Abstract
The Na+/H+-exchanger 3 (NHE3) is essential for regulation of Na+ transport in the renal and intestinal epithelium. Although changes in cell surface abundance control NHE3 function, the molecular signals that regulate NHE3 surface expression are not well defined. We found that overexpression of the calcineurin homologous protein-1 (CHP1) in opossum kidney cells increased NHE3 transport activity, surface protein abundance, and ezrin phosphorylation. CHP1 knockdown by small interfering RNA had the opposite effects. Overexpression of wild-type ezrin increased both NHE3 transport activity and surface protein abundance, confirming that NHE3 is downstream of ezrin. Expression of a pseudophosphorylated ezrin enhanced these effects, whereas expression of an ezrin variant that could not be phosphorylated prevented the downstream effects on NHE3. Furthermore, CHP1 knockdown reversed the activation of NHE3 by wild-type ezrin but not by the pseudophosphorylated ezrin. Taken together, these results demonstrate that CHP1 increases NHE3 abundance and constitutive function in a manner dependent on ezrin phosphorylation.
Na+/H+-exchanger 3 (NHE3), in the brush border membrane of renal proximal tubule cells,1,2 is of major importance in mediating the absorption of the bulk of filtered sodium and fluid.3,4 NHE3 is a member of the mammalian NHE superfamily that mediates electroneutral countertransport of H+ for Na+. At least ten NHE isoforms (NHE1–10), a cluster of distant NHE-related genes (NHA1–2), and one sperm-specific NHE are found in mammals.5–7 Gene disruption of NHE3 in mice causes hypotension, acidosis, and hypovolemia. NHE3 knockout mice have decreased renal absorption of Na+, fluid, and HCO3−, diarrhea, and universal mortality when fed with a low-salt diet.8
The current structural model of NHE3 predicts two major domains, an amino-terminal transmembrane domain and a carboxyl-terminal cytoplasmic domain.9 The latter functions as a regulatory domain involving the dynamic association with accessory proteins that form a protein network jointly modulating NHE3 expression, traffic, and activity. A number of NHE3 binding partners and regulatory proteins have been identified, such as megalin, PDZK1, DPPIV, PP2A, Hsp70, and Shank2.10–17 The functional roles of most of these associations remain elusive.
Moreover, NHE3 associates with the actin cytoskeleton by binding to ezrin, which provides a regulated linkage between the plasma membrane and the actin cytoskeleton.18–23 Ezrin is a member of the ezrin/radixin/moesin family and is highly enriched in the microvilli on the apical side of epithelial cells. NHE3 binds to ezrin both directly and indirectly. Direct binding of NHE3 to ezrin (amino acids 475 to 589 of the NHE3 C terminus24) likely affects many aspects of basal NHE3 trafficking, including delivery from the synthetic pathway, basal exocytosis, and movement of NHE3 along the brush border, that may contribute to NHE3 endocytosis.24 Indirect binding, via the PDZ-domain-containing proteins Na+/H+-exchanger regulatory factor (NHERF) 1 and 2 (amino acids 585 to 689 of the NHE3 C terminus25,26), mediates several aspects of NHE3 regulation, including regulation by intracellular Ca2 +,27,28 cAMP,25,26,29 cGMP,30 and lysophosphatidic acid.28,31 Ezrin also has been proposed to mediate the Na+/glucose-cotransporter-induced activation and translocation of NHE3.32
Ezrin is responsible for membrane targeting33,34 by direct association of its N terminus with the cytoplasmic domain of several integral membrane proteins (CD43, CD44, ICAM-1, -2, and -3, and NHE1 and 318,19,23); it also binds F-actin via its C terminus.35 In the cytoplasmic dormant form of ezrin, intramolecular association of the N terminus with the C terminus masks binding sites for membrane proteins and F-actin.33,36 Phosphoinositol-(4,5)-bisphosphate binding to the N terminus of ezrin followed by phosphorylation of threonine 567 at the C terminus of ezrin exposes both membrane protein and actin binding sites36 and thus activates ezrin.37 Ezrin in its closed conformation does not bind NHE3; only the open, active form of ezrin directly binds NHE3 in vitro.24
The calcineurin homologous protein (CHP) family belongs to an EF-hand (calcium-binding motifs composed of two helixes E and F joined by loop) subfamily of calcium-binding proteins that has similarity to the B regulatory subunit of the heterodimeric protein phosphatase calcineurin or Ca2+-calmodulin.38 The CHP family (CHP1–3) associates with NHE isoforms, including NHE3,38–41 in close proximity to the direct NHE3–ezrin binding region.42–44 CHP1 is a widely expressed and highly conserved cytosolic Ca2+-binding protein38,45 that regulates the phosphatase calcineurin46 and apoptosis-inducing protein kinase DRAK2 activity.47 CHP1 appears to be a crucial partner of several NHE isoforms for basal and regulated activity.38–40,48,49 Indeed, CHP1 sets the resting intracellular pH sensitivity of the transporter,39,50 permits NHE3 regulation by adenosine A1 receptors,48 and stabilizes NHE1 on the plasma membrane.49 CHP1 was found also to regulate targeting, docking, and fusion of transcytotic membrane vesicles45 and associate with microtubules and affect their organization.51,52 Similarly, CHP3 promotes NHE1 abundance, cell surface stability, and optimal transport function.41
These collective properties of CHP1 render it a multipotent regulatory protein with several functions and make it a plausible candidate to regulate NHE3 traffic. Here, we propose that CHP1 is an essential signal molecule that controls the level of basal NHE3 expression and function, and we present a model that links CHP1–ezrin–NHE3 in a regulatory complex.
Results
CHP1 Expression Modulates NHE3 Function
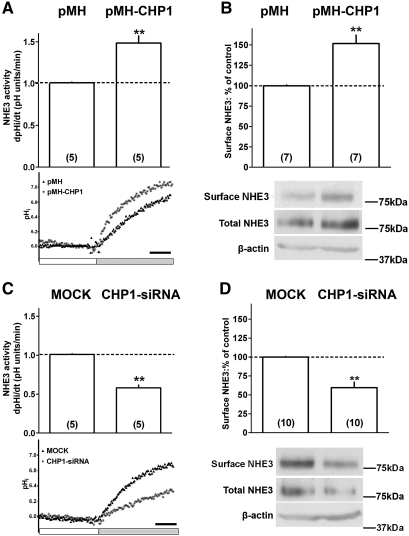
We selected the opossum kidney (OK) epithelial cell line for our study, because, unlike most other epithelial lines, OK cells express, among the NHE protein family members, exclusively NHE3,53,54 which greatly simplifies the analysis and interpretation of the data. OK cells used in this study also express endogenous CHP1.48 The effect of CHP1 expression on endogenous NHE3 activity and surface expression was evaluated. Overexpression of CHP1 (Supplemental Figure 1) increases both baseline NHE3 activity and apical surface antigen by 50% (Figure 1, A and B). Knockdown of CHP1, typically to 20%, by small interfering RNA (siRNA)48 (Supplemental Figure 1) inhibits both NHE3 baseline activity and reduces apical surface NHE3 antigen by 50% (Figure 1, C and D). We previously showed that CHP1 knockdown reduced total cellular NHE3.48 We confirmed that CHP1 knockdown reduces total NHE3 (Figure 1D) and found that CHP1 overexpression actually increases total NHE3 protein amount (Figure 1B). Therefore, the level of β-actin was used as a control for total protein. No changes in the β-actin amounts were detected in the conditions under study (Figure 1, B and D).
