Number Enhances the Innate Immune Response in the Kidney after IRI
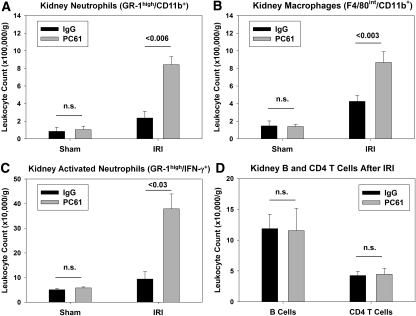
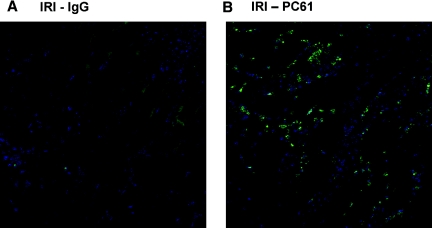
Twenty four hours after kidney IR or sham surgery, kidney leukocyte accumulation was determined by flow cytometry. There was no difference in kidney neutrophil (CD45+7-AAD−CD11b+GR-1high) or macrophage (CD45+7-AAD−F4/80intCD11b+) number after sham operation in the IgG- and PC61-treated WT mice (Figure 3, A and B). The number of neutrophils and macrophages in the kidney after IR in the PC61-treated mice (with significantly fewer Tregs) was significantly higher than the IgG-treated controls after IR (Figure 3, A and B). Previous studies from our laboratory demonstrated that GR-1highIFN-γ+ leukocytes are activated neutrophils that accumulate in the kidney early during reperfusion.6 Decreasing Treg numbers with PC61 caused a significant increase in the number of IFN-γ–producing activated neutrophils observed in the kidney after IR (Figure 3C). In contrast, the number of kidney CD3+CD4+ T cells and CD3−CD19+ B cells was not different at 24 h after IR (Figure 3D). Immunohistochemical analysis of kidney sections using the antibody 7/4 confirmed the flow cytometry results, demonstrating a marked increase in the number of kidney infiltrating neutrophils and recently emigrated monocytes in the outer medulla at 24 h after IR in PC61-treated mice (Figure 4). Real-time PCR analysis of total kidney RNA from IgG- or PC61-treated mice after sham or IR revealed that in addition to enhanced innate leukocyte accumulation, greater transcription of the innate inflammatory cytokines IL-6 and TNF-α was observed after IR in mice with fewer Tregs. Similarly TGF-β expression was increased in the kidney after IRI in the PC61-treated mice versus IgG-treated mice, whereas IL-10 expression was not different between the two treatment groups after IRI (Supplemental Figure 1).
Figure 3.
Partial Treg depletion enhances kidney leukocyte accumulation after IR. Five days after intravenous injection of 300 μg of PC61 or control IgG, C57Bl/6 mice underwent sham surgery or bilateral renal ischemia for 24 min. (A–C) After 24 h of reperfusion, renal leukocyte numbers were assessed by flow cytometry as described in the Concise Methods section. All groups were gated previously on CD45+ and 7-AAD− cell populations before the analysis shown. (D) CD4+ T cells were identified as CD3+/CD4+, and B cells were identified and CD3−CD19+. Data are means ± SEM; n = 6 to 11 per group. n.s., P > 0.05.
Figure 4.
Partial Treg depletion promotes kidney innate inflammatory cell accumulation after IR. (A and B) Five days after intravenous injection of control IgG (A) or PC61 (B), C57Bl/6 mice underwent renal ischemia for 24 min. After 24 h of reperfusion, renal neutrophil and recently emigrated monocyte accumulation was assessed by staining frozen sections with the 7/4 antibody (bright green). DAPI was used to identify nuclei (blue). Images are representative of at least three separate mice per group.
