Abstract
The incidence of many cancer types is significantly reduced in individuals with Down syndrome1–4 and it is proposed that this broad cancer protection is conferred by the elevated expression of one or more of the 231 supernumerary genes on the extra copy of chromosome 21. One such gene is the Down syndrome candidate region-1 (Dscr1, RCAN1), which encodes a protein that suppresses vascular endothelial growth factor (VEGF)-mediated angiogenic signalling via the calcineurin pathway5–10. Here we show that DSCR1 is elevated in Down syndrome individuals and a mouse model of Down syndrome. Further, we show that the modest elevation in expression afforded by a single extra transgenic copy of Dscr1 is sufficient to confer significant suppression of tumor growth in mice and that such resistance is a consequence of a deficit in tumor angiogenesis arising from suppression of the calcineurin pathway. We also provide evidence that attenuation of calcineurin activity by DSCR1 together with another chromosome 21 gene DYRK1A, may be sufficient to dramatically diminish angiogenesis. These data provide a mechanism for the reduced cancer incidence in Down syndrome and identifies the calcineurin signalling pathway and its regulators DSCR1 and DYRK1A as potential therapeutic targets in cancers arising in all individuals.
Down syndrome (DS), is the most common genetic cause of mental retardation in humans occurring in 1 out of 700 live births. Epidemiological studies suggest that while individuals with DS have increased risk of leukemia, they have a considerably reduced incidence of most solid tumors1–4. In the largest study to date involving 17,800 DS individuals, the mortality from cancers was <10% of expected4. Such data imply that one or more of the 231 trisomic genes on chromosome 21 is responsible for protecting these individuals against cancer. Of note, DS individuals also exhibit a reduced incidence of other angiogenesis-related diseases such as diabetic retinopathy11 and atherosclerosis12, suggesting that cancer protection in the DS population may be due in part, to angiogenesis suppression.
The Dscr1 gene lies on chromosome 21 and encodes a negative regulator of VEGF-calcineurin signalling in the endothelium5–10. Previous studies have demonstrated that gross over-expression of DSCR1 in endothelial cells blocks VEGF-mediated angiogenic responses in vitro7–10. We examined DSCR1 protein expression in DS human fetal tissues and observed a consistent 1.8-fold increase in DSCR1 levels in tissues from DS embryos, as well as elevated expression of DSCR1 fetal isoforms5, compared with those from age-matched non-DS fetuses (Fig. 1A). Substantial synteny exists in gene identity and order between much of human chromosome 21 and the mouse13. The Ts65Dn mouse model of DS is trisomic for 104 of the 231 genes on human chromosome 21 including DSCR114 (Supplemental Fig. 1A). We probed tissues from Ts65Dn mice to ascertain whether DSCR1 was also upregulated in the Ts65Dn mouse and found a 1.7-fold increase in DSCR1 protein expression compared with diploid littermates (Supplemental Fig. 1B). Thus, DSCR1 expression in Ts65Dn mice is elevated in an analogous fashion to DSCR1 expression in DS fetal tissues.
Figure 1. Dscr1 expression is upregulated in Down syndrome tissues and tumor angiogenesis is suppressed in Down syndrome models.
(a) Increased DSCR1 expression in human fetal Down syndrome (DS) tissues versus age-matched control (ctrl) tissues relative to β-actin. (b) Tumor growth is suppressed in the Ts65Dn Down syndrome mouse model. Values are mean ± s.e.m. n=10–12, *p<0.03; **p <0.01. (c) Microvessel density (MVD) per high-powered field (hpf) of tumors is quantified by anti-CD31 immunofluorescence. Bar, 20 µM. Values are mean ± s.e.m. *p <0.02; **p<0.01. (d) Angiogenesis in tumors from induced pluripotent stem cells (iPS) isolated from Down syndrome or cytogenetically normal cells was quantified by human specific anti-CD31 immunofluorescence. Arrows, hCD31-positive vessels. Values are mean ± s.e.m. n=3–6, **p<0.01.
We hypothesized that inhibition of tumorigenesis in the DS population may be partially due to suppression of tumor angiogenesis, thus predicting that the inhibitory effect of Dscr1 trisomy on tumor growth occurs within the host tumor microenvironment. To establish whether the Ts65Dn DS mouse, like DS individuals, exhibits generalized protection from cancers, we assayed the growth of two transplantable tumor models – Lewis lung carcinoma and B16F10 melanoma cells. We observed considerable growth suppression of both Lewis lung and B16F10 tumor cells in Ts65Dn mice relative to littermate controls (Fig. 1B), correlating with a significant decrease in microvessel density (Fig. 1C). Endothelial cells isolated from Ts65Dn mice demonstrated upregulation of Dscr1 mRNA in contrast to diploid littermates (Supplemental Fig. 1C) and were noticeably less responsive to VEGF-mediated proliferation in vitro (Supplemental Fig. 1D), further implicating an angiogenic defect in these mice. Thus, trisomy for orthologs of half the genes on human chromosome 21 was sufficient to slow ectopic tumor growth.