Figure 1.
Effect of CHP1 expression on surface NHE3 activity and protein. NHE3 activity was measured fluorimetrically as the rate of Na+-dependent pHi recovery. Representative traces and summary of data are shown. Onset of tracing is when cells were switched to Na+-free solution (white bar) after an acid load using the nigericin method (see Concise Methods for details). Intracellular pH was allowed to recover through NHE3 activity by the addition of Na+-containing solution (gray bar). Black bar indicates 25 s. NHE3 surface protein was quantified by cell surface biotinylation, affinity precipitation with streptavidin-bound agarose, and immunoblot using #3H3 anti-NHE3 antibody. Representative immunoblots and summary of data are shown. Antigen signals were normalized to β-actin. Results are expressed as dpHi/dt (pHi units/min) for NHE3 activity and percentage of control for NHE3 surface protein. Bars and error bars represent means and standard errors. The number of experiments performed under identical conditions is in parenthesis. **P < 0.01 ANOVA compared with control. CHP1-transfected cells (pMH-CHP1) were compared with empty-vector-transfected cells (pMH): (A) NHE3 activity. pMH is indicated by black triangles; pMH-CHP1 by asterisks. (B) NHE3 surface protein, NHE3 total protein, and β-actin total protein. CHP1-siRNA-transfected cells (CHP1-siRNA) were compared with mock-transfected cells (MOCK). Mock-transfected cells means transfected cells with scrambled siRNA: (C) NHE3 activity. Mock is indicated by black triangles; CHP1-siRNA by asterisks. (D) NHE3 surface protein, NHE3 total protein, and β-actin total protein.
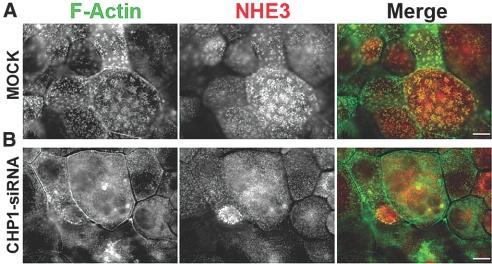
In OK cells, NHE3 is located in apical clusters,55 where it associates with F-actin filaments (Figure 2A). CHP1 colocalizes with NHE3 in these clusters (Figure 3A). CHP1 knockdown also decreased NHE3 localization in apical clusters and redistributed F-actin (Figure 2B). These findings indicate that CHP1 expression regulates NHE3 activity by modulating its amount at the cell surface and its localization in apical clusters.
Figure 2.
Effect of CHP1 knockdown on NHE3 localization in apical clusters. Colocalization of endogenous NHE3 and F-actin was analyzed by epifluorescence microscopy. NHE3 was stained with #3H3 antibody and F-actin by Alexa Fluor 488-conjugated with phalloidin. Colocalization is reflected by a yellow signal of the merged composite. Scale bar, 2 μm. (A) Mock-transfected cells (MOCK). (B) CHP1-siRNA-transfected cells (CHP1-siRNA).
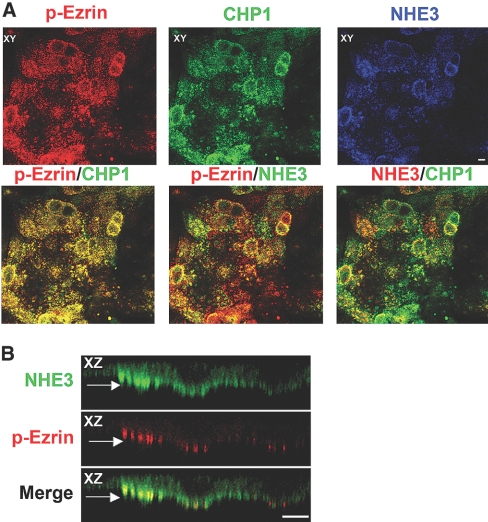
Figure 3.
Colocalization of NHE3 and CHP1 with endogenous phosphorylated ezrin. Localization of endogenous NHE3, CHP1, and phosphorylated ezrin by confocal microscopy. NHE3 was stained with #3H3 antiserum, CHP1 with anti-CHP polyclonal antibody, and phosphorylated ezrin with T567 phospho-specific polyclonal antibody. Colocalization of NHE3, CHP1, and phosphorylated ezrin is shown. Colocalization of two of the three molecules is indicated by the merged yellow color. Pseudocolor of green or red was assigned to NHE3 as indicated in the figure. XZ cross-sections and XY face views are highlighted. (A) Colocalization of endogenous NHE3, CHP1, and phosphorylated ezrin (p-Ezrin). (B) Detail of endogenous NHE3 and p-Ezrin colocalization in apical clusters as indicated by the arrow in the XZ plane. Scale bar, 2 μm.
CHP1 Expression Regulates the Amount of Phosphorylated Ezrin at Threonine 567
In support of the importance of ezrin and actin in NHE3 trafficking,24,32,43,56–59 we found an excellent correspondence between the clusters, presumably microvillar structures, of endogenous NHE3 and CHP1 and those of phosphorylated ezrin, suggesting that these molecules coexist in the same aggregated structures (yellow in Figure 3, A and B). We selectively examined phosphorylated ezrin by a T567 phospho-specific anti-ezrin antibody because the phosphorylation of T567 has been shown to play an important role in ezrin conformational activation.37,60,61 These data define that the phosphorylated form of ezrin colocalizes with NHE3 and CHP1, compatible with the hypothesis that phosphorylated ezrin, together with CHP1, may be part of a regulatory complex that controls NHE3 function. This hypothesis is further sustained by the fact that the regions of direct binding of ezrin and CHP1 to NHE3 are in close proximity.43
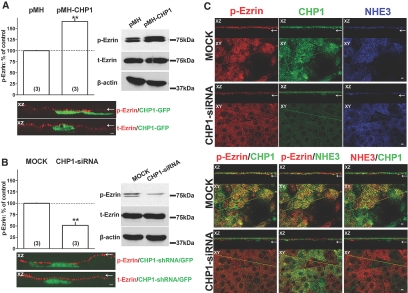
Common functions for EF-hand proteins such as CHP1 are regulation of protein phosphorylation, enzyme activities, or the organization of cytoskeleton constituents.62 To further test our hypothesis, we next evaluated whether expression of CHP1 alters the state of ezrin phosphorylation. Overexpression of CHP1 clearly induces an increase in phosphorylated ezrin by approximately 60% without changes in total ezrin or β-actin (Figure 4A). Conversely, CHP1 knockdown reduces phosphorylated ezrin by approximately 50% without changing total ezrin or β-actin (Figure 4B), and there is less accumulation of phosphorylated ezrin in apical clusters compared with that in mock-transfected cells (Figure 4C). These data support a role for CHP1 expression in regulating ezrin phosphorylation. As expected, NHE3 localization in apical clusters is reduced in CHP1-siRNA-transfected cells compared with that in mock-transfected cells (Figure 4C). Colocalization of CHP1 with phosphorylated ezrin or NHE3 in CHP1-siRNA- or mock-transfected OK cells also is shown in Figure 4C. Of note, reduction in CHP1 decreases the colocalization of NHE3 with phosphorylated ezrin (yellow in Figure 4C).