Mice Completely Deficient in Tregs Are More Susceptible to IRI
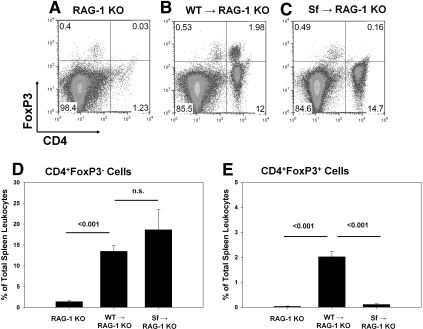
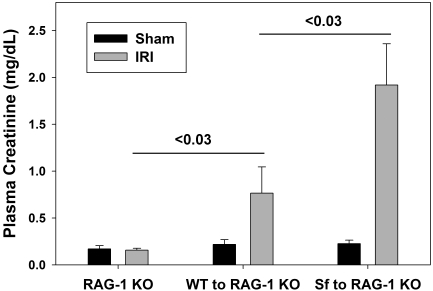
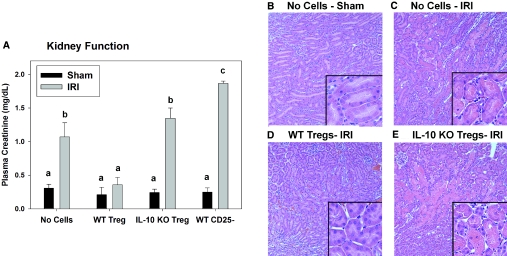
As an additional model to investigate the role of Tregs in IR, we used RAG-1 knockout (KO) mice, which lack T and B cells. RAG-1 KO mice were reconstituted with 5 or 10 million lymph node (LN) cells from either wild-type C57Bl/6 (WT) mice or Scurfy (Sf) mice, which lack expression of FoxP3 and thus CD4+CD25+FoxP3+ Tregs. Two weeks later, the efficiency of adoptive transfer was confirmed using spleen cells from the various groups of mice by flow cytometry (Figure 5). Tregs were absent from spleens of RAG-1 KO mice and RAG-1 KO mice reconstituted with Sf LN cells (Figure 5, Supplemental Figure 2). Additional groups of RAG-1 KO mice received isolated CD4+CD25+FoxP3+ Tregs from a WT mouse along with Sf cells. Reconstitution with WT LN cells or co-transfer of isolated Tregs with Sf LN cells resulted in the presence of a distinct CD4+FoxP3+ population in the spleen 2 wk after adoptive transfer (Figure 5, Supplemental Figure 2). RAG-1 KO mice that were not reconstituted with LN cells were protected from mild IRI (Figure 6) as described previously.3 Adoptive transfer of 10 million WT LN cells 2 wk before IR resulted in renal injury, and reconstitution of RAG-1 KO mice with 10 million Sf LN cells significantly exacerbated the severity of IRI compared with adoptive transfer of an equivalent number of WT LN cells (Figure 6; ATN scores: 10 million WT LN cells − IRI 2.0 ± 0.4 and 10 million Sf LN cells − IRI 3.6 ± 0.2, P < 0.05). To confirm that the potentiation of renal injury was due to the lack of Tregs in Sf → RAG-1 KO mice, we co-transferred isolated Tregs with 5 million Sf cells at a ratio of 1:5 (Tregs:Sf LN cells). The purity of the isolated Tregs was determined to be >85% CD4+CD25+, and of the CD4+CD25+ population, 80 to 95% expressed FoxP3 as determined by flow cytometry (Supplemental Figure 3). Co-transfer experiments confirmed the protective role of Tregs in this model; Treg repletion (1:5 ratio) significantly inhibited the IR-induced kidney dysfunction (Figure 7A) and tubular necrosis (Figure 7, B and C; ATN scores: 5 million Sf LN cells − IRI 1.3 ± 0.4 and 5 million Sf LN cells + 1 million Tregs 0.2 ± 0.1, P < 0.05). Co-transfer of Tregs at a 1:10 ratio was insufficient to prevent kidney IRI (data not shown).
Figure 5.
Effectiveness of LN cell transfer to RAG-1 KO recipients. (A through C) Representative flow cytometry results of splenocytes 2 wk after adoptive transfer of no cells (A), 10 million WT LN cells (B), or 10 million Sf LN cells (C). Total spleen cells were gated on the CD45+ and Fixable Green Live/Dead Stain–negative populations before the analysis shown. (D, E) Quantification of the percentage of CD45+ spleen cells that were CD4+FoxP3− or CD4+FoxP3+ 2 wk after adoptive transfer. Data are means ± SEM; n = 3 to 5 per group. n.s., P > 0.05 (D and E).
Figure 6.
Complete Treg deficiency potentiates kidney IRI. Two weeks after adoptive transfer of no cells, 10 million WT LN cells, or 10 million SF LN cells, RAG-1 KO mice underwent either sham surgery or 24 min of bilateral renal ischemia. Twenty-four hours after surgery, renal function was determined by measurement of PCr levels. Data are means ± SEM; n = 3 to 5 per group.
Figure 7.
