Abstract
Plants are continuously subjected to UV-B radiation (UV-B; 280–320 nm) as a component of sunlight causing damage to the genome. For elimination of DNA damage, a set of repair mechanisms, mainly photoreactivation, excision, and recombination repair, has evolved. Whereas photoreactivation and excision repair have been intensely studied during the last few years, recombination repair, its regulation, and its interrelationship with photoreactivation in response to UV-B-induced DNA damage is still poorly understood. In this study, we analyzed somatic homologous recombination in a transgenic Arabidopsis line carrying a β-glucuronidase gene as a recombination marker and in offsprings of crosses of this line with a photolyase deficient uvr2–1 mutant. UV-B radiation stimulated recombination frequencies in a dose-dependent manner correlating linearly with cyclobutane pyrimidine dimer (CPD) levels. Genetic deficiency for CPD-specific photoreactivation resulted in a drastic increase of recombination events, indicating that homologous recombination might be directly involved in eliminating CPD damage. UV-B irradiation stimulated recombination mainly in the presence of photosynthetic active radiation (400–700 nm) irrespective of photolyase activities. Our results suggest that UV-B-induced recombination processes may depend on energy supply derived from photosynthesis.
Depletion of stratospheric ozone levels leads to an increase of genotoxic solar UV-B radiation reaching the Earth's surface (1–4). Elevated UV-B radiation has multiple effects on the development and morphology of plants (for review see refs. 5 and 6), primarily resulting from DNA and photosynthetic damage, protein destruction (7), and signal transduction via a postulated UV-B photoreceptor (8). High fluences of UV-B introduce into the genome a number of different lesions, predominantly cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6–4) pyrimidinone adducts [(6–4) PPs] (9). The elimination of these DNA photoproducts is essential for cell survival because both of these classes of dimers act as blocks to DNA replication (10, 11) and transcription (12).
To avoid toxic effects of UV-induced DNA damage and to maintain genetic integrity, most organisms have developed a complex network of repair mechanisms involving photoreactivation, excision repair, and recombination repair (13). Photoreactivation is a light-dependent enzymatic process using UV-A (320–400 nm) and blue light (400–500 nm) to monomerize pyrimidine dimers whereas excision and recombination repair are light-independent processes. The interactions between photoreactivation and light-independent “dark” repair processes are not well understood. In Escherichia coli and Chlamydomonas reinhardtii, e.g., the presence of photolyase was proposed to have stimulatory effects on “dark” repair processes (14, 15).
Although plants are unique in their nature to be obligatorily exposed to UV-containing sun light, the understanding of DNA repair processes in plants lags far behind the knowledge of repair mechanisms in E. coli, yeast, and mammals. Studies on Arabidopsis mutants deficient in light-dependent repair of CPDs (16) or (6–4) PPs (17) revealed that photoreactivation represents the main repair pathway for these photoproducts (9). However, plants are also capable of repairing UV-induced dimers in the dark denoting that light-independent repair clearly takes place in plants (18). Recently, nucleotide excision repair, the most versatile “dark” repair mechanism, has been shown to also exist in plants (19, 20), yet only with a low capacity for repairing CPDs (21). In contrast, elimination of pyrimidine dimers via recombination has not yet been directly proven even though homologous recombination, a marker for recombination repair, was found strongly induced in somatic tissue after treatment with DNA-damaging agents, such as UV- and γ-radiation (22–25). To elucidate the role of recombination repair and its interaction with photoreactivation of UV-B-induced DNA damage, we studied homologous recombination between overlapping sequences of a β-glucuronidase marker gene in Arabidopsis (24), using a combination of a genetic with a photobiological approach. We found that the rate of recombination correlates linearly with the level of CPD formation and moreover, that it is strongly increased in photolyase-deficient uvr2–1 mutant plants. In addition, we observed that homologous recombination, a putative “dark” repair process for UV-induced DNA damage, is strongly influenced by light conditions applied to plants after exposure to UV radiation. These results suggest that recombination processes induced by UV-B radiation may require energy provided by photosynthetic activity.
Materials and Methods
Plant Material and Growth Conditions.