Figure 4.
Effect of CHP1 knockdown on ezrin phosphorylation and ezrin colocalization with NHE3 in apical clusters. In either CHP1-siRNA- or mock-transfected cell lysates, phosphorylated or total cellular ezrin protein abundance was measured by immunoblot. Antigen signals were normalized to β-actin. Representative immunoblots and summary of data are shown. Results are expressed as percentage of control of phosphorylated ezrin (p-Ezrin) or total ezrin (t-Ezrin). Anti-ezrin antibody for p-Ezrin detected two close bands in OK cells; changes in both bands were measured. Bars and error bars represent means and standard errors. The numbers in parentheses refer to the number of experiments performed under identical conditions. **P < 0.01 ANOVA compared with control. NS, statistically insignificant compared with control. CHP1-transfected cells (pMH-CHP1) were compared with empty-vector-transfected cells (pMH): (A) p-Ezrin, t-Ezrin, and β-actin proteins. CHP1-siRNA-transfected cells (CHP1-siRNA) were compared with mock-transfected cells (MOCK): (B) p-Ezrin, t-Ezrin, and β -actin proteins. Highlighted in A and B is the localization of p-Ezrin and t-Ezrin in cells expressing CHP1-GFP and CHP1-shRNA/GFP analyzed by confocal microscopy. Apical localization is indicated by an arrow in the XZ cross-sections. (C) Colocalization of endogenous NHE3, CHP1, and p-Ezrin in CHP1-siRNA- and mock-transfected cells analyzed by confocal microscopy. Colocalization of two of the three molecules is indicated by the merged yellow color. Pseudocolor of green or red was assigned to NHE3 as indicated in the figure. Highlighted in the figure are XZ cross-sections and XY face views. Apical localization is indicated by an arrow. Scale bar, 2 μm.
Expression of Ezrin or a Pseudophosphorylated Variant of Ezrin Activates NHE3
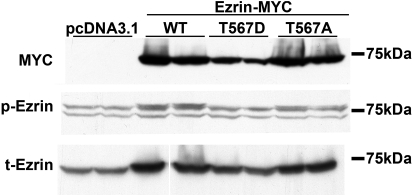
Ezrin activation is known to be promoted by phosphorylation of its C-terminal threonine 567.37,60,61 To further investigate the cascade of events that links CHP1, ezrin, and NHE3 in a regulatory complex, we manipulated ezrin phosphorylation by phospho-mimicry. We transiently expressed three ezrin constructs in OK cells: a full-length, wild-type ezrin (Ezrin-WT, Supplemental Figure 2A), an ezrin with a threonine to aspartic acid point mutation at position 567 (Ezrin-T567D, pseudophosphorylated ezrin,63 Supplemental Figure 3A), and an ezrin with a threonine to alanine point mutation at position 567 (Ezrin-T567A, nonphosphorylatable ezrin, which binds inefficiently to the actin cytoskeleton,63 Supplemental Figure 4A). The three ezrin constructs were either enhanced green fluorescent protein (eGFP)- or myc-tagged. Localization of ezrin and its mutated forms was detected by confocal microscopy in OK cells, and F-actin was used to visualize cell structure (Supplemental Figures, 2B, 3B, and 4B). Ezrin-WT is located mainly at the apical surface (Supplemental Figure 2); Ezrin-T567D and Ezrin-T567A are also recruited to the plasma membrane but do not exclusively localize on the apical surface (Supplemental Figures 3 and 4). Expression of the three ezrin constructs does not cause detectable changes in cell shape (Supplemental Figures 2B, 3B, and 4B), nor do eGFP-tagged ezrin constructs demonstrate a substantially distinct localization compared with that of myc-tagged constructs (Supplemental Figures 2C, 3C, and 4C). Ezrin-WT and -T567A show similar expression, whereas Ezrin-T567D is expressed at a lower level; an approximately twofold difference in expression was detected by immunoblot (Figure 5). eGFP-tagged constructs show a similar pattern of expression (Supplemental Figure 5A). Of note, some of the transfected Ezrin-WT is phosphorylated, as shown by an increase in ezrin phosphorylation in myc-tagged Ezrin-WT-expressing cells compared with empty-vector-transfected cells (Figure 5). In further support of this notion, some of the transfected eGFP-tagged Ezrin-WT (approximately 100 kD, Supplemental Figure 5A) is also phosphorylated, based on labeling by anti-phospho-ezrin antibody (Supplemental Figure 5B) and overlapping of eGFP-tagged Ezrin-WT localization with that of phosphorylated ezrin (yellow in Supplemental Figure 2D). As expected, the anti-phospho-ezrin antibody does not significantly detect Ezrin-T567D and Ezrin-T567A as shown by immunoblot (Figure 5 and Supplemental Figure 5B) or indirect immunofluorescence (lack of merged yellow in Supplemental Figures 3D and 4D). All three ezrin constructs are recognized by an anti-ezrin antibody for total ezrin (Figure 5 and Supplemental Figures 2E, 3E, 4E, and 5B). No changes in total endogenous ezrin are detected in cells expressing the three constructs (Supplemental Figure 5B). Jointly, these observations demonstrate that OK cells transiently expressing ezrin and its mutants represent a suitable cellular model to study the effects of ezrin overexpression. They do not show the dramatic morphologic changes seen in other cells producing Ezrin-T567D,63–65 probably because of a contained expression level of Ezrin-T567D. Occasionally, we observed that Ezrin-T567D in OK cells induced the formation of surface membrane projections (Supplemental Figure 3D).
Figure 5.