Treg repletion inhibits kidney IRI in Treg-deficient mice. Two weeks after adoptive transfer of no cells, 5 million WT or Sf LN cells, or 5 million Sf LN cells + 1 million isolated Tregs, RAG-1 KO mice underwent either sham surgery or 24 min of bilateral renal ischemia. Twenty-four hours after surgery, renal function was determined by measurement of PCr levels (A). Data are means ± SEM. *Significantly different from respective sham operated mice, P < 0.05 (A). (B, C) Twenty-four hours after surgery, the extent of outer medulla tubular necrosis was determined by H&E staining. Images are representative of 5 to 6 animals per group. Magnifications: ×200 in B and C; ×600 in insets.
Treg Deficiency Exacerbates Kidney Inflammatory Cell Accumulation after IR
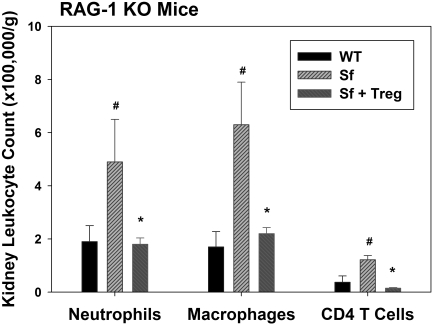
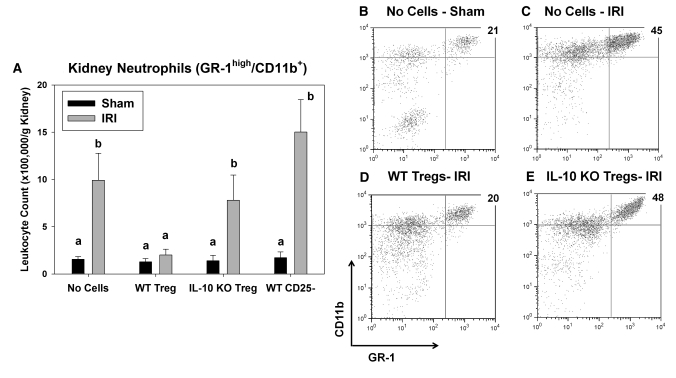
Twenty four hours after kidney IR or sham surgery, we determined kidney leukocyte accumulation by flow cytometry. The number of neutrophils (CD45+7-AAD−GR-1highCD11b+) and macrophages (CD45+7-AAD−F4/80intCD11b+) in the kidney after IR in the RAG-1 KO mice reconstituted with 5 million Sf LN cells was significantly higher than those reconstituted with 5 million WT LN cells after IR (Figure 8). Immunohistochemical analysis of kidney sections using the antibody 7/4 confirmed the flow cytometry results (Supplemental Figure 4). In contrast to PC61-treated mice, the number of CD4+ T cells in the kidney after IR was also higher in Treg-deficient mice. Co-transfer of Tregs with Sf LN cells completely blocked the increase in inflammatory leukocyte accumulation caused by Sf LN cell transfer alone (Figure 8).
Figure 8.
Treg repletion inhibits IR-induced leukocyte accumulation in Treg-deficient mice. Two weeks after adoptive transfer of 5 million WT or Sf LN cells or 5 million Sf LN cells + 1 million isolated Tregs (Sf + Treg), RAG-1 KO mice underwent 24 min of bilateral renal ischemia. Twenty-four hours after surgery, the accumulation of leukocytes in the kidney was determined by flow cytometry as described in the Concise Methods section. Data are means ± SEM. #P < 0.05 versus WT; *P < 0.05 versus Sf.
Tregs Directly Suppress Innate Responses to IRI in an IL-10–Dependent Manner
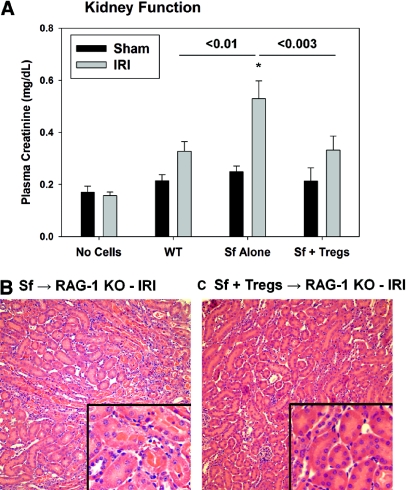
To test directly the ability of Tregs to suppress the innate response to kidney IRI and the role of IL-10 produced by Tregs, we adoptively transferred WT or IL-10 KO Tregs alone to RAG-1 KO mice before IRI. No difference in CD25 or FoxP3 expression levels was observed between WT and IL-10 KO Tregs (data not shown). In contrast to 24 min of ischemia, which did not result in a significant increase in PCr in RAG-1 KO mice (Figure 6), 28 min of ischemia caused an increase in kidney neutrophil accumulation, PCr levels, and ATN (Figures 9 and 10). Adoptive transfer of 1 × 106 WT CD4+CD25+ Tregs significantly reduced kidney injury, whereas the same number of CD4+CD25− T cells or CD4+CD25+ Tregs from IL-10 KO mice had no protective effect on IRI in RAG-1 KO mice (Figure 9; ATN scores: No cells − IRI 2.7 ± 0.4, 1 million WT Tregs − IRI 0.6 ± 0.3,a,b 1 million IL-10 KO Tregs − IRI 3.1 ± 0.2, aP < 0.02 versus no cells − IRI, bP < 0.001 versus IL-10 KO Tregs). WT Tregs but not IL-10 KO Tregs blocked the accumulation of GR-1highCD11b+ neutrophils in the kidney at 24 h after IRI (Figure 10).