The transgenic Arabidopsis (C24) line A651 (24) carrying a β-glucuronidase recombination substrate and a hygromycin resistance marker gene was used for all irradiation experiments, as well as for the cross with the photolyase deficient line uvr2–1 in the Landsberg erecta (Ler) background (16). The latter was provided by the Arabidopsis Seed Stock Center (Ohio State Univ., Columbus). The F3-generation was screened for homozygosity of the recombination substrate and of the uvr2–1 locus by growing plants and their offspring on hygromycin selection medium and, consequently, treatment with UV-B radiation (WG 295) for 1 h. Two lines were homozygous for uvr2–1 or UVR2–1 and the recombination substrate, respectively. To exclude the influence of the genetic background caused by the cross of two different Arabidopsis ecotypes (C24 and Ler), 10 control lines (homozygous for the hygromycin resistance marker and homo- or heterozygous for UVR2–1) were tested for their recombination frequency. All control lines showed similar basal and UV-B-induced recombination frequencies (data not shown). Before exposure to UV-B radiation, plants were grown in soil for 14 days in 24 h light of 15 W m−2 of photosynthetically active radiation (PAR), derived from cool white lamps (Biolux, Osram, Germany).
Light Treatments and Determination of Net Photosynthesis.
Arabidopsis plants were irradiated for various time intervals with UV-B from TL12 F40 lamps (Osram, Germany) filtered through 3-mm transmission cut-off filter glasses with half-maximal transmission at 295, 305, and 335 nm (WG 295, WG 305, and WG 335; Schott, Germany), respectively. Measurements of irradiance were performed by using an OL 754 UV-visible spectroradiometer (Optronic Laboratories, Orlando, FL). Unless indicated, plants were shifted immediately after UV-B treatment into continuous white light (15 W m−2) for another 3 days. The influence of different light qualities after UV-B irradiation on the recombination frequency was tested by exposing plants directly after irradiation for 24 h to either red light (λlmax = 658 nm, 6.4 W m−2), far red light (λlmax = 740 nm, 4.0 W m−2), or by incubation in darkness (23). For photoreactivation studies, plants were exposed, directly after UV-B irradiation, for 2 h to UV-A (10 W m−2, Osram L36W/73) combined with a WG 335 transmission cut-off filter and then shifted into red light for 22 h. After 24 h, all plants were transferred to continuous white light and kept growing for additional 2 days. Net photosynthesis in red or far-red-irradiated single Arabidopsis leaves at ambient CO2 concentration and constant temperature (25°C) was determined by using a HCM-1000 portable photosynthesis system (Walz, Effeltrich, Germany).
Determination of Pyrimidine Dimers.
Leaf material (400 mg) from 10, 14-day-old plants was used to extract genomic DNA. The extraction was performed with hexadecyltrimethylammonium bromide, and the amounts of CPDs were determined essentially as described (26). DNA dissolved in PBS (10 mM Na2HPO4 × 12 H2O/150 mM NaCl, pH 7.4) was heated in boiling water for 10 min. Then 400 ng of the denatured DNA in a volume of 50 μl was placed in quadruplicate in a 96-well microplate (Dynex M129B) and evaporated to dryness at 94°C for 1 h. After washing the wells with PBS-T (PBS supplemented with 0.5 ‰ Tween 20), 100 μl of 3% (wt/vol) BSA solution, and 50 μl of a 1,000-fold, diluted CPD-specific mouse monoclonal antiserum (Kamiya Biomedical, Thousand Oaks, CA) was added. Excess primary antiserum was removed by several washing steps with PBS and the peroxidase assay started after addition of horseradish peroxidase-linked rabbit antimouse antibody (1:2,000) and 100 μl of horseradish peroxidase-reaction mixture (0.04% o-phenylene diamine/0.007% H2O2 in citrate-phosphate buffer, pH 5.0). The horseradish peroxidase reaction was incubated at 37°C for 15 min and was stopped by addition of 50 μl of 2 M H2SO4. The absorbance of the reddish yellow reaction product represents the amount of CPDs in the DNA and was determined at 490 nm (A490) with a Dynex microplate reader (Dynex, Frankfurt, Germany).
Detection of Recombination Events.
Histochemical staining for detection of β-glucuronidase activity in Arabidopsis seedlings has been described previously (24, 27). For each sample, the average number of recombination events per plant was determined by quantifying recombination sectors on 120–150 plants.
Results
Recombination Frequency Is Stimulated by UV-B Radiation.