To validate that the compromised angiogenesis we observed in murine models of DS extended to human cells carrying trisomy 21, we compared microvessel density in teratomas derived from DS induced pluripotent stem (iPS) cells versus those from cytogenetically normal iPS cells from a healthy volunteer15. These iPS cell lines were inoculated intramuscularly into immunodeficient mice (Rag2−/−γc−/−), and angiogenesis assessed in the resulting tumors using a human-specific antibody to the endothelial marker CD3116. Microvessel density was significantly reduced in teratomas derived from Down syndrome iPS cells compared to iPS cells from the normal control (Fig. 1D and Supplemental Fig. 1E).
Given that Ts65Dn mice are trisomic for 104 genes, we next determined whether 3 copies of only Dscr1 would be sufficient to suppress tumorigenesis. We generated a Dscr1 transgenic mouse targeting a myc-tagged Dscr1 cDNA driven by its native promoter to the Hprt locus (Supplemental Fig. 2A, B). Expression of the targeted third copy of Dscr1 was verified by Western blot analysis on isolated endothelial cells (Supplemental Fig. 2C) and quantitative PCR analysis demonstrated a 2.4-fold increase in Dscr1 mRNA relative to littermate controls (Supplemental Fig. 2D). To ensure that one extra copy of Dscr1 was sufficient to restrain VEGF-calcineurin signalling, we examined NF-ATc1 subcellular localization as a measure of calcineurin activation (Fig. 2A). After VEGF treatment, endothelial cells isolated from Dscr1 transgenic mice exhibited predominantly cytoplasmic NF-ATc1 localization while wild-type endothelial cells displayed the expected nuclear localization (Fig. 2A). Dscr1 transgenic endothelial cells also exhibited decreased sensitivity to VEGF relative to wild-type endothelial cells, as assessed by VEGF-induced proliferation (Fig. 2B).
Figure 2. Targeted Dscr1 transgenic mice with three copies of Dscr1 show inhibition of tumor growth.
(a) NF-ATc1 nuclear import after VEGF treatment is suppressed in endothelial cells from Dscr1 transgenic mice. Bar, 5 µM. (b) VEGF-induced proliferation of endothelial cells from Dscr1 transgenic mice is significantly inhibited. *p<0.02; **p<0.03. (c) Tumor growth is suppressed in Dscr1 transgenic mice. n=8–12 mice per group, *p<0.05; **p<0.04. (d) Microvessel density (MVD) per high-powered field (hpf) of tumors is quantified by anti-CD31 immunofluorescence. Bar, 20 µM. Values are mean ± sem. *p<0.01. (e) Tumors from Dscr1 transgenic and wild-type mice are co-immunostained with anti-CD31 to detect endothelial cells and anti-Myc to detect the Dscr1 transgene. Bar, 20 µM.
Substantial growth inhibition of Lewis lung carcinoma and B16F10 melanoma cells was observed in Dscr1 transgenic mice (Fig. 2C) with a corresponding decrease in microvessel density (Fig. 2D) as compared with tumors from wild-type littermates. Quantification of endothelial cells in tumors isolated from Dscr1 transgenic and wild-type mice by flow cytometry showed a significant decrease in CD31+CD45− cells in tumors from Dscr1 transgenic mice (Supplemental Fig. 3A). Additionally, many of the CD31 reactive microvessels in tumors isolated from Dscr1 transgenic mice lacked functional lumens as evidenced by the absence of co-staining with circulating FITC-lectin and CD31-positivity (Supplemental Fig. 3B). Immunostaining with CD31 and Myc antibodies confirmed specific expression of the DSCR1 transgene in tumor endothelium of Dscr1 transgenic animals (Fig. 2E). Thus, a single extra copy of Dscr1 appears to be sufficient to blunt host angiogenic responses and suppress tumor angiogenesis and tumor growth. A subset of tumors initiate growth by coopting existing host vessels17 but the progressive growth and elaboration of new vessels accompanying tumor expansion requires neoangiogenesis. To ascertain whether excess DSCR1 suppresses initial vessel cooption or subsequent angiogenesis, Dscr1 transgenic and wild-type mice were inoculated with reduced numbers of Lewis lung carcinoma cells to generate slowly growing tumors. DSCR1 elevation inhibited the extended growth phase of transplanted tumors and not just their initial expansion (Supplemental Fig. 3C), supporting the notion that excess DSCR1 suppresses tumor angiogenesis.