Figure 9.
IL-10+ Tregs inhibit kidney IRI in lymphocyte-deficient RAG-1 KO mice. RAG-1 KO mice were administered PBS or 1 × 106 WT CD4+CD25+ Tregs, 1 × 106 IL-10 KO CD4+CD25+ Tregs, or 1 × 106 WT CD4+CD25− cells 18 h before 28 min of bilateral renal ischemia or sham surgery. (A) After 24 h of reperfusion, renal function was estimated by measurement of PCr, and ATN was assessed by H&E staining. (B–E) Data are means ± SEM; values with different superscripts are significantly different from each other. P < 0.05; n = 4 to 9 per group (A). Magnifications: ×200 in B through E; ×600 in insets.
Figure 10.
IL-10+ Tregs inhibit IR-induced neutrophil accumulation in lymphocyte-deficient RAG-1 KO mice. RAG-1 KO mice were administered PBS or 1 × 106 WT CD4+CD25+ Tregs, 1 × 106 IL-10 KO CD4+CD25+ Tregs, or 1 × 106 WT CD4+CD25− cells 18 h before 28 min of bilateral renal ischemia or sham surgery. (A) After 24 h of reperfusion, renal neutrophil accumulation was measured by flow cytometry as described in the Concise Methods section. (B through E) Total kidney cells were gated on the CD45+ and 7-AAD− populations before the analysis shown. Data are means ± SEM; values with different superscripts are significantly different from each other. P < 0.05; n = 4 to 9 per group (A).
Discussion
We have established a protective role for Tregs in kidney IRI using three different animal models to modulate Tregs. Use of PC61, which resulted in approximately 50% reduction in Tregs in the spleen, kidney, and blood, potentiated kidney IRI, as measured by PCr levels and ATN. Similar results were obtained using mice that completely lack Tregs (Sf LN cells → RAG-1 KO mice), and renal injury in these mice was significantly inhibited by adding Tregs along with Treg-deficient Sf cells. Adoptive transfer of WT Tregs alone blocked kidney IRI in lymphocyte-deficient RAG-1 KO mice. In contrast, IL-10 KO Tregs were ineffective at blocking IRI in RAG-1 KO mice, illustrating the important role for IL-10 from Tregs in protection from IRI. In each model of Treg deficiency, the innate immune response to kidney IRI was enhanced, an effect that was reversed by addition of WT Tregs. Collectively, these results demonstrate that Tregs function to protect the kidney from inflammation and dysfunction associated with IRI. In addition, our observations suggest that a major mechanism of Treg-mediated kidney protection is by inhibition of the innate immune response to kidney injury.
One method to investigate functions of Tregs is to deplete them partially using an anti-CD25 antibody (PC61), because Tregs express high levels of CD25 under normal conditions. In our hands, PC61 was effective at decreasing the total number and percentage of CD4+FoxP3+ Tregs in the kidney, spleen, and blood. One potential caveat of this method is that other leukocytes can also express CD25, including B cells, activated effector T cells, and NK cells. No significant effect on any of these non-Treg subsets was observed after PC61 administration. These results demonstrate that the use of PC61 specifically reduces Treg numbers in control mice without unintended effects on other leukocyte populations.