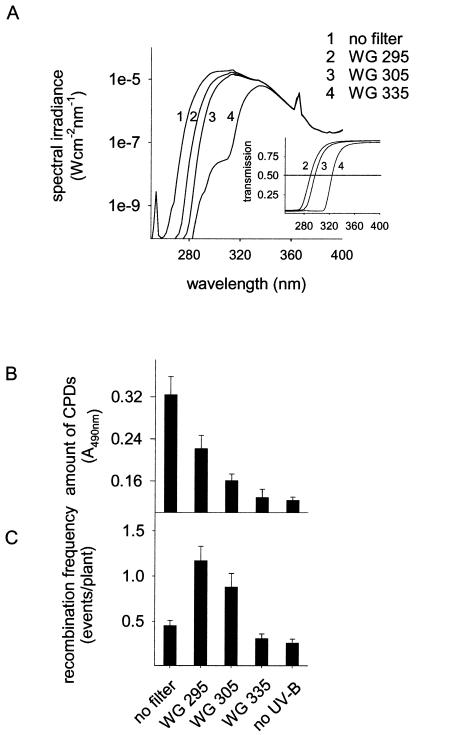
UV-B radiation has pleiotropic effects on plants including damage to nucleic acids and proteins (28). On the other hand, it also stimulates protection mechanisms, such as DNA repair pathways and flavonoid biosynthesis (6, 9). To determine the correlation between UV-B-induced DNA damage and recombination repair in plants, we induced different CPD levels in the plant genome by applying UV-B radiation in combination with different wavelength cut-off filters and monitored homologous recombination events. Three different filter glasses with transmission cut-offs of WG 335, WG 305, and WG 295, were used (Fig. 1A), allowing analysis of the influence of distinct UV-ranges on recombination. The influence of short UV-B and UV-C exposure on recombination was tested in the absence of any filter glass (Fig. 1A).
Figure 1.
Formation of CPDs and the induction of recombination frequency in dependence of UV-B spectral ranges. Plants were irradiated for 1 h (A) using cut-off filter glasses of different transmission (Inset). Amounts of CPDs (B) and recombination frequency (C) were determined under these light regimes in an Arabidopsis line carrying the GUS substrate as a marker for recombination events. Error bars represent the SE.
As a marker for DNA damage in the plants, we determined the concentration of CPDs using mAbs to thymidine dimers. In non-UV-treated plants, a background level of 0.12 ± 0.01 (A490 ± SEM) was measured. Exposure to the different UV-B doses for 1 h affected the CPD concentration and the recombination frequency in the respective plant populations to various extents (Fig. 1 B and C). Upon irradiation of the plants with the complete UV-B lamp spectrum containing wavelengths in the UV-B as well as in the UV-C range (i.e., in the absence of filter glasses; Fig. 1A), the CPD concentration increased 3-fold up to 0.32 A490 (± 0.03), whereas the recombination frequency was around background levels of 0.45 ± 0.06 (average number of recombination events per plant ± SEM). As leaves irradiated with UV-B and -C without filter glasses exhibited lesions within 3 days (data not shown), the damage caused by UV-C radiation might exceed the repair capacity and leads to the consequential decrease of recombination frequency. In contrast, exposing plants to wavelengths passing through the WG 295 filter led to an increase of the recombination frequency to 1.17 events/plant (± 0.16) and of the CPD concentration to 0.22 A490 (± 0.03). UV-B wavelengths passing through the WG 305 filter (Fig. 1A) also significantly increased recombination (P > 0.01, t test) as well as the amount of CPDs (Fig. 1B). Under the WG 335 filter, neither the CPD concentration nor the recombination frequency were influenced, indicating that UV-A and blue light stimulate recombination only to minimal extents.