Expression of myc-tagged Ezrin-WT and variants of ezrin in OK cells. Immunoblots were performed with anti-myc antibody to detect myc-tagged Ezrin (MYC), anti-phospho-ezrin antibody to detect phosphorylated ezrin (p-Ezrin), and anti-total-ezrin antibody to detect total ezrin (t-Ezrin). Representative immunoblot is shown in duplicate; four independent experiments showed similar results. In the bottom gel (t-Ezrin), a blank lane has been deleted as indicated by a white line. pcDNA 3.1, empty-vector-transfected cells; Ezrin-MYC, myc-tagged ezrin-transfected cells. Ezrin-WT (WT), Ezrin-T567D (T567D), Ezrin-T567A (T567A).
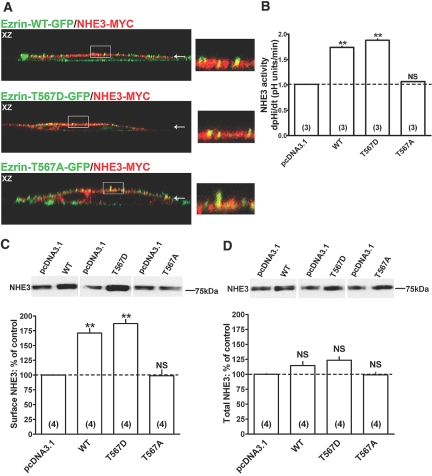
We next examined the effects of ezrin and its mutated forms on NHE3 function. Although we did not quantify whether the ezrin phosphorylation status affects NHE3 colocalization with ezrin, myc-tagged NHE3 colocalizes with each of the ezrin constructs at apical clusters (Figure 6A). Importantly, expression of the three ezrin constructs does not alter NHE3 distribution at the apical membrane, suggesting that their expression does not disrupt the epithelial polarity. Figure 6, B and C, shows that expression of Ezrin-WT increased NHE3 transport activity and surface expression. This effect was amplified by expression of Ezrin-T567D (Figure 6, B and C), especially considering that Ezrin-T567D expression is lower than that of Ezrin-WT. In contrast, expression of nonphosphorylatable Ezrin-T567A had no effect on NHE3 (Figure 6, B and C). Expression of ezrin and its mutants does not significantly affect total NHE3 (Figure 6D). Overall, these findings support the importance of ezrin phosphorylation in its ability to activate NHE3.
Figure 6.
Effect of Ezrin-WT and Ezrin-T567D expression on surface NHE3 activity and protein. (A) Colocalization of myc-tagged NHE3 (NHE3-MYC) with eGFP-tagged Ezrin-WT (Ezrin-WT-GFP), Ezrin-T567D (Ezrin-T567D-GFP), or Ezrin-T567A (Ezrin-T567A-GFP). In the XZ cross-sections, apical localization is indicated by an arrow. Colocalization is reflected by a yellow signal of the merged composite. The area indicated by a rectangle in the XZ cross-sections is magnified in the inset. NHE3 activity, NHE3 surface, and total protein were quantified as described in the legend of Figure 1. Myc-tagged Ezrin-WT (WT), Ezrin-T567D (T567D), and Ezrin-T567A (T567A) transfected cells were compared with empty-vector-transfected cells (pcDNA3.1): (B) NHE3 activity. (C) NHE3 surface protein. (D) NHE3 total protein. Representative immunoblots and summary of data are shown. Results are expressed as dpHi/dt (pHi units/min) for NHE3 activity and percentage of control for NHE3 surface and total protein. Bars and error bars represent means and standard errors. The number of experiments performed under identical conditions is in parentheses. **P < 0.01 ANOVA compared with control. NS, statistically insignificant compared with control.
CHP1 Knockdown Reverses Ezrin-Mediated Upregulation of NHE3 Function
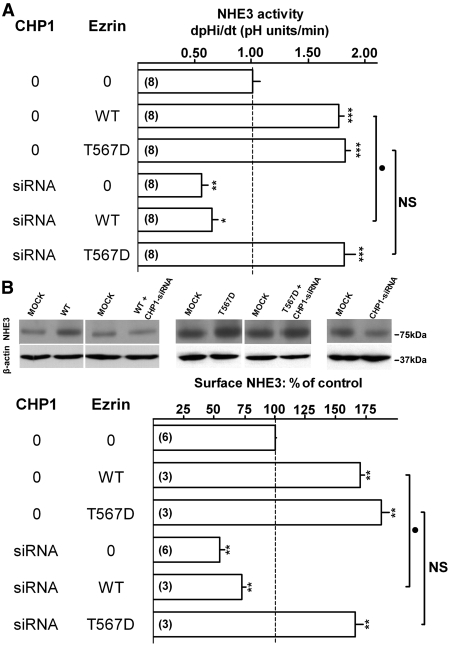
To further demonstrate the CHP1–ezrin–NHE3 signal cascade, we showed that the effect of expression of Ezrin-WT on surface NHE3 transport activity and protein abundance is abrogated when CHP1 is knocked down (Figure 7, A and B), implying that high levels of ezrin alone are insufficient to upregulate NHE3 without CHP1. Remarkably, with the pseudophosphorylated Ezrin-T567D, the presence of CHP1 is not essential for NHE3 activation (Figure 7, A and B). The nonphosphorylatable Ezrin-T567A was not included in this series of experiments because Ezrin-T567A expression does not affect NHE3 function. These findings confirmed that ezrin is a signal molecule downstream from CHP1 in activating NHE3 (Supplemental Figure 6).
Figure 7.
Effect of CHP1 knockdown on ezrin-mediated activation of NHE3 function. NHE3 activity, NHE3 surface protein, and β-actin total protein were quantified as described in the legend of Figure 1. Representative immunoblots and summary of data are shown. Antigen signals were normalized to β-actin. Results are expressed as dpHi/dt (pHi units/min) for NHE3 activity and percentage of control for NHE3 surface protein. Bars and error bars represent means and standard errors. The numbers in parentheses refer to the number of experiments performed under identical conditions. *P < 0.05/**P < 0.01/***P < 0.001 ANOVA compared with control. •P < 0.05 ANOVA cells transfected with Ezrin-WT (WT) compared with cells cotransfected with CHP1-siRNA and Ezrin-WT (WT). NS, evoked change in cells transfected with Ezrin-T567D (T567D) is not significantly different compared with cells cotransfected with CHP1-siRNA and Ezrin-T567D (T567D). 0 indicates transfection of scrambled siRNA in the CHP1 column or empty-vector in the ezrin column. Myc-tagged Ezrin-WT and variants were expressed in OK cells. (A) NHE3 activity. (B) NHE3 surface protein and β-actin total protein.