The second method used to study the role of Tregs in IR was the establishment of a mouse completely deficient in CD4+CD25+FoxP3+ cells. FoxP3 is a transcription factor that is vital for the regulatory functions of Tregs.7 Sf mice lack FoxP3 and develop severe autoimmune disease, resulting in premature death by 3 to 4 wk of age. Before death, Sf LN cells were harvested and used to reconstitute T cell– and B cell–deficient, RAG-1 KO mice. This strategy results in a mouse with an intact innate immune system, T cells and B cells, but devoid of FoxP3+ Tregs.25 These Treg-deficient mice were found to be significantly more sensitive to kidney IRI, adding further support to the hypothesis that Tregs are protective in this setting. To confirm the importance of Tregs, we co-administered highly pure isolated Tregs with Sf LN cells, which were sufficient to inhibit IRI significantly compared with Treg-deficient mice.
The model of adoptive transfer of CD4+CD25+ Tregs alone into RAG-1 KO mice, which contain only an innate immune system, has been used by others to demonstrate the potential for Tregs to inhibit the innate immune response directly.16,20 For example, Murphy et al.16 showed that adoptive transfer of CD4+CD25+ Tregs could suppress the innate response to burn injury, but an equivalent number of CD4+CD25− leukocytes could not. Kim et al.20 reported that both CD4+CD25+ Tregs and CD4+CD25− non-Tregs could prevent lethal overactivation of the innate immune response to infection. Similar to these two studies, we observed that CD4+CD25+ Tregs could protect RAG-1 KO mice from kidney IRI. In contrast to Kim et al.,20 CD4+CD25− cells did not offer protection from IRI. This difference may be explained by the methods used to activate the innate immune system in the different studies. In addition, the number of T cells (Tregs and non-Tregs) adoptively transferred was dramatically higher than in our study.
CD25 is the α-subunit of the IL-2 receptor, whose activation drives proliferation to sustain Treg numbers in vivo26–28 and is essential for maximal suppressive activity.29,30 In our model, targeting CD25 with PC61 was sufficient to exacerbate kidney IRI. In addition, in the adoptive transfer experiments, the Tregs, which were selected on the basis of CD25 expression, were competent to reduce kidney IRI in these models and prevent Sf LN cell–mediated multiorgan damage in a previous study.25 These findings suggest that Treg CD25 expression is an important phenotypic marker for protection against kidney IRI.
The role of the innate immune response in the pathogenesis of IRI is well established.4–6 Depletion of neutrophils or macrophages is sufficient to protect the kidney from IRI.4,5 In mice with decreased Tregs or mice completely deficient in Tregs, we observed significant increases in accumulation of neutrophils and macrophages in the kidney after IR. In addition, more neutrophils exhibiting an activated phenotype (IFN-γ producing) were detected in kidneys from mice with fewer Tregs after IR. We did not detect any difference in the numbers of adaptive immune cells in the kidney after IR in PC61-treated mice compared with controls. Examples of direct actions of Tregs on the innate immune response have been previously reported.14,16,18–20 To test whether Tregs could directly suppress the innate immune response to kidney IRI, we performed adoptive transfer of CD4+CD25+ Tregs or CD4+CD25− T cells into lymphocyte-deficient RAG-1 KO mice before IRI. We observed that the transfer of Tregs (CD4+CD25+) could prevent kidney neutrophil accumulation and IRI, but non-Tregs (CD4+CD25−) could not. These findings demonstrate that, in IR, Tregs can directly inhibit innate immune responses.
In Treg-deficient mice, we observed significantly greater CD4+ T cell accumulation after IR. This finding could be the result of the more highly activated phenotype of CD4+ T cells from Sf mice.25,31 Compared with T cells from a WT mouse, these Sf T cells express more activation markers and may be more highly responsive to IRI in the kidney. Another possibility is that a greater degree of Treg depletion than we achieved using PC61 is required to promote CD4+ T cell accumulation in the kidney after IR. In either case, the co-transfer of isolated Tregs with Sf LN cells prevented the kidney accumulation of CD4+ T cells, induced by Sf LN cell transfer alone, confirming that the enhanced lymphocyte accumulation was due to the lack of Tregs. It should be noted that our observations of infiltrating leukocytes after IR all were made at 24 h of reperfusion, and CD4+ T cells are reported to enter the kidney very early (within 30 min) during reperfusion and then exit shortly thereafter.6,32–34 Although the first two models used in this study leave open the possibility that Tregs act early to suppress initial CD4+ T cell activation (e.g., invariant NK T cells),6 results of the adoptive transfer of WT Tregs alone into RAG-1 KO mice before IRI demonstrate that Tregs can directly inhibit innate immune responses to IRI.