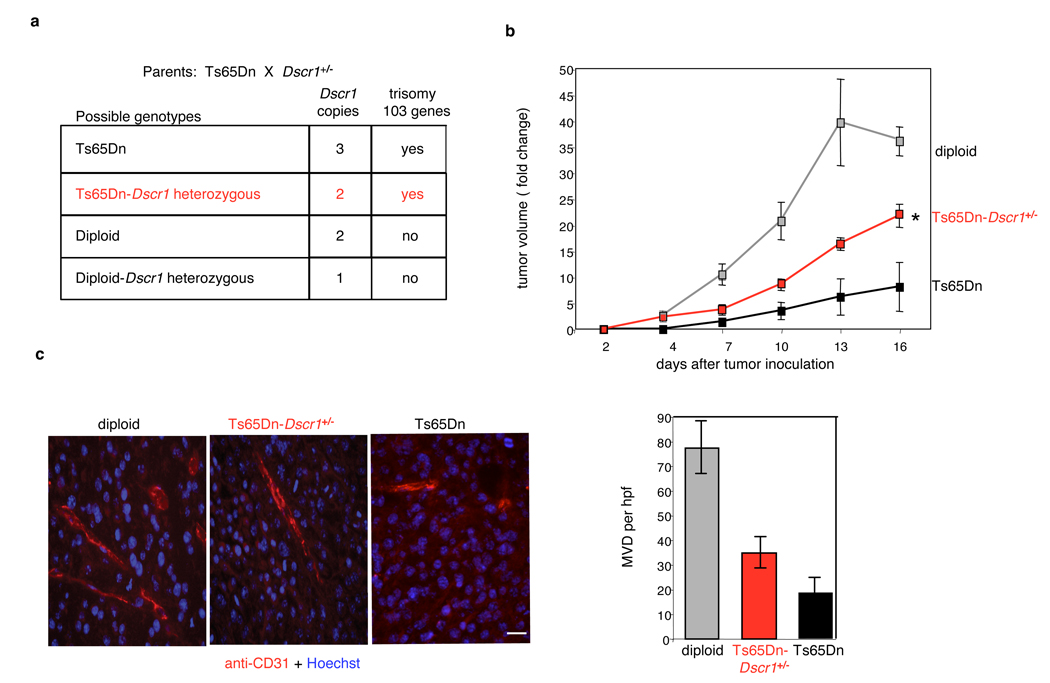
To confirm that tumor protection in the Ts65Dn mouse was specifically due to Dscr1 trisomy, we crossed the Ts65Dn mice to Dscr1+/− mice18 generating Ts65Dn/Dscr1+/− animals with 2 copies of Dscr1 but maintaining trisomy for the other 103 genes (Fig. 3A). After validating both segmental trisomy and Dscr1 status (Supplemental Fig. 4A and B), we compared flank tumor growth in diploid mice to that in Ts65Dn mice with 2 or 3 copies of Dscr1. Reduction to 2 Dscr1 copies in Ts65Dn/Dscr1+/− mice significantly abrogated the tumor protection observed in Ts65Dn parental mice (Fig. 3B). Loss of the protective effect against tumor growth was mirrored by a corresponding increase in microvessel density in tumors from Ts65Dn/ Dscr1+/− mice relative to their Ts65Dn littermates (Fig. 3C), confirming the pivotal role played by Dscr1 in tumor suppression in the Ts65Dn DS mouse model. Together, our data provide strong support for the notion that 1 extra copy of Dscr1 is necessary for maximal suppression of tumor growth via inhibition of tumor angiogenesis.
Figure 3. Trisomic expression of Dscr1 is necessary for significant suppression of tumor growth in the Ts65Dn Down syndrome mouse model.
(a) Possible genotypes arising from mating Ts65Dn and Dscr1+/− mice. (b) Suppression of B16F10 tumor growth in Ts65Dn Down syndrome mice is relieved upon loss of the third copy of Dscr1 (Ts65Dn-Dscr1+/−). Values are mean± sem, n=4–8 per group, *p<0.01. (c) Microvessel density (MVD) per high-powered field (hpf) is quantified by anti-CD31 immunofluorescence of tumors harvested from the indicated mice at comparable volumes (200–400 mm3). Bar, 20 µM. Values are mean ± sem.