Discussion
It was previously reported that CHP1 plays a key role in basal and regulated NHE1 activity38–40,49 and that expression of members of the CHP family stabilizes NHE1 protein at the cell surface.41,49 We have shown that CHP1 mediates the acute regulation of NHE3 by adenosine.48 Now we demonstrate that CHP1 is involved in the control of cell surface NHE3 activity and protein amount. Expression of CHP1 modulates NHE3 transport activity by affecting the basal amount of NHE3 at the cell surface and its localization in apical clusters. CHP1, however, seems to have more than one level of control on NHE3 expression, because total cellular NHE3 protein is reduced by CHP1 knockdown48 and increased by CHP1 overexpression. Akhter et al. have estimated that OK cells have 10 to 15% of total NHE3 on the brush border, indicating that most NHE3 protein is located in the cytosol.55 A 50% reduction of total NHE3 protein would cause a corresponding 50% reduction of surface NHE3 protein if the ratio between cytoplasmic and cell surface NHE3 is not changed by CHP1 expression. Ongoing studies aim to identify the mechanisms by which CHP1 modulates surface and total cellular NHE3 protein. The CHP1-dependent loss or gain of NHE3 protein might be caused by changes in NHE3 stability at the plasma membrane similar to what has been shown for the NHE1 protein.41,49
Our results indicate excellent colocalization of endogenous NHE3, CHP1, and phosphorylated ezrin in apical clusters, suggesting that these molecules coexist in the same aggregated structures and may function as a regulatory complex. We further propose a CHP1–ezrin–NHE3 signal cascade based on three lines of evidence.
First, ezrin activation is downstream from CHP1, because the amount of phosphorylated ezrin at threonine 567 is dependent on CHP1 expression level and CHP1 knockdown affects colocalization of phosphorylated ezrin with NHE3 in apical clusters. Importantly, it has been shown that CHP1 affects the GTPase-mediated stimulation of NHE1.38 GTPase proteins, such as RhoA, have been implicated in numerous studies in the regulation of NHE3 trafficking66–69 and ezrin phosphorylation,70 suggesting a mode of action that links CHP1, GTPase protein, ezrin, and NHE3 functions in a regulatory complex. This study does not address whether RhoA is involved in the CHP1–ezrin–NHE3 signal cascade, because although the involvement of RhoA GTPase in this pathway is plausible, other concomitant mechanisms of action cannot be ruled out, such as direct binding of CHP1 to the inactive form of ezrin to induce ezrin activation as was shown for S100 protein.71 Furthermore, several kinases and phosphatases are found to regulate ezrin/radixin/moesin protein phophorylation,19,61,72–76 and CHP1 is known to regulate protein kinase or phosphatase activity.46,47 In conjunction, these possible mechanisms render the model of how CHP1 affects ezrin activation very complex, and extensive studies are required to address it.
Second, NHE3 is downstream from ezrin, because ezrin, most likely via its phosphorylation, activates NHE3. The expression of three ezrin constructs in OK cells was characterized. Ezrin-WT is located mainly at the apical surface, as was shown previously in other cell models.37 Ezrin-T567D and Ezrin-T567A are both recruited to the plasma membrane, but neither Ezrin-T567D nor Ezrin-T567A locates exclusively on the apical surface. Similarly, in LLC-PK1 cells, Ezrin-T567D did not have restricted apical localization.37 Expression of Ezrin-T567A on the plasma membrane is not surprising, because the binding of unphosphorylated ezrin to the membrane seems to be mediated by phosphoinositol-(4,5)-bisphosphate.37,65,77 Expression of Ezrin-WT and its variants in OK cells seems not to affect cell morphology, as shown by F-actin staining, or cell polarity, as shown by preservation of apical localization of NHE3. Contrary to other cell models,63,64 in OK cells Ezrin-T567D expression does not appear to affect cell morphology and polarity, probably because of a low expression level of Ezrin-T567D in these cells compared with Ezrin-WT.
Ezrin-WT expression increases NHE3 surface activity and protein levels, an effect that is enhanced by expression of the pseudophosphorylated variant of ezrin. The finding that the nonphosphorylatable variant of ezrin does not affect NHE3 surface activity and amount of protein supports the concept that ezrin activation of NHE3 function is due to ezrin phosphorylation and not to ezrin overexpression. That ezrin has a specific role in maintaining NHE3 basal surface expression, however, is not completely unexpected. It was demonstrated that disruption of direct NHE3–ezrin interaction decreases NHE3 transport activity and the amount of NHE3 in the plasma membrane.24 In agreement with these findings, ezrin phosphorylation at threonine 567 is necessary for NHE3 recruitment to the apical membrane and NHE3-dependent intracellular pH (pHi) increase triggered by Na+/glucose cotransport.32
Third, ezrin is downstream from CHP1 in activating NHE3 because the effect of Ezrin-WT on NHE3 surface activity and protein abundance is abrogated when CHP1 is knocked down. This is concordant with the finding that a high level of ezrin alone is insufficient to activate NHE3. With overexpression of the pseudophosphorylated Ezrin-T567D, CHP1 knockdown does not affect the Ezrin-T567D-dependent activation of NHE3, indicating that once ezrin is phosphorylated the presence of CHP1 is not essential. Furthermore, the effect of ezrin phosphorylation on NHE3 expression is significantly higher than that induced by CHP1 overexpression alone, suggesting that some additional mechanisms between or in parallel with CHP1 and ezrin may attenuate the response of NHE3 to CHP1 expression. Of note, ezrin expression does not affect total expression of NHE3 but CHP1 knockdown does,48 indicating that regulation of ezrin phosphorylation by CHP1 expression is only one of several mechanisms by which CHP1 controls NHE3 function.
In summary, expression of CHP1 controls the transport activity and surface protein abundance of NHE3. Ezrin phosphorylation is necessary for the CHP1-dependent control of NHE3 function. We have shown previously that CHP1 is a NHE3 binding partner involved in the acute ligand-initiated regulation of NHE3.48 Here, we report that CHP1 expression is necessary not only for regulation of NHE3 but also for its constitutive function. We propose that the two accessory proteins CHP1 and ezrin control NHE3 function in a dependent manner (Supplemental Figure 6). Further studies are necessary to define additional molecular mechanisms by which CHP1 regulates NHE3.
Concise Methods
Antibodies and Other Reagents
All chemicals were obtained from Sigma (St. Louis, MO), except the cell culture reagents [Dulbecco modified Eagle medium, PBS, FCS, penicillin/streptomycin, and trypsin–EDTA] and the acetoxymethyl ester of 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF-AM) that were obtained from Invitrogen (Carlsbad, CA). The arginine- and lysine-reactive NHS-SS–biotin [succinimidyl 2-(biotinamido)-ethyl-1,3′ dithiopropionate] and streptavidin–agarose were from Pierce (Rockford, IL). The enhanced chemoluminescence system was from Amersham Biosciences (Piscataway, NJ). The following primary antibodies were used: monoclonal mouse anti-opossum NHE3 antibody (#3H3) to detect NHE3,78 anti-CHP rabbit polyclonal antibody (kindly provided by Dr. Diane L. Barber, University of California, San Francisco, CA) to detect CHP1,48 anti-ezrin rabbit polyclonal antibodies (Abcam, Cambridge, MA) to detect total and phospho-threonine 567 ezrin, anti-myc rabbit polyclonal antibody (Abcam, Cambridge, MA) to detect myc-tagged proteins, anti-GFP rabbit polyclonal antibody (Invitrogen, Carlsbad, CA) to detect eGFP-tagged proteins, and β-actin mouse monoclonal antibody (Sigma, St. Louis, MO) to detect β-actin.