We observed that kidney Treg numbers (per gram) are significantly decreased 24 h after IRI compared with sham-operated mice (1564 ± 152 versus 10,075 ± 3450; n = 4 and 3, respectively; P < 0.05). This result is in agreement recent findings of Liesz et al.,35 who reported that after ischemic stroke, FoxP3+ Tregs were undetectable in the ischemic brain until 3 d of reperfusion. Our observations may be due to the increased kidney levels of IL-6,36 which is known to suppress FoxP3 expression alone or in the presence of TGF-β,37,38 which is also elevated after IRI.39 These results suggest that the protection afforded by Tregs is due to the Tregs in the kidney during ischemia and early reperfusion (before the 24-h time point) and/or the global pool of Tregs (outside the kidney, up to 24 h of reperfusion).
Although TGF-β and IL-10 are products of Tregs, these cytokines are also produced by damaged renal tubular cells39 and by other T cells and macrophages,40 respectively. The involvement of all of these non-Tregs in kidney IRI is well documented3,4,39 and may explain our inability to detect decreases in IL-10 and TGF-β in Treg-deficient mouse kidneys after IRI.
A major mechanism of Treg-mediated suppression of innate inflammatory responses is IL-10 production.19,36 IL-10 inhibits leukocyte activation through suppression of Jak-Stat and NF-κB signaling pathways,40 and in mice, administration of exogenous IL-10 before IRI is sufficient to protect mice from kidney injury.41 For these reasons, we used Tregs isolated from the IL-10 KO mouse to determine its role in Treg-mediated protection from kidney IRI. The use of IL-10 KO Tregs completely abrogated the protection afforded to RAG-1 KO mice by adoptive transfer of the same number of WT Tregs, confirming that IL-10 from Tregs is indispensable for preventing kidney IRI. These results are similar to a recent report in an ischemic stroke model: WT Tregs prevented injury, whereas IL-10 KO Tregs had no effect.36
In conclusion, our findings demonstrate a potent protective role for CD4+CD25+FoxP3+ Tregs in kidney IRI. Measurements of infiltrating leukocytes in the postischemic kidney suggest that the protection is mediated, at least in part, by IL-10–dependent blockade of innate immune cell activation and accumulation.
Concise Methods
Mice and Adoptive Transfer
Male WT mice that were 6 to 8 wk of age and weighed 20 to 25 g were obtained from Charles River Laboratories (Wilmington, MA). Male B6.129S7-Rag1tm1Mom/J (RAG-1 KO) and B6.129P2-Il10tm1Cgn/J (IL-10 KO) mice that were 6 to 8 wk of age and weighed 20 to 25 g were obtained from the Jackson Laboratories (Bar Harbor, ME). Heterozygous female B6.Cg-Foxp3sf/x/J mice (Jackson Laboratories) were bred with male C57Bl/6 mice to produce Sf mice. C57Bl/6 and Sf LN cells were isolated as described previously.42 For adoptive transfer, 5 or 10 × 106 WT or Sf LN cells were administered by intravenous injection to RAG-1 KO mice 2 wk before IR. Tregs were isolated from spleen and LNs of WT or IL-10 KO mice using the Dynal (Carlsbad, CA) CD4+ negative selection kit and Miltenyi Biotec (Auburn, CA) CD25+ positive selection kit according to the manufacturer's protocols. In some experiments, isolated Tregs were mixed and co-transferred with Sf LN cells at a ratio of 1:5 or 1:10 (Tregs:total Sf LN cells). In other experiments, 1 × 106 WT or IL-10 KO CD4+CD25+ Tregs or WT CD4+CD25− leukocytes were administered by tail-vein injection to RAG-1 KO mice 18 h before IRI.
Surgical Protocol
For the renal IR protocol, bilateral flank incisions were made under anesthesia. Both renal pedicles were exposed and cross-clamped for 24 or 28 min, clamps were removed, and kidneys were allowed to reperfuse for 24 h as described previously by our laboratory.6 All animal experiments were approved by the University of Virginia Institutional Animal Care and Use Committee.
Assessment of Renal Function
PCr was measured after renal IR using a colorimetric assay (Sigma, St. Louis, MO),6 and outer medullary tubular necrosis was assessed by hematoxylin and eosin staining as described previously.43 Briefly, stained kidney sections were scored in a blinded manner, and each sample was assigned a number on the basis of the percentage of outer medulla tubules displaying evidence of tubular necrosis (presence of pink casts inside tubules). The scoring system used was as follows: 0, no damage; 1, <10%; 2, 10 to 25%; 3, 25 to 75%; and 4, >75%.