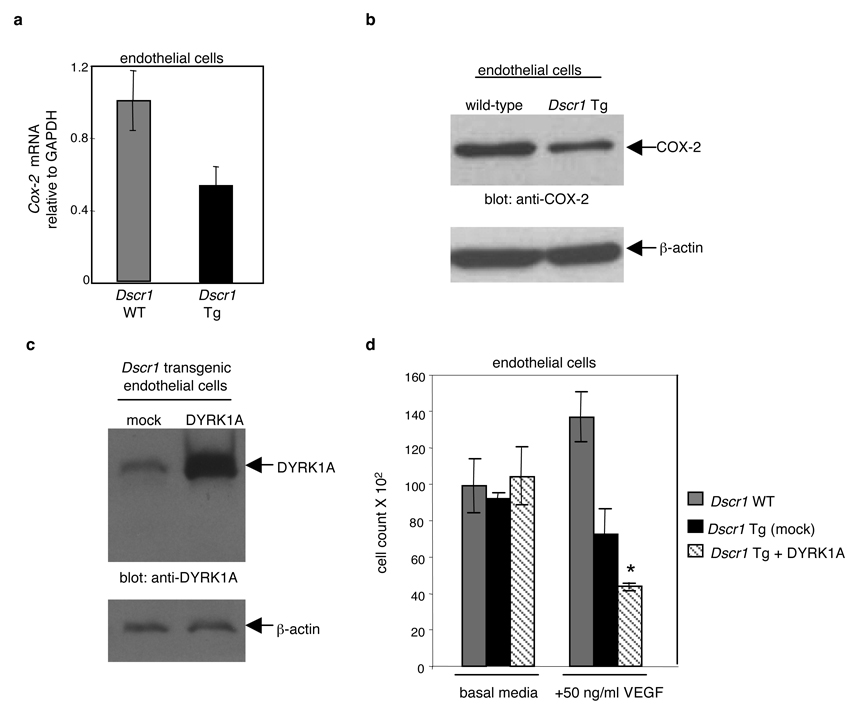
Increased dosage of Dscr1 might suppress angiogenesis by down-regulating expression of calcineurin-NFAT-dependent targets in endothelial cells. Cyclooxygenase 2 (COX-2) has been identified as a calcineurin-dependent gene and an important mediator of the angiogenic response to VEGF19. Quantitative PCR and Western blot analysis of COX-2 expression in Dscr1 transgenic endothelial cells demonstrated a substantial decrease in COX-2 levels relative to control littermates (Fig. 4A, B) suggesting that a modest increase in DSCR1 expression suppresses expression of COX-2 and likely other VEGF-responsive targets. While Dscr1 trisomy plays a significant role in preventing tumor angiogenesis in our DS mouse model, there are clearly other chromosome 21 genes that contribute to the tumor suppressive effects observed in the Ts65Dn mouse. Since increased dosage of Dscr1 attenuates VEGF-calcineurin-NFAT signalling, we examined the role of Dyrk1A, another chromosome 21 gene known to regulate NFAT signalling and contribute to other Down syndrome phenotypes20, 21. After confirmation of Dyrk1A expression in endothelial cells (Supplemental Fig. 4C), we examined the effects of Dyrk1A upregulation with Dscr1 trisomy after over-expression of Dyrk1A into Dscr1 transgenic endothelial cells (Fig 4C). VEGF-mediated endothelial proliferation was dramatically inhibited as a consequence of increased expression of both DYRK1A and DSCR1 (Fig. 4D) implying that upregulation of Dyrk1A may contribute to the remaining tumor suppression observed in the Ts65Dn-Dscr1+/− mouse with 2 copies of Dscr1. Collectively, our data demonstrate that the modest excess of DSCR1 afforded by a single extra copy, impedes VEGF-calcineurin signalling in endothelial cells. The consequent suppression of tumor angiogenesis and, ultimately, tumor growth provides a mechanistic basis for the remarkable protection from solid tumors observed in DS individuals. Of note, host Dscr1 copy number influences the growth and vascularization of allografted tumor cell lines confirming the cell intrinsic tumor suppressive effect of Dscr1 dosage by modulating angiogenesis within the host tumor microenvironment.
Figure 4. Calcineurin suppression by DSCR1 and DYRK1A attenuates endothelial cell activation.