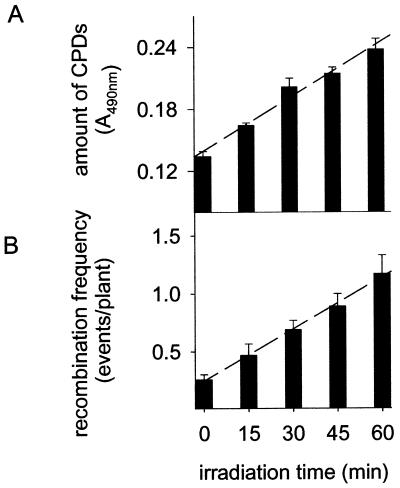
The main factor affecting recombination repair after UV-B irradiation seems to be the level of DNA damage in the genome because the recombination frequency correlates with the amount of CPDs. To study this correlation in more detail, we irradiated plants under WG 295 (Fig. 1A) for different time periods (15, 30, 45, and 60 min). The CPD concentration in the genome increased linearly with irradiation time (Fig. 2A). Interestingly, elevated levels of CPDs in UV-B-treated plants coincided strictly with increased recombination frequencies. The average number of recombination events per plant increased 4.5-fold after 60 min of UV-B irradiation compared to non-UV-B-irradiated control plants (Fig. 2B). The recombination frequency showed a similar linear dependence on the irradiation time as the CPD concentration. This strong correlation points to a possible cause–effect relationship between DNA damage and recombination.
Figure 2.
Dependence of CPD amounts and recombination frequencies on UV-B irradiation times. Plants were exposed to UV-B (described in Fig. 1A, WG 295) for various time intervals. Amounts of CPDs (A) and recombination frequency (B). Error bars represent the SE.
Absence of CPD-Specific Photolyase Activity Leads to Increased Homologous Recombination.
In plants photolyases play key roles in monomerizing both CPDs and (6–4) PPs, using radiation energy in the UV-A and blue wavelength range (320–500 nm) (9). To further study the correlation of CPD formation and recombination frequency upon UV-B irradiation, we analyzed homologous recombination in the photolyase-deficient Arabidopsis mutant line uvr2–1 that is inactive in repairing cyclobutane dimers (16). For this, the uvr2–1 mutant line was crossed with the transgenic marker line A651 carrying the β-glucuronidase (GUS) recombination substrate, and two lines, either homozygous for uvr2–1 and GUS (A94) or homozygous for UVR2–1 and GUS (A87), were selected. In the absence of UV-B, the background recombination frequency in both lines was similar to the parental line A651 (≈0.26 recombination events/plant; Fig. 3). However, irradiation with UV-B under WG 295 (Fig. 1A) stimulated recombination in line A94 (uvr2–1/GUS) much stronger than in A87 (UVR2–1/GUS) or in the parental line A651 (UVR2–1/GUS) line (Fig. 3; data not shown for line A651). On the average, UV-B-induced recombination in the uvr2–1 background was approximately four times higher than in the UVR2–1 background, irrespective of the irradiation time. Because the amount of CPDs accumulate equally in UV-irradiated photolyase-deficient and wild-type plants (16), the different recombination frequencies of these lines are not due to different damage patterns. These results confirm on a genetic basis that the lack of CPD-specific photolyase repair and the concomitant prolonged persistence of this type of DNA damage in the genome have a strong stimulatory influence on recombination processes in plants.
Figure 3.
Interaction between CPD photoreactivation and homologous recombination. White bars represent the photolyase mutant line A94 (uvr2–1/GUS) and black bars indicate the corresponding wild-type line A87 (UVR2–1/GUS). Recombination frequencies were determined in both lines after irradiation as described in Fig. 2.
UV-B-Induced Recombination Depends on PAR.