Flow Cytometry
Five-color flow cytometry was used to determine the number and phenotype of leukocytes in the kidney, spleen, and blood. Counting beads (Caltag, Carlsbad, CA) were used as described previously6 to determine the total number of CD45+ cells per gram of kidney or spleen tissue or 100 μl of blood. This leukocyte number was used to convert the percentages of leukocytes displaying certain characteristics into a leukocyte count used for comparisons between treatment groups. All samples were treated with anti-mouse CD16/CD32 (2.4G2) to block the nonspecific FcR binding, 7-AAD or LIVE/DEAD Fixable Green Dead Cell Stain (Invitrogen, Carlsbad, CA) to exclude dead cells and anti-CD45 to gate on the live leukocyte populations. CD4+CD25+FoxP3+ Tregs were stained, fixed, and permeabilized using the eBioscience (San Diego, CA) FoxP3 buffer set according to the manufacturer's protocol. Intracellular IFN-γ staining was performed using the BD Biosciences (San Jose, CA) Fix/Perm buffer set according to the manufacturer's protocol and as described previously by our laboratory.6 Flow cytometry data acquisition and analysis were performed on BD FACSCalibur using Flowjo software (Tree Star, Ashland, OR). Anti-mouse fluorophore-labeled antibodies (clones) used were as follows: CD45-PE (30-F11), CD45-FITC (30-F11), CD45-APC-AlexaFluor750 (30-F11), CD25-PE (PC61), CD4 PE-Cy5 (GK1.5), CD4 AlexaFluor647 (GK1.5), FoxP3-APC (FJK-16s), GR-1-FITC (RB6-8C5), CD11b-PE (M1/70), F4/80-APC (BM8), IFN-γ–AlexaFluor647 (XMG1.2), CD3-APC-AlexaFluor750 (17A2), NK1.1-PE (PK136), B220-APC-AlexaFluor750 (RA3-6B2), and CD8α-PE (53-6.7) from eBiosciences; CD19-PE (1D3) from BD Pharmingen (San Jose, CA); and CD25-PE (7D4) from Southern Biotech (Birmingham, AL).
Immunohistochemical Analysis
Kidneys were fixed overnight in Periodate-Lysine Paraformaldehyde (1%), incubated for 48 h in 30% sucrose at 4°C, then embedded and frozen in OCT compound (Ted Pella, Redding, CA). Frozen kidney sections (10 μm) from each mouse were blocked with goat serum and 2.4G2, then labeled with a FITC-conjugated antibody (clone 7/4; Cedarlane, Burlington, Ontario, Canada), which recognizes neutrophils and recently emigrated monocytes.44,45 Nuclei were visualized using DAPI. Specimens were mounted with ProLong Gold Antifade reagent (Molecular Probes, Eugene, OR) and examined using a Zeiss Axiovert 200 microscope with ApoTome (Zeiss, Thornwood, NY).
Real-Time Reverse Transcriptase–PCR
Kidney sections were immediately transferred into RNA Later (Ambion, Austin, TX). RNA and cDNA were prepared from these samples as described previously.43 Subsequently, real-time Reverse transcriptase–PCR was performed using a MyIQ Single Color Real-Time PCR Detection System (BioRad, Hercules, CA) as described previously.46 Primers used were as follows: Glyceraldehyde-3-phosphate dehydrogenase, 5′-ACGGCAAATTCAACGGCACAGTCA-3′ (forward) and 5′-TGGGGGCATCGGCAGAAGG-3′ (reverse); IL-6, 5′-TGGCTAAGGACCAAGACCATCCAA-3′ (forward) and 5′-AACGCACTAGGTTTGCCGAGTAGA-3′ (reverse); TNF-α, 5′-CCTCCCTCTCATCAGTTCTATGG-3′ (forward) and 5′-CGTGGGCTACAGGCTTGTC-3′ (reverse); TGF-β, 5′-TAAAGAGGTCACCCGCGTGCTAAT-3′ (forward) and 5′-ACTGCTTCCCGAATGTCTGACGTA-3′ (reverse); and IL-10, 5′-TGCACTACCAAAGCCACAAAGCAG-3′ (forward) and 5′-TGCAGTTATTGTCTTCCCGGCTGT-3′ (reverse).
Antibodies
The TIB-222 hybridoma was used by the Lymphocyte Culture Core Facility at the University of Virginia to produce the anti-mouse CD25 mAb (clone PC61). Control IgG ImmunoPure Rat IgG was purchased from Pierce (Rockford, IL).