(a, b) Cyclooxygenase-2 (COX-2) mRNA (a) and protein expression (b) was quantified by qPCR and Western blot analysis in endothelial cells isolated from Dscr1 wild-type (WT) and transgenic (Tg) mice. (c) Western blot analysis of endothelial cells isolated from Dscr transgenic mice and probed for DYRK1A after either mock infection (mock) or retroviral infection with Dyrk1A (DYRK1A). (d) Endothelial cells isolated from Dscr1 transgenic (Tg) mice with DYRK1A over-expression (+DYRK1A) demonstrate an even greater suppression of VEGF-mediated proliferation as compared to wild-type (WT) and DSCR1 over-expression alone (mock). Values are mean ± sem, *p<0.01.
Since human chromosome 21 harbors over 200 genes, it would be surprising if Dscr1 were the only chromosome 21 gene implicated in tumor suppression in DS individuals. Indeed our data suggests that increased dosage of Dyrk1A, appears to act in concert with Dscr1 to suppress tumor angiogenesis by further attenuating VEGF-calcineurin-NFAT signalling in endothelial cells. By disrupting the balance of NFAT phosphorylation, DYRK1A blocks transactivation of NFAT-dependent target genes20, 21. Other trisomy 21 genes may inhibit tumor development via cancer cell autonomous mechanisms. A recent study using the Ts65Dn mouse on the APCmin mice background demonstrated that 3 copies of the Ets2 proto-oncogene reduced the incidence of spontaneous intestinal tumors22. However, examination of a spontaneous tumor model prevents the distinction of Ets2 dosage effects on tumor initiation versus progression. In contrast, our work specifically investigates suppression of tumor progression as a consequence of Dscr1 trisomy. Additional studies are necessary to determine the magnitude and synergy of these genes in suppressing tumor growth in both the Ts65Dn mouse and the DS population.
Our studies implicate DSCR1-dependent inhibition of the VEGF-calcineurin-NFAT pathway in endothelial cells as a key component of the reduced cancer incidence in DS individuals. Hence, it is reasonable to speculate that cyclosporin A and FK506, immunosuppressive drugs that specifically inhibit calcineurin23, would also suppress tumor angiogenesis. Surprisingly, numerous clinical studies indicate that a significant increase in cancer incidence is a serious complication of transplant recipients receiving long-term immunosupressive therapy24. The mechanism behind this increased rate of cancer is not yet understood, however, such studies point to an important distinction between the calcineurin-inhibitory action of DSCR1 and those of cyclosporin A and FK506.
The cancer protection observed in DS individuals is remarkable given the heterogeneous mechanisms utilized amongst tumors. While DS individuals have less exposure to environmental and other factors that contribute to tumor incidence, the implication remains that one or more of the trisomic genes on chromosome 21 exert an anti-neoplastic effect, presumably by modulating some fundamental aspect of tumor initiation and/or progression. Microscopic avascular tumors represent the earliest stages of human neoplasias and are commonly observed in many organs upon autopsy25. This data suggests that progression into macroscopic tumors, not tumor initiation, may be rate limiting in human cancers. Such progression is critically dependent upon interactions between the incipient tumor and its microenvironment – most notably, activation of the “angiogenic switch”. The pivotal role played by DSCR1 in tumor angiogenesis makes it a compelling candidate for cancer protection in the DS population. Our data implicates VEGF-calcineurin-NFAT as a critical signalling axis in endothelial cells and that maximal suppression of calcineurin requires increased expression of both Dscr1 and Dyrk1A. Additional studies will be required to explore whether angiogenesis in general may also be impaired in the Ts65Dn mouse and in DS individuals. Finally, our studies in the Ts65Dn mouse and transgenic tri-allelic Dscr1 mouse demonstrate the in vivo relevance of a modest excess of Dscr1 arising from trisomy is sufficient to negatively regulate tumor growth by dampening VEGF-calcineurin signalling. By analogy, we conclude that elevated expression of Dscr1 in individuals with trisomy 21 is likely a significant contributor to the decreased cancer incidence in this population. It is perhaps, inspiring that the Down syndrome population provides us with novel insight into mechanisms that regulate cancer growth and, by so doing, identify potential targets for tumor prevention and therapy.
Methods Summary
Western blot analysis
Fourteen week-old human fetal kidney and liver tissue were isolated from control and DS tissue, lysed in RIPA buffer and quantified for protein concentration. Tissue lysates were separated by SDS-PAGE, probed with anti-DSCR1 mAb18 and detected via chemiluminescence. Blots were stripped and re-probed with beta actin. DSCR1 levels were quantified by densitometric analysis in the linear range and compared to beta actin. Ts65Dn mice, diploid littermates and Dscr1-null mice were sacrificed between 8–12 weeks of age, their brains dissected and probed as described above. Human microvascular endothelial cells were lysed and probed with anti-DYRK1A mAb.