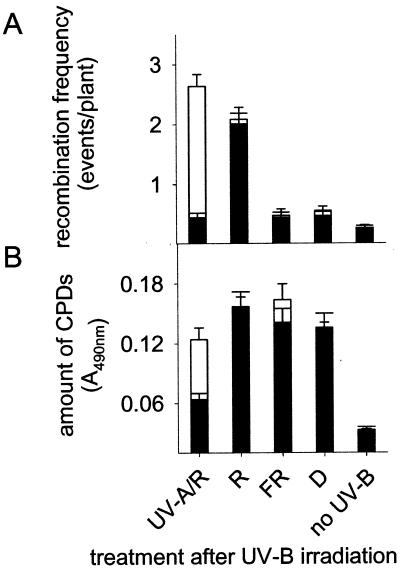
Photoreactivation in plants depends on energy provided by wavelengths in the UV-A- and blue-light range (9). UV-B-induced DNA damage cannot be photoreactivated under red light, far red light, or darkness. Under these conditions, CPDs are less efficiently repaired and, therefore, are longer persistent in the genome; this situation, therefore, mimics the one in photolyase-deficient plants. As a consequence, one would expect that under red light, far red light, or in darkness recombination is strongly increased. However, UVR2–1 lines, irradiated for 30 min with UV-B under WG 295 (Fig. 1A) and subsequently shifted either to darkness or far red light for 24 h did not exhibit higher recombination levels (P > 0.05, t test) than plants exposed to UV-A light (Fig. 4A), although CPDs were longer persistent in darkness and far red light (Fig. 4B). In contrast, exposure to red light for 24 h after UV-B treatment led to drastically enhanced recombination frequencies both in wild-type and mutant plants although comparable CPD levels were detected as found in far-red or dark-incubated plants (Fig. 4 A and B). When shifted to 2 h UV-A after UV-B irradiation, recombination was strongly stimulated only in uvr2–1 due the prolonged presence of nonrepaired CPDs in the photolyase-deficient mutant (Fig. 4 A and B). These data indicate that the photolyase activity in plants driven by UV-A may keep recombination levels low after UV-B irradiation (Fig. 3). In the absence of photolyase activity, i.e., in red light, far-red light, or darkness, as well as in the uvr2–1 mutant, a factor dependent on specific light conditions, such as photosynthesis, may cause the increase of recombination events. To test this hypothesis, gas exchange measurements determining the net photosynthesis were carried out with red and far red light-irradiated Arabidopsis leaves. The red light following the UV-B treatment emitted 25 μmol m−2 s−1 PAR and caused a rapid increase of the net rate of photosynthesis within 20 sec. Both, wild-type and uvr2–1 mutant plants reached a steady–state CO2 consumption between 0.57 and 1.11 μmol m−2 s−1 within 1 min. In contrast, transfer to darkness or far-red light resulted in CO2 production between −0.5 and −0.92 μmol m−2 s−1. These data suggest that photosynthetic processes or mechanisms regulated by PAR may influence UV-B-induced homologous recombination in plants.
Figure 4.
Dependence of recombination frequency (A) and amount of CPDs (B) on the light quality after UV-B irradiation. White bars represent the photolyase mutant A94 (uvr2–1/GUS), and black bars indicate the corresponding wild-type A87 (UVR2–1/GUS). Recombination frequencies were determined in both lines after irradiation with UV-B for 30 min (Fig. 1A, WG 295) and subsequent exposure to various light qualities as indicated. UV-A/R, 2 h UV-A, then transferred for 22 h to red light; R, red light; FR, far red light; D, darkness; no-UV-B, untreated control. The amount of CPDs in plants was determined 24 h after UV-B irradiation.
Discussion
We investigated the role of UV-B-induced DNA damage and its photoreactivation in intrachromosomal homologous recombination, using Arabidopsis thaliana carrying a recombination substrate and a cross of this line with the CPD-specific photolyase mutant uvr2–1 (16). By using transmission filter glasses with specific wavelength cut-offs, different UV-wavelength ranges were tested for their influence on recombination. Whereas UV-A radiation showed only a marginal influence both on the recombination frequency and on the CPD formation, radiation in the UV-B wavelength range drastically increased homologous recombination. Over the entire UV-B wavelength range, the rate of recombination correlated with the level of DNA damage, measured as CPD concentration. Although the UV-C containing radiation, as used here, induced high levels of damage into the genome of plants, it caused only a small increase in the recombination frequency. The low number of recombination events detected in plants exposed to UV-C containing radiation may result from the severe damage of UV-C radiation to plants, such as leaf necrosis, inhibited leaf growth, etc. (data not shown). Moreover, other primary UV-mediated effects, such as destruction of proteins involved in house-keeping functions including photosynthesis or DNA repair (28) may negatively affect the recombination machinery as well. In contrast, when plants are irradiated with low levels of UV-C, which do not cause lesions on leaves, the number of recombination events is strongly increased (24).
It has been suggested that homologous recombination is involved in repairing UV-B-induced DNA damage, such as CPDs, in plants (25). Using a more quantitative approach, we document here a linear correlation between the concentration of CPDs and the recombination frequency. This interrelationship strongly suggests that the level of CPDs in the genome of irradiated plants represents a main factor determining the activity of recombination after UV-B irradiation. Therefore, homologous recombination processes may be involved in repairing DNA lesions directly at damaged loci. Elimination of CPDs or other types of UV-B-induced DNA lesions via various DNA repair pathways, such as nucleotide excision repair, may create recombinogenic intermediates. Alternatively, or in addition, UV-B-promoted signaling pathways partially mediated by an UV-B photoreceptor (29, 30) may activate recombination processes by stimulating, e.g., transcription of DNA repair genes.