Cell Culture and Transient Transfection
OK cells were cultured in a mixture of Dulbecco modified Eagle medium (DMEM/Ham's F12), supplemented with 10% FCS, 2 mM glutamine, and 50 IU/ml penicillin and 50 μg/ml streptomycin. Cultures were incubated in a humidified 95% air/5% CO2 atmosphere at 37°C and subcultured weekly by trypsinization using 0.1% trypsin/0.5 mM EGTA in PBS.48,78 Cells generally reached confluence within 3 to 4 d, and confluent cells were serum-deprived for 2 d before assay. Studies on OK cells were performed between passages 23 and 57. OK cells were transiently transfected with plasmids, double-stranded siRNA, or both using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Transfection efficiency was monitored by transfection of the pEGFP-N1 vector (Clontech, Mountain View, CA) and was approximately 60 to 70%.
Plasmid Constructs and siRNA
The full-length human ezrin (GenBank Accession number BC013903) was cloned into pEGFP-N1 via XhoI and EcoRI sites using the following primers: CGCCTCGAGATGCCGAAACCAATCAATGTC (forward)/CTGAATTCGCAGGGCCTCGAACTCGTC (reverse). The mutations of threonine 567 to aspartic acid (T567D) and threonine 567 to alanine (T567A) were produced by site-directed mutagenesis using the primer CGGGACAAGTACAAGgacCTGCGGCAGATCCG to generate the T567D mutant and the primer CGGGACAAGTACAAGgccCTGCGGCAGATCCG to generate the T567A mutant. To generate the myc-tagged version of wild-type ezrin and the ezrin mutants, the pEGFP-N1 vector (Clontech, Mountain View, CA) was digested with AgeI and BsrGI and religated with the myc-containing oligonucleotide adaptor. To generate eGFP-tagged CHP1, the full-length mouse CHP1-encoding cDNA was cloned into pEGFP-N1 (Clontech, Mountain View, CA) via HindIII and BamHI restriction sites. All plasmids were sequence-verified. siRNA for CHP1 (CHP1-siRNA) was synthesized and characterized as described previously.48 Short hairpin RNA (shRNA) for CHP1 (CHP1-shRNA) was obtained by cloning the sequence used for CHP1-siRNA48 into the MSCV-LTRmiR30-PIG vector (Open Biosystems, Huntsville, AL) via XhoI and EcoRI restriction sites, according to the manufacturer's instructions. eGFP in the MSCV-LTRmiR30-PIG vector serves as a marker for CHP1-shRNA expression (CHP1-shRNA/GFP). CHP1-shRNA/GFP decreases the CHP1 expression level to a similar extent as CHP1-siRNA (not shown). The Silencer GFP (cycle 3) siRNA (Ambion, Austin, TX) was used as scrambled siRNA.
Measurement of Na+/H+ Exchange Activity
NHE3 activity in OK cells was measured fluorimetrically using the intracellularly trapped pH-sensitive dye BCECF-AM as previously shown.79 Briefly, cells grown on glass coverslips were loaded with 10 μM BCECF-AM (30 min at 37°C), and pHi was estimated from the ratio of fluorescence in a computer-controlled spectrofluorometer (λexcitation: 500 and 450 nm, λemission: 530 nm). The 500/450 nm fluorescence ratio was calibrated to pHi using K+/nigericin. Na+/H+ exchange activity was assayed as the initial rate of the Na+-dependent pHi increase after an acid load using nigericin in the absence of CO2/HCO3−. Comparisons were always made between cells of the same passage studied on the same day.
Quantification of Cell Surface and Total Antigen by Immunoblot
These assays were performed as described previously.48,78 OK cells were grown to confluence on 100-mm culture dishes. Cells were rinsed in Ca/Mg/PBS (containing 150 mM NaCl, 10 mM Na2HPO4, pH 7.4, 0.1 mM CaCl2, 1 mM MgCl2) and incubated with the arginine- and lysine-reactive NHS-SS–biotin (2 mg/ml) in buffer (containing 150 mM NaCl, 10 mM triethanolamine, pH 7.4, 2 mM CaCl2) for 1 h. After being quenched in Ca/Mg/PBS supplemented with 100 mM glycine, cells were lysed in radio-immunoprecipitation assay (RIPA) buffer (containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 5 mM EDTA, 1% (vol/vol) Triton X-100, 0.5% (w/v) deoxycholate, 0.1% (w/v) SDS), and centrifuged at 100,000 × g for 25 min, at 4°C. The protein content in the supernatants was quantified by the Bradford method (Bio-Rad, Hercules, CA). Equal amounts of cell lysate protein were equilibrated with streptavidin–agarose beads at 4°C. The beads were then washed sequentially with solution A (containing 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA), solution B (containing 50 mM Tris-HCl, pH 7.4, 500 mM NaCl), and solution C (containing 50 mM Tris-HCl, pH 7.4). Biotinylated proteins were released by incubation in 100 mM dithiothreitol, reconstituted in Laemmli's loading buffer, and subjected to SDS-PAGE and blotted with monoclonal mouse anti-opossum NHE3 (#3H3) antibody. For total protein detection, 50 μg of total cellular protein was resolved by SDS-PAGE.
Epifluorescence and Confocal Imaging
OK cells, plated on glass coverslip, were fixed (4% paraformaldehyde in PBS for 20 min) and permeabilized (0.2% saponin, 0.2% gelatin for 20 min). The cells were then incubated with primary antibodies in permeabilization buffer, followed by incubation with Cy2/Cy3/Cy5 conjugated donkey anti-mouse or donkey anti-rabbit polyclonal antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). F-Actin was labeled with Alexa Fluor 635 phalloidin according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Confocal fluorescence images were collected with a Leica SP2 laser-scanning confocal microscope using a 100×/1.4NA objective lens. Fluorescence of Cy2 (or eGFP), Cy3, and Cy5 (or Alexa 635) were sequentially excited by 488, 561, and 633 nm lasers to avoid cross-contamination between fluorophors. Emitted light was separated by an acousto-optical tunable filter (498 to 550 nm, Cy2 and eGFP; 590 to 625 nm, Cy3; 665 to 850 nm, Cy5 and Alexa-635) onto three photomultiplier channels. The epifluorescence images (Figure 2) were obtained with a Nikon TE2000-U microscope controlled by Metamorph 6.0 software. Apical clusters were visualized by a 60×/1.0NA objective lens focused on the apical surface of OK cells.