Tumor models
Six-ten week old mice were inoculated with 1–5 × 105 Lewis lung carcinoma or B16F10 melanoma cells in HBSS into the subcutaneous flank region as previously described6. Human iPS cell-derived teratomas were generated as previously described15.
Immunohistochemistry
Mice were euthanized and tumors harvested and fixed in neutral-buffered formalin for paraffin embedding. Sections were deparaffinized and epitopes unmasked as previously described6. Sections were immunostained with rat anti-CD31 mAb or anti-Myc pAb overnight at room temperature followed by incubation with goat anti-rat Alexa 594 and goat anti-rabbit Alexa 488, stained with Hoechst and analyzed using Axio Vision 4.0 software (Carl Zeiss Vision).
Endothelial cell isolation, immunofluorescence and proliferation
Four-week old Dscr1 transgenic or littermate control mice were euthanized, lungs removed, and endothelial cells isolated as previously described6. For immunofluorescence endothelial cells were plated on gelatin coated coverslips, fixed with paraformaldehyde, blocked, permeabilized and incubated with anti-NF-ATc1 mAb followed by goat anti-mouse Alexa 594. For proliferation assays endothelial cells were plated in triplicate in tissue culture wells coated with 0.2% gelatin as previously described6 and treated with the indicated concentrations of VEGF.
Statistical analysis
Data are shown as mean± s.e.m or s.d. as denoted. P values were calculated using Student’s t-test.
Methods
Generation of Ts65Dn mice and Ts65Dn-Dscr1 heterozygous mice
Two-month old diploid and Ts65Dn mice age 2–4 months old were obtained by breeding Ts65Dn females with C57BL/6C3H F1 males or acquired from Jackson Laboratories (Bar Harbor, ME). Mice were karyotyped with chromosomal spreads from the blood26, and metaphase chromosomal spreads were prepared and karyotypes evaluated. All protocols were approved by the Children’s Hospital or USUHS Institutional Animal Care and Use Committee. Experiments were performed without prior knowledge of genotypes.
The Ts65Dn/Dscr1+/− mice that were diploid for the Dscr1 gene and trisomic for the rest of the Ts65Dn segment of Chr.16 was generated as follows: Males heterozygous for the Dscr1 gene on a C57Bl/6 background were bred with Ts65Dn females. Litters were genotyped by fluorescence in situ hybridization (FISH) and PCR screening using tail DNA as previously described6, 27. In brief, nuclei of blood leukocytes stained by DAPI show red spots that reveal presence of chromosomes 16 and Ts65Dn segment detected by FISH with a probe produced by labeling of BAC 480C6 (Research Genetics), containing a fragment of Ts65Dn segment) with biotin-dUTP or digoxigenin-dUTP (Boehringer Mannheim) in a nick-translation reaction.
Dscr1 transgenic mice
A 593 bp cDNA fragment encoding the inducible murine Dscr1 isoform (Dscr1.Ex4) was subcloned with a 5’ 6-myc epitope into a a hypoxanthine phosphoribosyltransferase (Hprt) targeting vector with the Dscr1.Ex4 native 2 Kb promoter28. Allowing generation of single-copy, site-specific recombination at the Hprt locus reducing the risk of non-specific phenotypes due to site of insertion29. This construct was targeted into the Hprt locus by electroporation into Hprt-deficient embryonic stem cells (BK4 cells). Two independent recombinant embryonic stem cell clones screened by PCR and Southern blot were injected into blastocysts with both lines demonstrating germline transmission. Dscr1 transgenic mice were backcrossed onto a C57Bl/6 background for 6 generations.
Western blot analysis
Fourteen week-old human fetal kidney and liver tissue were isolated from control and DS tissue obtained from therapeutic miscarriages according to an approved IRB protocol at UCLA. Tissues were lysed in RIPA buffer and protein quantified by the Bio Rad DC Protein Assay. Five µg of tissues were, separated by SDS-PAGE, probed with anti-DSCR1 (clone 14B4) mAb18 and detected via chemiluminescence (ECL, Amersham). Blots were stripped and re-probed with beta actin. Levels of DSCR1 were quantified by densitometric analysis in the linear range and compared to beta actin. Ts65Dn mice, diploid littermates and Dscr1-null mice were sacrificed between 8–12 weeks of age, their brains dissected and probed as described above.