Other types of DNA damage not determined in our experiments may play an important role in the UV-B-mediated induction of recombination as well. The formation of (6–4) PPs in UV-irradiated DNA, for example, shows an identical action spectrum as that for the formation of CPDs (33). To test the role of CPDs as the major UV-B-induced DNA damage in the induction of recombination, we analyzed the CPD-specific photoreactivation mutant uvr2–1. Recombination events in this line increased with a similar linear relationship with the UV-B fluence as in the isogenic wild-type plant but were enhanced ≈4-fold under all UV-B conditions. This suggests that lack of photoreactivation may stimulate recombination processes via prolonged presence of CPDs in the genome (Fig. 3). Indeed, the prolonged presence of UV-induced DNA damage increased the recombination frequency in human excision repair deficient cells pointing to a network-like interplay of various DNA repair pathways (32).
Like nucleotide and base excision repair, recombination, up to now, has been classified as DNA “dark” repair pathway. After UV-B irradiation of wild-type plants, we found recombination only slightly increased in dark and in light (UV-A/R; Fig. 4) compared with non-UV-B-irradiated control plants. In the photolyase-deficient line, however, this increase was much more pronounced. If nonphotoreactivating red light was applied, recombination frequencies were enhanced in both lines. In contrast, far-red-irradiated or dark-incubated plants showed low levels of recombination frequencies. These findings point to a special role for PAR for efficient recombination repair after damaging UV-B irradiation. In dark or in far red light, plants may run short of energy because the repair of cellular structures may necessitate energy levels that cannot be replenished by photosynthetic activity. The lack of energy may affect the cellular metabolism, including cell cycle, transcription, replication, and recombination and may, therefore, influence recombination activity.
Interestingly, PAR leads to increased numbers of recombination events only after UV-irradiation but not after methyl methanesulfonate treatment. The 100 ppm methyl methanesulfonate increased recombination in UVR2–1/GUS and uvr2–1/GUS homozygous plants similarly by a factor of ≈9.6–10, irrespective of the presence or absence of light (unpublished data). This result suggests that the light dependence of recombination repair is restricted to UV-induced DNA damage, indicating that besides PAR additional factors regulated in a light- or energy-dependent manner may promote recombination. These factors, however, have yet to be determined.
In this study, we analyzed the interaction between recombination and photoreactivation repair in plants. The lack of photolyase or the inhibition of photoreactivation have a strong stimulatory effect on homologous recombination processes in the presence of PAR. We show that the regulation of UV-induced recombination is clearly correlated with the number of CPD lesions in the genomic DNA and that interactions with photoreactivation of this damage exists in plants.
Acknowledgments
We thank J. Lucht, G. Neuhaus-Url, T. Boller, and O. Mittelsten Scheid for helpful suggestions during preparation of the manuscript. We also thank all members of our groups for stimulating discussions during the work and C. Ramos and V. Gloeckler for their technical help. This research was supported by the Foundation of the Chemical Industry of Basel. Novartis Research Foundation is acknowledged for their support of this work.