Statistical Analysis
Results are represented as mean ± standard error. Quantitative differences between control and test conditions were assessed statistically by ANOVA. A probability (P) <0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Dr. Diane L. Barber (University of California, San Francisco, CA) for the anti-CHP antiserum. We thank Mr. Mark Jevons (University of Newcastle, Newcastle upon Tyne, U.K.) for his help in performing preliminary experiments for this project. This work was supported by the American Heart Association Texas Affiliate (0325098Y to F.D.S.), the National Institutes of Health (DK-54396 and DK-48482), the Simmons Family Foundation, and Seed Funds from the Pak Center of Mineral Metabolism and Clinical Research. Some of the data were presented in abstract form at the annual Experimental Biology meeting, April 5 to 9, 2008; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS: NHE3: A Na+/H+ exchanger isoform of renal brush border. Am J Physiol 265: F736–F742, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW: Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Preisig PA, Ives HE, Cragoe EJ, Jr, Alpern RJ, Rector FC, Jr: Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest 80: 970–978, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preisig PA, Rector FC, Jr: Role of Na+-H+ antiport in rat proximal tubule NaCl absorption. Am J Physiol 255: F461–F465, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Brett CL, Donowitz M, Rao R: Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288: C223–C239, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Orlowski J, Grinstein S: Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr Opin Cell Biol 19: 483–492, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SH, Kim T, Park ES, Yang S, Jeong D, Choi Y, Rho J: NHE10, a novel osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival. Biochem Biophys Res Commun 369: 320–326, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE: Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Orlowski J, Grinstein S: Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447: 549–565, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Biemesderfer D, Nagy T, DeGray B, Aronson PS: Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem 274: 17518–17524, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Li X, Zhang H, Cha B, Leu S, Akhter S, Donowitz M: Heat shock cognate protein 70 (Hsc70) interacts with Na+/H+ exchanger 3 (NHE3) and this interaction is necessary for NHE3 activity. Gastroenterology 126: A43, 2004 [Google Scholar]
- 12.Quinones H, McLeroy P, Hu MC, Price E, Mumby MC, Moe OW: Protein phosphatases (PP2A) and the acute regulation of Na+/H+ exchanger NHE3 by dopamine (DA): direction interaction of PP2A with NHE3. J Am Soc Nephrol 12: 8A, 2001 [Google Scholar]
- 13.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, Murer H: PDZK1: I. A major scaffolder in brush borders of proximal tubular cells. Kidney Int 64: 1733–1745, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS: Association of Na+-H+ exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem 276: 46671–46677, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Girardi AC, Knauf F, Demuth HU, Aronson PS: Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA: Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol 294: F414–F422, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, Moe OW, Lee J, Kim E, Lee MG: Shank2 associates with and regulates Na+/H+ exchanger 3. J Biol Chem 281: 1461–1469, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Bretscher A, Chambers D, Nguyen R, Reczek D: ERM-merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 16: 113–143, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Louvet-Vallee S: ERM proteins: From cellular architecture to cell signaling. Biol Cell 92: 305–316, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Gautreau A, Louvard D, Arpin M: ERM proteins and NF2 tumor suppressor: The yin and yang of cortical actin organization and cell growth signaling. Curr Opin Cell Biol 14: 104–109, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hughes SC, Fehon RG: Understanding ERM proteins—The awesome power of genetics finally brought to bear. Curr Opin Cell Biol 19: 51–56, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Fievet B, Louvard D, Arpin M: ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta 1773: 653–660, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bretscher A, Edwards K, Fehon RG: ERM proteins and merlin: Integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M: The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: Dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell 17: 2661–2673, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M: cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun CH, Lamprecht G, Forster DV, Sidor A: NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem 273: 25856–25863, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Lee-Kwon W, Park JB, Ryu SH, Yun CH, Donowitz M: Ca2+-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3–E3KARP-α-actinin-4 complex for oligomerization and endocytosis. J Biol Chem 277: 23714–23724, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M: Ca2+-dependent inhibition of NHE3 requires PKCα which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol 285: C1527–C1536, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Weinman EJ, Steplock D, Donowitz M, Shenolikar S: NHERF associations with sodium–hydrogen exchanger isoform 3 (NHE3) and ezrin are essential for cAMP-mediated phosphorylation and inhibition of NHE3. Biochemistry 39: 6123–6129, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M: cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem 280: 16642–16650, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Choi JW, Lee-Kwon W, Jeon ES, Kang YJ, Kawano K, Kim HS, Suh PG, Donowitz M, Kim JH: Lysophosphatidic acid induces exocytic trafficking of Na+/H+ exchanger 3 by E3KARP-dependent activation of phospholipase C. Biochim Biophys Acta 1683: 59–68, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Shiue H, Palkon S, Wang Y, Cullinan P, Burkhardt JK, Musch MW, Chang EB, Turner JR: Ezrin regulates NHE3 translocation and activation after Na+-glucose cotransport. Proc Natl Acad Sci USA 101: 9485–9490, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M: Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol 120: 129–139, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niggli V, Andreoli C, Roy C, Mangeat P: Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett 376: 172–176, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Turunen O, Wahlstrom T, Vaheri A: Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol 126: 1445–1453, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gary R, Bretscher A: Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell 6: 1061–1075, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M: Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 164: 653–659, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin X, Barber DL: A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Natl Acad Sci USA 93: 12631–12636, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang T, Su X, Wakabayashi S, Shigekawa M: Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem 276: 17367–17372, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Inoue H, Nakamura Y, Nagita M, Takai T, Masuda M, Nakamura N, Kanazawa H: Calcineurin homologous protein isoform 2 (CHP2), Na+/H+ exchangers-binding protein, is expressed in intestinal epithelium. Biol Pharm Bull 26: 148–155, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Zaun HC, Shrier A, Orlowski J: Calcineurin B homologous protein 3 promotes the biosynthetic maturation, cell surface stability, and optimal transport of the Na+/H+ exchanger NHE1 isoform. J Biol Chem 283: 12456–12467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammar YB, Takeda S, Hisamitsu T, Mori H, Wakabayashi S: Crystal structure of CHP2 complexed with NHE1-cytosolic region and an implication for pH regulation. EMBO J 25: 