Statistical Analysis
Comparisons of two treatment groups were made using an unpaired t test to determine statistical significance. ANOVA was performed for comparisons of three or more groups, and the Holm-Sidak procedure was used for pair-wise comparisons using the SigmaStat statistical software (San Jose, CA). P < 0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants DK56223, DK58413, DK62324, T32 DK072922, PO1 HL073361, DE-017579, and AR-051203.
We are grateful to Dr. Kenneth Tung and Hui Qiao for the use of the TIB-222 hybridoma and assistance with purification of the PC61 mAb. We also thank Dr. Peter Lobo for helpful discussions and suggestions about this project.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: The role of CD4+ T cells and IFN-gamma. J Immunol 176: 3108–3114, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Day YJ, Huang L, Ye H, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV: Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K: Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–1232, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Buckner JH, Ziegler SF: Regulating the immune system: The induction of regulatory T cells in the periphery. Arthritis Res Ther 6: 215–222, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR: T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol 177: 6780–6786, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K, Rao VP, Poutahidis T, Rickman BH, Ohtani M, Xu S, Rogers AB, Ge Z, Horwitz BH, Fujioka T, Erdman SE, Fox JG: CTLA-4 blockade abrogates protection by regulatory T cells in a mouse model of microbially-induced innate immune-driven colitis. Infect Immun 76: 5834–5842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huehn J, Hamann A: Homing to suppress: address codes for Treg migration. Trends Immunol 26: 632–636, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Mira E, Leon B, Barber DF, Jimenez-Baranda S, Goya I, Almonacid L, Marquez G, Zaballos A, Martinez AC, Stein JV, Ardavin C, Manes S: Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol 181: 3524–3534, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP: Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol 66: 222–230, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralainirina N, Poli A, Michel T, Poos L, Andres E, Hentges F, Zimmer J: Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol 81: 144–153, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Murphy TJ, Choileain NN, Zang Y, Mannick JA, Lederer JA: CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol 174: 2957–2963, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F: CD4+CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197: 111–119, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, Alexander SI, Harris DC: CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol 17: 2731–2741, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lewkowicz P, Lewkowicz N, Sasiak A, Tchorzewski H: Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol 177: 7155–7163, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX: Adaptive immune cells temper initial innate responses. Nat Med 13: 1248–1252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, Soloski MJ, Rabb H: Normal mouse kidneys contain activated and CD3+CD4−CD8− double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM: Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol 178: 4136–4146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai H, Saio M, Nonaka K, Suwa T, Umemura N, Ouyang GF, Nakagawa J, Tomita H, Osada S, Sugiyama Y, Adachi Y, Takami T: Depletion of CD4+CD25+ regulatory T cells enhances interleukin-2-induced antitumor immunity in a mouse model of colon adenocarcinoma. Cancer Sci 98: 416–423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KS: Cutting edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J Immunol 180: 4366–4370, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Sharma R, Jarjour WN, Zheng L, Gaskin F, Fu SM, Ju ST: Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J Autoimmun 29: 10–19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida AR, Legrand N, Papiernik M, Freitas AA: Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol 169: 4850–4860, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY: A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6: 1142–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Bagavant H, Jarjour WN, Sung SS, Ju ST: The role of Fas in the immune system biology of IL-2R alpha knockout mice: Interplay among regulatory T cells, inflammation, hemopoiesis, and apoptosis. J Immunol 175: 1965–1973, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T, Orinska Z, Bulfone-Paus S, Scheffold A: IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol 38: 1643–1653, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ: Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med 196: 851–857, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma R, Ju AC, Kung JT, Fu SM, Ju ST: Rapid and selective expansion of nonclonotypic T cells in regulatory T cell-deficient, foreign antigen-specific TCR-transgenic scurfy mice: Antigen-dependent expansion and TCR analysis. J Immunol 181: 6934–6941, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H: Phenotypic and functional characterization of of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW: S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69: 1601–1608, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R: Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 15: 192–199, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK: Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, Bromberg JS: Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol 182: 259–273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basile DP, Rovak JM, Martin DR, Hammerman MR: Increased transforming growth factor-beta 1 expression in regenerating rat renal tubules following ischemic injury. Am J Physiol Renal Physiol 270: F500–F509, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A: Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA: Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118–2128, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Sharma R, Zheng L, Guo X, Fu SM, Ju ST, Jarjour WN: Novel animal models for Sjogren's syndrome: Expression and transfer of salivary gland dysfunction from regulatory T cell-deficient mice. J Autoimmun 27: 289–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S: Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol 33: 2090–2097, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Henderson RB, Hobbs JAR, Mathies M, Hogg N: Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 102: 328–335, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, Lynch K, Okusa MD: Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int 75: 167–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.