Abbreviations
- UV-B
UV-B radiation
- CPD
cyclobutane pyrimidine dimer
- [6–4] PP
pyrimidine [6–4] pyrimidinone dimer
- PAR
photosynthetic active radiation
- GUS
β-glucuronidase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230251897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230251897
References
- 1.Kerr J, McElroy C. Science. 1993;262:1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- 2.Madronich S, McKenzie R L, Björn L O, Caldwell M M. J Photochem Photobiol B. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie R, Connor B, Bodeker G. Science. 1999;285:1709–1711. doi: 10.1126/science.285.5434.1709. [DOI] [PubMed] [Google Scholar]
- 4.Waibel A E, Peter T, Carslaw K S, Oelhaf H, Wetzel G, Crutzen P J, Tsias A, Reimer E, Fischer H. Science. 1999;283:2064–2069. doi: 10.1126/science.283.5410.2064. [DOI] [PubMed] [Google Scholar]
- 5.Jordan B R. Adv Botanical Res. 1996;22:98–162. [Google Scholar]
- 6.Jansen M A K, Gaba V, Greenberg B M. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- 7.Pratt L H, Butler W L. Photochem Photobiol. 1970;11:503–509. doi: 10.1111/j.1751-1097.1970.tb06021.x. [DOI] [PubMed] [Google Scholar]
- 8.Batschauer A. Planta. 1998;206:479–492. doi: 10.1007/s004250050425. [DOI] [PubMed] [Google Scholar]
- 9.Britt A B. Trends Plant Sci. 1999;4:20–24. doi: 10.1016/s1360-1385(98)01355-7. [DOI] [PubMed] [Google Scholar]
- 10.LeClerc J E, Borden A, Lawrence C W. Proc Natl Acad Sci USA. 1991;88:9685–9689. doi: 10.1073/pnas.88.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbett A H, Zechiedrich E L, Lloyd R S, Osheroff N. J Biol Chem. 1991;266:19666–19671. [PubMed] [Google Scholar]
- 12.Buchholz G, Ehmann B, Wellmann E. Plant Physiol. 1995;108:227–234. doi: 10.1104/pp.108.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 14.Sancar A, Franklin K A, Sancar G B. Proc Natl Acad Sci USA. 1984;81:7397–7401. doi: 10.1073/pnas.81.23.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlcek D, Podstavkova S, Miadokova E. Mutat Res. 1995;336:251–256. doi: 10.1016/0921-8777(94)00056-c. [DOI] [PubMed] [Google Scholar]
- 16.Landry L G, Stapleton A E, Lim J, Hoffman P, Hays J B, Walbot V, Last R L. Proc Natl Acad Sci USA. 1997;94:328–332. doi: 10.1073/pnas.94.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C Z, Yee J, Mitchell D L, Britt A B. Proc Natl Acad Sci USA. 1997;94:7441–7445. doi: 10.1073/pnas.94.14.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britt A B. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:75–100. doi: 10.1146/annurev.arplant.47.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Swoboda I, Bhalla P L, Sijbers A M, Zhao C, Ong E K, Hoeijmakers J H, Singh M B. Plant J. 1998;13:823–829. doi: 10.1046/j.1365-313x.1998.00081.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Hossain G S, Islas-Osuna M A, Mitchell D L, Mount D W. Plant J. 2000;21:519–528. doi: 10.1046/j.1365-313x.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- 21.Gallego F, Fleck O, Li A, Wyrzykowska J, Tinland B. Plant J. 2000;21:507–518. doi: 10.1046/j.1365-313x.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- 22.Tovar J, Lichtenstein C. Plant Cell. 1992;4:319–332. doi: 10.1105/tpc.4.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebel E G, Masson J, Bogucki A, Paszkowski J. Proc Natl Sci USA. 1993;90:422–426. doi: 10.1073/pnas.90.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puchta H, Swoboda P, Hohn B. Plant J. 1995;7:203–210. [Google Scholar]
- 25.Ries G, Heller W, Puchta H, Seidlitz H, Sandermann H, Hohn B. Nature (London) 2000;406:98–101. doi: 10.1038/35017595. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno T, Matsunaga T, Ihara M, Nikaido O. Mutat Res. 1993;254:175–184. doi: 10.1016/0921-8777(91)90009-e. [DOI] [PubMed] [Google Scholar]
- 27.Swoboda P, Gal S, Hohn B, Puchta H. EMBO J. 1994;13:484–489. doi: 10.1002/j.1460-2075.1994.tb06283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stapleton A E. Plant Cell. 1992;4:1353–1358. doi: 10.1105/tpc.4.11.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christie J, Jenkins G. Plant Cell. 1996;8:1555–1567. doi: 10.1105/tpc.8.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frohnmeyer H, Bowler C, Xhu G, Yamagata H, Schaefer E, Chua N-H. Plant J. 1998;13:763–772. [Google Scholar]
- 31.Matsunaga T, Hieda K, Nikaido O. Photochem Photobiol. 1991;54:403–410. doi: 10.1111/j.1751-1097.1991.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsujimura T, Maher V M, Godwin A R, Liskaz R M, McCormick J J. Proc Natl Acad Sci USA. 1990;87:1566–1570. doi: 10.1073/pnas.87.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancar A, Tang M S. Photochem Photobiol. 1993;57:905–921. doi: 10.1111/j.1751-1097.1993.tb09233.x. [DOI] [PubMed] [Google Scholar]