2315–2325, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donowitz M, Li X: Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Mishima M, Wakabayashi S, Kojima C: Solution structure of the cytoplasmic region of Na+/H+ exchanger 1 complexed with essential cofactor calcineurin B homologous protein 1. J Biol Chem 282: 2741–2751, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Barroso MR, Bernd KK, DeWitt ND, Chang A, Mills K, Sztul ES: A novel Ca2+-binding protein, p22, is required for constitutive membrane traffic. J Biol Chem 271: 10183–10187, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Sikkink RA, Rusnak F, Barber DL: Inhibition of calcineurin phosphatase activity by a calcineurin B homologous protein. J Biol Chem 274: 36125–36131, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Kuwahara H, Kamei J, Nakamura N, Matsumoto M, Inoue H, Kanazawa H: The apoptosis-inducing protein kinase DRAK2 is inhibited in a calcium-dependent manner by the calcium-binding protein CHP. J Biochem 134: 245–250, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Di Sole F, Cerull R, Babich V, Quinones H, Gisler SM, Biber J, Murer H, Burckhardt G, Helmle-Kolb C, Moe OW: Acute regulation of Na/H exchanger NHE3 by adenosine A1 receptors is mediated by calcineurin homologous protein. J Biol Chem 279: 2962–2974, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Matsushita M, Sano Y, Yokoyama S, Takai T, Inoue H, Mitsui K, Todo K, Ohmori H, Kanazawa H: Loss of calcineurin homologous protein-1 in chicken B lymphoma DT40 cells destabilizes Na+/H+ exchanger isoform-1 protein. Am J Physiol Cell Physiol 293: C246–C254, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Pang T, Hisamitsu T, Mori H, Shigekawa M, Wakabayashi S: Role of calcineurin B homologous protein in pH regulation by the Na+/H+ exchanger 1: Tightly bound Ca2+ ions as important structural elements. Biochemistry 43: 3628–3636, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Timm S, Titus B, Bernd K, Barroso M: The EF-hand Ca2+-binding protein p22 associates with microtubules in an N-myristoylation-dependent manner. Mol Biol Cell 10: 3473–3488, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrade J, Zhao H, Titus B, Timm Pearce S, Barroso M: The EF-hand Ca2+-binding protein p22 plays a role in microtubule and endoplasmic reticulum organization and dynamics with distinct Ca2+-binding requirements. Mol Biol Cell 15: 481–496, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helmle-Kolb C, Counillon L, Roux D, Pouyssegur J, Mrkic B, Murer H: Na/H exchange activities in NHE1-transfected OK-cells: Cell polarity and regulation. Pflugers Arch 425: 34–40, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Helmle-Kolb C, Montrose MH, Stange G, Murer H: Regulation of Na+/H+ exchange in opossum kidney cells by parathyroid hormone, cyclic AMP and phorbol esters. Pflugers Arch 415: 461–470, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Akhter S, Kovbasnjuk O, Li X, Cavet M, Noel J, Arpin M, Hubbard AL, Donowitz M: Na+/H+ exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol Cell Physiol 283: C927–C940, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Cha B, Kenworthy A, Murtazina R, Donowitz M: The lateral mobility of NHE3 on the apical membrane of renal epithelial OK cells is limited by the PDZ domain proteins NHERF1/2, but is dependent on an intact actin cytoskeleton as determined by FRAP. J Cell Sci 117: 3353–3365, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Shiue H, Musch MW, Wang Y, Chang EB, Turner JR: Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J Biol Chem 280: 1688–1695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurashima K, D'Souza S, Szaszi K, Ramjeesingh R, Orlowski J, Grinstein S: The apical Na+/H+ exchanger isoform NHE3 is regulated by the actin cytoskeleton. J Biol Chem 274: 29843–29849, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Cha B, Donowitz M: The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins. Clin Exp Pharmacol Physiol 35: 863–871, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Simons PC, Pietromonaco SF, Reczek D, Bretscher A, Elias L: C-terminal threonine phosphorylation activates ERM proteins to link the cell's cortical lipid bilayer to the cytoskeleton. Biochem Biophys Res Commun 253: 561–565, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S: Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donato R: S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33: 637–668, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Gautreau A, Louvard D, Arpin M: Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol 150: 193–203, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou R, Zhu L, Kodani A, Hauser P, Yao X, Forte JG: Phosphorylation of ezrin on threonine 567 produces a change in secretory phenotype and repolarizes the gastric parietal cell. J Cell Sci 118: 4381–4391, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Zhu L, Hatakeyama J, Poon K, Chen C, Shastri A, Forte JG: Comparative study of ezrin phosphorylation among different tissues: more is good; too much is bad. Am J Physiol Cell Physiol 295: C192–C202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szaszi K, Kurashima K, Kapus A, Paulsen A, Kaibuchi K, Grinstein S, Orlowski J: RhoA and Rho kinase regulate the epithelial Na+/H+ exchanger NHE3: Role of myosin light chain phosphorylation. J Biol Chem 275: 28599–28606, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Hayashi H, Szaszi K, Coady-Osberg N, Furuya W, Bretscher AP, Orlowski J, Grinstein S: Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J Gen Physiol 123: 491–504, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexander RT, Furuya W, Szaszi K, Orlowski J, Grinstein S: Rho GTPases dictate the mobility of the Na/H exchanger NHE3 in epithelia: Role in apical retention and targeting. Proc Natl Acad Sci USA 102: 12253–12258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Huang HC, Yin H, Alpern RJ, Preisig PA: RhoA required for acid-induced stress fiber formation and trafficking and activation of NHE3. Am J Physiol Renal Physiol 293: F1054–F1064, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Matsui T, Yonemura S, Tsukita S: Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr Biol 9: 1259–1262, 1999 [DOI] [PubMed] [Google Scholar]
- 71.Koltzscher M, Neumann C, Konig S, Gerke V: Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P. Mol Biol Cell 14: 2372–2384, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K: Association of the myosin-binding subunit of myosin phosphatase and moesin: Dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J Cell Biol 141: 409–418, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hishiya A, Ohnishi M, Tamura S, Nakamura F: Protein phosphatase 2C inactivates F-actin binding of human platelet moesin. J Biol Chem 274: 26705–26712, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Oshiro N, Fukata Y, Kaibuchi K: Phosphorylation of moesin by Rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem 273: 34663–34666, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Pietromonaco SF, Simons PC, Altman A, Elias L: Protein kinase C-θ phosphorylation of moesin in the actin-binding sequence. J Biol Chem 273: 7594–7603, 1998 [DOI] [PubMed] [Google Scholar]
- 76.Tsukita S, Yonemura S: Cortical actin organization: Lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem 274: 34507–34510, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Yonemura S, Matsui T, Tsukita S: Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: An essential role for polyphosphoinositides in vivo. J Cell Sci 115: 2569–2580, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Di Sole F, Cerull R, Babich V, Casavola V, Helmle-Roth C, Burckhardt G: Short- and long-term A3 adenosine receptor activation inhibits the Na+/H+ exchanger NHE3 activity and expression in opossum kidney cells. J Cell Physiol 216: 221–233, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Moe OW, Miller RT, Horie S, Cano A, Preisig PA, Alpern RJ: Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest 88: 1703–1708, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.