Human microvascular endothelial cells (Cambrex) were cultured as previously described6, lysed, separated by SDS-PAGE and probed with anti-DYRK1A pAb (Abcam). Primary mouse endothelial cells from the indicated genotypes were lysed, separated by SDS-PAGE and probed with anti-Myc (9E10) mAb or anti-COX2 mAb (Alexis).
Immunohistochemistry and microvessel density
Mice were euthanized and tumors harvested and fixed in neutral-buffered formalin for paraffin embedding. Paraffin embedded sections were deparaffinized and epitopes unmasked as previously described6. Sections were immunostained with rat anti-CD31 mAb (1:50; Pharmingen) overnight at room temperature followed by incubation for 2 hours with goat anti-rat Alexa 594 conjugated secondary antibody (1:1000; Molecular Probes), and analyzed using Axio Vision 4.0 software. Microvessel density was quantified after CD31 immunostaining of 5 sections per tumor, 5 mice per cohort and quantified as previously described6.
Quantification of microvessel density in tumors by flow cytometry
Tumor-bearing mice were anesthetized with avertin and the vasculature perfused for 3 minutes at 120 mm Hg pressure with saline from a 18-gauge cannula inserted into the aorta via an incision in the left ventricle. Tumors were dissected, minced with scissors, and then digested in PBS supplemented with 2 mg/ml of collagenase/dispase (Roche) for 45 minutes at 37°C. Dissociated cells were resuspended with PBS containing 10% FBS and 106 cells were stained with anti-mouse CD31-FITC conjugated antibody plus anti-mouse CD45-PerCP conjugated antibody (BD Pharmingen) for 15 minutes on ice. Cells were washed in PBS, examined in a FACScan flow cytometer, and analyzed using CellQuest software (BD Biosciences).
FITC-lectin perfusion
Mice were anesthetized with isofluorane. FITC-labeled Lycopersicon esculentum lectin (100 µg in 100 µl of 0.9% NaCl; Vector Laboratories, Burlingame, CA) was injected into the femoral vein and allowed to circulate for 3 minutes before perfusion of fixative. The chest was opened and the vasculature perfused for 3 minutes at a pressure of 120 mm Hg with fixative (4% paraformaldehyde in PBS) from a 18-gauge cannula inserted into the aorta via an incision in the left ventricle. The right atrium was incised to create a route for the fixative to escape. After removal, tissues were processed for immunohistochemistry.
Immunofluorescence
10×105 endothelial cells/ml were plated onto gelatin-coated glass coverslips overnight. Cells were fixed with 4% paraformaldehyde for 10 min followed by blocking in 3% milk in TBS-T for 45 min. Anti-VEGF-R2 mAb (1:500; Cell Signaling) or anti-mouse NF-ATc1 (1:200) or isotype matched control antibodies was added for 1 hr. Cells were washed with PBS-T before the addition of rabbit anti-mouse-Alexa594 (1:500; Molecular Probes) for 30 min protected from light. Nuclei were stained with 1% Hoechst dye for 1 minute. Cells were washed with PBS-T, mounted and imaged on a fluorescence microscope.
Quantification of Dscr1 and Cox-2 mRNA in endothelial cells
Total RNA was isolated from 5×105 endothelial cells using the RNeasy kit (Qiagen). cDNA was generated from 5 ug total RNA using SuperScript III First –Strand Synthesis system (Invitrogen) and subjected to real-time PCR with PerfeCTa™ SYBR Green SuperMix (Quanta Biosciences) using the RNA Engine Opticon Monitor 2 System (MJ Research Inc). Dscr1 was amplified with forward primer: 5’-AGCTCCCTGATTGCTTGTGT-3’and reverse primer: 5’-AGGAACTCGGTCTTGTGCAG-3’. COX-2 was amplified with forward primer: 5’-CCCCCACAGTCAAAGACACT-3’and reverse primer: 5’-AGTTGCTCATCACCCCACTC-3’. GAPDH was amplified with forward primer: 5’-TGAAGGTCGGTGTGAACGGATTTGG-3’ and reverse primer: 5’-CATGTAGGCCATGAGGTCCACCAC-3’.
Retroviral expression of DYRK1A
Dyrk1A cDNA was obtained from Open Biosystems, excised with EcoR1 and cloned into pBABE. DYRK1A retrovirus was generated by transfecting GPG293 cells as previously described30. Virus was used to infect 2×105 human microvascular endothelial cells or primary mouse endothelial cells by serial incubations with DYRK1A virus.
Supplementary Material
Acknowledgements
We thank Gerard Evan, Carla Kim, Karen Cichowski and Joseph Italiano for critical discussions and advice. This work was supported by the Howard Hughes Medical Institute (G.Q.D.), Harvard Stem Cell Institute (G.Q.D.), the NIH Director’s Pioneer Award (G.Q.D.), NHLBI (W.C.A.), Jerome Lejeune Foundation (Z.G.), USUHS (Z.G.), the Smith Family Medical Foundation (S.R.), the Garrett B. Smith Foundation (S.R.) and the Platypus Sports Foundation (S.R.).
References
- 1.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumors in individuals with Down’s syndrome. Lancet. 2000;355:165. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 2.Satge D, et al. A lack of neuroblastoma in Down syndrome: a study from 11 European countries. Cancer Res. 1998;58:448. [PubMed] [Google Scholar]
- 3.Patja K, Pukkala E, Sund R, Iivanainen M, Kaski M. Cancer incidence of persons with Down syndrome in Finland: a population-based study. Int. J. Cancer. 2006;118:1769. doi: 10.1002/ijc.21518. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 5.Fuentes JJ, Pritchard MA, Estivill X. Genomic organization, alternative splicing, and expression patterns of the DSCR1 (Down syndrome candidate region 1) gene. Genomics. 1997;44:358. doi: 10.1006/geno.1997.4866. [DOI] [PubMed] [Google Scholar]
- 6.Ryeom S, et al. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420. doi: 10.1016/j.ccr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Hesser BA, et al. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood. 2004;104:149. doi: 10.1182/blood-2004-01-0273. [DOI] [PubMed] [Google Scholar]
- 8.Iizuka M, Abe M, Shiiba K, Sasaki I, Sato Y. Down syndrome candidate region 1, a downstream target of VEGF, participates in endothelial cell migration and angiogenesis. J. Vasc. Res. 2004;41:334. doi: 10.1159/000079832. [DOI] [PubMed] [Google Scholar]
- 9.Minami T, et al. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J. Biol. Chem. 2004;279:50537. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- 10.Yao YG, Duh EJ. VEGF selectively induces Down syndrome critical region 1 gene expression in endothelial cells: a mechanism for feedback regulation of angiogenesis? Biochem. Biophys. Res. Comm. 2004;321:648. doi: 10.1016/j.bbrc.2004.06.176. [DOI] [PubMed] [Google Scholar]
- 11.Fulcher T, et al. Diabetic retinopathy in Down's syndrome. Brit. J. Opthalmol. 1998;82:407. doi: 10.1136/bjo.82.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdoch JC, Rodger JC, Rao SS, Fletcher CD, Dunnigan MG. Down's syndrome: an atheroma-free model? Br Med J. 1977;2:226. doi: 10.1136/bmj.2.6081.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson LE, Richtsmeier JT, Leszl J, Reeves RH. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science. 2004;306:687. doi: 10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves RH, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nature Genet. 1995;11:177. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 15.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melero-Martin JM, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 18.Ryeom S, Greenwald RJ, Sharpe AH, McKeon F. The threshold pattern of calcineurin-dependent gene expression is altered by loss of the endogenous inhibitor calcipressin. Nature Immunol. 2003;4:874. doi: 10.1038/ni966. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez GL, et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J. Exp. Med. 2001;193:607. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arron JR, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 21.Gwack Y, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 22.Sussan TE, Yang A, Li F, Ostrowski MC, Reeves RH. Trisomy represses Apc(Min)-mediated tumours in mouse models of Down's syndrome. Nature. 2008;451:73. doi: 10.1038/nature06446. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 24.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 25.Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237. doi: 10.1056/NEJM199304293281706. [DOI] [PubMed] [Google Scholar]
- 26.Harashima C, et al. Elevated expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in cerebellar unipolar brush cells of a Down syndrome mouse model. Cell Mol Neurobiol. 2006;267:19. doi: 10.1007/s10571-006-9066-4. [DOI] [PubMed] [Google Scholar]
- 27.Shi YP, Huang TT, Carlson EJ, Epstein CJ. The mapping of transgenes by fluorescence in situ hybridization on G-banded mouse chromosomes. Mammalian Genome. 1994;5:337. doi: 10.1007/BF00356551. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, et al. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 29.Bronson SK, et al. Single-copy transgenic mice with chosen-site integration. Proc. Natl. Acad. Sci. USA. 1996;93:9067. doi: 10.1073/pnas.93.17.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuriato S, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc. Natl. Acad. Sci. USA. 2006;103:16266. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.